Impact of High-Pressure Processing on Prevention of Quality Loss and Spoilage Bacteria Diversity in Precooked Baby Clam (Paphia undulata) During Refrigerated Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Microbial Media
2.2. Treatment of Precooked Baby Clam Edible Portion with and Without HPP
2.3. Physical Analyses
2.4. Chemical Analyses
2.4.1. pH Value
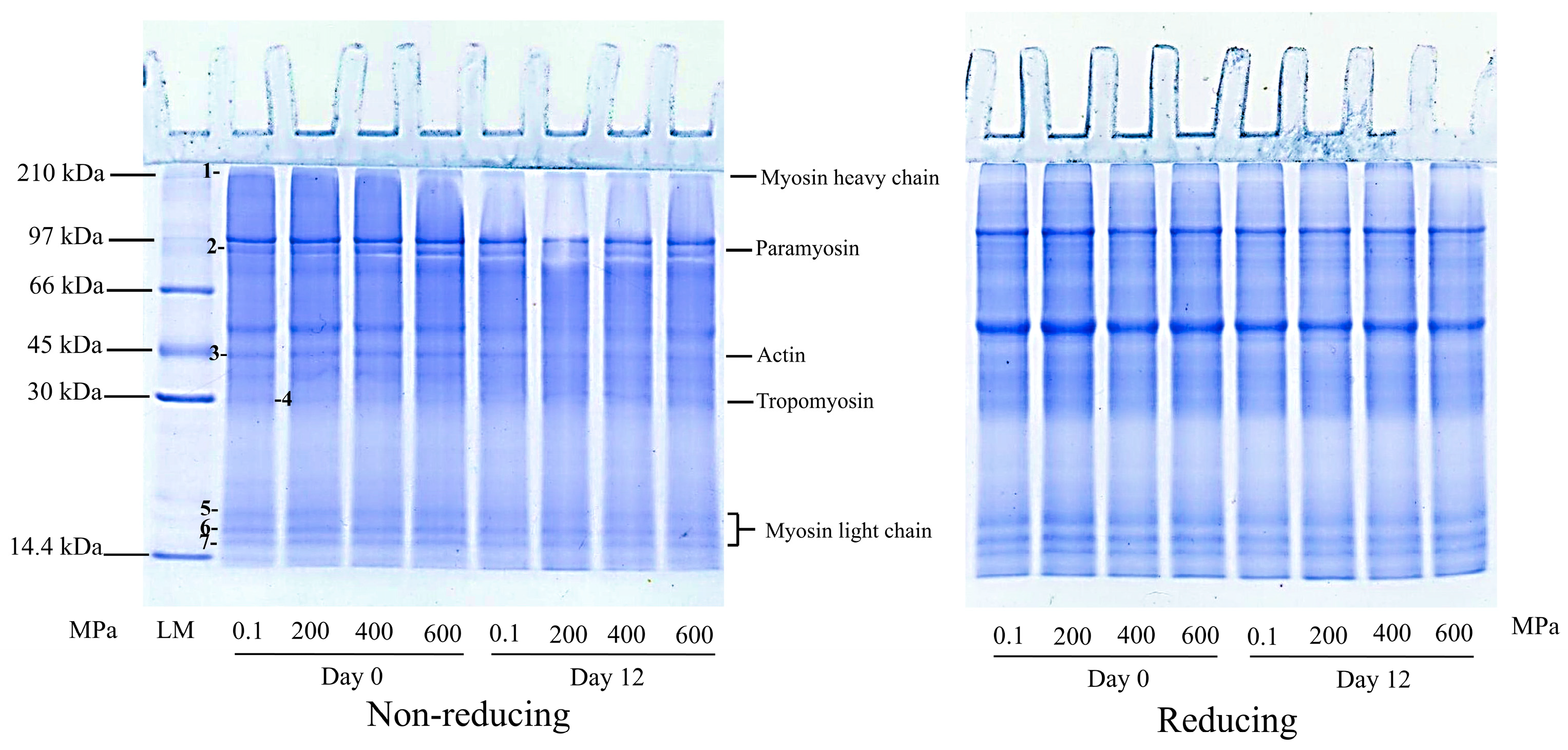
2.4.2. Protein Patterns
2.4.3. Total Volatile Basic-Nitrogen (TVB-N) and Trimethylamine-Nitrogen (TMA-N) Contents
2.4.4. Volatile Compounds
2.5. Microbiological Analysis
2.6. Next-Generation Sequencing
2.7. Sensory Evaluation
2.8. Experimental Design and Statistical Analyses
3. Results and Discussion
3.1. Appearance of Precooked Baby Clam Edible Portion Subjected to HPP at Various Pressure Levels
3.2. Protein Patterns of Precooked Baby Clam Edible Portion Subjected to HPP at Various Pressure Levels
3.3. Chemical Quality of Precooked Baby Clam Edible Portion Subjected to HPP at Various Pressure Levels
3.4. Microbiological Quality
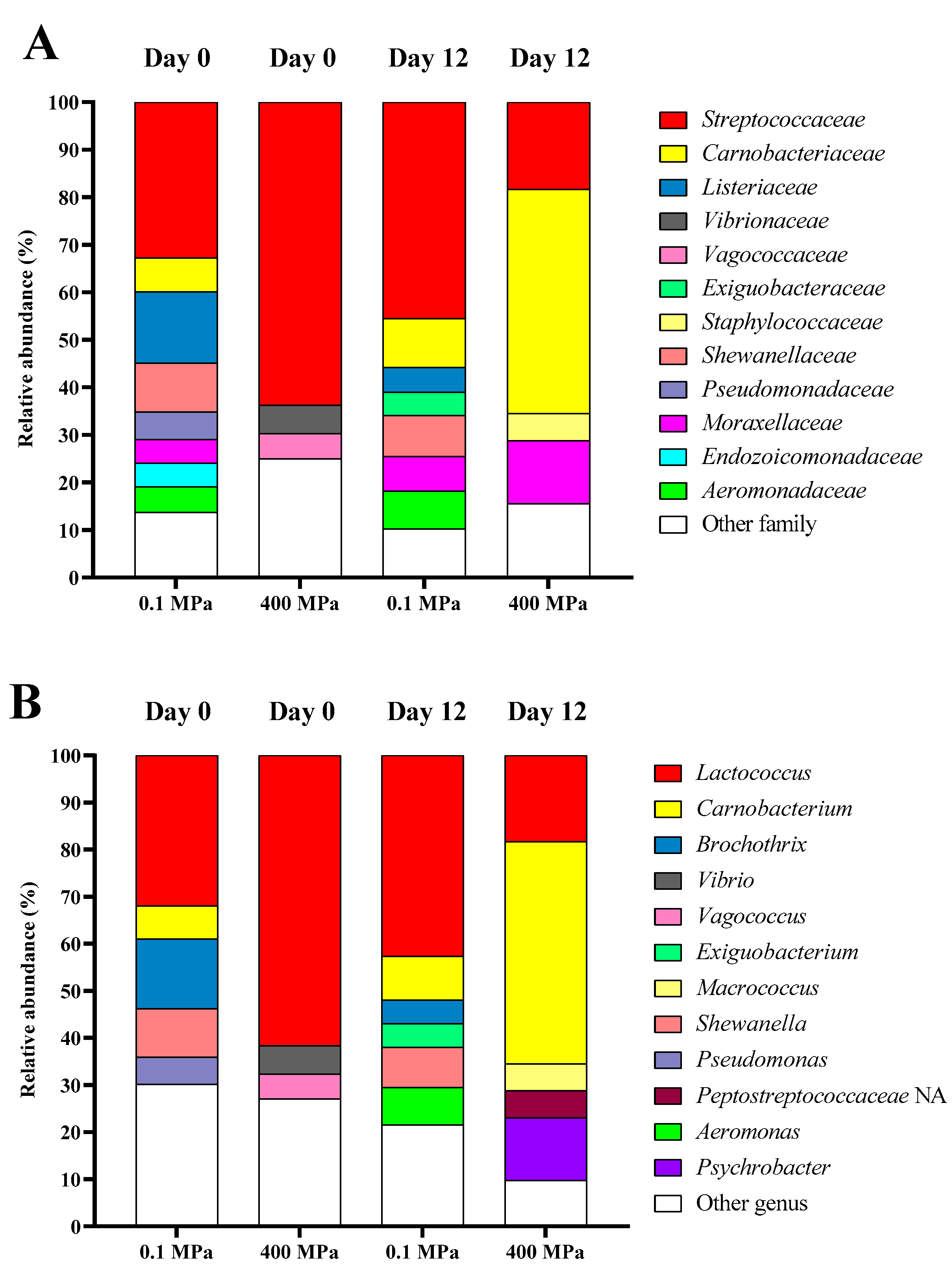
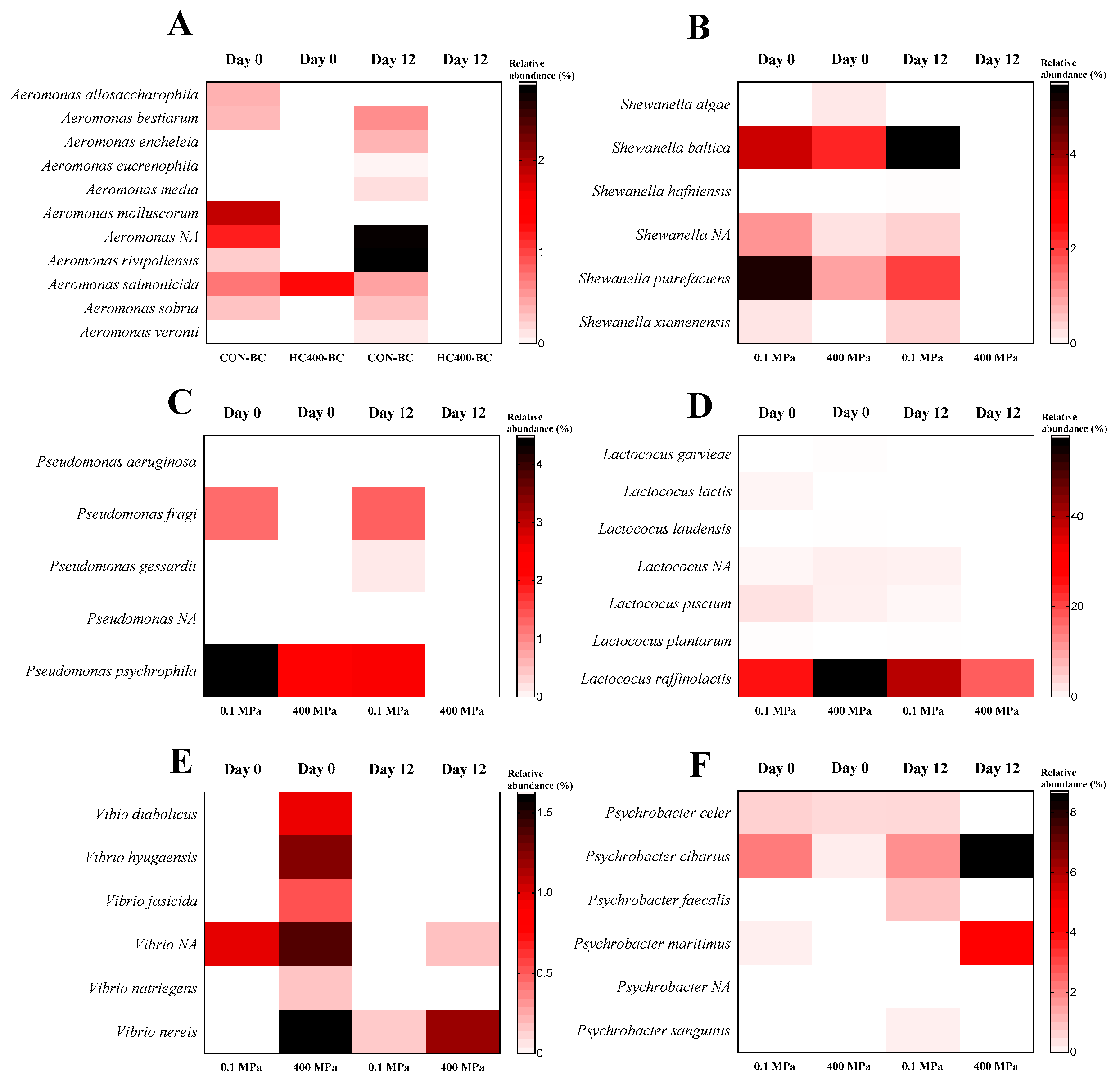
3.5. Bacterial Diversity of Precooked Baby Clam Edible Portion Subjected to HPP Under Selected Conditions
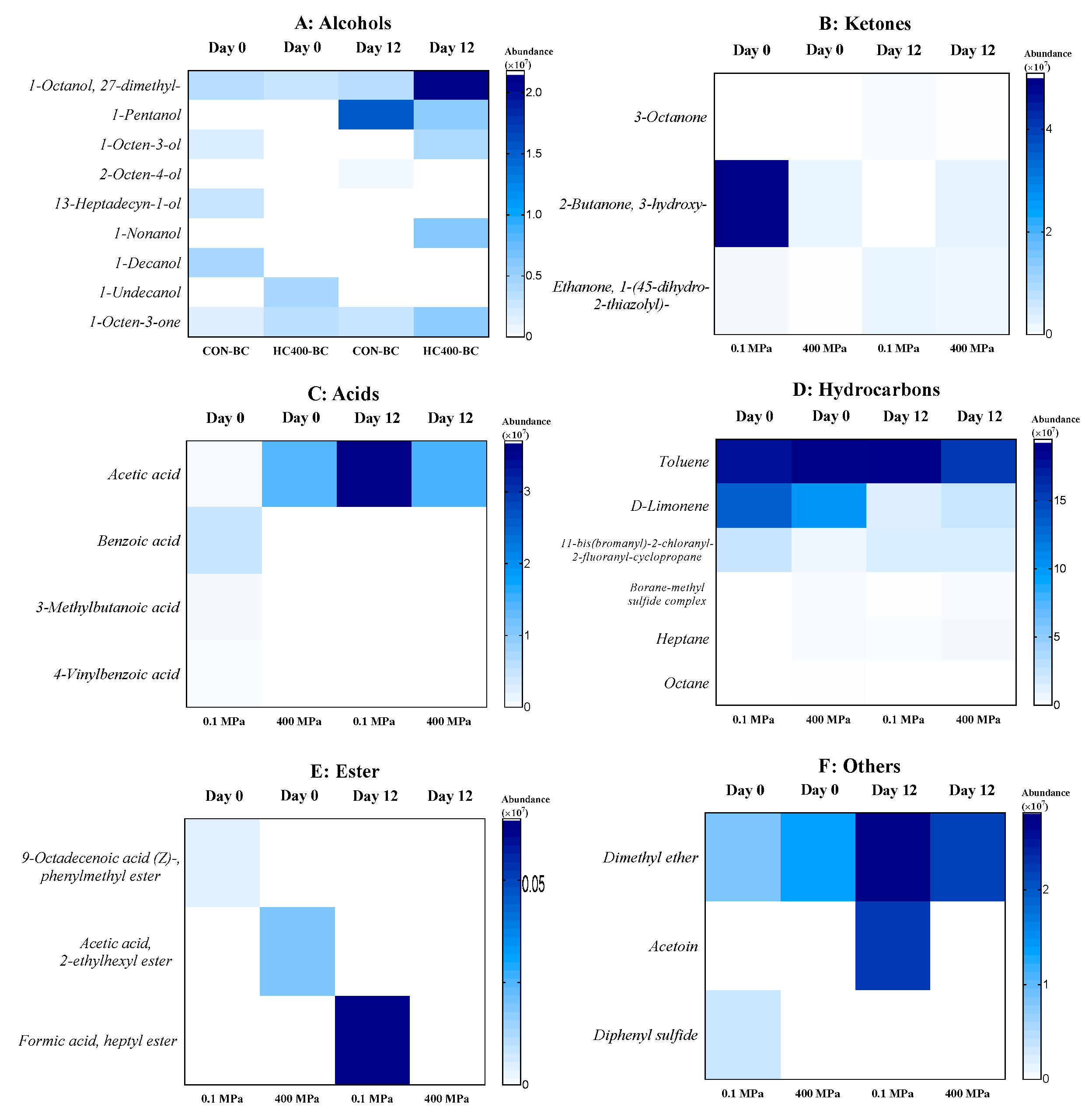
3.6. Volatile Organic Compounds of Precooked Baby Clam Edible Portion Subjected to HPP Under Selected Conditions
3.7. Acceptability of Precooked Baby Clam Edible Portion Subjected to HPP Under Selected Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Zhao, T.; Zhou, L.; Han, G.; Shen, Y.; Ke, C. Thermal Tolerance Traits of the Undulated Surf Clam Paphia undulata Based on Heart Rate and Physiological Energetics. Aquaculture 2019, 498, 343–350. [Google Scholar] [CrossRef]
- Department of Fisheries. Fisheries Statistics of Thailand: Fisheries Development and Planning Division; Thailand department of fisheries, ministry of agriculture and cooperatives: Bangkok, Thailand, 2023. [Google Scholar]
- Zhang, P.; Yongo, E.; Feng, H.; Pan, S.; Sun, A.; Zhou, L.; Guo, Z.; Ke, C. Effects of Substrate, Temperature, Salinity, Size and Transportation on Burrowing Capacity of Juvenile Undulated Surf Clam Paphia undulata. Aquac. Res. 2022, 53, 2796–2805. [Google Scholar] [CrossRef]
- Ashie, J.P.; Smith, B.K.S.; Haard, N.F. Spoilage and Shelf-life Extension of Fresh Fish and Shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, L.; Ke, Z.; Chen, H.; Zheng, Y.; Yang, P.; Xiang, X.; Zhou, X.; Jin, Y.; Deng, S.; et al. A Comparative Study on the Taste Quality of Mytilus coruscus under Different Shucking Treatments. Food Chem. 2023, 412, 135480. [Google Scholar] [CrossRef] [PubMed]
- Lohalaksanadech, S.; Lohalaksnadech, D.; Sujarit, C. Effect of Lactic Acid and Hot Water Treatments on Quality of Shucked Hard Clams (Meretrix casta) during Refrigerated Storage. J. Sustain. Sci. Manag. 2021, 16, 30–37. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Chen, H.; Cai, B.; Wan, P.; Zhu, X.; Sun, H.; Sun, H.; Pan, J. Identification of Odor Volatile Compounds and Deodorization of Paphia undulata Enzymatic Hydrolysate. J. Ocean Univ. China 2016, 15, 1101–1110. [Google Scholar] [CrossRef]
- Françoise, L. Occurrence and Role of Lactic Acid Bacteria in Seafood Products. Food Microbiol. 2010, 27, 698–709. [Google Scholar] [CrossRef]
- Lin, C.-S.; Lee, Y.-C.; Kung, H.-F.; Cheng, Q.-L.; Ou, T.-Y.; Chang, S.K.C.; Tsai, Y.-H. Inactivation of Microbial Loads and Retardation of Quality Loss in Asian Hard Clam (Meretrix lusoria) Using High-Hydrostatic-Pressure Processing during Refrigerated Storage. Food Control 2022, 133, 108583. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.P.; Kelly, A.L. Changes in the Microbiological and Physicochemical Quality of High-Pressure-Treated Oysters (Crassostrea gigas) during Chilled Storage. Food Control 2008, 19, 1139–1147. [Google Scholar] [CrossRef]
- Palamae, S.; Patil, U.; Suyapoh, W.; Sornying, P.; Buatong, J.; Zhang, B.; Benjakul, S. Elucidation of High-Pressure Processing toward Microbial Inhibition, Physicochemical Properties, Collagen Fiber and Muscle Structure of Blood Clam Edible Portion. Food Chem. 2024, 455, 139840. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Cui, Y.; Lin, X.-D.; Yu, J.-F.; Liao, X.-J.; Ling, J.-G.; Shang, H.-T. Impact of High Hydrostatic Pressure on the Shelling Efficacy, Physicochemical Properties, and Microstructure of Fresh Razor Clam (Sinonovacula constricta). J. Food Sci. 2018, 83, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Srivastav, P.P.; Pathak, S.S. Kinetics of Total Volatile Basic Nitrogen and Trimethylamine Formation in Stored Rohu (Labeo rohita) Fish. J. Aquat. Food Prod. Technol. 2019, 28, 452–464. [Google Scholar] [CrossRef]
- Palamae, S.; Patil, U.; Saetang, J.; Detcharoen, M.; Suyapoh, W.; Ma, L.; Zhang, B.; Benjakul, S. Asian Green Mussel Treated with Sous Vide and Chitooligosaccharide-Catechin Conjugate: Chemical, Physical, Microbiological, Histological Properties and Quality Changes during Refrigerated Storage. Food Control 2025, 173, 111206. [Google Scholar] [CrossRef]
- Patil, U.; Palamae, S.; Nazeer, R.A.; Zhang, B.; Benjakul, S. Combined Hurdle Effects of Pulsed Electric Field and Ultraviolet-C Irradiation on Microbial Load Reduction and Composition of Hemeproteins from Asian Seabass Gills. Food Control 2024, 164, 110591. [Google Scholar] [CrossRef]
- Morten, C.M.; Thomas Carr, B.; Thomas Carr, B. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; De Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, R.; Gui, M.; Jiang, Z.; Li, P. Identification of the Specific Spoilage Organism in Farmed Sturgeon (Acipenser Baerii) Fillets and Its Associated Quality and Flavour Change during Ice Storage. Foods 2021, 10, 2021. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Hu, W. Changes in Microbiological, Physicochemical and Muscle Proteins of Post Mortem Large Yellow Croaker (Pseudosciaena crocea). Food Control 2013, 34, 514–520. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total Volatile Basic Nitrogen and Trimethylamine in Muscle Foods: Potential Formation Pathways and Effects on Human Health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3620–3666. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Investigation of Quality Attributes of Ice-Stored Pacific White Shrimp (Litopenaeus vannamei) as Affected by Sequential Minimal Ozone Treatment. LWT-Food Sci. Technol. 2014, 57, 538–547. [Google Scholar] [CrossRef]
- Vongsawasdi, P.; Nopharatana, M.; Khueankhancharoen, J.; Changyoug, C. Effect of Modified Atmosphere Packaging on Qualities and Shelf Life of Precooked Baby Clam (Paphia undulata). Kasetsart J. (Nat. Sci.) 2011, 45, 530–538. [Google Scholar]
- Bongiorno, T.; Tulli, F.; Comi, G.; Sensidoni, A.; Andyanto, D.; Iacumin, L. Sous Vide Cook-Chill Mussel (Mytilus galloprovincialis): Evaluation of Chemical, Microbiological and Sensory Quality during Chilled Storage (3 °C). LWT-Food Sci. Technol. 2018, 91, 117–124. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Trimethylamine N-Oxide in Aquatic Foods. J. Agric. Food Chem. 2024, 72, 14498–14520. [Google Scholar] [CrossRef]
- Bono, G.; Badalucco, C. Combining Ozone and Modified Atmosphere Packaging (MAP) to Maximize Shelf-Life and Quality of Striped Red Mullet (Mullus surmuletus). LWT-Food Sci. Technol. 2012, 47, 500–504. [Google Scholar] [CrossRef]
- He, H.; Adams, R.M.; Farkas, D.F.; Morrissey, M.T. Use of High-Pressure Processing for Oyster Shucking and Shelf-Life Extension. J. Food Sci. 2002, 67, 640–645. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Hwang, J.-S.; Chi, H.-Y.; Lai, K.-M. Effect of Different High Pressure Treatments on Shucking, Biochemical, Physical and Sensory Characteristics of Oysters to Elaborate a Traditional Taiwanese Oyster Omelette. J. Sci. Food Agric. 2010, 90, 530–535. [Google Scholar] [CrossRef]
- Smelt, J.P.P.M.; Rijke, A.G.F.; Hayhurst, A. Possible Mechanism of High Pressure Inactivation of Microorganisms. Int. J. High Press. Res. 1994, 12, 199–203. [Google Scholar] [CrossRef]
- Nikparvar, B.; Subires, A.; Capellas, M.; Hernandez-Herrero, M.; Crauwels, P.; Riedel, C.U.; Bar, N. A Diffusion Model to Quantify Membrane Repair Process in Listeria monocytogenes Exposed to High Pressure Processing Based on Fluorescence Microscopy Data. Front. Microbiol. 2021, 12, 598739. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Tsai, Y.-H.; Chen, S.-L.; Kung, H.-F.; Arakawa, O.; Wei, C.-I. Inactivation and Damage of Histamine-Forming Bacteria by Treatment with High Hydrostatic Pressure. Foods 2020, 9, 266. [Google Scholar] [CrossRef]
- Palamae, S.; Temdee, W.; Buatong, J.; Suyapoh, W.; Sornying, P.; Tsai, Y.-H.; Benjakul, S. Use of High Pressure Processing in Combination with Acidic Electrolyzed Water Depuration for the Shelf-Life Extension of Blood Clam (Tegillarca granosa). Food Control 2024, 156, 110160. [Google Scholar] [CrossRef]
- Khan, M.A.; Parrish, C.C.; Shahidi, F. Enumeration of Total Heterotrophic and Psychrotrophic Bacteria Using Different Types of Agar to Evaluate the Microbial Quality of Blue Mussels (Mytilus edulis) and Sea Scallops (Placopecten magellanicus). Food Res. Int. 2005, 38, 751–758. [Google Scholar] [CrossRef]
- Gilstrap, O.; Liu, C.; Nindo, C.; Parveen, S. Pilot Scale Assessment of High-Pressure Processing (HPP) to Enhance Microbiological Quality and Shelf Life of Fresh Ready-to-Eat (RTE) Blue Crab Meat. Microorganisms 2023, 11, 2909. [Google Scholar] [CrossRef] [PubMed]
- Palamae, S.; Mittal, A.; Zhang, B.; Benjakul, S. Chitooligosaccharide-Catechin Conjugate and High-Pressure Processing: Microbial Control and Quality Preservation of Baby Clam during Refrigerated Storage. Food Biosci. 2024, 62, 105493. [Google Scholar] [CrossRef]
- DeWitt, C.A.M.; Oliveira, A.C.M. Modified Atmosphere Systems and Shelf Life Extension of Fish and Fishery Products. Foods 2016, 5, 48. [Google Scholar] [CrossRef]
- Parlapani, F.F. Microbial Diversity of Seafood. Curr. Opin. Food Sci. 2021, 37, 45–51. [Google Scholar] [CrossRef]
- Sameli, N.; Sioziou, E.; Bosnea, L.; Kakouri, A.; Samelis, J. Assessment of the Spoilage Microbiota during Refrigerated (4 °C) Vacuum-Packed Storage of Fresh Greek Anthotyros Whey Cheese without or with a Crude Enterocin A-B-P-Containing Extract. Foods 2021, 10, 2946. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.-J.E.; Boziaris, I.S. Microbiological Spoilage and Volatiles Production of Gutted European Sea Bass Stored under Air and Commercial Modified Atmosphere Package at 2 °C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Ayseli, M.T. A Systematic Review on Nano-Delivery Systems Enriched with Aromatic Compounds: Flavor, Odor, and Chemical Quality Perspectives in Fish. Food Chem. Adv. 2024, 5, 100750. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhu, C.; Zhang, D.; Liu, H. Effects of High-Pressure Processing on Aquatic Products with an Emphasis on Sensory Evaluation. Int. J. Food Sci. Technol. 2022, 57, 6980–6996. [Google Scholar] [CrossRef]


| Parameters | Samples | Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 |
|---|---|---|---|---|---|---|---|
| TVB-N content (mg N/100 g) | 0.1 MPa | 1.11 ± 0.14 aF | 2.13 ± 0.15 aE | 3.30 ± 0.09 aD | 5.46 ± 0.08 aC | 10.95 ± 0.12 aB | 13.06 ± 0.32 aA |
| 200 MPa | 0.91 ± 0.09 abF | 1.26 ± 0.04 aE | 2.37 ± 0.10 aD | 4.22 ± 0.08 aC | 5.34 ± 0.07 aB | 7.56 ± 0.05 bA | |
| 400 MPa | 0.82 ± 0.01 bE | 0.94 ± 0.05 bE | 1.87 ± 0.04 bD | 2.79 ± 0.01 bC | 3.36 ± 0.05 bB | 4.88 ± 0.07 cA | |
| 600 MPa | 0.79 ± 0.00 bE | 0.92 ± 0.01 bE | 1.85 ± 0.03 bD | 2.77 ± 0.04 bC | 3.35 ± 0.01 bB | 4.20 ± 0.09 dA | |
| TMA-N content (mg N/100 g) | 0.1 MPa | 0.33 ± 0.12 aF | 0.78 ± 0.01 aE | 1.13 ± 0.11 aD | 2.45 ± 0.07 aC | 5.46 ± 0.11 aB | 7.48 ± 0.21 aA |
| 200 MPa | 0.35 ± 0.06 aE | 0.57 ± 0.00 bDE | 0.75 ± 0.09 bD | 1.04 ± 0.08 bC | 3.49 ± 0.12 bB | 4.05 ± 0.13 bA | |
| 400 MPa | 0.27 ± 0.03 aE | 0.43 ± 0.09 bDE | 0.64 ± 0.04 bD | 1.04 ± 0.00 bC | 1.74 ± 0.12 cB | 2.26 ± 0.10 cA | |
| 600 MPa | 0.25 ± 0.05 aE | 0.40 ± 0.10 bDE | 0.62 ± 0.08 bD | 0.95 ± 0.05 bC | 1.64 ± 0.10 cB | 2.03 ± 0.09 dA | |
| pH | 0.1 MPa | 6.75 ± 0.01 aA | 6.62 ± 0.02 bB | 6.50 ± 0.03 cC | 6.20 ± 0.04 bD | 5.75 ± 0.03 cE | 5.39 ± 0.02 dF |
| 200 MPa | 6.76 ± 0.04 aA | 6.70 ± 0.01 aB | 6.60 ± 0.02 bC | 6.25 ± 0.02 bD | 5.85 ± 0.01 bE | 5.56 ± 0.03 cF | |
| 400 MPa | 6.77 ± 0.02 aA | 6.75 ± 0.03 aAB | 6.70 ± 0.02 aB | 6.63 ± 0.02 aB | 6.56 ± 0.02 aB | 6.50 ± 0.01 bB | |
| 600 MPa | 6.76 ± 0.03 aA | 6.74 ± 0.01 aAB | 6.70 ± 0.01 aB | 6.60 ± 0.01 aC | 6.61 ± 0.04 aC | 6.57 ± 0.01 aC |
| Attributes | Day 0 | Day 12 | |
|---|---|---|---|
| 0.1 MPa | 400 MPa | 400 MPa | |
| Appearance | 7.45 ± 0.96 a | 7.43 ± 0.98 a | 7.11 ± 1.00 a |
| Color | 7.21 ± 0.96 a | 7.15 ± 0.94 a | 7.00 ± 0.93 a |
| Smell | 7.17 ± 0.85 a | 7.22 ± 0.91 a | 7.10 ± 0.87 a |
| Texture | 6.95 ± 0.73 a | 7.38 ± 0.64 a | 7.00 ± 0.85 a |
| Taste | 6.98 ± 0.84 a | 7.13 ± 0.82 a | 6.90 ± 0.85 a |
| Overall | 7.21 ± 0.91 a | 7.25 ± 0.82 a | 7.02 ± 0.89 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palamae, S.; Patil, U.; Sinlapapanya, P.; Hong, H.; Zhao, Y.; Zhang, B.; Benjakul, S. Impact of High-Pressure Processing on Prevention of Quality Loss and Spoilage Bacteria Diversity in Precooked Baby Clam (Paphia undulata) During Refrigerated Storage. Foods 2025, 14, 1421. https://doi.org/10.3390/foods14081421
Palamae S, Patil U, Sinlapapanya P, Hong H, Zhao Y, Zhang B, Benjakul S. Impact of High-Pressure Processing on Prevention of Quality Loss and Spoilage Bacteria Diversity in Precooked Baby Clam (Paphia undulata) During Refrigerated Storage. Foods. 2025; 14(8):1421. https://doi.org/10.3390/foods14081421
Chicago/Turabian StylePalamae, Suriya, Umesh Patil, Pitima Sinlapapanya, Hui Hong, Yadong Zhao, Bin Zhang, and Soottawat Benjakul. 2025. "Impact of High-Pressure Processing on Prevention of Quality Loss and Spoilage Bacteria Diversity in Precooked Baby Clam (Paphia undulata) During Refrigerated Storage" Foods 14, no. 8: 1421. https://doi.org/10.3390/foods14081421
APA StylePalamae, S., Patil, U., Sinlapapanya, P., Hong, H., Zhao, Y., Zhang, B., & Benjakul, S. (2025). Impact of High-Pressure Processing on Prevention of Quality Loss and Spoilage Bacteria Diversity in Precooked Baby Clam (Paphia undulata) During Refrigerated Storage. Foods, 14(8), 1421. https://doi.org/10.3390/foods14081421










