Effect of Terroir on Phenolic Content and Aroma Properties of Grapes and Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Conditions and Materials
2.2. Vinification
2.3. Determination of Soil Samples and Microenvironmental Indicators
2.3.1. Soil Collection
2.3.2. Determination
2.4. Physicochemical Parameters of Grapes
2.4.1. Sugar and Acid Contents
2.4.2. Extraction of Phenolic Compounds
2.4.3. Determination of Phenolic Compounds
2.5. Monomeric Sugar
2.5.1. Extraction of Samples
2.5.2. Determination of Samples
2.6. Organic Acid
2.6.1. Extraction of Samples
2.6.2. Determination of Samples
2.7. Monomeric Anthocyanins
2.7.1. Extraction of Grape Skins
2.7.2. Determination
2.8. Monomeric Phenols
2.8.1. Extraction of Monomeric Phenols from Grape Skins
2.8.2. Extraction of Monomeric Phenols from Wine
2.8.3. Qualitative and Quantitative Analysis
2.9. Volatile Compounds
2.9.1. Extraction of Volatile Compounds
2.9.2. Qualitative and Quantitative Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Ecological Factors in Different Production Areas
3.1.1. Climate Conditions
3.1.2. Analysis of Soil Nutrient Indicators
3.2. Analysis of All Grape Indicators in the Wine Regions and Vineyards
3.3. Oenology and Physicochemical Indicators of Wine
3.4. Analysis of Wine Indicators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Sun, Q.; Chang, Q. Soil types effect on grape and wine composition in Helan Mountain area of Ningxia. PLoS ONE 2015, 10, e0116690. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Gu, X.; Sun, X.; Jin, G.; Zhang, J.; Ma, W. Flavor Chemical Profiles of Cabernet Sauvignon Wines: Six Vintages from 2013 to 2018 from the Eastern Foothills of the Ningxia Helan Mountains in China. Foods 2021, 11, 22. [Google Scholar] [CrossRef]
- Foroni, F.; Vignando, M.; Aiello, M.; Parma, V.; Paoletti, M.G.; Squartini, A.; Rumiati, R.I. The smell of terroir! Olfactory discrimination between wines of different grape variety and different terroir. Food Qual. Prefer. 2017, 58, 18–23. [Google Scholar] [CrossRef]
- Meinert, L.D. The Science of Terroir. Elements 2018, 14, 153–158. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario-A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef]
- Cagnasso, E.; Rolle, L.; Caudana, A.; Gerbi, V. Relationship Between Grape Phenolic Maturity and Red Wine Phenolic Composition. Ital. J. Food Sci. 2008, 20, 365–380. [Google Scholar]
- Glories, Y. The phenolic compounds in grapes and wine. J. Int. Des Sci. Vigne Vin 2002, 36. [Google Scholar]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Vitic. 2006, 57, 54–59. [Google Scholar] [CrossRef]
- Coletta, A.; Toci, A.T.; Pati, S.; Ferrara, G.; Grieco, F.; Tufariello, M.; Crupi, P. Effect of soil management and training system on Negroamaro wine aroma. Foods 2021, 10, 454. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Cheng, H.; Yang, Y. Variation of soil organic matter with particle size in the wind erosion region of northern China. CATENA 2024, 241, 108025. [Google Scholar] [CrossRef]
- Miller, R.O.; Kissel, D.E. Comparison of Soil pH Methods on Soils of North America. Soil. Sci. Soc. Am. J. 2010, 74, 310–316. [Google Scholar] [CrossRef]
- Saha, U.; Biswas, A. Comparison of Diffusion-Conductimetric and Distillation-Titration Methods in Analyzing Ammonium- and Nitrate-Nitrogen in the KCl-Extracts of Georgia Soils. Commun. Soil. Sci. Plant Anal. 2018, 49, 63–75. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, L.; Sun, Y.; Gao, S. Soil nutrients and enzyme activities based on millet continuous cropping obstacles. Sci. Rep. 2024, 14, 17329. [Google Scholar] [CrossRef]
- Chaitanya, T.; Padmaja, G.; Rao, P.C. Improving the Efficiency of Ammonium Acetate Extraction of Soil Potassium by Saturation Extract Method. Curr. J. Appl. Sci. Technol. 2020, 39, 121–136. [Google Scholar] [CrossRef]
- OIV. International Code of Oenological Practices. Available online: http://www.oiv.int/oiv/info/enpratiquesoenologiques (accessed on 1 August 2023).
- Li, W.; Yao, H.; Chen, K.; Ju, Y.; Min, Z.; Sun, X.; Fang, Y. Effect of foliar application of fulvic acid antitranspirant on sugar accumulation, phenolic profiles and aroma qualities of Cabernet Sauvignon and Riesling grapes and wines. Food Chem. 2021, 351, 129308. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Antioxidant potential of white grape pomaces: Phenolic composition and antioxidant capacity measured by spectrophotometric and cyclic voltammetry methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- Cliff, M.A.; King, M.C.; Schlosser, J. Anthocyanin, phenolic composition, colour measurement and sensory analysis of BC commercial red wines. Food Res. Int. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Restuccia, D.; Sicari, V.; Pellicanò, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Vojnoski, B.; Dörnyei, Á.; Márk, L.; Dimovska, V.; Stafilov, T.; Kilár, F. Identification of polyphenolic compounds in red and white grape varieties grown in R. macedonia and changes of their content during ripening. Food Res. Int. 2011, 44, 2851–2860. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, X.; Zhang, A.; Wang, P.; Chen, Q.; Ma, T.; Fang, Y. Foliar Phenylalanine Application Promoted Antioxidant Activities in Cabernet Sauvignon by Regulating Phenolic Biosynthesis. J. Agric. Food Chem. 2020, 68, 15390–15402. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Buica, A. Comparison of an Offline SPE–GC–MS and Online HS–SPME–GC–MS Method for the Analysis of Volatile Terpenoids in Wine. Molecules 2020, 25, 657. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Schreiner, R.P.; Qian, M.C. Soil Nitrogen, Phosphorus, and Potassium Alter β-Damascenone and Other Volatiles in Pinot noir Berries. Am. J. Enol. Vitic. 2018, 69, 157–166. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhan, J.; Huang, W. Effects of ecological factors on quality of winegrape and wine. Food Sci. 2006, 27, 791–797. [Google Scholar]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Soil pH Buffering Capacity and Nitrogen Availability Following Compost Application in a Tropical Acid Soil. Compos. Sci. Util. 2017, 26, 1–15. [Google Scholar] [CrossRef]
- Riesterer-Loper, J.; Workmaster, B.A.; Atucha, A. Impact of Fruit Zone Sunlight Exposure on Ripening Profiles of Cold Climate Interspecific Hybrid Winegrapes. Am. J. Enol. Vitic. 2019, 70, 286–296. [Google Scholar] [CrossRef]
- Tian, Z.; Li, J.; Jia, X.; Yang, F.; Wang, Z. Assimilation and Translocation of Dry Matter and Phosphorus in Rice Genotypes Affected by Salt-Alkaline Stress. Sustainability 2016, 8, 568. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, R.; Qin, Q.; Sun, Q. Soil affected the variations in grape and wine properties along the eastern foot of Helan Mountain, China. Acta Agric. Scand. Sect. B-Soil. Plant Sci. 2019, 69, 494–502. [Google Scholar] [CrossRef]
- Dienes-Nagy, Á.; Marti, G.; Breant, L.; Lorenzini, F.; Fuchsmann, P.; Baumgartner, D.; Rösti, J. Identification of putative chemical markers in white wine (Chasselas) related to nitrogen deficiencies in vineyards. OENO One 2020, 54, 583–599. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, J.; Jiang, T.; Ma, T.; Wang, R. Effect of nitrogen regulation on berry quality and flavonoids during veraison stage. Food Sci. Nutr. 2021, 9, 5448–5456. [Google Scholar] [CrossRef] [PubMed]
- Retallack, G.J.; Burns, S.F. The effects of soil on the taste of wine. GSA Today 2016, 26, 4–9. [Google Scholar] [CrossRef]
- Keller, M.; Arnink, K.J.; Hrazdina, G. Interaction of nitrogen availability during bloom and light intensity during veraison. I. Effects on grapevine growth, fruit development, and ripening. Am. J. Enol. Vitic. 1998, 49, 333–340. [Google Scholar] [CrossRef]
- Chan, K.Y.; Fahey, D.J. Effect of composted mulch application on soil and wine grape potassium status. Soil. Res. 2011, 49, 455. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Schachtman, D.P.; Treeby, M.T.; Thomas, M.R. A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Aust. J. Grape Wine Res. 2003, 9, 154–168. [Google Scholar] [CrossRef]
- Erian, A.M.; Sauer, M. Utilizing yeasts for the conversion of renewable feedstocks to sugar alcohols—A review. Bioresour. Technol. 2022, 346, 126296. [Google Scholar] [CrossRef]
- King, E.S.; Dunn, R.L.; Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Prefer. 2013, 28, 235–243. [Google Scholar] [CrossRef]
- Liebert, H.P. Effect of sugar on the glycerol content and the redox potential of wine. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Zweite Nat. Abt. Allg. Landwirtsch. Tech. Mikrobiol. 1970, 125, 289–294. [Google Scholar]
- Jensen, J.S.; Demiray, S.; Egebo, M.; Meyer, A.S. Prediction of Wine Color Attributes from the Phenolic Profiles of Red Grapes (Vitis vinifera). J. Agric. Food Chem. 2008, 56, 1105–1115. [Google Scholar] [CrossRef]
- Kozina, B.; Karoglan, M.; Jeromel, A.; Maslov, L. Effect of Grapevine Solarization on the Anthocyanins Composition of Pinot Noir Wines. Cereal Res. Commun. 2008, 36, 47–50. [Google Scholar]
- Yan, Y.; Song, C.; Falginella, L.; Castellarin, S.D. Day Temperature Has a Stronger Effect Than Night Temperature on Anthocyanin and Flavonol Accumulation in ‘Merlot’ (Vitis vinifera L.) Grapes During Ripening. Front. Plant Sci. 2020, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-Z.; Wang, C.; Xie, S.; Zhang, Z.-W. Effects of leaf removal and cluster thinning on berry quality of Vitis vinifera cultivars in the region of Weibei Dryland in China. J. Integr. Agric. 2018, 17, 1620–1630. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Peng, C.T.; Wen, Y.; Tao, Y.S.; Lan, Y.Y. Modulating the formation of Meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef]
- Falqué, E.; Fernández, E.; Dubourdieu, D. Differentiation of white wines by their aromatic index. Talanta 2001, 54, 271–281. [Google Scholar] [CrossRef]
- Prata-Sena, M.; Castro-Carvalho, B.M.; Nunes, S.; Amaral, B.; Silva, P. The terroir of Port wine: Two hundred and sixty years of history. Food Chem. 2018, 257, 388–398. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Barbe, J.-C.; Darriet, P.; Geffroy, O.; Gomès, E.; Guillaumie, S.; Helwi, P.; Laboyrie, J.; Lytra, G.; Le Menn, N.; et al. Recent advancements in understanding the terroir effect on aromas in grapes and wines: This article is published in cooperation with the XIIIth International Terroir Congress November 17-18 2020, Adelaide, Australia. Guest editors: Cassandra Collins and Roberta De Bei. OENO One 2020, 54, 985–1006. [Google Scholar] [CrossRef]
- White, R.E. The Value of Soil Knowledge in Understanding Wine Terroir. Front. Environ. Sci. 2020, 8, 12. [Google Scholar] [CrossRef]
- Tufariello, M.; Pati, S.; D’Amico, L.; Bleve, G.; Losito, I.; Grieco, F. Quantitative issues related to the headspace-SPME-GC/MS analysis of volatile compounds in wines: The case of Maresco sparkling wine. LWT 2019, 108, 268–276. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Camara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. C R C Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, K.; Mutturi, S. Recent advances in the microbial production of squalene. World J. Microbiol. Biotechnol. 2022, 38, 91. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.P.; Mira de Orduña, R.; Pilone, G.J.; Liu, S.-Q. Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhong, K.; Cui, X.; Ma, Y.; Hou, Z.; Sun, Y.; Han, G. Acetaldehyde accumulation during wine micro oxygenation: The influence of microbial metabolism. Food Control 2022, 142, 109227. [Google Scholar] [CrossRef]
- Guittin, C.; Maçna, F.; Picou, C.; Perez, M.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. New Online Monitoring Approaches to Describe and Understand the Kinetics of Acetaldehyde Concentration during Wine Alcoholic Fermentation: Access to Production Balances. Fermentation 2023, 9, 299. [Google Scholar] [CrossRef]
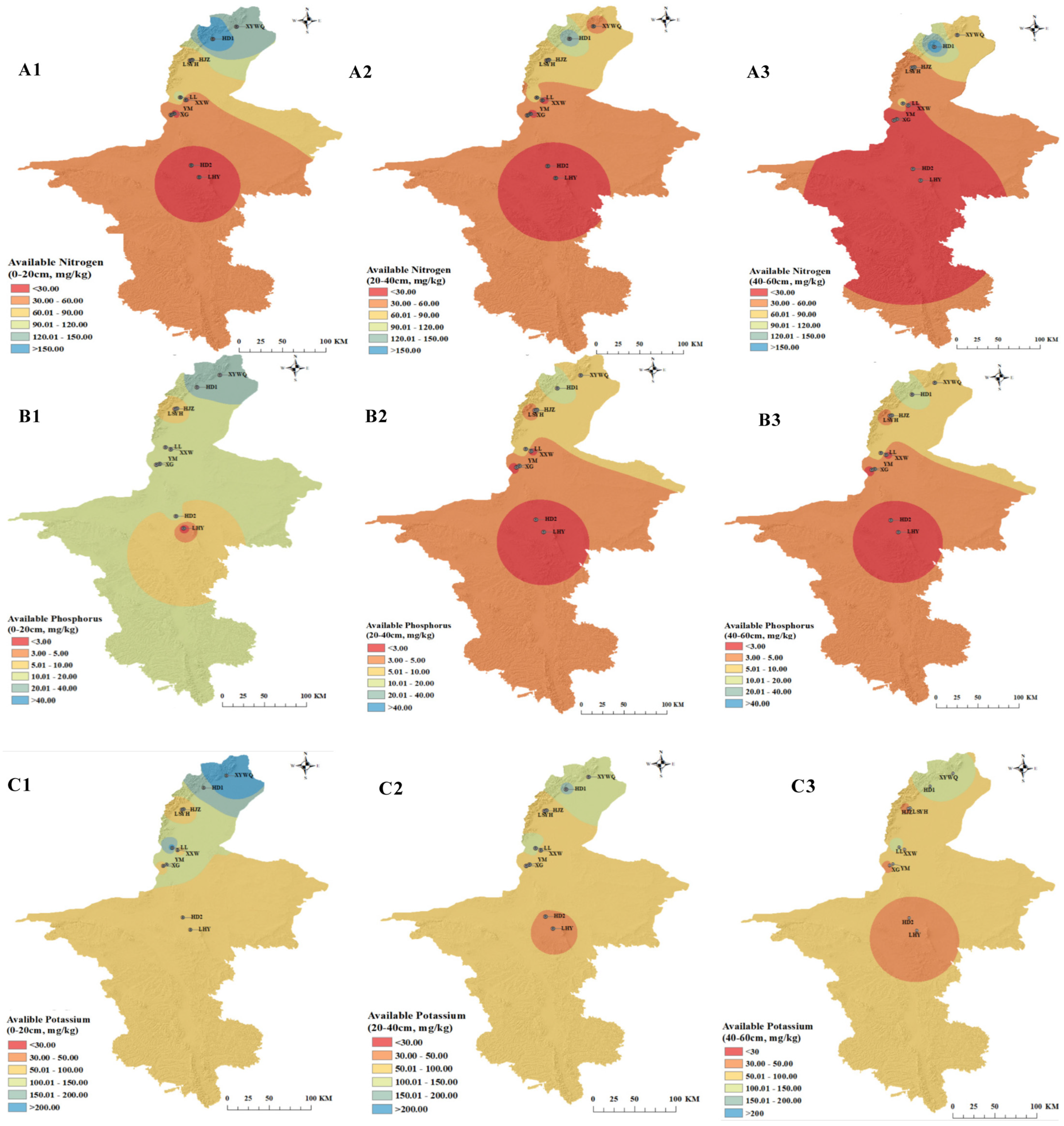


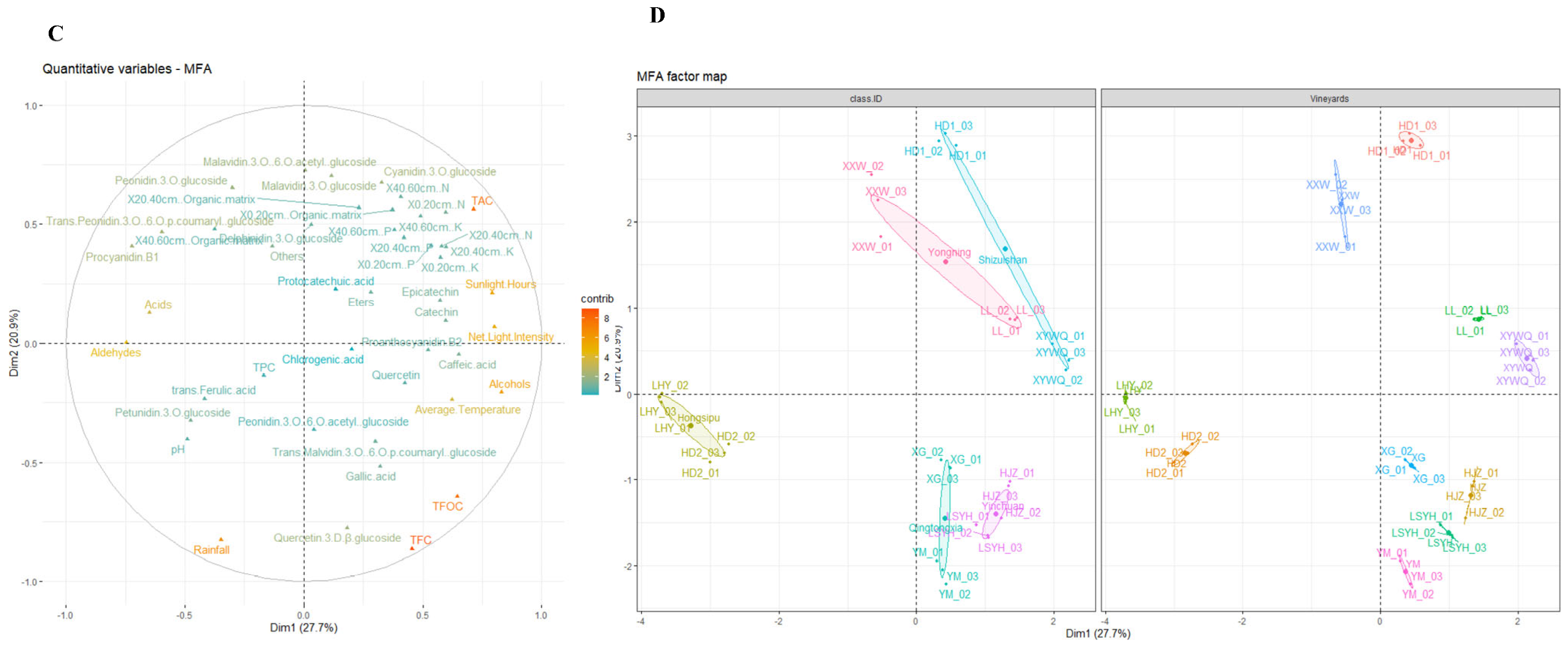
| Wine Regions | Vineyards | Average Temperature (°C) | Average Humidity (%) | Rainfall (mm) | Net Light Intensity (Net, J/m2/d) | Sunlight Hours (h) | pH |
|---|---|---|---|---|---|---|---|
| Shizuishan | XYWQ | 26.1 | 44.8 | 24.77 | 17,000,185.37 | 182.95 | 8.20 |
| HD1 | 25.1 | 52.9 | 7.97 | ||||
| Yinchuan | LSYH | 27.4 | 44.9 | 33.09 | 17,482,354.61 | 179.64 | 8.16 |
| HJZ | 26.5 | 44.5 | 8.23 | ||||
| Yongning | LL | 26.5 | 46.8 | 28.22 | 16,831,369.98 | 178.75 | 8.08 |
| XXW | 26.2 | 48.6 | 8.37 | ||||
| Qingtongxia | YM | 25.4 | 51.7 | 32.92 | 16,434,452.67 | 180.85 | 8.42 |
| XG | 25.8 | 47.4 | 8.35 | ||||
| Hongsipu | LHY | 24.7 | 46.0 | 32.17 | 15,922,102 | 176 | 8.43 |
| HD2 | 25.5 | 46.7 | 8.35 |
| Shizuishan | Yinchuan | Yongning | Qingtongxia | Hongsipu | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XYWQ | HD1 | LSYH | HJZ | LL | XXW | YM | XG | LHY | HD2 | |
| 0~20 cm | 30.85 ± 0.25 b | 44.96 ± 0.39 a | 11.64 ± 0.19 c | 6.98 ± 0.03 f | 9.86 ± 0.24 d | 4.73 ± 0.09 g | 6.87 ± 0.07 f | 4.45 ± 0.03 g | 2.71 ± 0.02 h | 9.02 ± 0.01 e |
| 20~40 cm | 9.02 ± 0.01 d | 45.91 ± 0.08 a | 10.58 ± 0.16 b | 7.7 ± 0.16 e | 9.78 ± 0.11 c | 2.95 ± 0.04 j | 6.82 ± 0.1 f | 4.19 ± 0.02 h | 3.3 ± 0.01 i | 5.19 ± 0.03 g |
| 40~60 cm | 13.13 ± 0.18 c | 44.88 ± 0.44 b | 9.19 ± 0.14 d | 6.34 ± 0.02 e | 8.9 ± 0.25 d | 4.86 ± 0.08 f | 4.05 ± 0.11 f | 2.8 ± 0.01 g | 46.53 ± 0.85 a | 4.22 ± 0 f |
| Shizuishan | Yinchuan | Yongning | Qingtongxia | Hongsipu | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XYWQ | HD1 | LSYH | HJZ | LL | XXW | YM | XG | LHY | HD2 | |
| Grapes | ||||||||||
| TA (g/L) | 3.25 ± 0.00 g | 4.57 ± 0.01 c | 3.08 ± 0.00 h | 3.59 ± 0.00 f | 3.14 ± 0.01 h | 4.39 ± 0.00 d | 3.87 ± 0.03 e | 3.77 ± 0.04 e | 5.26 ± 0.02 a | 5.15 ± 0.02 b |
| TSS (g/L) | 25.16 ± 0.03 e | 25.70 ± 0.01 d | 27.61 ± 0.01 c | 28.03 ± 0.02 b | 28.07 ± 0.01 b | 19.56 ± 0.00 g | 27.62 ± 0.01 c | 28.64 ± 0.01 a | 22.58 ± 0.03 f | 25.68 ± 0.01 d |
| pH | 3.74 ± 0.01 c | 3.81 ± 0.01 a | 3.76 ± 0.01 bc | 3.64 ± 0.00 d | 3.79 ± 0.01 ab | 3.74 ± 0.01 c | 3.62 ± 0.01 de | 3.59 ± 0.00 e | 3.50 ± 0.00 g | 3.55 ± 0.00 f |
| YAN (mg/L) | 180.50 ± 1.50 de | 235.50 ± 0.50 a | 215.50 ± 1.50 b | 200.00 ± 3.00 c | 230.50 ± 2.50 a | 200.00 ± 3.00 c | 174.00 ± 4.00 ef | 169.50 ± 2.50 ef | 167.50 ± 1.50 f | 189.50 ± 0.50 cd |
| TPC (mg/g) | 52.02 ± 6.01 ab | 46.64 ± 4.72 b | 52.91 ± 3.50 ab | 43.09 ± 3.62 b | 46.09 ± 1.90 b | 45.12 ± 2.28 b | 60.41 ± 6.80 a | 60.16 ± 3.72 a | 52.02 ± 5.85 ab | 47.07 ± 5.65 b |
| TFC (mg/g) | 38.92 ± 0.76 b | 42.39 ± 0.19 a | 35.25 ± 3.63 cd | 33.30 ± 1.34 d | 28.26 ± 0.14 e | 39.80 ± 2.20 ab | 33.53 ± 1.21 d | 25.85 ± 0.42 e | 37.81 ± 0.34 bc | 35.33 ± 0.30 cd |
| TFOC (mg/g) | 11.99 ± 0.06 f | 12.02 ± 0.08 f | 13.44 ± 0.00 d | 13.81 ± 0.07 c | 10.92 ± 0.04 h | 13.06 ± 0.04 e | 13.96 ± 0.02 b | 11.36 ± 0.11 g | 12.10 ± 0.00 f | 14.22 ± 0.00 a |
| TAC (mg/g) | 29.99 ± 0.11 b | 23.62 ± 0.02 cd | 24.00 ± 0.20 cd | 22.50 ± 0.18 de | 20.96 ± 0.16 e | 22.60 ± 0.04 de | 25.20 ± 1.29 c | 25.43 ± 0.17 c | 34.33 ± 2.12 a | 17.99 ± 0.65 f |
| Wine | ||||||||||
| Alcoholic degree (%) | 14.75 ± 0.04 f | 14.88 ± 0.34 e | 15.18 ± 0.01 d | 16.52 ± 0.04 a | 15.53 ± 0.08 c | 12.74 ± 0.01 h | 15.47 ± 0.02 c | 15.76 ± 0.00 b | 14.87 ± 0.02 e | 13.19 ± 0.00 g |
| Reducing substances (g/L) | 2.65 ± 0.07 c | 2.77 ± 0.06 a | 2.54 ± 0.06 de | 2.41 ± 0.01 g | 2.41 ± 0.01 g | 2.51 ± 0.01 ef | 2.37 ± 0.01 g | 2.72 ± 0.01 b | 2.57 ± 0.01 d | 2.47 ± 0.01 f |
| pH | 3.60 ± 0.00 c | 3.44 ± 0.04 f | 3.69 ± 0.00 a | 3.60 ± 0.01 c | 3.62 ± 0.01 b | 3.45 ± 0.00 f | 3.58 ± 0.00 d | 3.62 ± 0.01 b | 3.37 ± 0.01 g | 3.51 ± 0.00 e |
| Glycerol (g/L) | 8.85 ± 0.15 b | 9.45 ± 0.55 a | 9.13 ± 0.05 a | 9.55 ± 0.05 a | 9.55 ± 0.15 a | 8.65 ± 0.15 b | 9.30 ± 0.10 a | 8.60 ± 0.10 b | 7.95 ± 0.15 c | 8.85 ± 0.25 b |
| TPC (mg/L) | 365.54 ± 2.21 b | 335.26 ± 2.27 d | 344.24 ± 1.31 c | 394.28 ± 5.94 a | 243.39 ± 1.81 g | 307.8 ± 4.19 f | 315.24 ± 0.63 e | 239.54 ± 0.73 g | 360.92 ± 2.02 b | 347.32 ± 0.96 c |
| TFC (mg/L) | 1650.81 ± 25.97 c | 1177.97 ± 18.91 f | 1978.3 ± 14.29 b | 2022.24 ± 4.68 ab | 1666.96 ± 8.10 c | 1265.83 ± 20.39 e | 2048.98 ± 13.51 a | 1708.98 ± 11.77 c | 1311.67 ± 29.22 e | 1590.55 ± 16.43 d |
| TFOC (mg/L) | 317.11 ± 6.97 b | 277.16 ± 1.02 d | 315.42 ± 4.28 b | 322.03 ± 11.04 ab | 323.47 ± 0.38 ab | 288.44 ± 0.94 c | 328.80 ± 1.00 a | 317.42 ± 3.45 b | 277.77 ± 1.39 d | 297.06 ± 2.36 c |
| TAC (mg/L) | 161.53 ± 3.76 c | 162.97 ± 2.41 c | 147.32 ± 0.17 d | 150.40 ± 5.09 d | 176.84 ± 4.90 b | 180.94 ± 8.02 a | 135.71 ± 1.25 e | 147.58 ± 0.17 d | 108.92 ± 1.75 f | 112.33 ± 3.91 f |
| Compounds | Threshold (mg/L) | Odor Descriptor | Shizuishan | Yinchuan | Yongning | Qingtongxia | Hongsipu | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XYWQ | HD1 | LSYH | HJZ | LL | XXW | YM | XG | LHY | HD2 | |||
| Alcohols | ||||||||||||
| Isobutanol | 1.11 | fusel, alcohol A | 3.97 ± 0.06 d | 4.41 ± 0.14 c | 4.79 ± 0.02 b | 6.28 ± 0.16 a | 4.77 ± 0.23 b | 4.01 ± 0.02 d | 4.87 ± 0.17 b | 4.73 ± 0.08 b | 2.93 ± 0.07 e | 3.72 ± 0.29 d |
| Isoamyl alcohol | 1.22 | cheese, solvent B | 122.60 ± 1.11 d | 111.70 ± 5.90 e | 121.52 ± 0.88 d | 150.48 ± 0.03 a | 130.62 ± 0.95 bc | 111.79 ± 0.20 e | 128.81 ± 0.23 c | 134.58 ± 3.58 bc | 96.04 ± 0.36 f | 107.94 ± 2.50 e |
| 4-Methyl-1-pentanol | - | - | 0.09 ± 0.00 a | 0.09 ± 0.00 a | 0.06 ± 0.00 e | 0.08 ± 0.00 c | 0.06 ± 0.00 d | 0.04 ± 0.00 g | 0.05 ± 0.00 f | 0.05 ± 0.00 f | 0.05 ± 0.00 f | 0.08 ± 0.00 b |
| 3-methyl-1-pentanol | 1.35 | vinous, herbaceous | 0.25 ± 0.03 c | 0.26 ± 0.02 bc | 0.25 ± 0.01 c | 0.29 ± 0.01 b | 0.26 ± 0.01 c | 0.20 ± 0.00 d | 0.26 ± 0.01 c | 0.26 ± 0.00 bc | 0.32 ± 0.01 a | 0.29 ± 0.01 b |
| 1-Hexanol | 8.00 | green, grass | 3.36 ± 0.18 c | 4.31 ± 0.04 b | 3.57 ± 0.02 c | 2.70 ± 0.09 d | 4.52 ± 0.20 b | 4.96 ± 0.04 a | 2.89 ± 0.05 d | 3.39 ± 0.18 c | 3.40 ± 0.07 c | 2.93 ± 0.09 d |
| 3-Hexen-1-ol | 0.40 | Green, floral | 0.05 ± 0.00 c | 0.09 ± 0.00 a | 0.08 ± 0.00 b | 0.03 ± 0.00 ef | 0.04 ± 0.00 e | 0.04 ± 0.00 d | 0.03 ± 0.00 g | 0.00 ± 0.00 h | 0.03 ± 0.00 f | 0.02 ± 0.00 g |
| (R,R)-2,3-Butanediol | 1.62 | Rubber G | 0.56 ± 0.01 b | 0.45 ± 0.01 c | 0.77 ± 0.01 a | 0.57 ± 0.03 b | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| 1-Nonanol | 0.60 | Green | 0.16 ± 0.00 c | 0.12 ± 0.01 e | 0.10 ± 0.00 f | 0.05 ± 0.00 h | 0.19 ± 0.01 b | 0.27 ± 0.00 a | 0.14 ± 0.00 d | 0.12 ± 0.00 e | 0.13 ± 0.01 e | 0.07 ± 0.00 g |
| Phenethyl alcohol | - | - | 33.30 ± 0.37 b | 22.56 ± 1.35 de | 22.95 ± 0.52 d | 15.55 ± 0.31 g | 37.06 ± 0.03 a | 28.81 ± 0.12 c | 22.25 ± 0.56 de | 33.19 ± 0.41 b | 19.10 ± 0.09 f | 21.58 ± 0.24 e |
| Heptaethylene glycol | - | - | 0.09 ± 0.00 a | 0.00 ± 0.00 ef | 0.01 ± 0.00 de | 0.01 ± 0.00 cd | 0.00 ± 0.00 f | 0.02 ± 0.00 b | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.01 ± 0.00 c |
| 1-Butanol | 150.00 | Medicinal, alcohol | 0.28 ± 0.00 cd | 0.16 ± 0.03 f | 0.44 ± 0.02 b | 0.57 ± 0.02 a | 0.30 ± 0.01 c | 0.20 ± 0.00 e | 0.26 ± 0.01 d | 0.31 ± 0.01 c | 0.16 ± 0.01 f | 0.16 ± 0.00 f |
| 1-Octanol | 0.01 | intense citrus, roses | 0.13 ± 0.00 d | 0.18 ± 0.00 a | 0.13 ± 0.00 d | 0.05 ± 0.00 f | 0.13 ± 0.00 d | 0.15 ± 0.00 c | 0.06 ± 0.00 f | 0.10 ± 0.00 e | 0.17 ± 0.02 ab | 0.16 ± 0.00 bc |
| Subtotal | 164.84 ± 1.62 b | 144.32 ± 7.48 e | 154.66 ± 0.36 cd | 176.67 ± 0.03 a | 177.95 ± 1.44 a | 150.50 ± 0.13 d | 159.62 ± 1.04 bc | 176.72 ± 3.43 a | 122.32 ± 0.47 g | 136.97 ± 2.44 f | ||
| Esters | ||||||||||||
| Ethyl Acetate | 7.50 | pineapple, fruity, balsamic | 11.63 ± 1.01 b | 9.78 ± 0.28 c | 4.64 ± 0.07 e | 8.86 ± 0.31 cd | 12.74 ± 0.83 a | 8.52 ± 0.25 d | 9.48 ± 0.30 cd | 11.43 ± 0.64 b | 11.59 ± 0.38 b | 8.95 ± 0.13 cd |
| Isoamyl acetate | 0.003 | banana, fruity, sweet | 1.11 ± 0.17 d | 1.87 ± 0.04 a | 0.40 ± 0.04 h | 0.97 ± 0.00 de | 1.72 ± 0.12 b | 0.62 ± 0.02 g | 1.04 ± 0.05 de | 1.36 ± 0.03 c | 0.77 ± 0.02 fg | 0.90 ± 0.02 ef |
| Hexyl acetate | 1.29 | apple, cherry, pear, floral B | 0.12 ± 0.00 b | 0.12 ± 0.01 b | 0.00 ± 0.00 f | 0.06 ± 0.00 d | 0.13 ± 0.00 a | 0.07 ± 0.00 c | 0.07 ± 0.00 c | 0.03 ± 0.00 e | 0.00 ± 0.00 f | 0.00 ± 0.00 f |
| Ethyl lactate | 1.37 | lactic, raspberry, fruity, buttery B | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 0.02 ± 0.00 d | 0.00 ± 0.00 e | 0.08 ± 0.00 c | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 0.13 ± 0.00 a | 0.10 ± 0.00 b |
| Ethyl octanoate | 1.45 | pineapple, pear, floral, fruity, brandy B | 10.61 ± 0.26 b | 6.90 ± 0.08 c | 3.15 ± 0.04 i | 31.31 ± 0.01 a | 6.93 ± 0.06 c | 4.08 ± 0.06 g | 3.49 ± 0.04 h | 5.95 ± 0.01 e | 5.46 ± 0.17 f | 6.33 ± 0.10 d |
| Phenethyl acetate | - | - | 0.66 ± 0.00 f | 0.75 ± 0.02 e | 0.67 ± 0.00 f | 0.81 ± 0.00 d | 1.63 ± 0.03 a | 0.80 ± 0.00 d | 1.46 ± 0.00 c | 1.54 ± 0.02 b | 0.61 ± 0.01 g | 0.59 ± 0.01 g |
| Ethyl caprate | - | - | 5.26 ± 0.06 a | 3.33 ± 0.16 b | 0.99 ± 0.01 i | 1.21 ± 0.04 h | 2.93 ± 0.00 c | 1.63 ± 0.01 fg | 1.66 ± 0.00 f | 2.36 ± 0.03 e | 1.52 ± 0.02 g | 2.64 ± 0.05 d |
| Ethyl butyrate | 0.02 | strawberry, apple, banana | 0.69 ± 0.01 a | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.48 ± 0.01 c | 0.53 ± 0.01 b |
| Ethyl Hexanoate | 5 | fruity, green apple; floral, violet | 2.69 ± 0.00 a | 1.55 ± 0.02 b | 0.77 ± 0.01 g | 0.80 ± 0.01 g | 1.42 ± 0.04 c | 0.98 ± 0.02 f | 0.81 ± 0.00 g | 1.06 ± 0.04 e | 1.26 ± 0.01 d | 1.05 ± 0.02 e |
| 3-Hexen-1-ol | 0.40 | Green, floral | 0.05 ± 0.00 c | 0.09 ± 0.00 a | 0.08 ± 0.00 b | 0.03 ± 0.00 ef | 0.04 ± 0.00 e | 0.04 ± 0.00 d | 0.03 ± 0.00 g | 0.00 ± 0.00 h | 0.03 ± 0.00 f | 0.02 ± 0.00 g |
| Ethyl nonanoate | 1.30 | waxy, fruity | 0.07 ± 0.00 a | 0.03 ± 0.00 b | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.02 ± 0.00 c | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| Diethyl succinate | 500.00 | Light fruity | 0.10 ± 0.00 g | 0.24 ± 0.01 e | 0.15 ± 0.00 f | 0.10 ± 0.00 g | 0.29 ± 0.01 c | 0.29 ± 0.01 c | 0.10 ± 0.00 g | 0.27 ± 0.00 d | 0.32 ± 0.00 b | 0.40 ± 0.01 a |
| Subtotal | 32.99 ± 0.97 b | 24.66 ± 0.30 d | 10.84 ± 0.10 g | 44.19 ± 0.36 a | 27.81 ± 1.02 c | 17.13 ± 0.35 f | 18.14 ± 0.39 f | 24.00 ± 0.72 d | 22.17 ± 0.22 e | 21.52 ± 0.17 e | ||
| Acids | ||||||||||||
| Isobutyric acid | 200.00 | Fatty | 0.05 ± 0.00 c | 0.05 ± 0.00 c | 0.14 ± 0.05 a | 0.08 ± 0.00 bc | 0.10 ± 0.00 b | 0.07 ± 0.00 bc | 0.08 ± 0.00 bc | 0.08 ± 0.00 bc | 0.06 ± 0.00 c | 0.08 ± 0.00 bc |
| Hexanoic acid | 3.00 | Cheese, rancid, fatty | 0.56 ± 0.00 b | 0.48 ± 0.01 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.48 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 1.58 ± 0.15 a |
| Octanoic acid | 0.50 | rancid, harsh, cheese, fatty acid | 2.21 ± 0.01 d | 2.69 ± 0.15 c | 1.28 ± 0.02 fg | 0.41 ± 0.00 i | 1.41 ± 0.02 f | 1.74 ± 0.01 e | 1.17 ± 0.02 gh | 1.06 ± 0.04 h | 3.23 ± 0.02 b | 4.52 ± 0.09 a |
| Syrene | 1.40 | resin, floral | 0.53 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 2-methylbutanoic acid | 1.66 | - | 0.00 ± 0.00 i | 0.00 ± 0.00 i | 0.63 ± 0.01 a | 0.40 ± 0.00 f | 0.51 ± 0.00 d | 0.60 ± 0.00 b | 0.46 ± 0.00 e | 0.53 ± 0.02 c | 0.35 ± 0.00 h | 0.38 ± 0.00 g |
| Subtotal | 3.36 ± 0.02 c | 3.22 ± 0.15 c | 2.05 ± 0.08 e | 0.89 ± 0.01 g | 2.02 ± 0.02 e | 2.40 ± 0.01 d | 2.19 ± 0.02 e | 1.67 ± 0.06 f | 3.63 ± 0.02 b | 6.56 ± 0.24 a | ||
| Aldehydes | ||||||||||||
| acetaldehyde | 0.71 | fruity, pungent, green, grassy, apple F | 0.46 ± 0.02 c | 0.41 ± 0.00 d | 0.45 ± 0.02 c | 0.31 ± 0.01 e | 0.48 ± 0.02 c | 0.56 ± 0.01 b | 0.55 ± 0.01 b | 0.41 ± 0.02 d | 1.72 ± 0.05 a | 0.54 ± 0.01 b |
| 2,3-Pentanedione | - | - | 2.44 ± 0.02 b | 2.51 ± 0.04 b | 2.56 ± 0.00 b | 1.37 ± 0.03 b | 3.46 ± 0.01 b | 23.16 ± 15.46 a | 3.21 ± 0.26 b | 3.48 ± 0.00 b | 3.62 ± 0.04 b | 3.31 ± 0.02 b |
| Total | 204.10 | 175.13 | 170.56 | 223.42 | 211.71 | 193.76 | 183.71 | 206.28 | 153.45 | 168.90 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phajon, Y.; Tan, H.; Liu, B.; Zhang, Y.; Ju, Y.; Shen, T.; Xu, M.; Fang, Y. Effect of Terroir on Phenolic Content and Aroma Properties of Grapes and Wines. Foods 2025, 14, 1409. https://doi.org/10.3390/foods14081409
Phajon Y, Tan H, Liu B, Zhang Y, Ju Y, Shen T, Xu M, Fang Y. Effect of Terroir on Phenolic Content and Aroma Properties of Grapes and Wines. Foods. 2025; 14(8):1409. https://doi.org/10.3390/foods14081409
Chicago/Turabian StylePhajon, Yuyuen, Hongbing Tan, Bochen Liu, Yang Zhang, Yanlun Ju, Tian Shen, Meilong Xu, and Yulin Fang. 2025. "Effect of Terroir on Phenolic Content and Aroma Properties of Grapes and Wines" Foods 14, no. 8: 1409. https://doi.org/10.3390/foods14081409
APA StylePhajon, Y., Tan, H., Liu, B., Zhang, Y., Ju, Y., Shen, T., Xu, M., & Fang, Y. (2025). Effect of Terroir on Phenolic Content and Aroma Properties of Grapes and Wines. Foods, 14(8), 1409. https://doi.org/10.3390/foods14081409





