Spicy Food Ingredient from Red Habanero By-Product Obtained by Ultrasound-Assisted Extraction
Abstract
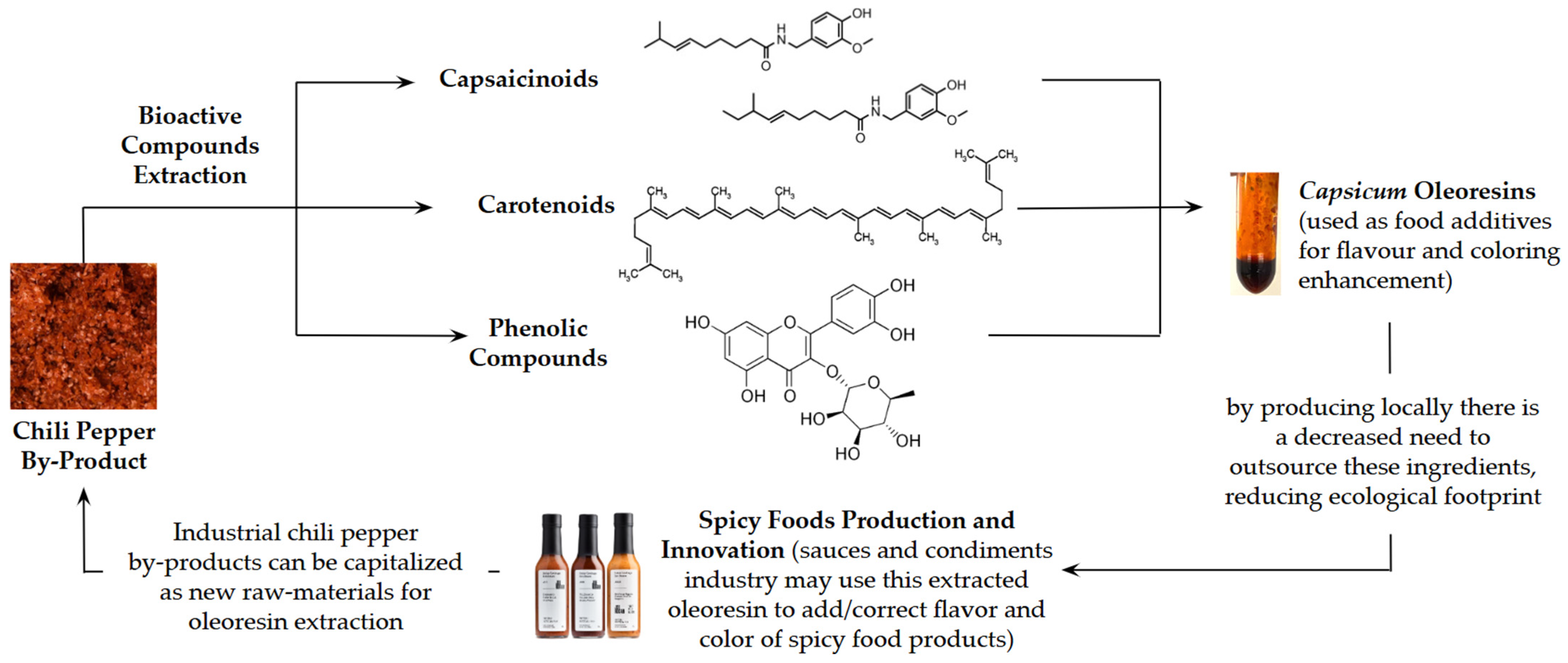
1. Introduction
- Study the effects of UAE time and Acoustic Power Density (APD) on process efficiency parameters.
- Study the effects of UAE time and APD on capsaicinoids, total phenolics, antioxidant activity, color, and peroxide value of the oleoresin produced.
- Generate mathematical models for each response using experimental design and response surface methodology (RSM), and optimize UAE processing conditions.
- Experimentally validate the responses for the predicted optimum ultrasound conditions.
- Compare results obtained with optimized UAE conditions with reflux-assisted extraction (RAE), as described by AOAC for capsaicinoids extraction.
2. Materials and Methods
2.1. Red Habanero Chili Pepper By-Product
2.2. Experimental Design to Study the Effect of Acoustic Power Density and Ultrasound Extraction Time on Several Parameters
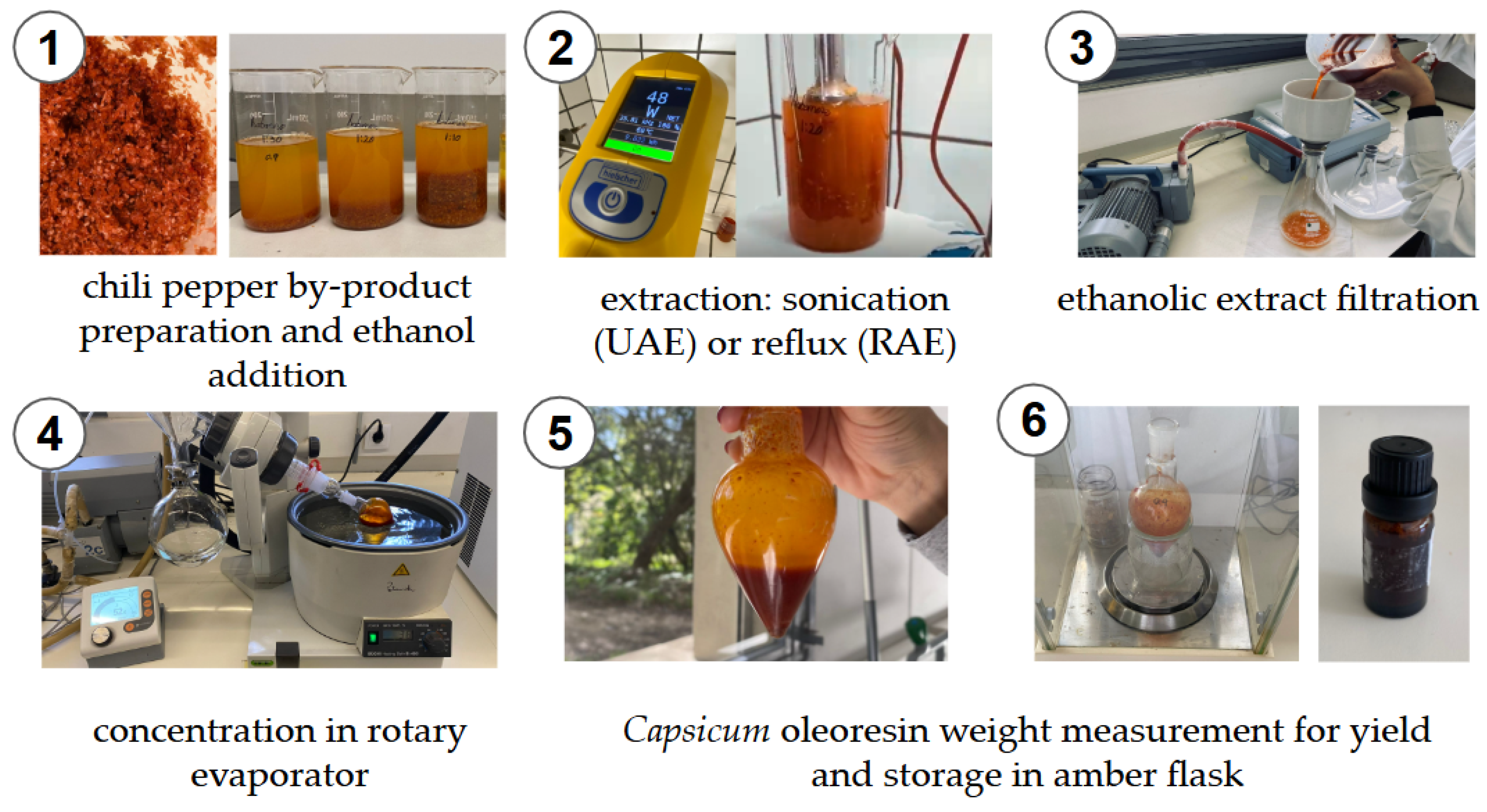
2.3. Extraction Processes
2.3.1. Ultrasound Assisted Extraction (UAE)
2.3.2. Reflux Assisted Extraction (RAE)
2.3.3. Extraction Yield Determination
2.4. Physicochemical Parameters Analysis
2.4.1. Capsaicinoids
2.4.2. Total Phenolic Content
2.4.3. Antioxidant Activity
FRAP Assay
DPPH Radical Scavenging Activity Assay
2.4.4. Diluted Capsicum Oleoresin Color Parameters
2.4.5. Peroxide Value
2.5. Statistical Analysis
3. Results and Discussion
3.1. Ultrasound-Assisted Extraction Conditions Responses and Impact on Oleoresin Quality
3.1.1. Energy Consumption, Temperature, and Extraction Yield
3.1.2. Capsaicinoids, Total Phenolic Content, and Antioxidant Activity of Oleoresin
3.1.3. Oleoresin Color Parameters
3.1.4. Peroxide Value
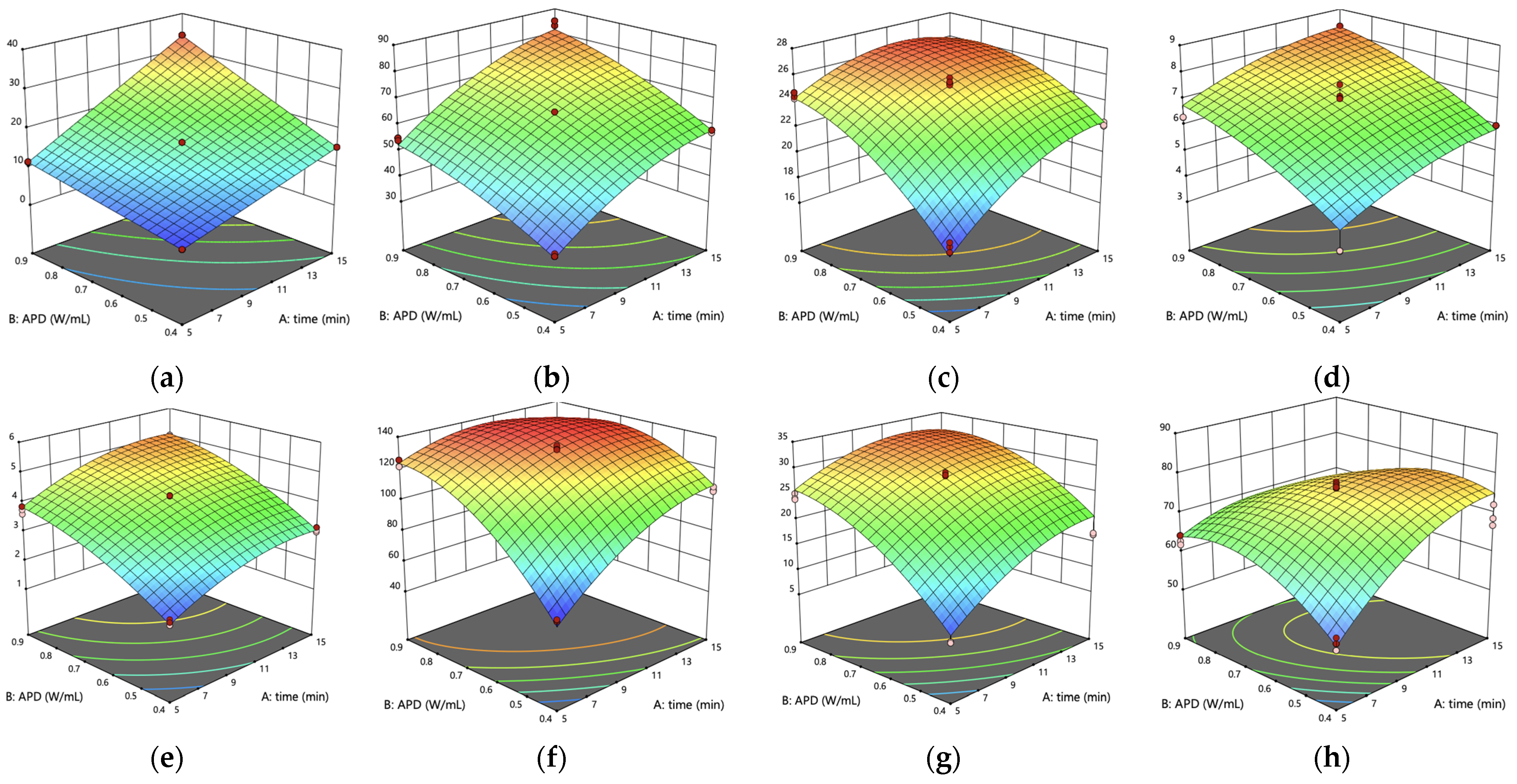
3.2. Mathematical Models and Surface Plots for Each Parameter
3.3. Experimental Validation of Optimum Ultrasound-Assisted Extraction (UAE) Conditions and Comparison with Reflux-Assisted Extraction (RAE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Run: | 1 | 2 | 3 | 4 | 5 | 6 |
| Time (min) | 5 | 5 | 15 | 15 | 10 | 10 |
| APD (w/mL) | 0.90 | 0.40 | 0.40 | 0.90 | 0.65 | 0.65 |
| PV (meqO2/kg) | 0.55 ± 0.26 | 0.41 ± 0.04 | 0.57 ± 0.34 | 0.41 ± 0.09 | 0.30 ± 0.07 | 0.43 ± 0.30 |
| Run: | 7 | 8 | 9 | 10 | 11 | 12 |
| Time (min) | 10 | 10 | 17 | 3 | 10 | 10 |
| APD (w/mL) | 0.65 | 0.65 | 0.65 | 0.65 | 1.00 | 0.30 |
| PV (meqO2/kg) | 0.31 ± 0.03 | 0.31 ± 0.21 | 0.25 ± 0.00 | 0.43 ± 0.17 | 0.50 ± 0.29 | 0.37 ± 0.03 |
| Note: APD = acoustic power density; PV = Peroxide value. All responses are presented as the mean ± standard deviation of three real replicates (n = 3). | ||||||
References
- Gruber, G.K.; Lima, B.D.O.; Chiarello, L.M.; Gonçalves, M.J.; Botton, V. Functional and nutritional properties of chilli sauce: Propriedades funcionais e nutricionais do molho de pimenta. Braz. J. Dev. 2022, 8, 64065–64080. [Google Scholar] [CrossRef]
- Costa, J.; Sepúlveda, M.; Gallardo, V.; Cayún, Y.; Santander, C.; Ruíz, A.; Reyes, M.; Santos, C.; Cornejo, P.; Lima, N.; et al. Antifungal Potential of Capsaicinoids and Capsinoids from the Capsicum Genus for the Safeguarding of Agrifood Production: Advantages and Limitations for Environmental Health. Microorganisms 2022, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, G.D.; Lončar, A.; Ranilović, J.; Šubarić, D.; Panjičko, M. The Influence of Polyphenolic Compounds on Anaerobic Digestion of Pepper Processing Waste during Biogas and Biomethane Production. Processes 2024, 12, 913. [Google Scholar] [CrossRef]
- Azabou, S.; Taheur, F.B.; Jridi, M.; Bouaziz, M.; Nasri, M. Discarded seeds from red pepper (Capsicum annuum) processing industry as a sustainable source of high added-value compounds and edible oil. Environ. Sci. Pollut. Res. 2017, 24, 22196–22203. [Google Scholar] [CrossRef]
- Cvetković, T.; Ranilović, J.; Jokić, S. Quality of Pepper Seed By-Products: A Review. Foods 2022, 11, 748. [Google Scholar] [CrossRef]
- Sala, S.; Anton, A.; McLaren, S.J.; Notarnicola, B.; Saouter, E.; Sonesson, U. In quest of reducing the environmental impacts of food production and consumption. J. Clean. Prod. 2017, 140, 387–398. [Google Scholar] [CrossRef]
- Cortés-Ferré, H.E.; Guajardo-Flores, D.; Romero-De La Vega, G.; Gutierrez-Uribe, J.A. Recovery of Capsaicinoids and Other Phytochemicals Involved with TRPV-1 Receptor to Re-valorize Chili Pepper Waste and Produce Nutraceuticals. Front. Sustain. Food Syst. 2021, 4, 588534. [Google Scholar] [CrossRef]
- Yasin, M.; Li, L.; Donovan-Mak, M.; Chen, Z.-H.; Panchal, S.K. Capsicum Waste as a Sustainable Source of Capsaicinoids for Metabolic Diseases. Foods 2023, 12, 907. [Google Scholar] [CrossRef]
- Shu, J.; Yin, Y.; Liu, Z. Integrated Processes Turning Pepper Sauce Waste into Valuable By-Products. Foods 2022, 12, 67. [Google Scholar] [CrossRef]
- Cerecedo-Cruz, L.; Azuara-Nieto, E.; Hernández-Álvarez, A.J.; González-González, C.R.; Melgar-Lalanne, G. Evaluation of the oxidative stability of Chipotle chili (Capsicum annuum L.) oleoresins in avocado oil. Grasas Aceites. 2018, 69, e240. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Mandal, P.K.; Han, K.-H.; Fukushima, M.; Choi, K.; Kim, C.-J.; Lee, C.-H. Capsaicin and tocopherol in red pepper seed oil enhances the thermal oxidative stability during frying. J. Food Sci. Technol. 2010, 47, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Sanati, S.; Razavi, B.M.; Hosseinzadeh, H. A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iran. J. Basic Med. Sci. 2018, 21, 439–448. [Google Scholar] [CrossRef]
- Janssens, P.L.H.R.; Hursel, R.; Martens, E.A.P.; Westerterp-Plantenga, M.S. Acute Effects of Capsaicin on Energy Expenditure and Fat Oxidation in Negative Energy Balance. PLoS ONE 2013, 8, e67786. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef]
- Kennedy, L.E.; Abraham, A.; Kulkarni, G.; Shettigar, N.; Dave, T.; Kulkarni, M. Capsanthin, a Plant-Derived Xanthophyll: A Review of Pharmacology and Delivery Strategies. AAPS PharmSciTech. 2021, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Tieman, D.; Rathinasabapathi, B. Capsanthin/Capsorubin Synthase Expression in Tomato Alters Carotenoid Pools, Enhancing Provitamin A and Flavor Volatiles. 2024. Available online: http://biorxiv.org/lookup/doi/10.1101/2024.09.27.615503 (accessed on 23 January 2025).
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. Carotenoids exclusively synthesized in red pepper (capsanthin and capsorubin) protect human dermal fibroblasts against UVB induced DNA damage. Photochem. Photobiol. Sci. 2016, 15, 1204–1211. [Google Scholar] [CrossRef]
- Eraslan, E.; Erden, Y.; Oruc, S.; Bircan, B.; Gunay, S. Capsanthin induces death in human prostate cancer cell lines by inducing DNA damage. EuroBiotech J. 2022, 6, 99–104. [Google Scholar] [CrossRef]
- Vulić, J.; Šeregelj, V.; Kalušević, A.; Lević, S.; Nedović, V.; Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G. Bioavailability and Bioactivity of Encapsulated Phenolics and Carotenoids Isolated from Red Pepper Waste. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef]
- Echave, J.; Pereira, A.G.; Carpena, M.; Ángel Prieto, M.; Simal-Gandara, J. Capsicum Seeds as a Source of Bioactive Compounds: Biological Properties, Extraction Systems, and Industrial Application. In Capsicum; Dekebo, A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Lekmine, S.; Benslama, O.; Bensalah, B.; Touzout, N.; Moussa, H.; Tahraoui, H.; Ola, M.S.; Hafsa, H.; Zhang, J.; Amrane, A. Bioactive Phenolics of Hyoscyamus muticus L. Subsp. Falezlez: A Molecular and Biochemical Approach to Antioxidant and Urease Inhibitory Activities. Int. J. Mol. Sci. 2025, 26, 370. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ferre, H.E.; Martínez-Avila, M.; Antunes-Ricardo, M.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Gutierrez-Uribe, J.A. In Vitro Evaluation of Anti-Inflammatory Activity of “Habanero” Chili Pepper (Capsicum chinense) Seeds Extracts Pretreated with Cellulase. 2022. Available online: https://www.researchsquare.com/article/rs-1871277/v1 (accessed on 6 October 2024).
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annuum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Procopio, F.R.; Ferraz, M.C.; Paulino, B.N.; Do Amaral Sobral, P.J.; Hubinger, M.D. Spice oleoresins as value-added ingredient for food industry: Recent advances and perspectives. Trends Food Sci. Technol. 2022, 122, 123–139. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Jiménez-Fernández, M.; Azuara, E. Oleoresins from Capsicum spp.: Extraction Methods and Bioactivity. Food Bioprocess Technol. 2017, 10, 51–76. [Google Scholar] [CrossRef]
- Olivas-Méndez, P.; Chávez-Martínez, A.; Santellano-Estrada, E.; Guerrero Asorey, L.; Sánchez-Vega, R.; Rentería-Monterrubio, A.L.; Chávez-Flores, D.; Tirado-Gallegos, J.M.; Méndez-Zamora, G. Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annuum) on Beef Hamburgers. Foods 2022, 11, 2018. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Cordova, M.G.; Jacobo, Á.S.; Cansino, N.D.S.C.; Moreno, E.R.; Rojas, Q.Y.Z.; Ortega, J.A.-A.; García, E.A. Capsaicin, Dihydrocapsaicin Content and Antioxidants Properties of Habanero Pepper (Capsicum chinense Jacq.) Oleoresin During Storage. Emir. J. Food Agric. 2021, 33, 583–588. [Google Scholar] [CrossRef]
- Scoville, W.L. Note on Capsicums. J. Am. Pharm. Assoc. 1912, 1, 453–454. [Google Scholar] [CrossRef]
- Lozada, D.N.; Coon, D.L.; Guzmán, I.; Bosland, P.W. Heat profiles of ‘superhot’ and New Mexican type chile peppers (Capsicum spp.). Sci. Hortic. 2021, 283, 110088. [Google Scholar] [CrossRef]
- Olguín-Rojas, J.A.; Vázquez-León, L.A.; Palma, M.; Fernández-Ponce, M.T.; Casas, L.; Fernández Barbero, G.; Rodríguez-Jimenes, G.D.C. Re-Valorization of Red Habanero Chili Pepper (Capsicum chinense Jacq.) Waste by Recovery of Bioactive Compounds: Effects of Different Extraction Processes. Agronomy 2024, 14, 660. [Google Scholar] [CrossRef]
- Lu, M.; Ho, C.-T.; Huang, Q. Extraction, bioavailability, and bioefficacy of capsaicinoids. J. Food Drug Anal. 2017, 25, 27–36. [Google Scholar] [CrossRef]
- Barbero, G.; Liazid, A.; Palma, M.; Barroso, C. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of the compounds and characteristics of pepper seed oil by pressure-assisted, ultrasound-assisted and conventional solvent extraction. Innov. Food Sci. Emerg. Technol. 2019, 54, 78–86. [Google Scholar] [CrossRef]
- Peshkovsky, A.S. From Research to Production. In Ultrasound: Advances for Food Processing and Preservation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 409–423. [Google Scholar]
- Johnson, J.B.; Mani, J.S.; Naiker, M. Correlations between Capsaicin, Dihydrocapsaicin and Phenolic Content in Habanero Chillies. In Proceedings of the 2nd International Electronic Conference on Foods—Future Foods and Food Technologies for a Sustainable World, Online, 15–30 October 2021; p. 30. [Google Scholar]
- Carreira-Casais, A.; Carpena, M.; Pereira, A.G.; Chamorro, F.; Soria-Lopez, A.; Perez, P.G.; Otero, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Critical Variables Influencing the Ultrasound-Assisted Extraction of Bioactive Compounds—A Review. In Proceedings of the 1st International Electronic Conference on Chemical Sensors and Analytical Chemistry, Online, 1–15 July 2021; p. 50. [Google Scholar]
- Majid, H.; Silva, F.V.M. Optimisation of ultrasound assisted extraction of antiacetylcholinesterase and antioxidant compounds from manuka (Leptospermum scoparium) for use as a phytomedicine against Alzheimer’s disease. N. Z. J. For. Sci. 2020, 50, 12. [Google Scholar] [CrossRef]
- Alzaabi, S.A.; Chia, W.Y.; Show, P.L. Exploring the potential of circular economy in the food sector. Syst. Microbiol. Biomanufacturing 2024, 4, 620–630. [Google Scholar] [CrossRef]
- Accorsi, R.; Manzini, R.; Pini, C.; Penazzi, S. On the design of closed-loop networks for product life cycle management: Economic, environmental and geography considerations. J. Transp. Geogr. 2015, 48, 121–134. [Google Scholar] [CrossRef]
- EU DIRECTIVE. Directive 2009/32/EC of the European Parliament and of the Council on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients. Available online: http://data.europa.eu/eli/dir/2009/32/oj (accessed on 1 October 2024).
- AOAC 995.03; Capsaicinoids in Capsicums and Their Extractives; AOAC: Rockville, MD, USA, 2000; pp. 14–16.
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2018, 101, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Castellar, M.; Obón, J.; Fernández-López, J. The isolation and properties of a concentrated red-purple betacyanin food colourant from Opuntia stricta fruits. J. Sci. Food Agric. 2006, 86, 122–128. [Google Scholar] [CrossRef]
- Ying, Q.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzińska, M. Phytochemical Content, Oxidative Stability, and Nutritional Properties of Unconventional Cold-pressed Edible Oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef]
- Cárdenas-Castro, A.P.; Perales-Vázquez, G.D.C.; De La Rosa, L.A.; Zamora-Gasga, V.M.; Ruiz-Valdiviezo, V.M.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Sauces: An undiscovered healthy complement in Mexican cuisine. Int. J. Gastron. Food Sci. 2019, 17, 100154. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Hernández-Ogarcía, S.G. Effect of Cooking Processes on the Contents of Two Bioactive Carotenoids in Solanum lycopersicum Tomatoes and Physalis ixocarpa and Physalis philadelphica Tomatillos. Molecules 2007, 12, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Atalay, A.B.; İNanç, A.L. The Applications of Green Extraction: Production and Quality Characterization of Seed Oils Extracted from Red Pepper (Capsicum Annuum L.) Waste. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Doğa Derg. 2023, 26, 150–160. [Google Scholar] [CrossRef]
- Astráin-Redín, L.; Ciudad-Hidalgo, S.; Raso, J.; Condón, S.; Cebrián, G.; Álvarez, I. Application of High-Power Ultrasound in the Food Industry. In Sonochemical Reactions; Karakuş, S., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Marhamati, M.; Kheirati Kakhaki, Z.; Rezaei, M. Advance in Ultrasound Assisted Extraction of Edible Oils: A Review. J. Nutr. Health 2020, 8, 220–230. [Google Scholar] [CrossRef]
- Rafajlovska, V.; Slaveska-Raicki, R.; Klopcevska, J.; Srbinosk, M. Extraction of Oleoresin from Pungent Red Paprika Under Different Conditions. In Mass Transfer in Chemical Engineering Processes; Marko, J., Ed.; InTech: Houston, TX, USA, 2011. [Google Scholar]
- Orellana-Escobedo, L.; Garcia-Amezquita, L.E.; Olivas, G.I.; Ornelas-Paz, J.J.; Sepulveda, D.R. Capsaicinoids content and proximate composition of Mexican chili peppers (Capsicum spp.) cultivated in the State of Chihuahua: Contenido de capsaicinoides y composición proximal de chiles mexicanos (Capsicum spp.) cultivados en el estado de Chihuahua. CyTA J. Food. 2013, 11, 179–184. [Google Scholar] [CrossRef]
- Gallego, M.R. Oleorresinas de capsicum en la industria alimentaria. Rev. Lasallista Investig. 2006, 3, 43–47. [Google Scholar]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]

| Factorial Points | Central Points | Star Points | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| A | −1 | −1 | 1 | 1 | 0 | 0 | 0 | 0 | α | −α | 0 | 0 |
| B | 1 | −1 | −1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | α | −α |
| time | 5 | 5 | 15 | 15 | 10 | 10 | 10 | 10 | 17 | 3 | 10 | 10 |
| APD | 0.90 | 0.40 | 0.40 | 0.90 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 1.00 | 0.30 |
| Run | UAE Conditions | Responses | ||||
| Time (min) | APD (W/mL) | Efficiency Parameters | ||||
| E (W·h) | T (°C) | Y (%) | ||||
| 01 | 5 | 0.90 | 11.37 ± 0.07 | 54.67 ± 0.58 | 24.40 ± 0.24 | |
| 02 | 5 | 0.40 | 5.06 ± 0.05 | 34.33 ± 0.58 | 17.50 ± 0.36 | |
| 03 | 15 | 0.40 | 15.25 ± 0.00 | 57.67 ± 0.58 | 22.22 ± 0.16 | |
| 04 | 15 | 0.90 | 33.92 ± 0.14 | 82.67 ± 2.52 | 25.16 ± 0.36 | |
| 05 | 10 | 0.65 | 16.39 ± 0.10 | 64.33 ± 0.58 | 25.13 ± 0.16 | |
| 06 | 10 | 0.65 | 16.39 ± 0.10 | 63.67 ± 0.58 | 25.36 ± 0.47 | |
| 07 | 10 | 0.65 | 16.39 ± 0.10 | 64.67 ± 0.58 | 25.17 ± 0.16 | |
| 08 | 10 | 0.65 | 16.39± 0.10 | 64.00 ± 0.00 | 25.21 ± 0.24 | |
| 09 | 17 | 0.65 | 27.86 ± 0.16 | 73.0 ± 1.00 | 25.67 ± 0.41 | |
| 10 | 3 | 0.65 | 4.92 ± 0.03 | 33.67 ± 0.58 | 19.29 ± 0.19 | |
| 11 | 10 | 1.00 | 25.06 ± 0.10 | 71.33 ± 0.58 | 26.54 ± 0.49 | |
| 12 | 10 | 0.30 | 7.56 ± 0.10 | 43.67 ± 1.15 | 18.23 ± 0.37 | |
| Run | UAE Conditions | Responses | ||||
| Time (min) | APD (W/mL) | Chemical parameters | ||||
| CAP | TPC | FRAP | DPPH | |||
| 01 | 5 | 0.90 | 6.31 ± 0.01 | 3.74 ± 0.13 | 122.91 ± 2.33 | 24.38 ± 0.61 |
| 02 | 5 | 0.40 | 3.60 ± 0.02 | 1.90 ± 0.10 | 61.87 ± 0.59 | 6.33 ± 0.14 |
| 03 | 15 | 0.40 | 5.98 ± 0.00 | 3.06 ± 0.08 | 106. 99 ± 1.49 | 16.57 ± 0.19 |
| 04 | 15 | 0.90 | 8.14 ± 0.22 | 5.00 ± 0.02 | 118.03 ± 0.78 | 27.52 ± 0.14 |
| 05 | 10 | 0.65 | 7.29 ± 0.36 | 4.20 ± 0.03 | 132.73 ± 1.78 | 28.38 ± 0.76 |
| 06 | 10 | 0.65 | 7.02 ± 0.12 | 4.20 ± 0.03 | 131.43 ± 0.94 | 27.17 ± 0.29 |
| 07 | 10 | 0.65 | 6.94 ± 0.10 | 4.21 ± 0.02 | 131.99 ± 0.38 | 27.92 ± 0.29 |
| 08 | 10 | 0.65 | 6.71 ± 0.30 | 4.21 ± 0.02 | 131.01 ± 1.29 | 27.87 ± 0.81 |
| 09 | 17 | 0.65 | 7.29 ± 0.02 | 4.31 ± 0.03 | 134.40 ± 3.49 | 32.64 ± 0.45 |
| 10 | 3 | 0.65 | 6.04 ± 0.06 | 2.56 ± 0.11 | 87.63 ± 1.12 | 16.47 ± 0.24 |
| 11 | 10 | 1.00 | 8.28 ± 0.54 | 5.05 ± 0.43 | 133.81 ± 1.74 | 30.78 ± 0.49 |
| 12 | 10 | 0.30 | 4.90 ± 0.25 | 2.07 ± 0.06 | 70.52 ± 0.59 | 12.94 ± 0.45 |
| Run | UAE Conditions | Responses | ||||
| Time (min) | APD (W/mL) | Color parameters | ||||
| L* | a* | b* | ΔE | |||
| 01 | 5 | 0.90 | 86.51 ± 1.23 | 12.90 ± 2.59 | 65.68 ± 1.00 | 62.92 ± 1.22 |
| 02 | 5 | 0.40 | 91.26 ± 0.96 | 10.55 ± 0.63 | 56.63 ± 1.61 | 52.75 ± 1.51 |
| 03 | 15 | 0.40 | 84.62 ± 0.88 | 14.47 ± 1.53 | 71.52 ± 2.29 | 69.20 ± 2.70 |
| 04 | 15 | 0.90 | 90.53 ± 1.27 | 10.38 ± 1.20 | 59.77 ± 4.64 | 55.85 ± 4.50 |
| 05 | 10 | 0.65 | 80.90 ± 0.96 | 16.76 ± 1.39 | 76.05 ± 0. 42 | 74.96 ± 0.50 |
| 06 | 10 | 0.65 | 78.94 ± 1.32 | 18.63 ± 0.59 | 77.42 ± 0.78 | 77.25 ± 0.80 |
| 07 | 10 | 0.65 | 80.64 ± 1.11 | 17.89 ± 1.73 | 76.50 ± 0.96 | 75.74 ± 1.61 |
| 08 | 10 | 0.65 | 82.45 ± 2.50 | 16.97 ± 0.92 | 76.46 ± 1.95 | 75.01 ± 1.97 |
| 09 | 17 | 0.65 | 88.80 ± 0.76 | 12.66 ± 2.32 | 62.31 ± 1.09 | 59.16 ± 1.32 |
| 10 | 3 | 0.65 | 89.65 ± 0.79 | 12.11 ± 0.62 | 59.29 ± 1.44 | 55.97 ± 1.19 |
| 11 | 10 | 1.00 | 88.04 ± 1.14 | 13.55 ± 1.15 | 76.89 ± 0.40 | 73.41 ± 0.72 |
| 12 | 10 | 0.30 | 88.61 ± 1.51 | 12.67 ± 0.57 | 62.84 ± 0.32 | 59.68 ± 0.72 |
| Responses | Units | R2 | Prediction Equation |
|---|---|---|---|
| Energy Consumption | (W·h) | 1.00 | –0.19 + 0.03(A) + 0.29(B) + 2.47 (AB) |
| Final Temperature | °C | 0.99 | –23.49 + 6.07(A) + 92.54(B) + 0.93(AB) − 0.20(A2) − 45.70(B2) |
| Extraction Yield | % (d.b.) | 0.97 | –6.12 + 2.01(A) + 49.11(B) − 0.79(AB) − 0.06(A2) − 23.34(B2) |
| Capsaicinoids Content | mg NVA/g (d.b.) | 0.87 | –1.41 + 0.41(A) + 12.38(B) − 0.01(A2) − 5.79(B2) |
| Total Phenolic Content | mg GAE/g (d.b.) | 0.98 | –3.59 + 0.45(A) + 11.17(B) − 0.02(A2) − 5.50(B2) |
| FRAP | μmol FeSO4 eq/g, (d.b.) | 0.97 | –167.35 + 18.40(A) + 515.15(B) − 10.00(AB) − 0.46(A2) − 256.91(B2) |
| DPPH | μmol TEAC/g, (d.b.) | 0.90 | –46.15 + 4.25(A) + 119.10(B) − 1.42(AB) − 0.12(A2) − 58.09(B2) |
| Color Variation (ΔE) | — | 0.92 | –28.78 + 1.98(A) + 70.36(B) − 0.05(A2) − 37.16(B2) |
| Ultrasound Optimized Extraction Conditions (Time: 8 min|APD: 0.87 W/mL) | Reflux Extraction (Time: 300 min|85 °C) | ||
|---|---|---|---|
| Responses | Predicted Confidence Interval (95%) | Experimental Values * | Experimental Values * |
| E (W·h) | [17.39–17.63] | 17.44 ± 0.06 a | 387.87 ± 0.29 b |
| T (°C) | [62.64–66.74] | 66.00 ± 1.00 a | 82.63 ± 3.72 b |
| Y (%) | [25.14–26.60] | 26.03 ± 0.65 a | 20.88 ± 0.66 b |
| CAP (mg NVA/g d.b.) | [6.66–8.19] | 7.47 ± 0.32 a | 7.61 ± 0.64 a |
| TPC (mg GAE/g d.b.) | [4.32–4.69] | 4.29 ± 0.26 a | 2.41 ± 0.04 b |
| FRAP (μmol FeSO4 eq/g d.b.) | [128.86–140.05] | 138.77 ± 3.84 a | 115.06 ± 4.51 b |
| DPPH (μmol TEAC/g d.b.) | [26.42–33.29] | 32.91 ± 2.88 a | 12.33 ± 0.62 b |
| Color Variation (ΔE) | [62.67–76.69] | 75.38 ± 3.95 a | 65.86 ± 0.88 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, A.; Silva, A.F.R.; Ramos, M.P.; Komora, N.; Silva, F.V.M.; Fradinho, P. Spicy Food Ingredient from Red Habanero By-Product Obtained by Ultrasound-Assisted Extraction. Foods 2025, 14, 1407. https://doi.org/10.3390/foods14081407
Toscano A, Silva AFR, Ramos MP, Komora N, Silva FVM, Fradinho P. Spicy Food Ingredient from Red Habanero By-Product Obtained by Ultrasound-Assisted Extraction. Foods. 2025; 14(8):1407. https://doi.org/10.3390/foods14081407
Chicago/Turabian StyleToscano, António, Andreia F. R. Silva, Maria P. Ramos, Norton Komora, Filipa V. M. Silva, and Patrícia Fradinho. 2025. "Spicy Food Ingredient from Red Habanero By-Product Obtained by Ultrasound-Assisted Extraction" Foods 14, no. 8: 1407. https://doi.org/10.3390/foods14081407
APA StyleToscano, A., Silva, A. F. R., Ramos, M. P., Komora, N., Silva, F. V. M., & Fradinho, P. (2025). Spicy Food Ingredient from Red Habanero By-Product Obtained by Ultrasound-Assisted Extraction. Foods, 14(8), 1407. https://doi.org/10.3390/foods14081407








