Improvement of Physiological Metabolism and Flavor Quality of Eriocheir sinensis Ovaries by Dietary Supplementation with Antarctic Krill Meal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Diet Formulation and Preparation
2.3. Experimental Design and Feeding Trials
2.4. Ovarian Tissue Collection
2.5. GC-IMS Analysis of Volatile Composition
2.6. Nontargeted and Widely Targeted Metabolomics Analysis
2.6.1. Metabolome Extraction
Extraction of Hydrophilic Metabolites
Extraction of Hydrophobic Metabolites
2.6.2. Chromatography and Mass Spectrometry Acquisition Conditions
Analysis Conditions for Hydrophilic Substances
Analysis Conditions for Hydrophobic Substances
2.6.3. Identification of Differential Metabolites
2.7. Statistical Analysis
3. Results and Discussion
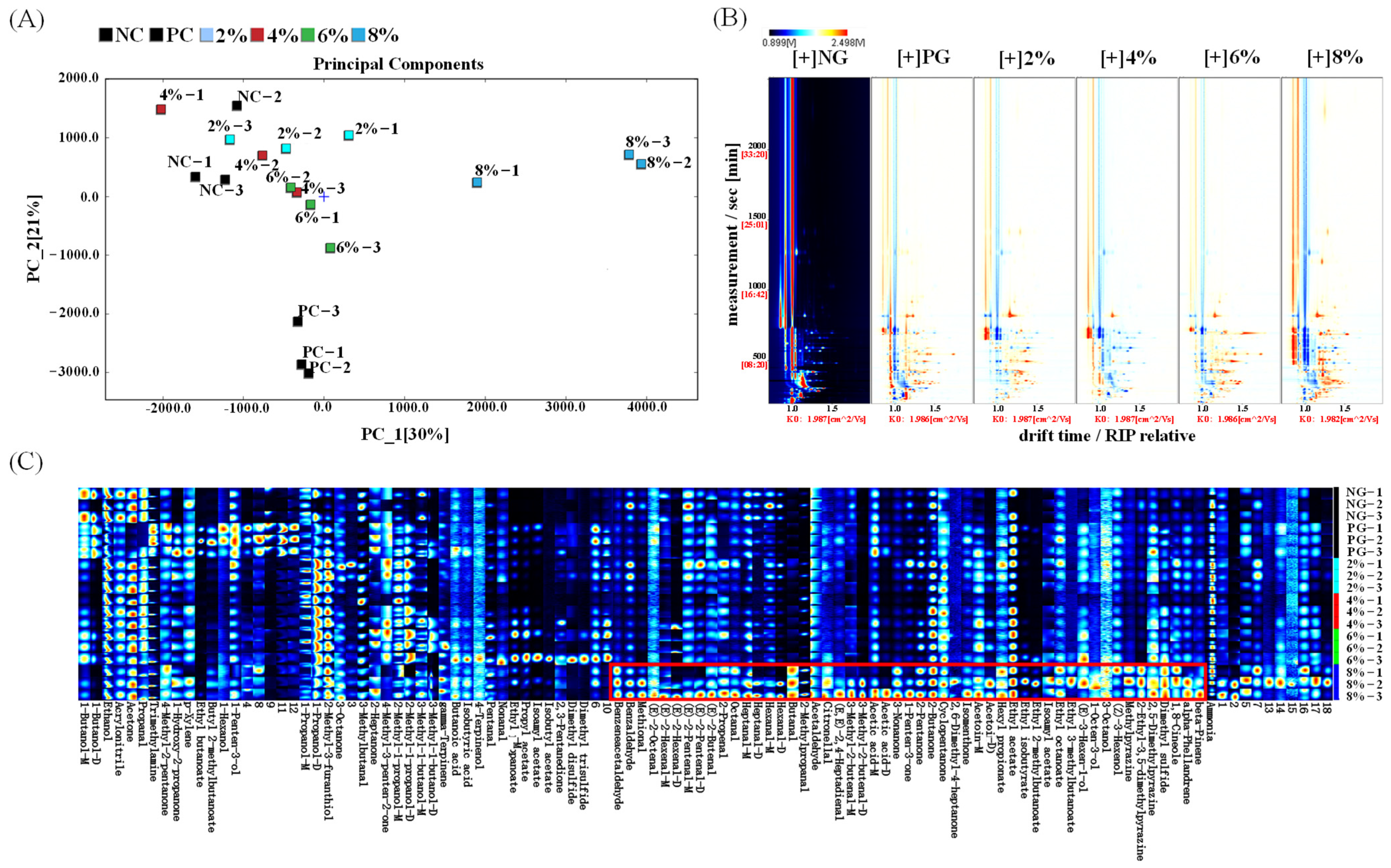
3.1. Analysis of Ovarian Volatile Odor Components
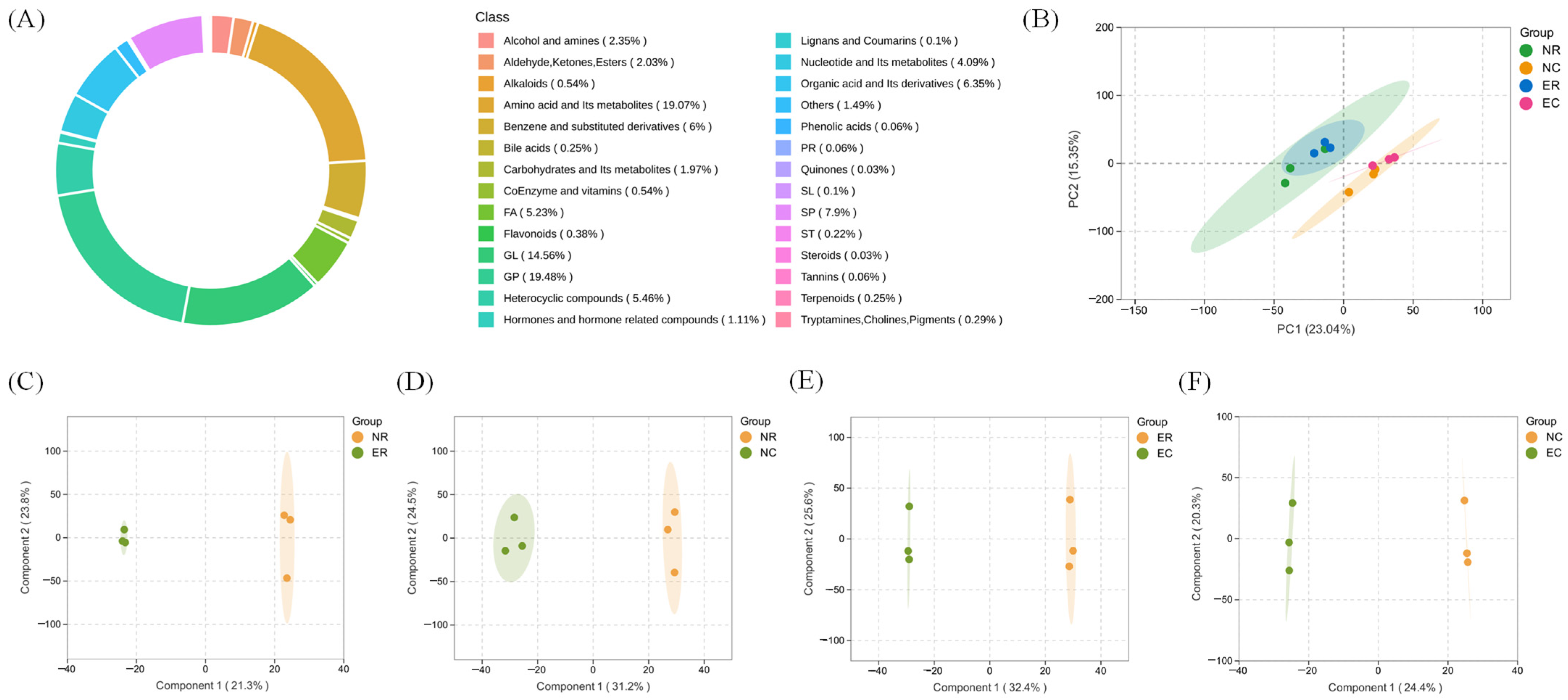
3.2. Multivariate Statistical Analysis of Metabolites
3.3. Metabonomic Analysis Based on Group 1 (ER vs. NR)
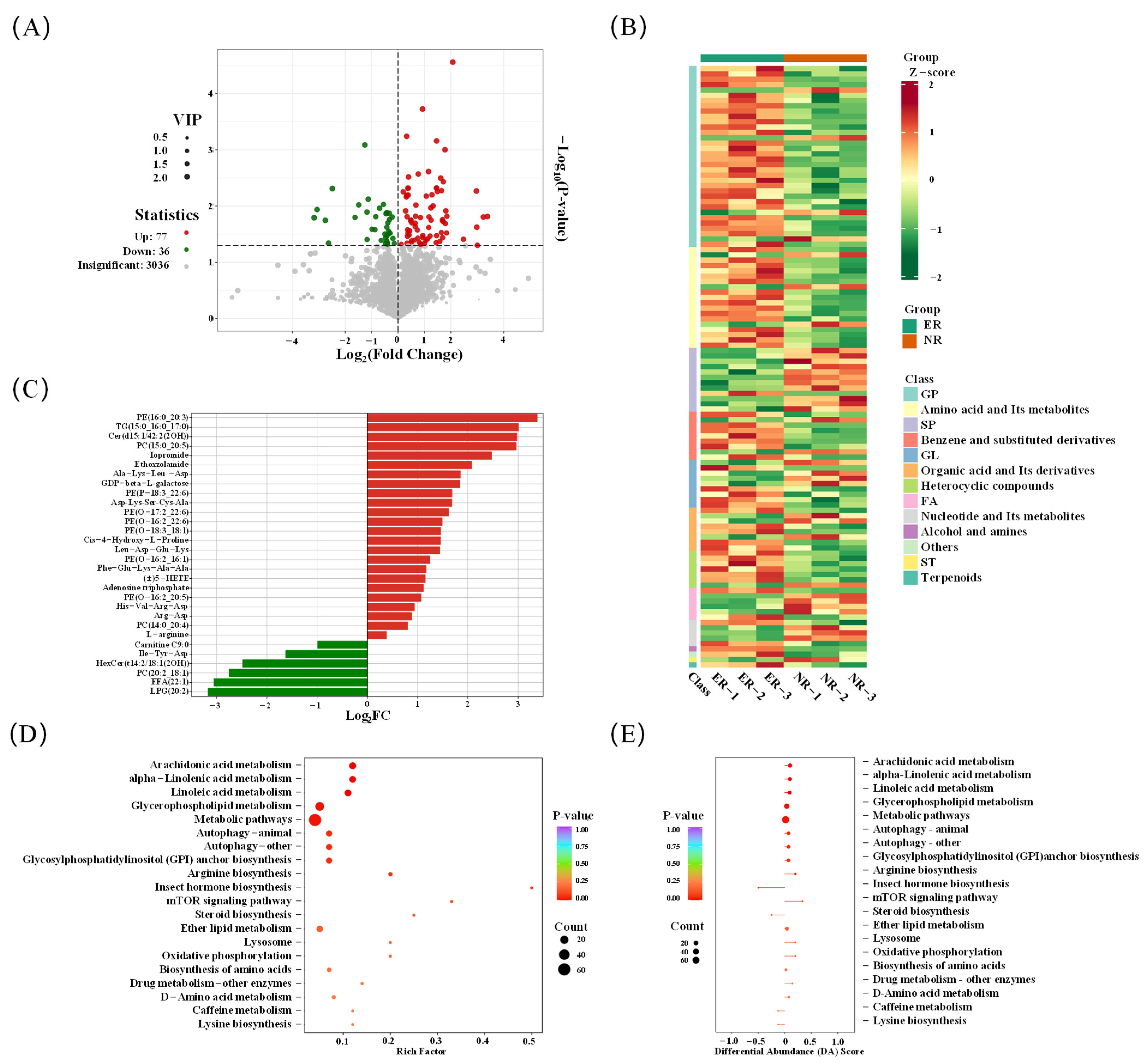
3.3.1. Screening of Differential Metabolites
3.3.2. Analysis of Lipid in Group 1
3.3.3. Analysis of Amino Acids and Nucleotides
3.3.4. Enrichment Analysis of KEGG Metabolic Pathway
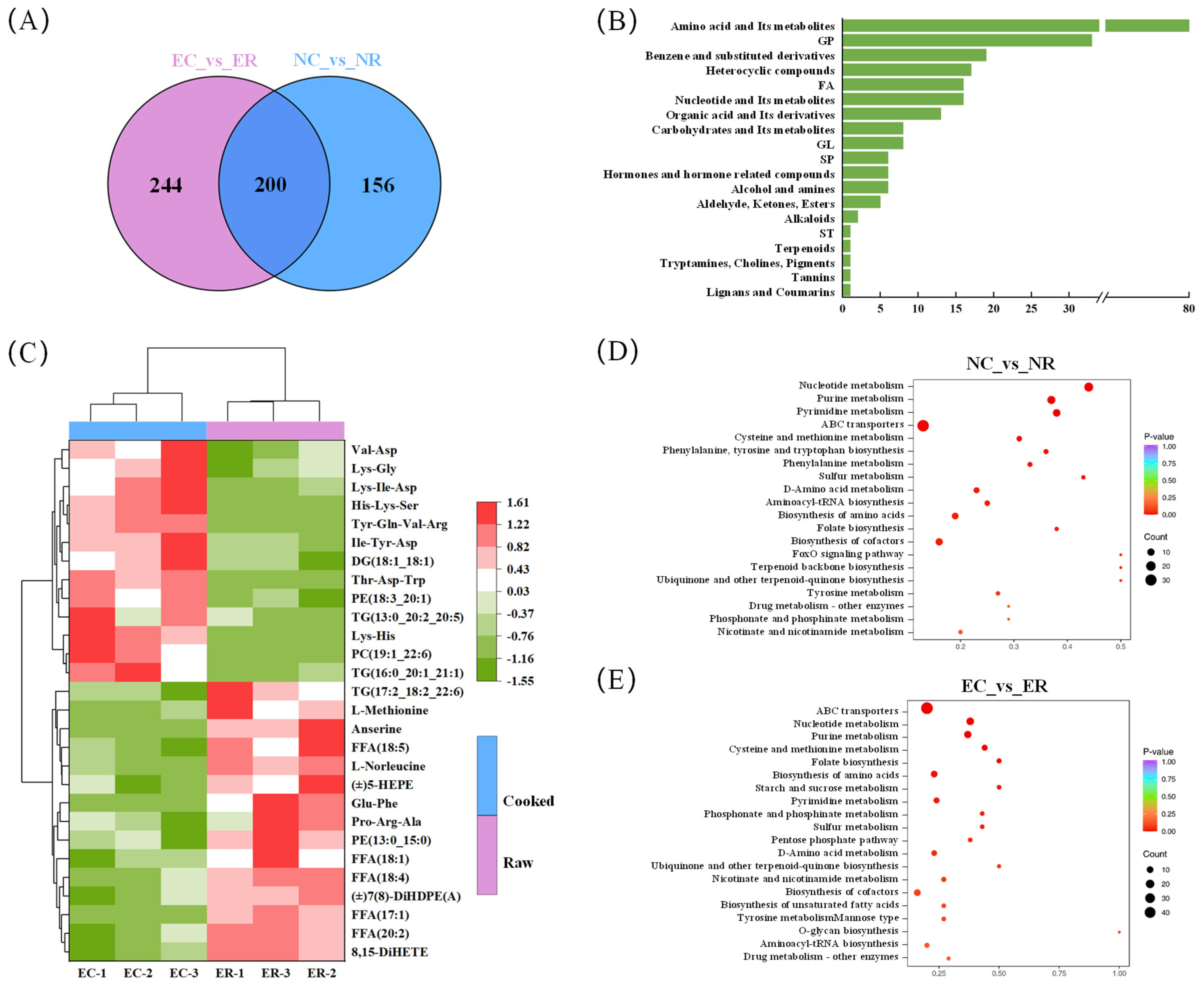
3.4. Analysis of Thermal Reaction Pathways Based on Group 2 (NC vs. NR) and Group 3 (EC vs. ER)
3.4.1. Analysis of Lipid
3.4.2. Analysis of Amino Acid and Its Metabolites
3.5. Analysis of Taste Related Metabolites Based on Group 4 (EC vs. NC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhang, R.; Jiang, X.; Wu, X.; Wang, X. Dietary supplementation with synthetic astaxanthin and DHA interactively regulates physiological metabolism to improve the color and odor quality of ovaries in adult female Eriocheir sinensis. Food Chem. 2024, 430, 137020. [Google Scholar] [PubMed]
- Pan, J.; Wu, X.G.; Zhao, H.L.; He, J.; Jiang, X.D.; Wang, Y.P.; Cheng, Y.X. Effects of three feeding modes on the culture performance of adult pond-reared Chinese mitten crab (Eriocheir sinensis) during the second year culture. Freshw. Fish. 2016, 46, 87–93. [Google Scholar]
- Tang, S.; Wang, J.J.; Li, Y.; Malakar, P.K.; Zhao, Y. Recent advances in the use of antarctic krill (Euphausia superba) as a sustainable source of high-quality protein: A comprehensive review. Trends Food Sci. Technol. 2024, 152, 104684. [Google Scholar]
- Kaur, K.; Kortner, T.M.; Benitez-Santana, T.; Burri, L. Effects of Antarctic krill products on feed intake, growth performance, fillet quality, and health in salmonids. Aquac. Nutr. 2022, 2022, 3170854. [Google Scholar]
- Sun, P.; Zhang, X.; Ren, X.; Cao, Z.; Zhao, Y.; Man, H.; Li, D. Effect of basic amino acid pretreatment on the quality of canned Antarctic krill. Food Bioprocess Technol. 2022, 16, 1690–1702. [Google Scholar]
- Wei, Y.; Chen, H.; Jia, M.; Zhou, H.; Zhang, Y.; Xu, W.; Zhang, W.; Mai, K. Effects of dietary Antarctic krill Euphausia superba meal on growth performance and muscle quality of triploid rainbow trout Oncorhynchus mykiss farmed in sea water. Aquaculture 2019, 509, 72–84. [Google Scholar]
- Tharaka, K.; Benitez-Santana, T.; Gunathilaka, B.E.; Kim, M.; Lee, C.; Shin, J.; Lee, K. Evaluation of Antarctic krill (Euphausia superba) meal supplementation in diets for olive flounder (Paralichthys olivaceus). Aquac. Res. 2020, 51, 2291–2302. [Google Scholar]
- Torrecillas, S.; Montero, D.; Carvalho, M.; Benitez-Santana, T.; Izquierdo, M. Replacement of fish meal by Antarctic krill meal in diets for European sea bass Dicentrarchus labrax: Growth performance, feed utilization and liver lipid metabolism. Aquaculture 2021, 545, 737166. [Google Scholar]
- Wang, X.Y. Effects of Antarctic Krill Meal on Growth Performance, Protein Metabolism, and Antioxidant Capacity in Juvenile 649 Chinese Mitten Crab (Eriocheir sinensis). Master’s Thesis, East China Normal University, Shanghai, China, 2020. [Google Scholar]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138. [Google Scholar]
- Ahn, H.-S.; Yeom, J.; Yu, J.; Kwon, Y.-I.; Kim, J.-H.; Kim, K. Convergence of plasma metabolomics and proteomics analysis to discover signatures of high-grade serous ovarian cancer. Cancers 2020, 12, 3447. [Google Scholar] [CrossRef]
- Feng, L.; Wang, S.; Chen, H.; Sun, J.; Zhang, N.; Zhang, H. Fatty acid composition and key aroma components of two different cold pressed sesame oils. J. Food Compos. Anal. 2025, 139, 107143. [Google Scholar]
- Song, J.; Wang, H.; Wu, X.; Wang, X.; Shi, W. The flavor of gonad and meat of female Portunus Trituberculatus cultured in indoor and outdoor. J. Food Biochem. 2019, 43, e12743. [Google Scholar]
- Iglesias, J.; Gallardo, J.M.; Medina, I. Determination of carbonyl compounds in fish species samples with solid-phase microextraction with on-fibre derivatization. Food Chem. 2010, 123, 771–778. [Google Scholar]
- Jeleń, H.; Gracka, A. Characterization of aroma compounds: Structure, physico-chemical and sensory properties. In Flavour: From Food to Perception; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 126–153. [Google Scholar]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar]
- Reineccius, G.; Peterson, D. Principles of food flavor analysis. In Instrumental Assessment of Food Sensory Quality; Woodhead Publishing: Sawston, UK, 2013; pp. 53–102. [Google Scholar]
- Nogueira, M.S.; Scolaro, B.; Milne, G.L.; Castro, I.A. Oxidation products from omega-3 and omega-6 fatty acids during a simulated shelf life of edible oils. LWT-Food Sci. Technol. 2019, 101, 113–122. [Google Scholar]
- Song, S.; Zhang, X.; Hayat, K.; Liu, P.; Jia, C.; Xia, S.; Xiao, Z.; Tian, H.; Niu, Y. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 2011, 124, 203–209. [Google Scholar] [CrossRef]
- Ambasankar, K.; Dayal, J.S.; Vasagam KP, K.; Sivaramakrishnan, T.; Sandeep, K.P.; Panigrahi, A.; Raja, R.A.; Burri, L.; Vijayan, K.K. Growth, fatty acid composition, immune-related gene expression, histology and haematology indices of Penaeus vannamei fed graded levels of Antarctic krill meal at two different fishmeal concentrations. Aquaculture 2022, 553, 738069. [Google Scholar]
- Jin, S.; Yue, D.; Fu, H.; Jiang, S.; Xiong, Y.; Qiao, H.; Wu, Y. Effects of dietary supplementation with 17β-estradiol and 17α-methyltestosterone on growth performance and gonadal development of the juvenile oriental river prawn (Macrobrachium nipponense). Aquac. Rep. 2022, 23, 101042. [Google Scholar]
- Song, D.; Shi, B.; Ding, L.; Jin, M.; Sun, P.; Jiao, L.; Zhou, Q. Regulation of dietary phospholipids on growth performance, antioxidant activities, phospholipid metabolism and vitellogenesis in prereproductive phase of female swimming crabs, Portunus trituberculatus. Aquaculture 2019, 511, 734230. [Google Scholar]
- Pizzini, A.; Lunger, L.; Demetz, E.; Hilbe, R.; Weiss, G.; Ebenbichler, C.; Tancevski, I. The role of omega-3 fatty acids in reverse cholesterol transport: A review. Nutrients 2017, 9, 1099. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar]
- Fuke, S.; Konosu, S. Taste-active components in some foods: A review of Japanese research. Physiol. Behav. 1991, 49, 863–868. [Google Scholar]
- Qi, C.; Wang, X.; Han, F.; Jia, Y.; Lin, Z.; Wang, C.; Lu, J.; Yang, L.; Wang, X.; Li, E.; et al. Arginine supplementation improves growth, antioxidant capacity, immunity and disease resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish. Immunol. 2019, 93, 463–473. [Google Scholar]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chem. 2020, 320, 126656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, M.; Zheng, Y.; Xu, C.-H.; Tao, N.-P.; Wu, X.; Wang, X. Brackish water improves the taste quality in meat of adult male Eriocheir sinensis during the postharvest temporary rearing. Food Chem. 2021, 343, 128409. [Google Scholar] [PubMed]
- Zhang, L.; Yin, M.; Zheng, Y.; Tao, N.-P.; Wu, X.; Wang, X. Short-term rearing in brackish water regulates the taste-related metabolites of abdomen muscle for adult male Eriocheir sinensis. LWT-Food Sci. Technol. 2021, 142, 110898. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, C.; Rao, W.; Chen, P.; Lei, K.; Mai, K.; Zhang, W. Dietary phospholipids improve growth performance and change the lipid composition and volatile flavor compound profiles in the muscle of abalone Haliotis discus hannai by affecting the glycerophospholipid metabolism. Aquac. Rep. 2023, 30, 101567. [Google Scholar]
- Liu, Q.; Lin, J.; Zhao, W.; Lei, M.; Yang, J.; Bai, W. The dynamic changes of flavors and UPLC-Q-Exactive-Orbitrap-MS based lipidomics in mackerel (Scomberomorus niphonius) during dry-cured processing. Food Res. Int. 2023, 163, 112273. [Google Scholar]
- Ecker, J.; Liebisch, G. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid 564 and sphingolipid species. Prog. Lipid Res. 2014, 54, 14–31. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid 562 oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Wang, S.; Adhikari, K.; Hung, Y. Acceptability and preference drivers of freshly roasted peanuts. J. Food Sci. 2017, 82, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological implications of lipid oxidation products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar]
- Shibata, A.; Uemura, M.; Hosokawa, M.; Miyashita, K. Formation of acrolein in the autoxidation of triacylglycerols with different fatty acid compositions. J. Am. Oil Chem. Soc. 2015, 92, 1661–1670. [Google Scholar]
- Rivas-Cañedo, A.; Martínez-Onandi, N.; Gaya, P.; Nuñez, M.; Picon, A. Effect of high-pressure processing and chemical composition on lipid oxidation, aminopeptidase activity and free amino acids of Serrano dry-cured ham. Meat Sci. 2021, 172, 108349. [Google Scholar]
- Zhang, S.-S.; Guo, S.; Zheng, Z.-J.; Liu, S.-J.; Hou, Y.-F.; Ho, C.-T.; Bai, N.-S. Characterization of volatiles in Allium tenuissimum L. flower by headspace-gas chromatography-olfactometry-mass spectrometry, odor activity values, and the omission and recombination experiments. LWT-Food Sci. Technol. 2021, 151, 112144. [Google Scholar]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Liu, W.; Zhao, L. Novel umami ingredients: Umami peptides and their taste. J. Food Sci. 2017, 82, 16–23. [Google Scholar]
- Kajiya, K.; Arino, M.; Koshio, A.; Minami, Y. Composition and taste of beef, pork, and duck meat and bioregulatory functions of imidazole dipeptides in meat. Sci. Rep. 2023, 13, 2125. [Google Scholar]
- Yang, M.; Pan, T.; Li, T.; Duan, G.; Jiang, H.; Ling, J. The effects of dietary L-theanine on the growth performance, non-specific immunity, antioxidant status, and intestinal microflora of female Chinese mitten crabs (Eriocheir sinensis). Aquac. Rep. 2024, 34, 101923. [Google Scholar]
- Zhang, L.; Tao, N.-P.; Wu, X.; Wang, X. Metabolomics of the hepatopancreas in Chinese mitten crabs (Eriocheir sinensis). Food Res. Int. 2022, 152, 110914. [Google Scholar]
- Zhang, R.; Zhang, L.; Wu, X.; Wang, X. Metabolomics analysis reveals bitter taste formation in off-season crab hepatopancreas marketed in June of the lunar calendar. J. Agric. Food Chem. 2024, 72, 27575–27586. [Google Scholar]
- Gigl, M.; Frank, O.; Barz, J.; Gabler, A.; Hegmanns, C.; Hofmann, T. Identification and quantitation of reaction products from quinic acid, quinic acid lactone, and chlorogenic acid with Strecker aldehydes in roasted coffee. J. Agric. Food Chem. 2021, 69, 1027–1038. [Google Scholar] [PubMed]

| Ingredients | NG | PG | 2% | 4% | 6% | 8% |
|---|---|---|---|---|---|---|
| Peeled soybean meal (46%) | 12.00 | 6.00 | 10.50 | 9.00 | 7.50 | 6.00 |
| Soy protein concentrate | 4.00 | 0.00 | 3.00 | 2.00 | 1.00 | 0.00 |
| Fermented soybean meal | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 |
| Fish meal | 12.00 | 20.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Rapeseed meal (36%) | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Peanut meal | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Chicken meal | 8.00 | 7.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Antarctic krill meal | 0.00 | 0.00 | 2.00 | 4.00 | 6.00 | 8.00 |
| Flour | 17.20 | 20.60 | 18.20 | 19.20 | 20.20 | 21.20 |
| Gluten meal | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Bentonite | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Penaeus polymorpha | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Vitamin premix | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Calcium dihydrogen phosphate | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Choline chloride (60%) | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Vitamin C (35%) | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin E (50%) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Phospholipid oil | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Refined fish oil | 4.60 | 4.60 | 4.60 | 4.60 | 4.60 | 4.60 |
| Soybean oil | 4.60 | 4.20 | 4.10 | 3.60 | 3.10 | 2.60 |
| Total (%) | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Theoretical protein level (%) | 38.53 | 38.52 | 38.54 | 38.55 | 38.56 | 38.57 |
| Theoretical total fat level (%) | 14.00 | 14.02 | 13.99 | 13.99 | 13.98 | 13.97 |
| Theoretical energy value (MJ/kg) | 19.11 | 19.06 | 19.10 | 19.09 | 19.08 | 19.06 |
| Astaxanthin (mg/kg) | 0.00 | 0.00 | 2.30 | 4.60 | 6.90 | 9.20 |
| Lysine | 2.14 | 2.19 | 2.14 | 2.15 | 2.15 | 2.15 |
| Methionine | 0.67 | 0.75 | 0.69 | 0.71 | 0.73 | 0.74 |
| Class | Compounds | Formula | Taste Attribute | FC | Type |
|---|---|---|---|---|---|
| Free amino acids | Histidine | C6H9N3O2 | Bitter | 2.131 | Up |
| L-theanine | C7H14N2O3 | Sweet/Unami | 1.294 | Up | |
| D-phenylalanine | C9H11NO2 | Bitter | 1.060 | Up | |
| 2-phenylglycine | C8H9NO2 | Bitter | 8.541 | Up | |
| L-leucine | C6H13NO2 | Bitter | 0.652 | Down | |
| L-norleucine | C6H13NO2 | Bitter | 0.762 | Down | |
| Nucleotide and its metabolites | 5′-deoxy-5′-fluoroadenosine | C10H12FN5O3 | - | 1.527 | Up |
| Stavudine | C10H12N2O4 | - | 1.498 | Up | |
| Arainosine | C10H12N4O5 | Bitter | 1.433 | Up | |
| Dihydrozeatin riboside | C15H23N5O5 | - | 1.151 | Up | |
| 2′-O-methylguanosine | C11H15N5O5 | Unami | 0.514 | Down | |
| 2-methylguanosine | C11H15N5O5 | Unami | 0.630 | Down | |
| 1-methylguanosine | C11H15N5O5 | Unami | 0.630 | Down | |
| Nicotinamide riboside chloride | C11H15CN2O5 | - | 0.696 | Down | |
| Organic acid and its derivatives | Streptamine 4-phosphate | C6H15N2O7P | - | 2.645 | Up |
| 3-methyl-2-oxovaleric acid | C6H10O3 | Bitter | 2.097 | Up | |
| 3-Furoic acid | C5H4O3 | Sour | 1.666 | Up | |
| Ethylsalicylate | C9H10O3 | Sweet/Bitter | 1.239 | Up | |
| Piperonylic acid | C8H6O4 | Bitter | 1.224 | Up | |
| 2′-Cytidylic acid | C9H14N3O8P | Bitter | 0.468 | Down | |
| 2-hydroxyethanesulfonate | C2H6O4S | Sour | 0.540 | Down | |
| 2-O-(alpha-D-glucosyl)-sn-glycerol 3-phosphate | C9H19O11P | - | 0.595 | Down | |
| N-acetyl-alpha-D-glucosamine 1-phosphate | C8H16NO9P | - | 0.681 | Down | |
| Sterol ester | Glycocholic acid | C26H43NO6 | Bitter | 1.834 | Up |
| Free fatty acids | α-linolenic acid | C14H28O2 | Bitter | 1.442 | Up |
| Myristic acid | C14H28O2 | Bitter | 1.381 | Up | |
| Oxylipins | 10-hydroxystearic acid | C18H36O3 | Bitter | 1.445 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhang, R.; Qiu, Z.; Shi, Y.; Zhu, S.; Wu, X.; Wang, X.; Zhang, L. Improvement of Physiological Metabolism and Flavor Quality of Eriocheir sinensis Ovaries by Dietary Supplementation with Antarctic Krill Meal. Foods 2025, 14, 1287. https://doi.org/10.3390/foods14081287
Zhou S, Zhang R, Qiu Z, Shi Y, Zhu S, Wu X, Wang X, Zhang L. Improvement of Physiological Metabolism and Flavor Quality of Eriocheir sinensis Ovaries by Dietary Supplementation with Antarctic Krill Meal. Foods. 2025; 14(8):1287. https://doi.org/10.3390/foods14081287
Chicago/Turabian StyleZhou, Siqi, Renyue Zhang, Zehui Qiu, Yuyao Shi, Shaicheng Zhu, Xugan Wu, Xichang Wang, and Long Zhang. 2025. "Improvement of Physiological Metabolism and Flavor Quality of Eriocheir sinensis Ovaries by Dietary Supplementation with Antarctic Krill Meal" Foods 14, no. 8: 1287. https://doi.org/10.3390/foods14081287
APA StyleZhou, S., Zhang, R., Qiu, Z., Shi, Y., Zhu, S., Wu, X., Wang, X., & Zhang, L. (2025). Improvement of Physiological Metabolism and Flavor Quality of Eriocheir sinensis Ovaries by Dietary Supplementation with Antarctic Krill Meal. Foods, 14(8), 1287. https://doi.org/10.3390/foods14081287







