Enhancement of Nutritional Value and Sensory Characteristics of Quinoa Fermented Milk via Fermentation with Specific Lactic Acid Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Strain Activation and Culture
2.3. Quinoa Fermentation
2.4. Color Measurement
2.5. Extraction and Measurement of Protein
2.6. Extraction and Measurement of Amylose and Amylopectin
2.7. Extraction and Measurement of Total Sugar
2.8. Extraction and Measurement of Dietary Fiber
2.9. Extraction and Determination of Free and Bound Phenolics
2.10. Determination of Antioxidant Activities of Free Phenolics Extracted from Fermented and Unfermented Quinoa
2.10.1. DPPH Radical Scavenging Ability
2.10.2. ABTS Radical Scavenging Ability
2.11. In Vitro Gastrointestinal Digestion and Bioaccessibility
2.12. Determination of Saponins
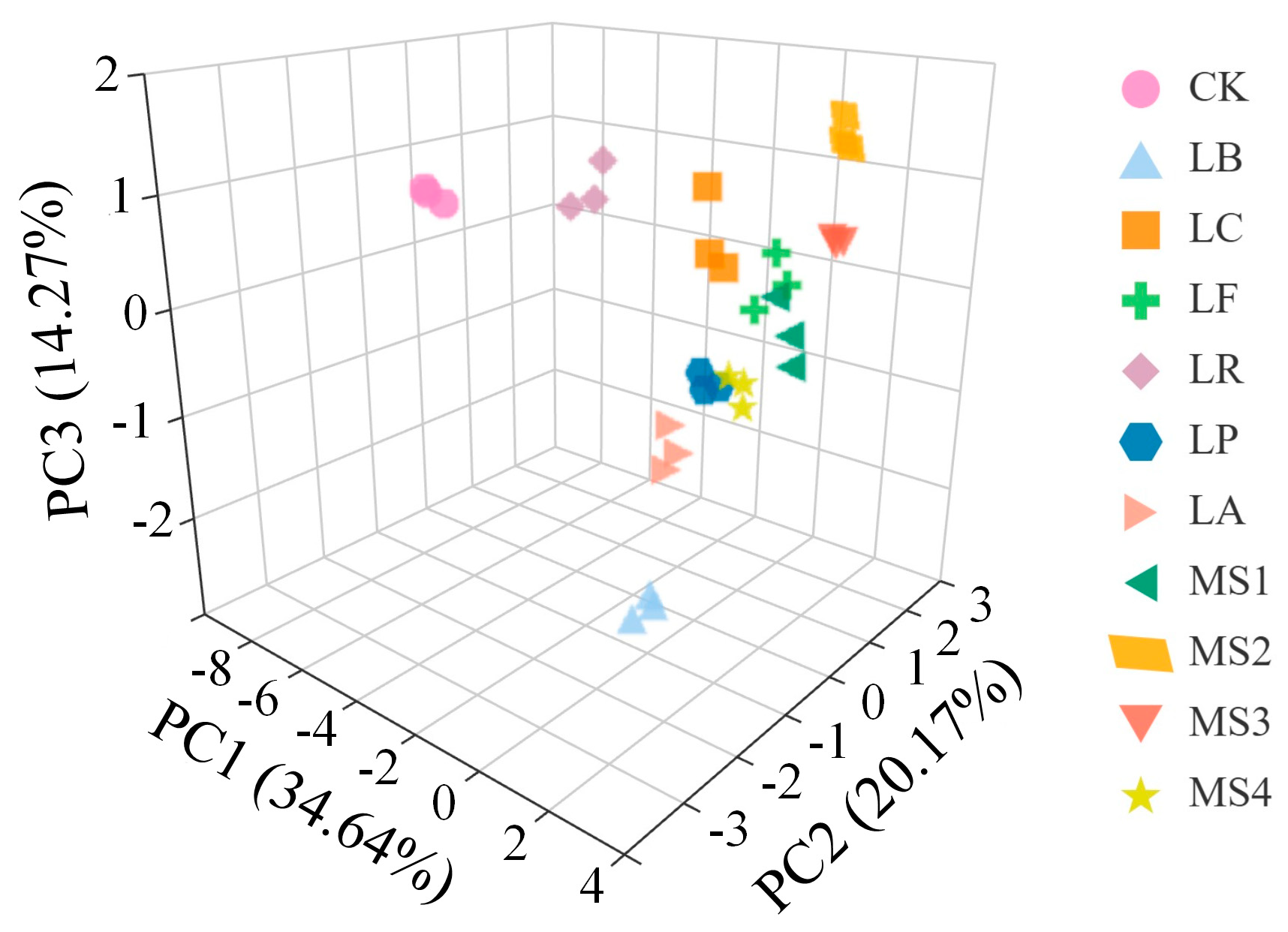
2.13. Strain Screening Using PCA
2.14. Preparation and Analysis of Quinoa Fermented Milk
2.14.1. Determination of pH and Titratable Acidity (TA)
2.14.2. Rheological Analysis of Quinoa Fermented Milk
2.14.3. Sensory Evaluation Measurements
2.14.4. Electronic Tongue Determination
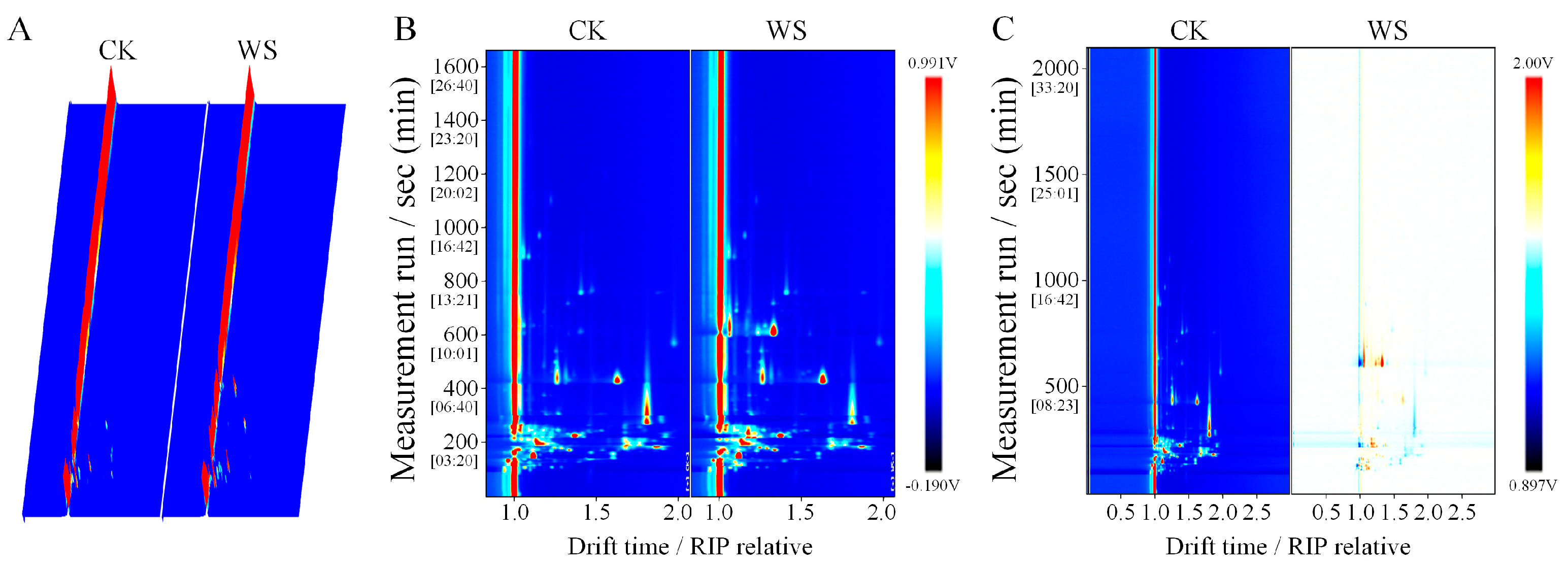
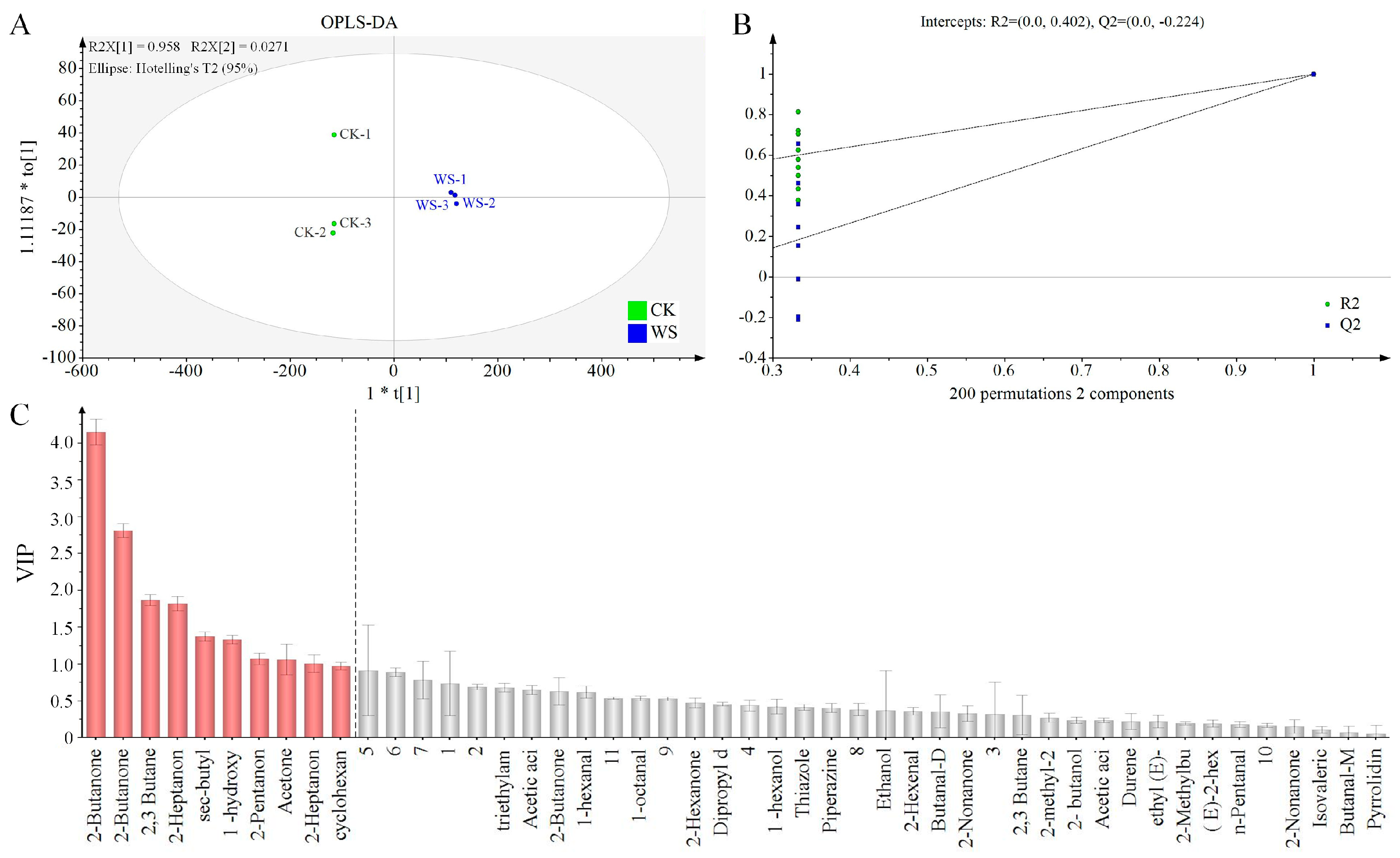
2.14.5. GC-IMS Measurement
2.14.6. Determination of Viable Bacterial Count and Color
2.14.7. Determination of WHC and Suspension Stability
2.15. Statistical Analysis
3. Results and Discussion
3.1. Impact of Fermentation Using Different Strains on Quinoa Color
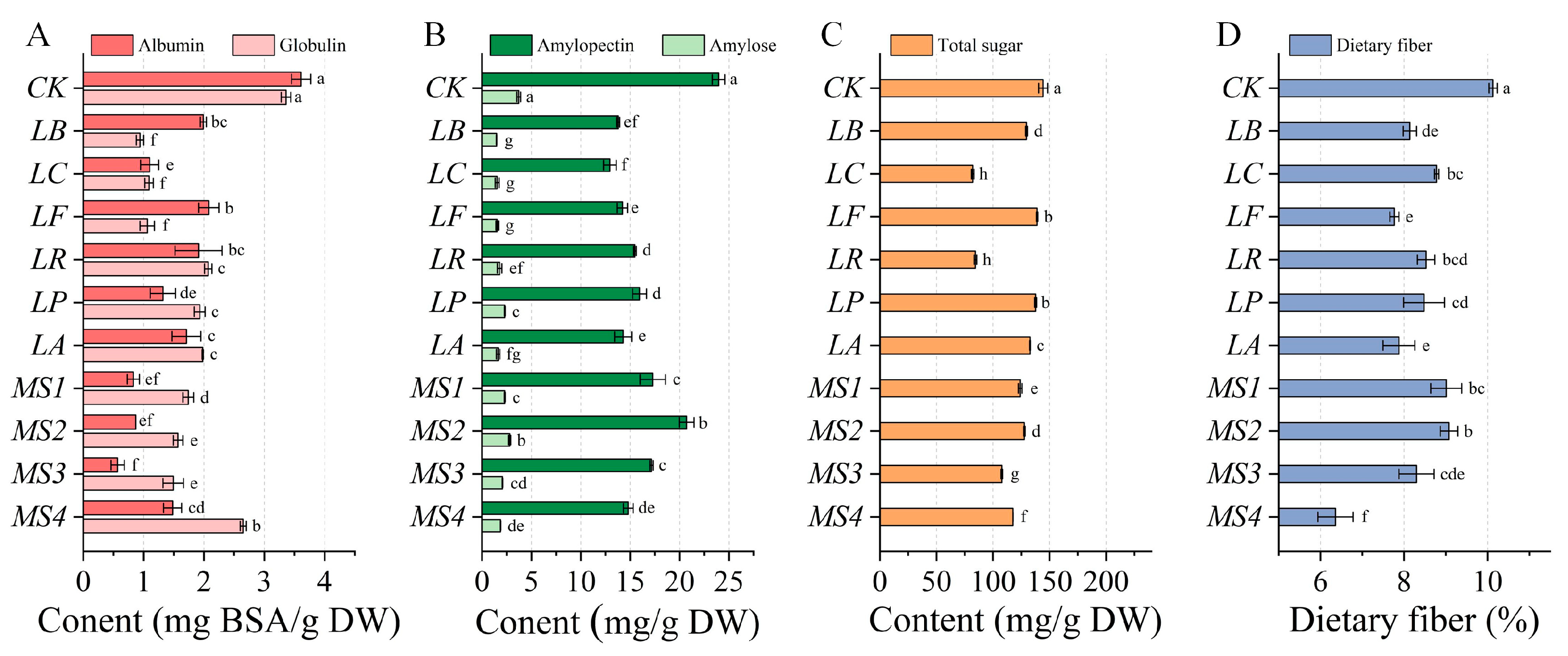
3.2. Effects of Fermentation Using Various Strains on Nutrient Content of Quinoa
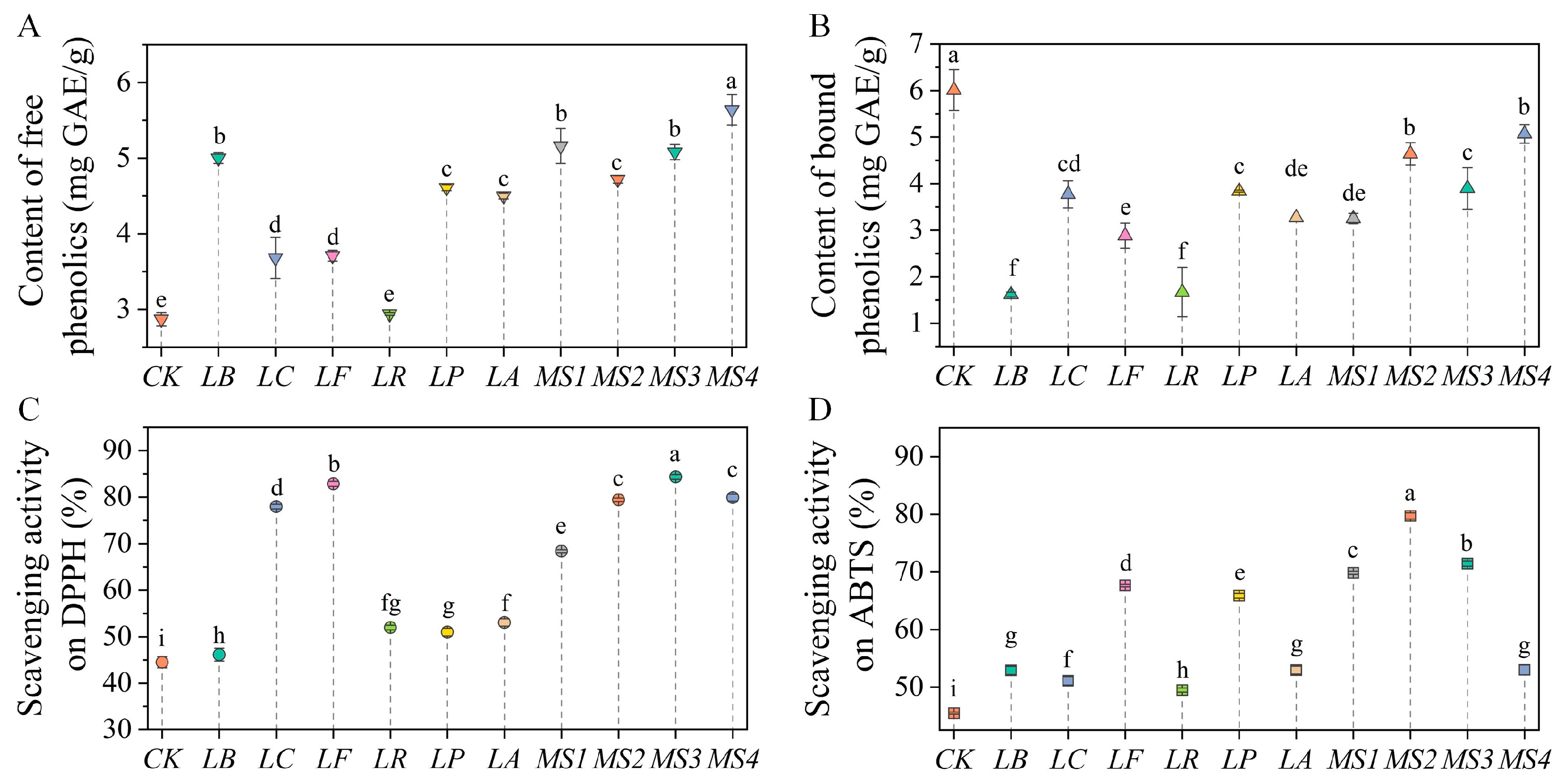
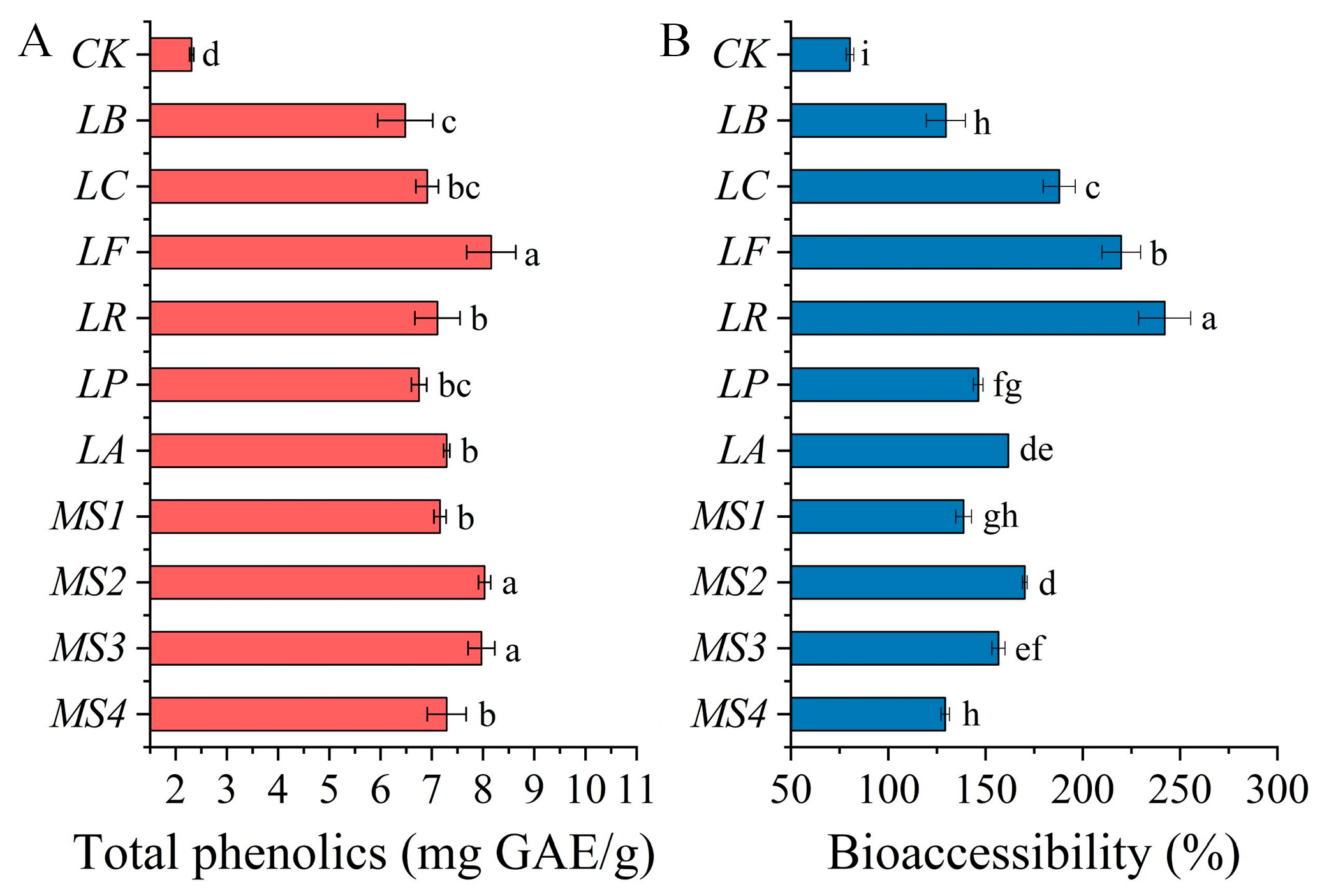
3.3. Effects of Fermentation on Phenolic Content and Antioxidant Ability of Quinoa
3.4. Effects of Fermentation on Bioaccessibility of Phenolics in Quinoa
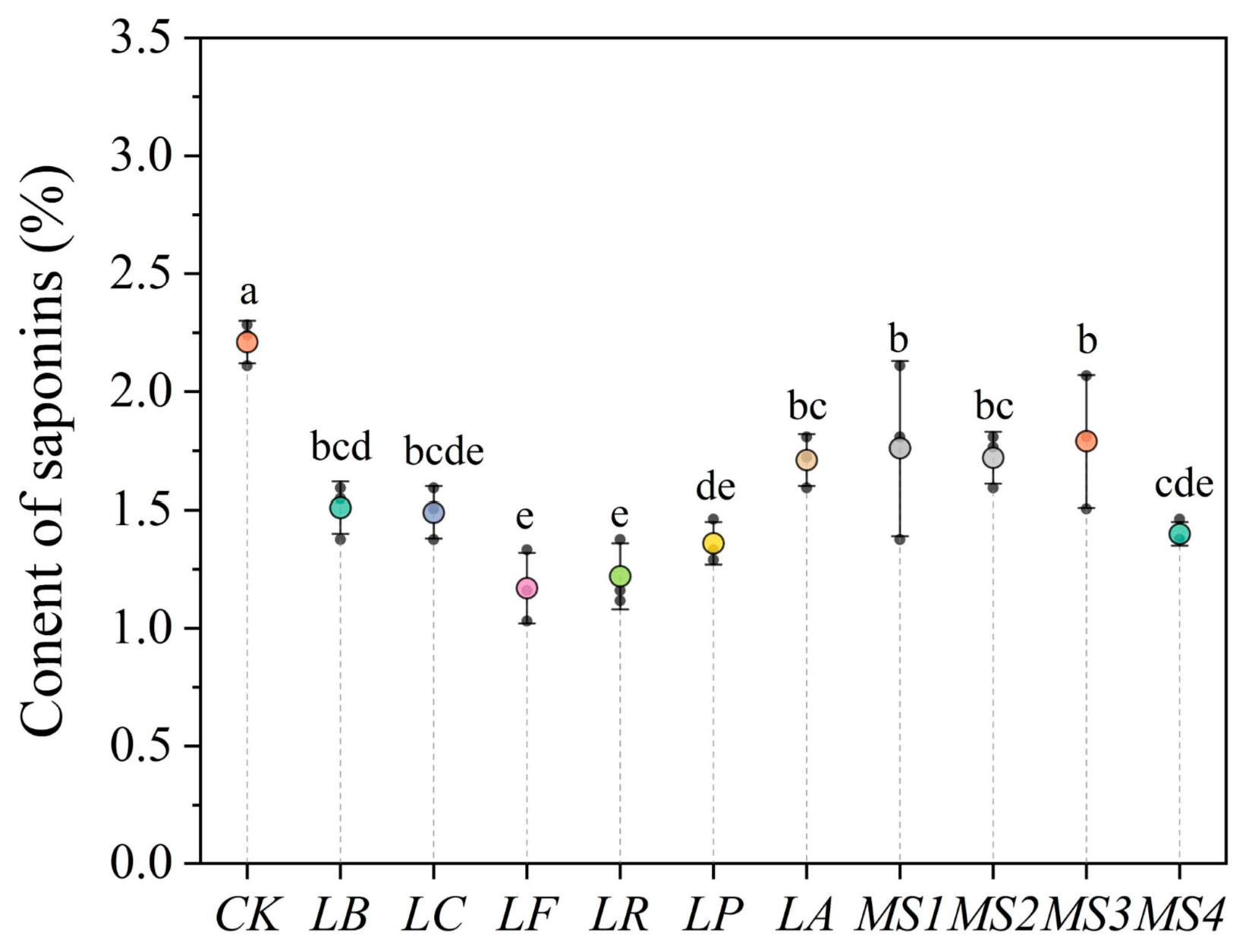
3.5. Effects of Fermentation Using Various Strains on Saponin Content in Quinoa
3.6. Selection of Better Starter Culture for Quinoa Fermentation Based on Principal Component Analysis
3.7. Physical and Chemical Indicators of Quinoa Fermented Milk
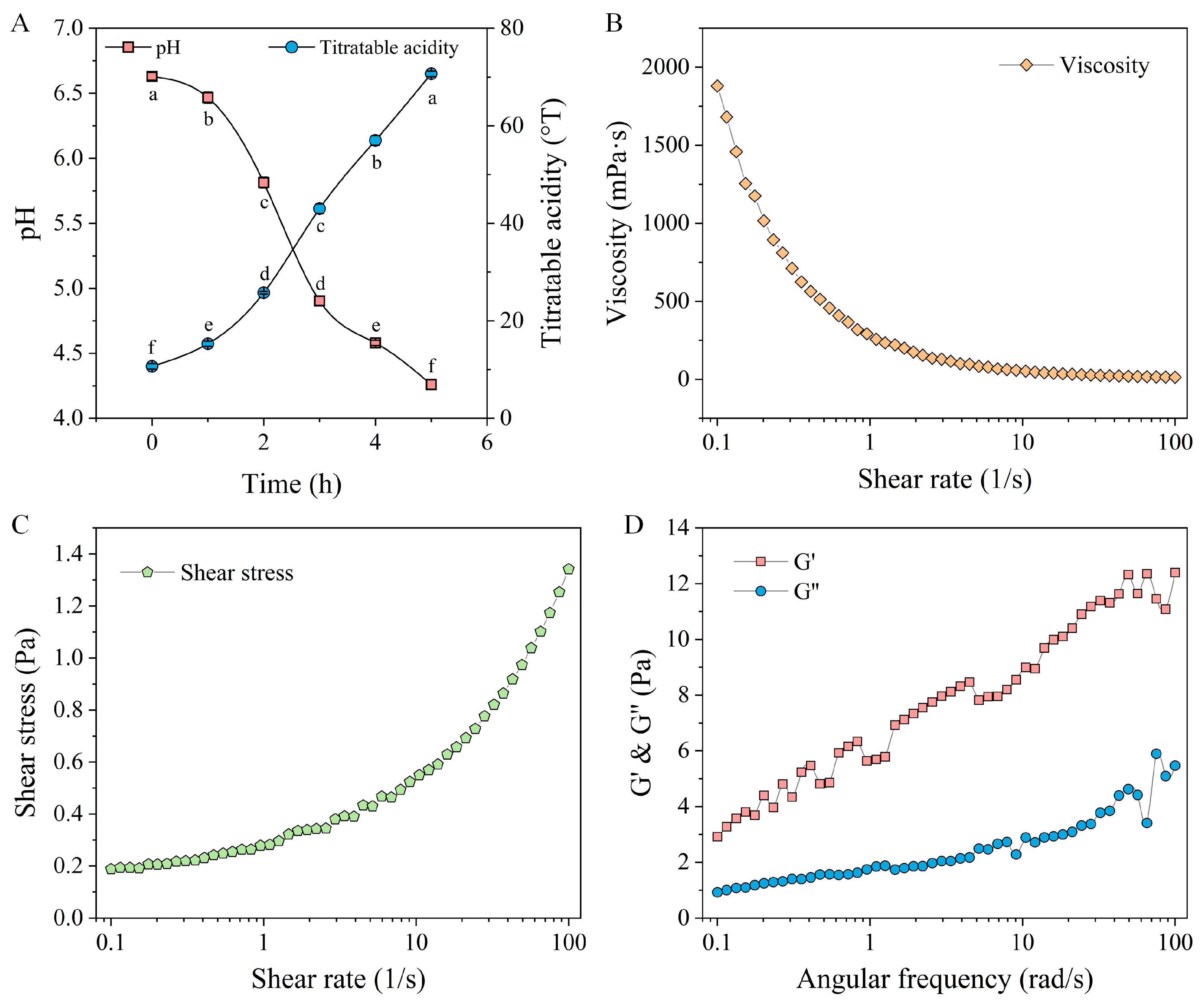
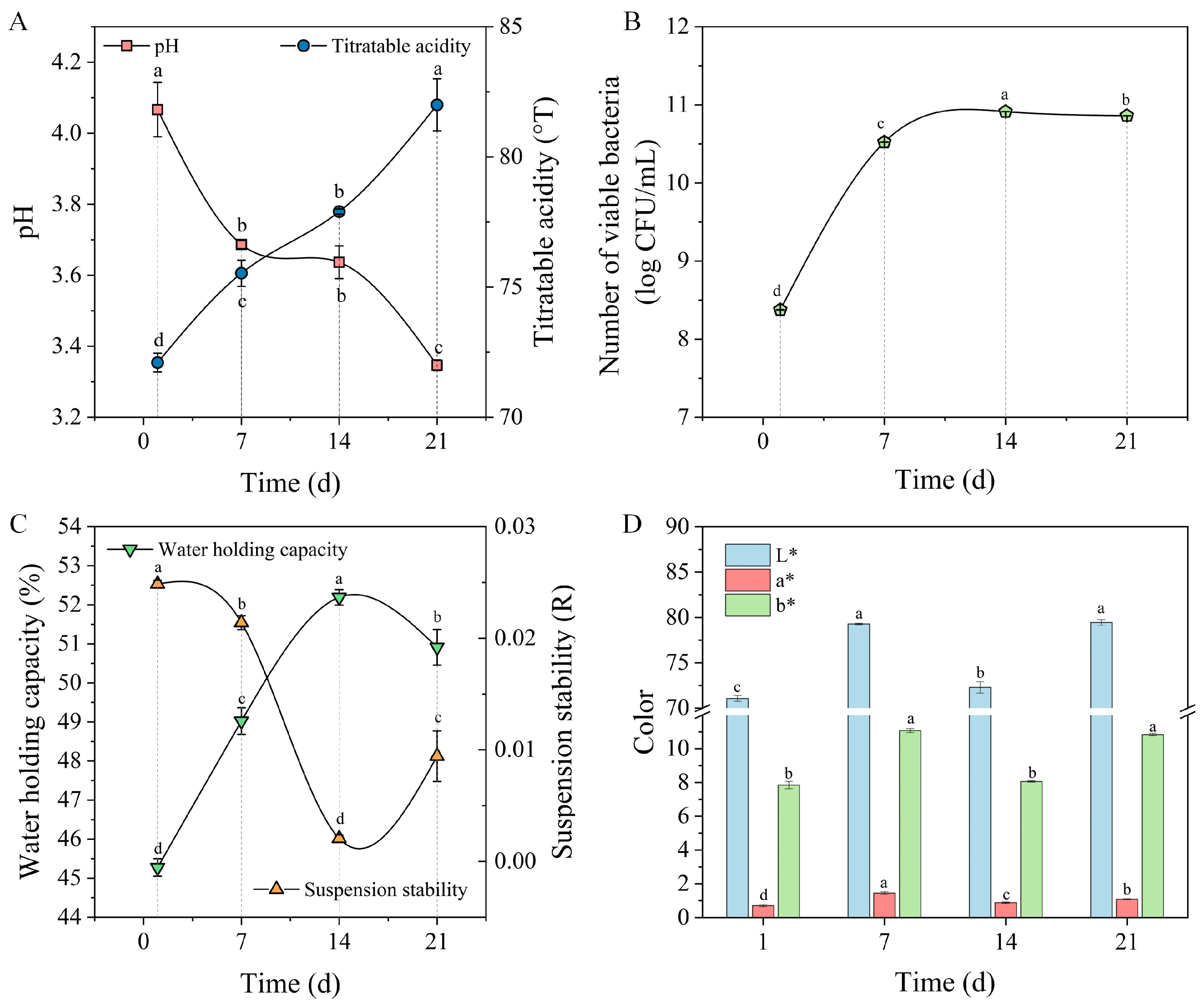
3.7.1. Variations in pH and TA During Fermentation Stage
3.7.2. Rheological Properties
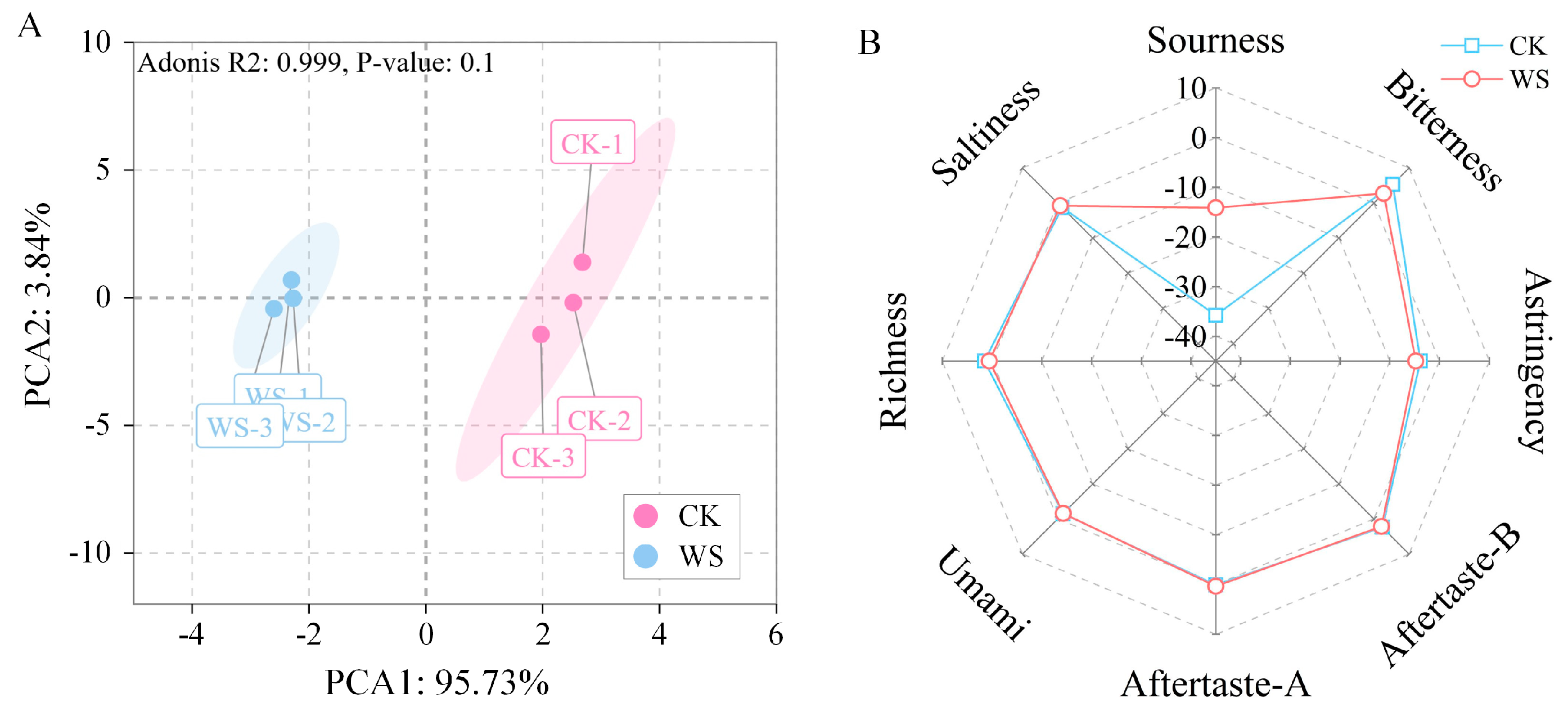
3.7.3. Sensory Profiling
3.7.4. Stability Evaluation During Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelshafy, A.M.; Rashwan, A.K.; Osman, A.I. Potential food applications and biological activities of fermented quinoa: A review. Trends Food Sci. Technol. 2024, 144, 104339. [Google Scholar] [CrossRef]
- Maradini, A.M.; Pirozi, M.R.; Borges, J.T.D.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar]
- Huang, K.; Liu, Y.; Zhang, Y.; Cao, H.; Luo, D.-k.; Yi, C.; Guan, X. Formulation of plant-based yoghurt from soybean and quinoa and evaluation of physicochemical, rheological, sensory and functional properties. Food Biosci. 2022, 49, 101831. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Xing, B.; Sun, M.H.; Zhou, B.W.; Ren, G.X.; Qin, P.Y. Changes in bio-accessibility, polyphenol profile and antioxidants of quinoa and djulis sprouts during in vitro simulated gastrointestinal digestion. Food Sci. Nutr. 2020, 8, 4232–4241. [Google Scholar] [CrossRef]
- Reddy, M.V.K.; Dubey, P.K.; Mishra, A.A.; Ahada Sabeel, V. Potential processing techniques for safe utilisation of pseudo cereals in the food system. J. Food Compos. Anal. 2024, 135, 106609. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Iafelice, G.; Verardo, V.; Marconi, E.; Caboni, M.F. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd). Food Chem. 2014, 157, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V. Impact of Fermentation on Phenolic Compounds and Antioxidant Capacity of Quinoa. Fermentation 2021, 7, 20. [Google Scholar] [CrossRef]
- Canaviri-Paz, P.; Oscarsson, E.; Kjellström, A.; Olsson, H.; Jois, C.; Håkansson, Å. Effects on Microbiota Composition after Consumption of Quinoa Beverage Fermented by a Novel Xylose-Metabolizing L. plantarum Strain. Nutrients 2021, 13, 3318. [Google Scholar] [CrossRef]
- Ibrahem, E.; Mohamed, G. Effect of Different preparatory processes on Improving the Nutritional Value of Quinoa (Chenopodium quinoa, Willd) and Studying its Effect on Obese Anemic Rats. Egypt. J. Nutr. Health 2021, 16, 71–94. [Google Scholar] [CrossRef]
- Li, S.L.; Chen, C.; Ji, Y.X.; Lin, J.; Chen, X.M.; Qi, B. Improvement of nutritional value, bioactivity and volatile constituents of quinoa seeds by fermentation with Lactobacillus casei. J. Cereal Sci. 2018, 84, 83–89. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.Q.; Mesmari, N.; Dahmani, S.; Al Dhaheri, A.S.; Kizhakkayil, J. In vitro investigation of bioactivities of solid-state fermented lupin, quinoa and wheat using Lactobacillus spp. Food Chem. 2019, 275, 50–58. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Gaide, I.; Basinskiene, L. Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread. Foods 2021, 10, 171. [Google Scholar] [CrossRef]
- Muñoz Pabon, K.S.; Hoyos Concha, J.L.; Solanilla Duque, J.F. Quinoa extruded snacks with probiotics: Physicochemical and sensory properties. Front. Sustain. Food Syst. 2022, 6, 935425. [Google Scholar] [CrossRef]
- Le, L.Q.; Gong, X.X.; An, Q.; Xiang, D.B.; Zou, L.; Peng, L.X.; Wu, X.Y.; Tan, M.L.; Nie, Z.L.; Wu, Q.; et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021, 357, 129752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, T.; Zhang, Y.L.; Chen, Y.J.; Ge, X.F.; Sui, W.J.; Zhu, Q.M.; Geng, J.T.; Zhang, M. Release of bound polyphenols from wheat bran soluble dietary fiber during simulated gastrointestinal digestion and colonic fermentation in vitro. Food Chem. 2023, 402, 134111. [Google Scholar] [CrossRef]
- Gao, Y.; Ping, H.; He, Z.Y.; Liu, J.; Zhao, M.; Ma, Z.H. Characterization of the active components and bioaccessibility of phenolics in differently colored foxtail millets. Food Chem. 2024, 452, 139355. [Google Scholar] [CrossRef]
- Shi, F.F.; Wang, L.; Li, S.R. Enhancement in the physicochemical properties, antioxidant activity, volatile compounds, and non-volatile compounds of watermelon juices through Lactobacillus plantarum JHT78 fermentation. Food Chem. 2023, 420, 136146. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhu, X.L.; Huang, Q.; Zhang, L.; Liu, X.H.; Liu, R.; Lu, Q. Antioxidant and anti-inflammatory activities of rape bee pollen after fermentation and their correlation with chemical components by ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry-based untargeted metabolomics. Food Chem. 2023, 409, 135342. [Google Scholar] [CrossRef]
- Starzynska-Janiszewska, A.; Stodolak, B.; Dulinski, R.; Fernández-Fernández, C.; Martín-García, B.; Gómez-Caravaca, A.M. Evaluation of saponin and phenolic profile of quinoa seeds after fungal fermentation. J. Cereal Sci. 2023, 111, 103656. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Liu, Y.F.; Zhang, Q.Y.; Li, Y.X.; Wu, Z.Q. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
- León-Roque, N.; Aguilar-Tuesta, S.; Quispe-Neyra, J.; Mamani-Navarro, W.; Alfaro-Cruz, S.; Condezo-Hoyos, L. A green analytical assay for the quantitation of the total saponins in quinoa (Chenopodium quinoa Willd.) based on macro lens-coupled smartphone. Talanta 2019, 204, 576–585. [Google Scholar] [PubMed]
- Zhang, J.; Zhong, Y.; Wang, D.; Deng, Y.; Li, Y.; Liu, C.; Wang, J.L.T. Effect of mixed fermentation of Lactiplantibacillus plantarum and Lactiplantibacillus pentosus on phytochemical and flavor characteristics of Wallace melon juice. J. Sci. Food Agric. 2024, 104, 3776–3787. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.X.; Shen, Y.B.; Yu, H.Y.; He, Y.J.; Chen, C. Effects of 4 Probiotic Strains in Coculture with Traditional Starters on the Flavor Profile of Yogurt. J. Food Sci. 2017, 82, 1693–1701. [Google Scholar] [CrossRef]
- Mu, H.; Dai, T.; Huang, S.; Wu, K.; Wang, M.; Tan, C.; Zhang, F.; Sheng, J.; Zhao, C. Physical and Chemical Properties, Flavor and Organoleptic Characteristics of a Walnut and Purple Rice Fermented Plant Drink. Foods 2024, 13, 400. [Google Scholar] [CrossRef]
- Hurtado-Murillo, J.; Franco, W.; Contardo, I. Impact of homolactic fermentation using Lactobacillus acidophilus on plant-based protein hydrolysis in quinoa and chickpea flour blended beverages. Food Chem. 2025, 463, 141110. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, D.; Kumar, G.; Devkota, L.; Subedi, D.; Dhital, S. The choice of probiotics affects the rheological, structural, and sensory attributes of lupin-oat-based yoghurt. Food Hydrocoll. 2024, 156, 110353. [Google Scholar] [CrossRef]
- Weijia, W.; Xiaoxiayue, G.; Aiguo, L.; Lizeng, L.; Pengcheng, W.; Yi, Y. Quality comparison of lactose free yoghurt and ordinary yoghurt with different heat treatment. Food Ferment. Ind. 2021, 5, 99–104. [Google Scholar]
- Yuan, K.; Wu, G.; Li, X.; Zeng, Y.; Wen, X.; Liu, R.; Jiang, X.; Tian, L.; Sun, J.; Bai, W. Anthocyanins degradation mediated by β-glycosidase contributes to the color loss during alcoholic fermentation in a structure-dependent manner. Food Res. Int. 2024, 175, 113732. [Google Scholar] [CrossRef]
- Fernández, M.; Martín, A.; Benito, M.J.; Casquete, R.; Recio, I.; Córdoba, M.D.G. Influence of starter cultures on the generation of antioxidant nitrogen compounds in Iberian dry-fermented sausages. Int. J. Food Sci. Technol. 2016, 51, 435–443. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Guixing, R.; Cong, T.; Xin, F.; Shengyuan, G.; Gang, Z.; Lizhen, Z.; Zou, L.; Peiyou, Q. Nutrient composition, functional activity and industrial applications of quinoa (Chenopodium quinoa Willd.). Food Chem. 2023, 410, 135290. [Google Scholar]
- Vicente-Sánchez, M.L.; Castro-Alija, M.J.; Jiménez, J.M.; María, L.V.; Jose, C.M.; Pastor, R.; Albertos, I. Influence of salinity, germination, malting and fermentation on quinoa nutritional and bioactive profile. Crit. Rev. Food Sci. Nutr. 2024, 64, 7632–7647. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C.G. Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.H.; Malleshi, N.G.; Koseki, T. Physico-chemical characteristics and non-starch polysaccharide contents of Indica and Japonica brown rice and their malts. Lwt-Food Sci. Technol. 2010, 43, 784–791. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, X.S.; Cao, X.R.; Iftikhar, M.; Wang, J. Semi-solid state fermentation and enzymatic hydrolysis impeded the destroy of wheat bran on gluten polymerization. LWT-Food Sci. Technol. 2018, 98, 306–313. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Soltani, M.; Hekmat, S.; Ahmadi, L. Microbial and sensory evaluation of probiotic yoghurt supplemented with cereal/pseudo-cereal grains and legumes. Int. J. Dairy Technol. 2018, 71, 141–148. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.H.; Chen, P.X.; Zhang, H.; Liu, R.H.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and α-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chu, G.Q.; Yang, P.; Liu, Y.Y.; Zhang, Y.; Guan, X.; Li, S.; Song, H.D.; Zhang, Y. Benefits of Monascus anka solid-state fermentation for quinoa polyphenol bioaccessibility and the anti-obesity effect linked with gut microbiota. Food Funct. 2024, 15, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Zare, F.; Champagne, C.P.; Simpson, B.K.; Orsat, V.; Boye, J.I. Effect of the addition of pulse ingredients to milk on acid production by probiotic and yoghurt starter cultures. LWT-Food Sci. Technol. 2012, 45, 155–160. [Google Scholar] [CrossRef]
- Michalak, M.; Kubik-Komar, A.; Waśko, A.; Polak-Berecka, M. Starter culture for curly kale juice fermentation selected using principal component analysis. Food Biosci. 2020, 35, 100602. [Google Scholar] [CrossRef]
- Aktar, T. Physicochemical and sensory characterisation of different yoghurt production methods. Int. Dairy J. 2022, 125, 105245. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Formation and Physical Properties of Yogurt. Asian-Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Wang, X.Y.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105153. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Kanawjia, S.K.; Khetra, Y.; Puri, R. Effect of potassium-based emulsifying salts on sensory, textural, and functional attributes of low-sodium processed Mozzarella cheese. Dairy Sci. Technol. 2015, 95, 265–278. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef]
- Pachekrepapol, U.; Kokhuenkhan, Y.; Ongsawat, J. Formulation of yogurt-like product from coconut milk and evaluation of physicochemical, rheological, and sensory properties. Int. J. Gastron. Food Sci. 2021, 25, 100393. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Wang, C.; Tan, Q.; Liu, Y.; Chen, H.; Zhang, Y.; Zhang, Y.; Liu, S.; Suo, H. Improvement of brown yogurt quality by Lactiplantibacillus plantarum S58 and oat β-glucan: Physicochemical properties, sensory quality, and metabolic changes. Food Biosci. 2024, 62, 105308. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, K.; Zhang, Y.; Cao, H.; Luo, D.K.; Yi, C.; Guan, X. Manufacture and characterization of a novel dairy-free quinoa yogurt fermented by modified commercial starter with Weissella confusa. Food Chem. X 2023, 19, 100823. [Google Scholar] [CrossRef]
- Hoxha, R.; Evstatieva, Y.; Nikolova, D. Physicochemical, Rheological, and Sensory Characteristics of Yogurt Fermented by Lactic Acid Bacteria with Probiotic Potential and Bioprotective Properties. Foods 2023, 12, 2552. [Google Scholar] [CrossRef] [PubMed]
- Sudun; Wulijideligen; Arakawa, K.; Miyamoto, M.; Miyamoto, T. Interaction between lactic acid bacteria and yeasts in airag, an alcoholic fermented milk. Anim. Sci. J. 2013, 84, 66–74. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Shah, N.P. Probiotics—From Metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Brüls, M.; Foroutanparsa, S.; Olsthoorn, M.; Tas, R.P.; Voets, I.K. Investigating the impact of exopolysaccharides on yogurt network mechanics and syneresis through quantitative microstructural analysis. Food Hydrocoll. 2024, 150, 109629. [Google Scholar] [CrossRef]
- Nionelli, L.; Coda, R.; Curiel, J.A.; Poutanen, K.; Marco, G.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar]

| Group | Inoculation Ratio | Quinoa Mass (g) | Volume of Bacterial Suspension (mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LB | LC | LF | LR | LP | LA | Sterile Water | |||
| CK | — | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 30 |
| LB | Lactobacillus bulgaricus | 100 | 5 | 0 | 0 | 0 | 0 | 0 | 25 |
| LC | Lactobacillus casei | 100 | 0 | 5 | 0 | 0 | 0 | 0 | 25 |
| LF | Lactobacillus fermentum | 100 | 0 | 0 | 5 | 0 | 0 | 0 | 25 |
| LR | Lactobacillus rhamnosus | 100 | 0 | 0 | 0 | 5 | 0 | 0 | 25 |
| LP | Lactobacillus plantarum | 100 | 0 | 0 | 0 | 0 | 5 | 0 | 25 |
| LA | Lactobacillus acidophilus | 100 | 0 | 0 | 0 | 0 | 0 | 5 | 25 |
| MS1 | LA:LP:LB 1:1:1 | 100 | 1.667 | 0 | 0 | 0 | 1.667 | 1.667 | 25 |
| MS2 | LR:LF:LC 1:1:1 | 100 | 0 | 1.667 | 1.667 | 1.667 | 0 | 0 | 25 |
| MS3 | LA:LP:LB:LR:LF:LC 1:1:1:1:1:1 | 100 | 0.833 | 0.833 | 0.833 | 0.833 | 0.833 | 0.833 | 25 |
| MS4 | LR:LF:LC:LP 1:1:1:1 | 100 | 0 | 1.25 | 1.25 | 1.25 | 1.25 | 0 | 25 |
| Group | L* | a* | b* | ΔE |
|---|---|---|---|---|
| CK | 68.63 ± 0.17 a | 3.67 ± 0.15 f | 12.32 ± 0.62 bcd | |
| LB | 58.90 ± 0.05 c | 5.24 ± 0.29 cd | 12.97 ± 0.28 bc | 9.88 |
| LC | 60.31 ± 0.08 b | 4.62 ± 0.05 e | 11.99 ± 0.02 cd | 8.38 |
| LF | 53.50 ± 1.15 g | 6.13 ± 0.40 ab | 11.51 ± 0.23 d | 15.35 |
| LR | 54.39 ± 0.32 f | 6.21 ± 0.19 ab | 12.46 ± 0.65 bcd | 14.47 |
| LP | 59.85 ± 0.20 b | 5.02 ± 0.15 d | 12.01 ± 0.53 cd | 8.89 |
| LA | 56.64 ± 0.11 d | 5.52 ± 0.24 c | 12.46 ± 0.63 bcd | 12.13 |
| MS1 | 54.92 ± 0.76 f | 6.15 ± 0.16 ab | 12.78 ± 0.99 bcd | 13.94 |
| MS2 | 54.79 ± 0.29 f | 5.60 ± 0.07 c | 12.29 ± 0.50 bcd | 13.97 |
| MS3 | 55.69 ± 0.17 e | 5.95 ± 0.03 b | 13.32 ± 0.60 ab | 13.18 |
| MS4 | 56.41 ± 0.13 de | 6.37 ± 0.22 a | 13.97 ± 0.21 a | 12.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Liao, J.; Wang, T.; Zhao, H. Enhancement of Nutritional Value and Sensory Characteristics of Quinoa Fermented Milk via Fermentation with Specific Lactic Acid Bacteria. Foods 2025, 14, 1406. https://doi.org/10.3390/foods14081406
Zhao L, Liao J, Wang T, Zhao H. Enhancement of Nutritional Value and Sensory Characteristics of Quinoa Fermented Milk via Fermentation with Specific Lactic Acid Bacteria. Foods. 2025; 14(8):1406. https://doi.org/10.3390/foods14081406
Chicago/Turabian StyleZhao, Li, Juan Liao, Tingyu Wang, and Haijiao Zhao. 2025. "Enhancement of Nutritional Value and Sensory Characteristics of Quinoa Fermented Milk via Fermentation with Specific Lactic Acid Bacteria" Foods 14, no. 8: 1406. https://doi.org/10.3390/foods14081406
APA StyleZhao, L., Liao, J., Wang, T., & Zhao, H. (2025). Enhancement of Nutritional Value and Sensory Characteristics of Quinoa Fermented Milk via Fermentation with Specific Lactic Acid Bacteria. Foods, 14(8), 1406. https://doi.org/10.3390/foods14081406





