Ripening-Related Changes in Color and Bioactive Compounds of Diospyros kaki: Preliminary Insights on Its Antifungal Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Double-Phase Extraction
2.4. Colorimetric CIEL*a*b* Analysis
2.5. HPLC-DAD Analysis
2.6. In Vitro Antifungal Evaluation
2.6.1. Antifungal Susceptibility Test
2.6.2. Anti-Adhesion Assay
2.6.3. Antibiofilm Activity
2.7. In Vivo Antifungal Evaluation
Galleria mellonella Survival Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Extraction Procedures
3.2. Colorimetric Analysis
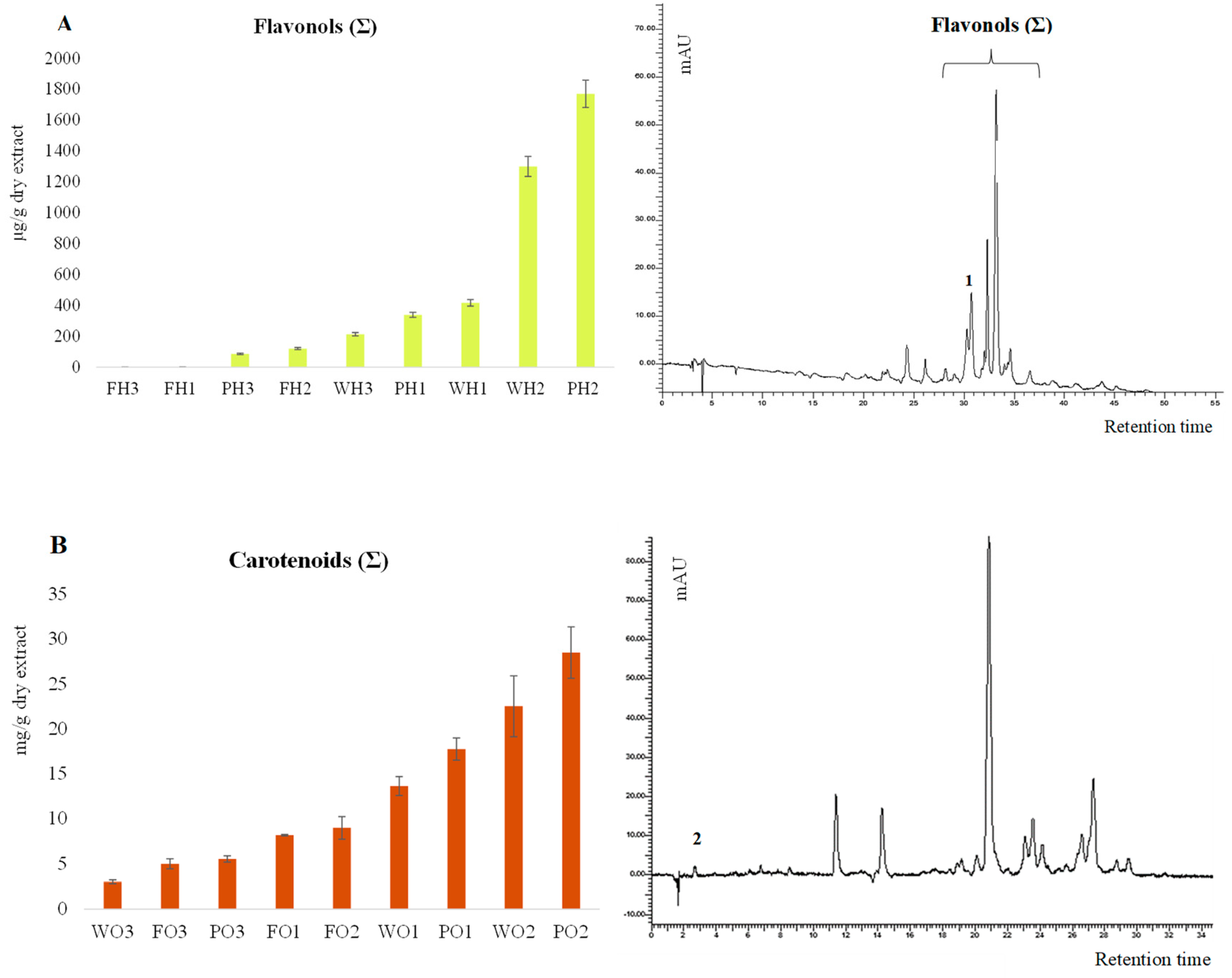
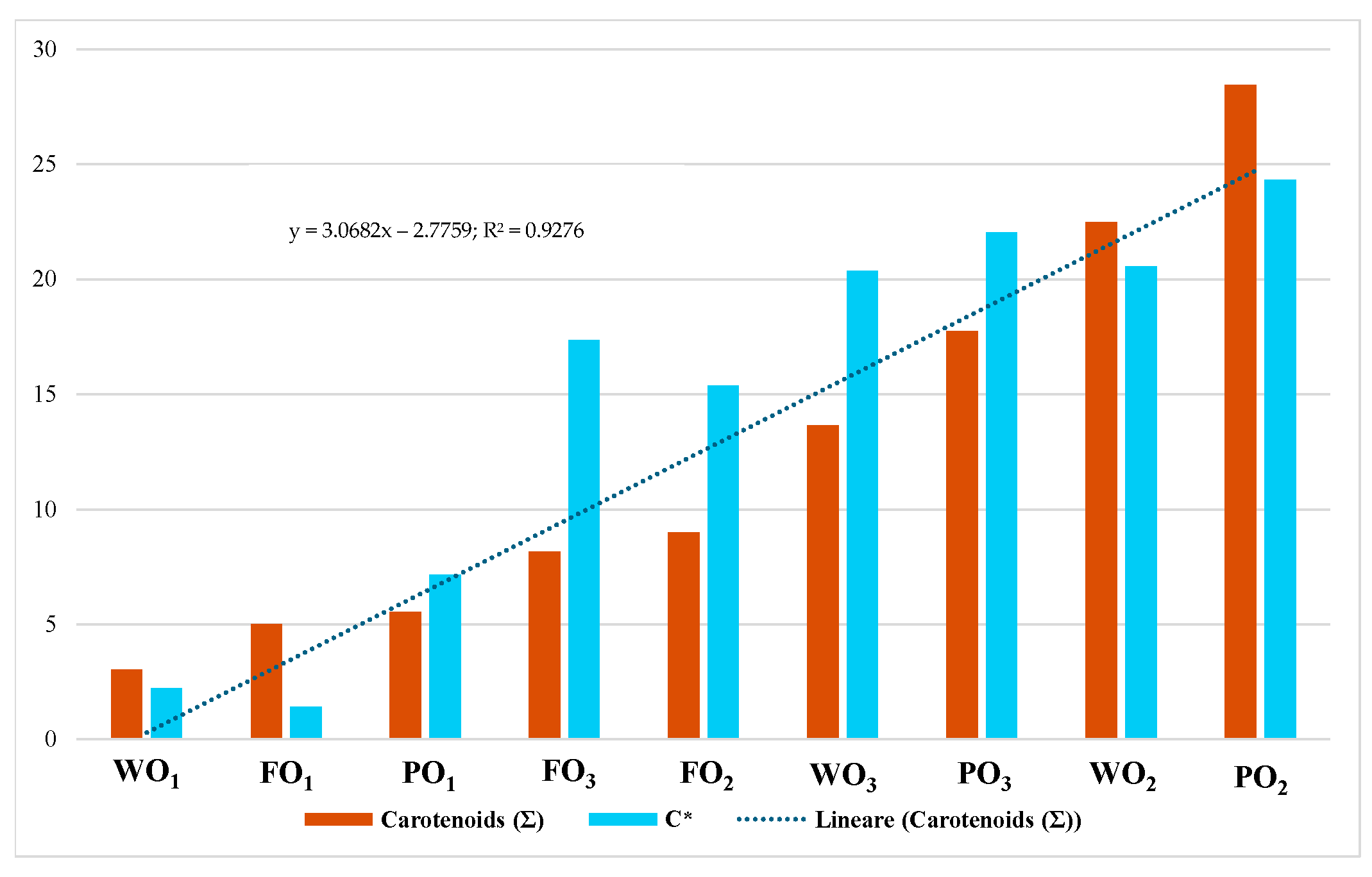
3.3. HPLC-DAD Analysis
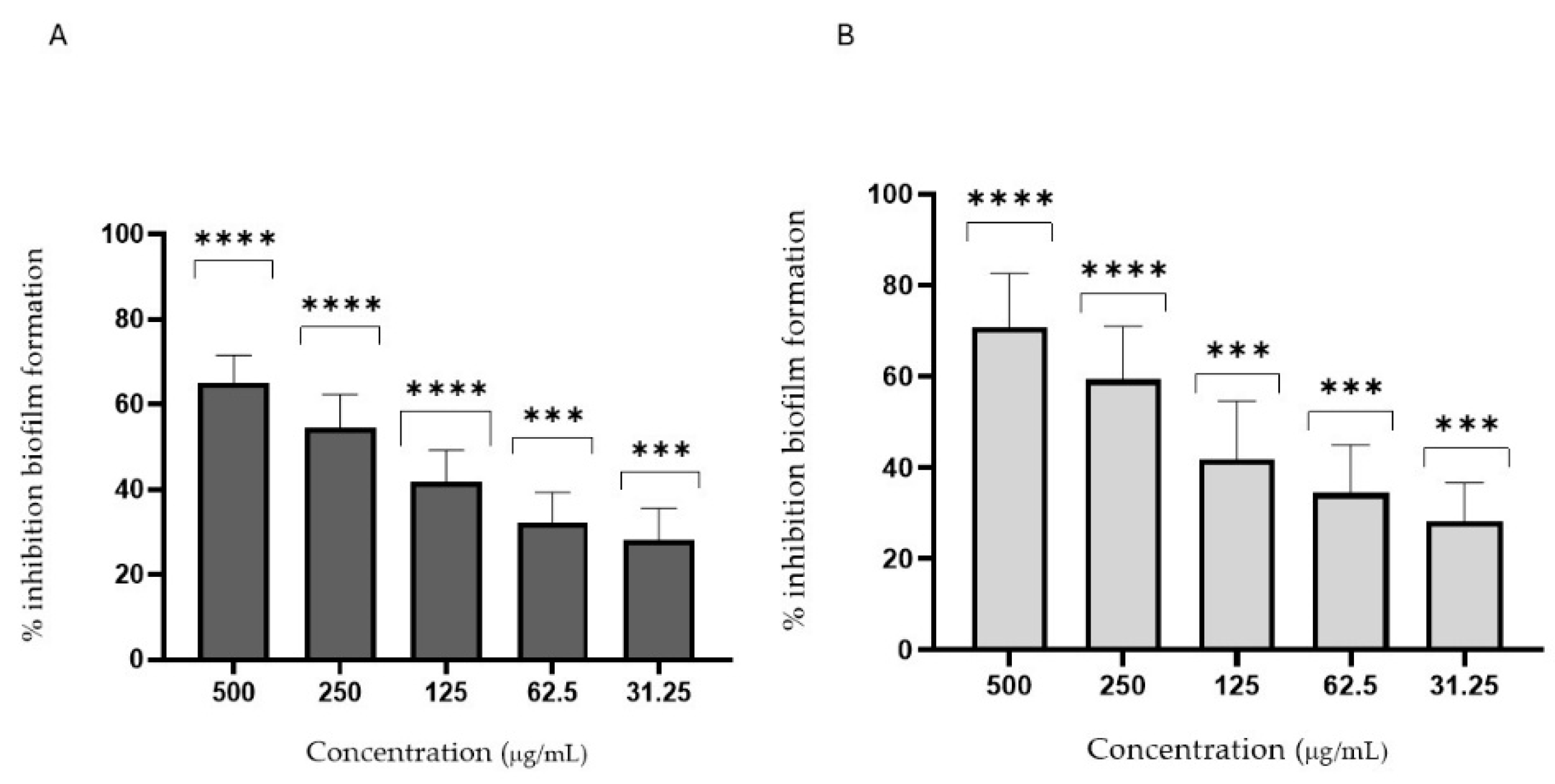
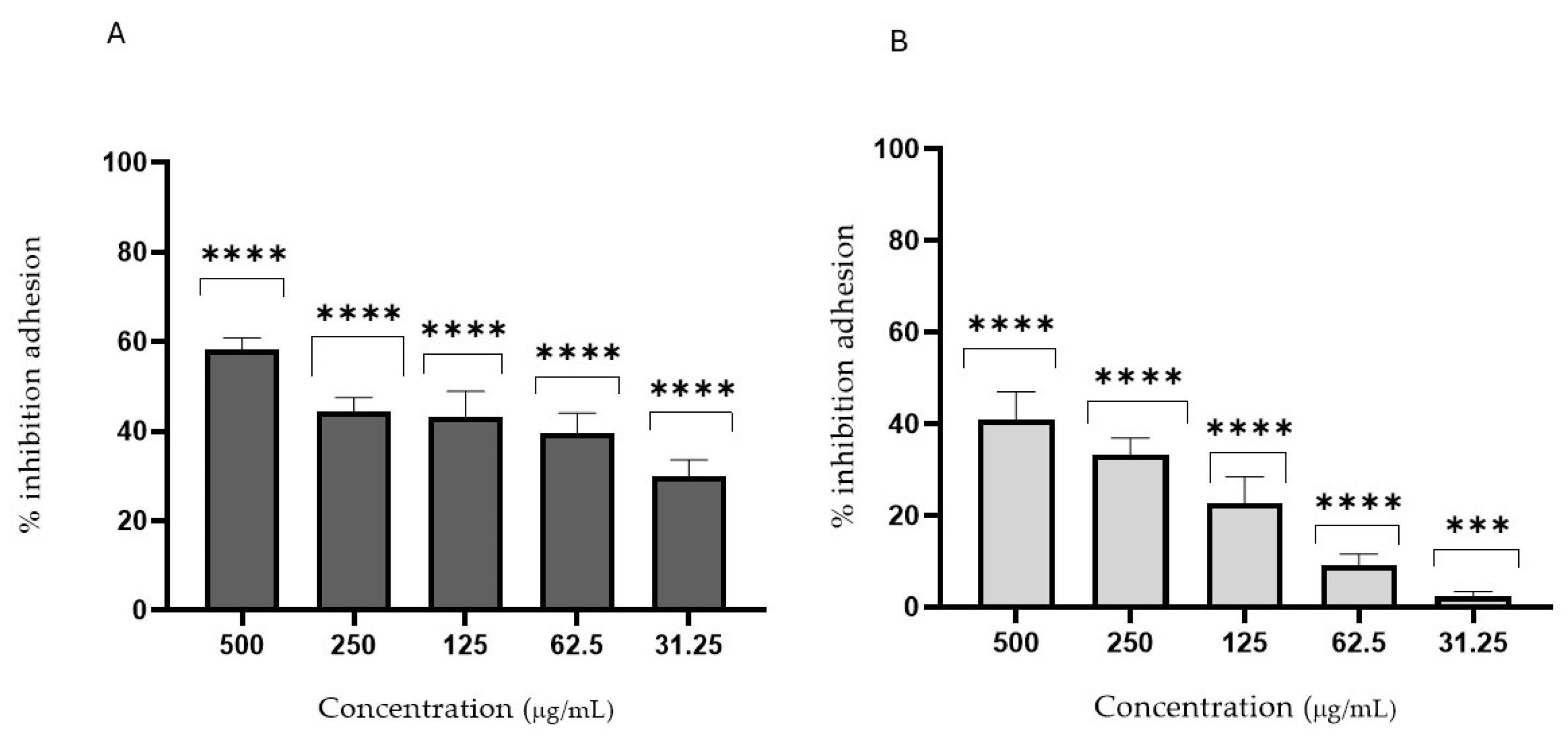
3.4. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matheus, J.R.V.; Andrade, C.J.D.; Miyahira, R.F.; Fai, A.E.C. Persimmon (Diospyros kaki L.): Chemical properties, bioactive compounds and potential use in the development of new products—A review. Food Rev. Intern. 2022, 38, 384–401. [Google Scholar] [CrossRef]
- Yoo, S.K.; Kim, J.M.; Park, S.K.; Kang, J.Y.; Han, H.J.; Park, H.W.; Kim, C.-W.; Lee, U.; Heo, H.J. Chemical compositions of different cultivars of astringent persimmon (Diospyros kaki Thunb.) and the effects of maturity. Korean J. Food Sci. Technol. 2019, 51, 248–257. [Google Scholar]
- Choudhary, R.; Singh, A.; Upadhyay, A.; Singh, R.; Thangalakshmi, S.; Dar, A.H.; Bajpai, V.K.; Shukla, S. Exotic god fruit, persimmon (Diospyros kaki): Pharmacological importance and human health aspects. Foods 2023, 4, e52. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 2015, 14, 542. [Google Scholar]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. From Diospyros kaki L.(persimmon) phytochemical profile and health impact to new product perspectives and waste valorization. Nutrients 2021, 13, 3283. [Google Scholar] [CrossRef]
- Bianchini, S.; Bovio, F.; Negri, S.; Bisson, L.; Piccinelli, A.L.; Rastrelli, L.; Forcella, M.; Fusi, P. Methanolic Extract of the Nutritional Plant (Diospyros kaki Thunb.) Exhibits Anticancer Activity by Inducing Mitochondrial Dysfunction in Colorectal Cancer Cells. Nutrients 2024, 16, 3742. [Google Scholar] [CrossRef] [PubMed]
- Rashed, K.; Ćirić, A.; Glamočlija, J.; Soković, M. Antibacterial and antifungal activities of methanol extract and phenolic compounds from Diospyros virginiana L. Ind. Crops Prod. 2014, 59, 210–215. [Google Scholar] [CrossRef]
- Dhawefi, N.; Jedidi, S.; Rtibi, K.; Jridi, M.; Sammeri, H.; Abidi, C.; Zouari, N.; Sebai, H. Antidiarrheal, antimicrobial, and antioxidant properties of the aqueous extract of Tunisian persimmon (Diospyros kaki Thunb.) Fruits. J. Med. Food 2021, 24, 1100–1112. [Google Scholar] [CrossRef]
- Wang, R.; Shi, X.; Li, K.; Bunker, A.; Li, C. Activity and potential mechanisms of action of persimmon tannins according to their structures: A review. Inter. J. Biol. Macromol. 2023, 242, 125120. [Google Scholar] [CrossRef]
- Malik, S.; Ahmad, D.; Durrani, I.S.; Khan, M.S. Determination of antimicrobial potential of economically important persimmon (Diospyros kaki) and date plum (Diospyros lotus). Sarhad J. Agric. 2025, 41, 457–465. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, M.; Sahin, I.; Kucukbayrak, A.; Ozdemir, D.; Tevfik Yavuz, M.; Oksuz, S.; Cakir, S. Hand carriage of Candida species and risk factors in hospital personnel. Mycoses 2007, 50, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Osme, A.; De Salvo, C.; Zoli, E.; Herrada, J.; McCormick, T.S.; Ghannoum, M.; Cominelli, F.; Di Martino, L. Candida tropicalis Affects Candida albicans Virulence by Limiting Its Capacity to Adhere to the Host Intestinal Surface, Leading to Decreased Susceptibility to Colitis in Mice. J. Fungi 2024, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Padala, S.R.; Kaur, G.; Kullaa, A. Candida albicans induces oral microbial dysbiosis and promotes oral diseases. Microorganisms 2024, 12, 2138. [Google Scholar] [CrossRef]
- Ramage, G.; Kean, R.; Rautemaa-Richardson, R.; Williams, C.; Lopez-Ribot, J.L. Fungal biofilms in human health and disease. Nat. Rev. Microbiol. 2025, 1–16. [Google Scholar] [CrossRef]
- Sharma, K.; Parmanu, P.K.; Sharma, M. Mechanisms of antifungal resistance and developments in alternative strategies to combat Candida albicans infection. Arch. Microbiol. 2024, 206, 95. [Google Scholar] [CrossRef]
- Cairone, F.; Simonetti, G.; Orekhova, A.; Casadei, M.A.; Zengin, G.; Cesa, S. Health potential of Clery strawberries: Enzymatic inhibition and anti-Candida activity evaluation. Molecules 2021, 26, 1731. [Google Scholar] [CrossRef]
- Kreulen, I.A.; de Jonge, W.J.; van den Wijngaard, R.M.; van Thiel, I.A. Candida spp. in human intestinal health and disease: More than a gut feeling. Mycopathologia 2023, 188, 845–862. [Google Scholar] [CrossRef]
- Patsilinakos, A.; Ragno, R.; Carradori, S.; Petralito, S.; Cesa, S. Carotenoid content of Goji berries: CIELAB, HPLC-DAD analyses and quantitative correlation. Food Chem. 2018, 268, 49–56. [Google Scholar] [CrossRef]
- Cairone, F.; Garzoli, S.; Menghini, L.; Simonetti, G.; Casadei, M.A.; Di Muzio, L.; Cesa, S. Valorization of kiwi peels: Fractionation, bioactives analyses and hypotheses on complete peels recycle. Foods 2022, 11, 589. [Google Scholar] [CrossRef]
- Fraschetti, C.; Goci, E.; Nicolescu, A.; Cairone, F.; Carradori, S.; Filippi, A.; Palmieri, V.; Mocan, A.; Cesa, S. Pomegranate Fruit Cracking during Maturation: From Waste to Valuable Fruits. Foods 2023, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Cairone, F.; Carradori, S.; Locatelli, M.; Casadei, M.A.; Cesa, S. Reflectance colorimetry: A mirror for food quality—A mini review. Eur. Food Res. Technol. 2020, 246, 259–272. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI standard M27; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Ourabah, A.; Atmani-Kilani, D.; Debbache-Benaida, N.; Kolesova, O.; Azib, L.; Yous, F.; Benloukil, M.; Botta, B.; Atmani, D.; Simonetti, G. Anti-Candida albicans biofilm activity of extracts from two selected indigenous Algerian plants: Clematis flammula and Fraxinus angustifolia. J. Herb. Med. 2020, 20, 100319. [Google Scholar] [CrossRef]
- Sturabotti, E.; Camilli, A.; Georgian Moldoveanu, V.; Bonincontro, G.; Simonetti, G.; Valletta, A.; Serangeli, I.; Miranda, E.; Amato, F.; Marrani, A.G.; et al. Targeting the Antifungal Activity of Carbon Dots against Candida albicans Biofilm Formation by Tailoring Their Surface Functional Groups. Chem. A Eur. J. 2024, 30, e202303631. [Google Scholar] [CrossRef]
- Cesa, S.; Cairone, F.; De Monte, C. Polyphenols and Flavonoids: Chemical, Pharmacological and Therapeutic Aspects. In Flavonoids and Phenolics; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 1–26. [Google Scholar]
- Travičić, V.; Cvanić, T.; Vidović, S.; Pezo, L.; Hidalgo, A.; Šovljanski, O.; Ćetković, G. Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction. Foods 2024, 13, 2863. [Google Scholar] [CrossRef]
- González, C.M.; García, A.L.; Llorca, E.; Hernando, I.; Atienzar, P.; Bermejo, A.; Moraga, G.; Quiles, A. Carotenoids in dehydrated persimmon: Antioxidant activity, structure, and photoluminescence. LWT 2021, 142, 111007. [Google Scholar] [CrossRef]
- Giordani, E.; Doumett, S.; Nin, S.; Del Bubba, M. Selected primary and secondary metabolites in fresh persimmon (Diospyros kaki Thunb.): A review of analytical methods and current knowledge of fruit composition and health benefits. Food Res. Inter. 2011, 44, 1752–1767. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Medina, V.; Carmona, L.; Bruno, M.; Al-Babili, S.; Zacarías, L. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J. Exp. Bot. 2013, 64, 4461–4478. [Google Scholar] [CrossRef]
- Cairone, F.; Fraschetti, C.; Menghini, L.; Zengin, G.; Filippi, A.; Casadei, M.A.; Cesa, S. Effects of processing on chemical composition of extracts from sour cherry fruits, a neglected functional food. Antioxidants 2023, 12, 445. [Google Scholar] [CrossRef]
- Heredia, A.; Peinado, I.; Barrera, C.; Grau, A.A. Influence of process variables on colour changes, carotenoids retention and cellular tissue alteration of cherry tomato during osmotic dehydration. J. Food Compos. Anal. 2009, 22, 285–294. [Google Scholar] [CrossRef]
- Ranganath, K.G. Pigments that colour our fruits: An overview. Erwerbs-Obstbau 2022, 64, 535–547. [Google Scholar] [CrossRef]
- Adams, J.B. Effect of enzymatic reactions on color of fruits and vegetables. In Enzymes in Fruit and Vegetable Processing; CRC Press: Boca Raton, FL, USA, 2010; pp. 33–58. [Google Scholar]
- Marzorati, S.; Schievano, A.; Idà, A.; Verotta, L. Carotenoids, chlorophylls and phycocyanin from Spirulina: Supercritical CO2 and water extraction methods for added value products cascade. Green Chem. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Muñoz, A.; Sánchez-Hernández, S.; Samaniego-Sánchez, C.; Giménez-Martínez, R.; Olalla-Herrera, M. Differences in the phenolic profile by UPLC coupled to high resolution mass spectrometry and antioxidant capacity of two diospyros kaki varieties. Antioxidants 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K.; Palaiogiannis, D.; Lalas, S.I. Maximizing the Extraction of Bioactive Compounds from Diospyros Kaki Peel through the Use of a Pulsed Electric Field and Ultrasound Extraction. Biomass 2023, 3, 422–440. [Google Scholar] [CrossRef]
- Yan, H.; Pengfei, W.; Brennan, H.; Ping, Q.; Bingxiang, L.; Feiyan, Z.; Hongbo, C.; Haijiang, C. Diversity of carotenoid composition, sequestering structures and gene transcription in mature fruits of four Prunus species. Plant. Physiol. Biochem. 2020, 151, 113–123. [Google Scholar] [CrossRef]
- Gea-Botella, S.; Agulló, L.; Martí, N.; Martínez-Madrid, M.C.; Lizama, V.; Martín-Bermudo, F.; Berna, G.; Saura, D.; Valero, M. Carotenoids from persimmon juice processing. Food Res. Intern. 2021, 141, 109882. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Cilla, A.; Barberá, R.; Zacarías, L. Carotenoid bioaccessibility in pulp and fresh juice from carotenoid-rich sweet oranges and mandarins. Food Funct. 2015, 6, 1950–1959. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Y.; Li, Z.; Fu, Y.; Fu, Z.; Xu, S.; Li, J.; Yan, J.; Yang, X. ZmcrtRB3 Encodes a Carotenoid Hydroxylase that Affects the Accumulation of α-carotene in Maize KernelF. J. Integr. Plant Biol. 2012, 54, 260–269. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Ann. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Giuffrida, D.; Mangraviti, D.; Rigano, F.; Mondello, L.; Arlorio, M.; Coïsson, J.D. Characterization of peel and pulp proanthocyanidins and carotenoids during ripening in persimmon “Kaki Tipo” cv, cultivated in Italy. Food Res. Inter. 2019, 120, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef]
- Förster, C.; Handrick, V.; Ding, Y.; Nakamura, Y.; Paetz, C.; Schneider, B.; Arlorio, M.; Köllner, T.G. Biosynthesis and antifungal activity of fungus-induced O-methylated flavonoids in maize. Plant Physiol. 2022, 188, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Simonetti, G.; Palocci, C.; Valletta, A.; Kolesova, O.; Chronopoulou, L.; Donati, L.; Di Nitto, A.; Brasili, E.; Tomai, P.; Gentili, A.; et al. Anti-Candida biofilm activity of pterostilbene or crude extract from non-fermented grape pomace entrapped in biopolymeric nanoparticles. Molecules 2019, 24, 2070. [Google Scholar] [CrossRef]
- Li, K.; Diao, Y.; Zhang, H.; Wang, S.; Zhang, Z.; Yu, B.; Huang, S.; Yang, H. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. promote cutaneous wound healing in rats. BMC Complement. Altern. Med. 2011, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Liu, Y.; Liu, Y.; Lv, R.; Sun, W.; Ding, W.; Cai, Y.; Li, W.; Liu, X.; Qu, W. Candida albicans biofilms: Antifungal resistance, immune evasion, and emerging therapeutic strategies. Int. J. Antimicrob. Agents 2022, 60, 106673. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2020, 121, 3390–3411. [Google Scholar] [CrossRef]
- Giammarino, A.; Bellucci, N.; Angiolella, L. Galleria mellonella as a model for the study of fungal pathogens: Advantages and disadvantages. Pathogens 2024, 13, 233. [Google Scholar] [CrossRef]




| Samples | Extraction Yield % | L* | a* | b* | C*ab | hab |
|---|---|---|---|---|---|---|
| W1 | - $ | 51.62 ± 1.21 a | 26.46 ± 0.65 a | 45.83 ± 1.80 a | 52.92 ± 0.20 a | 59.99 ± 0.45 a |
| W2 | - | 31.22 ± 0.30 b | 23.66 ± 6.38 a | 27.28 ± 4.39 b | 36.13 ± 7.50 b | 49.40 ± 3.15 a |
| W3 | - | 30.94 ± 9.23 b | 24.90 ± 4.36 a | 24.41 ± 5.25 b | 35.20 ± 0.56 b | 44.35 ± 11.09 a |
| F1 | - | 54.05 ± 0.52 a | 23.68 ± 1.17 a | 42.35 ± 1.79 a | 48.52 ± 0.90 a | 60.79 ± 1.48 a |
| F2 | - | 28.74 ± 1.70 b | 18.21 ± 0.26 a | 23.61 ± 0.82 b | 29.82 ± 0.49 b | 52.35 ± 1.37 b |
| F3 | - | 26.81 ± 4.88 b | 19.24 ± 4.15 a | 22.91 ± 4.60 b | 30.23 ± 0.84 b | 49.83 ± 11.67 b |
| P1 | - | 37.95 ± 1.32 a | 31.66 ± 0.32 a | 35.14 ± 1.21 a | 47.30 ± 0.43 a | 47.98 ± 1.29 a |
| P2 | - | 36.09 ± 2.21 a | 32.34 ± 0.74 a | 32.42 ± 3.39 a | 45.84 ± 1.88 a | 45.00 ± 3.65 a |
| P3 | - | 33.31 ± 9.78 a | 34.11 ± 1.51 a | 33.46 ± 12.95 a | 48.13 ± 10.07 a | 43.40 ± 10.00 a |
| WH1 | 4.68 | 52.43 ± 0.25 a | −1.77 ± 1.38 a | 1.35 ± 0.56 a | 2.23 ± 1.09 a | 142.61 ± 0.22 a |
| WH2 | 6.47 | 59.86 ± 2.87 a | −1.88 ± 0.45 a | 2.49 ± 0.97 a | 3.12 ± 1.04 a | 127.85 ± 4.37 b |
| WH3 | 5.09 | 58.17 ± 0.91 a | −2.23 ± 0.21 a | 4.22 ± 1.75 b | 4.79 ± 1.63 a | 119.24 ± 8.08 b |
| FH1 | 3.54 | 50.31 ± 1.05 a | −1.07 ± 0.62 a | −0.82 ± 1.49 b | 1.35 ± 0.66 b | 217.66 ± 1.45 a |
| FH2 | 10.02 | 60.97 ± 1.37 b | −1.91 ± 0.52 a | 3.30 ± 1.52 a | 3.82 ± 1.58 a | 121.14 ± 5.24 b |
| FH3 | 11.03 | 64.04 ± 2.86 b | −1.47 ± 0.14 a | 1.27 ± 1.93 b | 2.27 ± 0.98 a | 150.51 ± 46.75 b |
| PH1 | 5.58 | 50.80 ± 1.33 a | −1.52 ± 0.07 a | 0.78 ± 1.09 a | 1.71 ± 0.89 a | 152.78 ± 1.11 a |
| PH2 | 8.34 | 56.46 ± 0.07 b | −3.38 ± 0.02 b | 8.40 ± 2.28 b | 9.07 ± 2.12 b | 112.54 ± 5.44 a |
| PH3 | 13.83 | 62.36 ± 2.50 b | −3.55 ± 0.28 b | 7.69 ± 1.63 b | 8.47 ± 1.60 b | 115.08 ± 2.99 a |
| WO1 | 0.02 | 49.52 ± 1.45 a | −1.62 ± 0.23 a | 1.52 ± 1.35 a | 2.22 ± 0.14 a | 136.81 ± 1.63 a |
| WO2 | 0.09 | 51.64 ± 2.12 a | −4.43 ± 0.57 b | 20.07 ± 1.81 b | 20.56 ± 1.89 b | 102.42 ± 0.45 a |
| WO3 | 0.04 | 54.68 ± 1.40 a | −4.97 ± 0.20 b | 19.77 ± 1.11 b | 20.38 ± 0.73 b | 104.11 ± 1.14 a |
| FO1 | 0.01 | 50.66 ± 0.86 a | −1.39 ± 1.43 a | 0.17 ± 1.16 a | 1.40 ± 0.44 a | 172.92 ± 1.93 a |
| FO2 | 0.04 | 53.01 ± 1.03 a | −4.00 ± 0.74 b | 14.86 ± 4.82 b | 15.40 ± 4.84 b | 105.41 ± 2.11 b |
| FO3 | 0.04 | 56.50 ± 0.26 a | −4.58 ± 1.88 b | 16.72 ± 1.75 b | 17.34 ± 0.38 b | 105.31 ± 1.31 b |
| PO1 | 0.02 | 51.27 ± 1.15 a | −2.67 ± 1.82 a | 6.64 ± 0.56 a | 7.15 ± 1.22 a | 111.92 ± 1.38 a |
| PO2 | 0.09 | 48.70 ± 1.56 a | −3.91 ± 0.22 a | 24.02 ± 2.02 b | 24.34 ± 2.03 b | 99.25 ± 0.25 a |
| PO3 | 0.08 | 54.35 ± 1.26 a | −5.00 ± 0.66 b | 21.45 ± 1.34 b | 22.03 ± 1.38 b | 103.13 ± 0.30 a |
| µg/g Dry Extract | Rutin | Flavonols (Σ) | mg/g Dry Extract | Lutein | Carotenoids (Σ) |
|---|---|---|---|---|---|
| PH1 | 86.4 ± 2.6 a | 86.4 ± 4.3 a | PO1 | BDL * | 5.54 ± 0.36 a |
| PH2 | 176.5 ± 5.3 b | 1767.9 ± 88.3 b | PO2 | 0.20 ± 0.02 a | 28.47 ± 2.85 b |
| PH3 | 118.7 ± 3.6 b | 338.7 ± 16.9 b | PO3 | 0.57 ± 0.03 a | 17.74 ± 1.24 b |
| FH1 | 116.1 ± 0.1 a | 116.1 ± 0.1 a | FO1 | 0.01 ± 0.002 a | 5.00 ± 0.56 a |
| FH2 | 121.5 ± 3.6 a | 121.5 ± 6.1 a | FO2 | 0.09 ± 0.01 a | 9.00 ± 1.26 a |
| FH3 | 118.2 ± 0.1 a | 118.2 ± 0.1 a | FO3 | 0.11 ± 0.01 a | 8.18 ± 0.08 b |
| WH1 | 213.6 ± 6.4 a | 213.6 ± 10.7 a | WO1 | 0.11 ± 0.01 a | 3.03 ± 0.20 a |
| WH2 | 247.4 ± 7.4 a | 1299.1 ± 65.0 b | WO2 | 0.30 ± 0.05 b | 22.49 ± 3.37 b |
| WH3 | 155.4 ± 4.7 b | 416.5 ± 20.8 b | WO3 | 0.39 ± 0.02 b | 13.64 ± 1.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cairone, F.; Angiolella, L.; Bertini, F.; Iazzetti, A.; Fabrizi, G.; Petralito, S.; Cesa, S.; Simonetti, G. Ripening-Related Changes in Color and Bioactive Compounds of Diospyros kaki: Preliminary Insights on Its Antifungal Activity. Foods 2025, 14, 1332. https://doi.org/10.3390/foods14081332
Cairone F, Angiolella L, Bertini F, Iazzetti A, Fabrizi G, Petralito S, Cesa S, Simonetti G. Ripening-Related Changes in Color and Bioactive Compounds of Diospyros kaki: Preliminary Insights on Its Antifungal Activity. Foods. 2025; 14(8):1332. https://doi.org/10.3390/foods14081332
Chicago/Turabian StyleCairone, Francesco, Letizia Angiolella, Francesca Bertini, Antonia Iazzetti, Giancarlo Fabrizi, Stefania Petralito, Stefania Cesa, and Giovanna Simonetti. 2025. "Ripening-Related Changes in Color and Bioactive Compounds of Diospyros kaki: Preliminary Insights on Its Antifungal Activity" Foods 14, no. 8: 1332. https://doi.org/10.3390/foods14081332
APA StyleCairone, F., Angiolella, L., Bertini, F., Iazzetti, A., Fabrizi, G., Petralito, S., Cesa, S., & Simonetti, G. (2025). Ripening-Related Changes in Color and Bioactive Compounds of Diospyros kaki: Preliminary Insights on Its Antifungal Activity. Foods, 14(8), 1332. https://doi.org/10.3390/foods14081332











