Isolation of Lactic Acid Bacteria from Naturally Ensiled Rosa roxburghii Tratt Pomace and Evaluation of Their Ensiling Potential and Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Spontaneous RRTP Silage Preparation and Sampling
2.3. Isolation of Acid-Producing Strains from RRTP
2.4. Phenotype Characterization of the Isolated RRTP LAB Strains
2.5. Genotypic Identification of Presumptive LAB Isolates
2.6. Evaluation of the Ensiling Potential of Isolated LAB
2.6.1. Determination of Growth Curve and Acid-Producing Ability
2.6.2. Antibacterial Activity of Isolated LAB
2.6.3. Ethanol, Acid, Osmotic Pressure, and Bile Salt Tolerance
2.7. Antioxidant Activity
2.7.1. Preparation of Cell-Free Supernatant (CFS), Intact Cells (ICs), and Cell-Free Extract (CFE)
2.7.2. Superoxide Dismutase (SOD) Activity
2.7.3. DPPH Free Radical Scavenging Activity
2.7.4. Ferric Reducing Antioxidant Power (FRAP)
2.8. Safety Evaluation of Isolated LAB
2.8.1. Antibiotic Sensitivity
2.8.2. Hemolysis Test
2.9. Data Analysis
3. Results and Discussion
3.1. Isolation and Phenotypic Characterization of Presumptive LAB Isolates
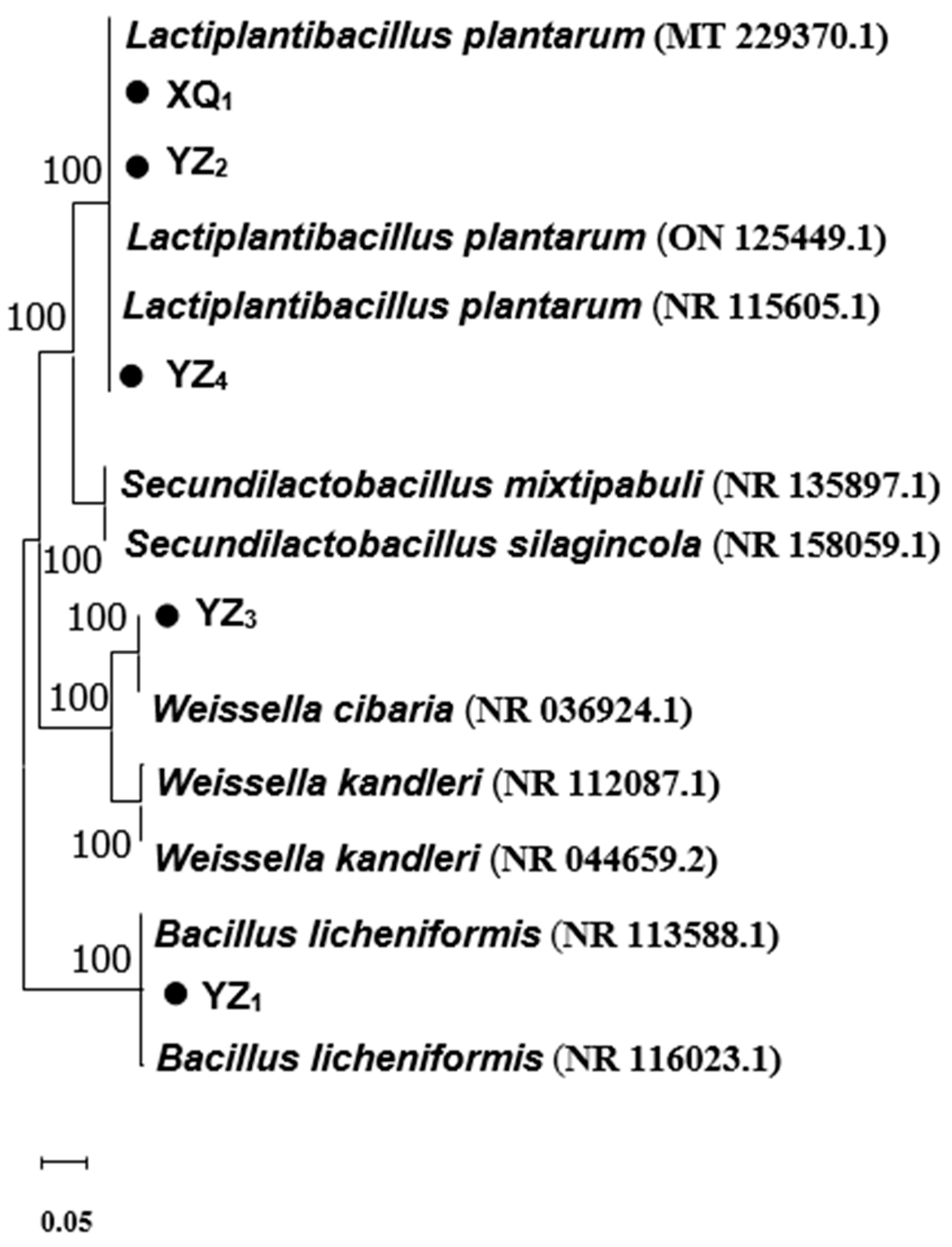
3.2. Genotypic Identification
3.3. Ensiling Potential
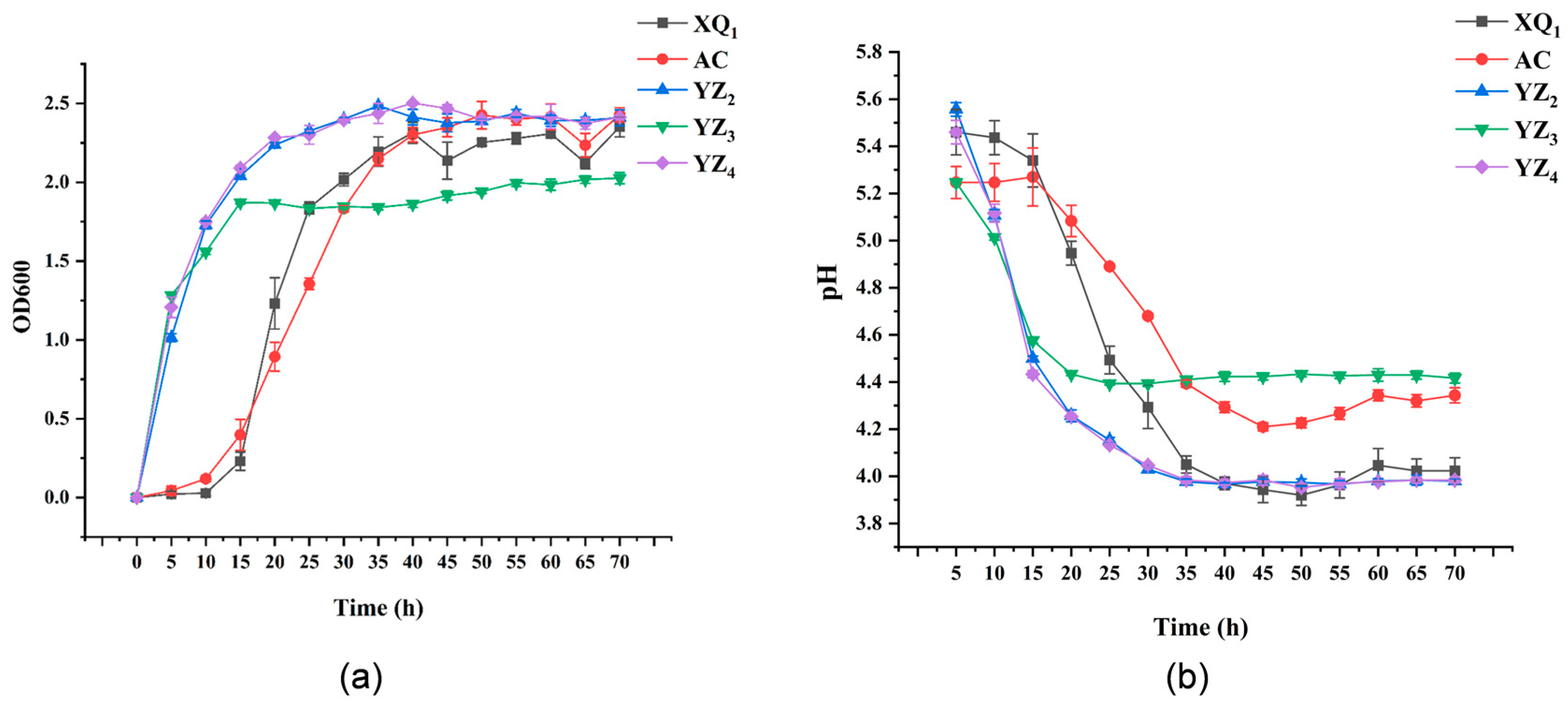
3.3.1. Growth and Acid Production Capacity
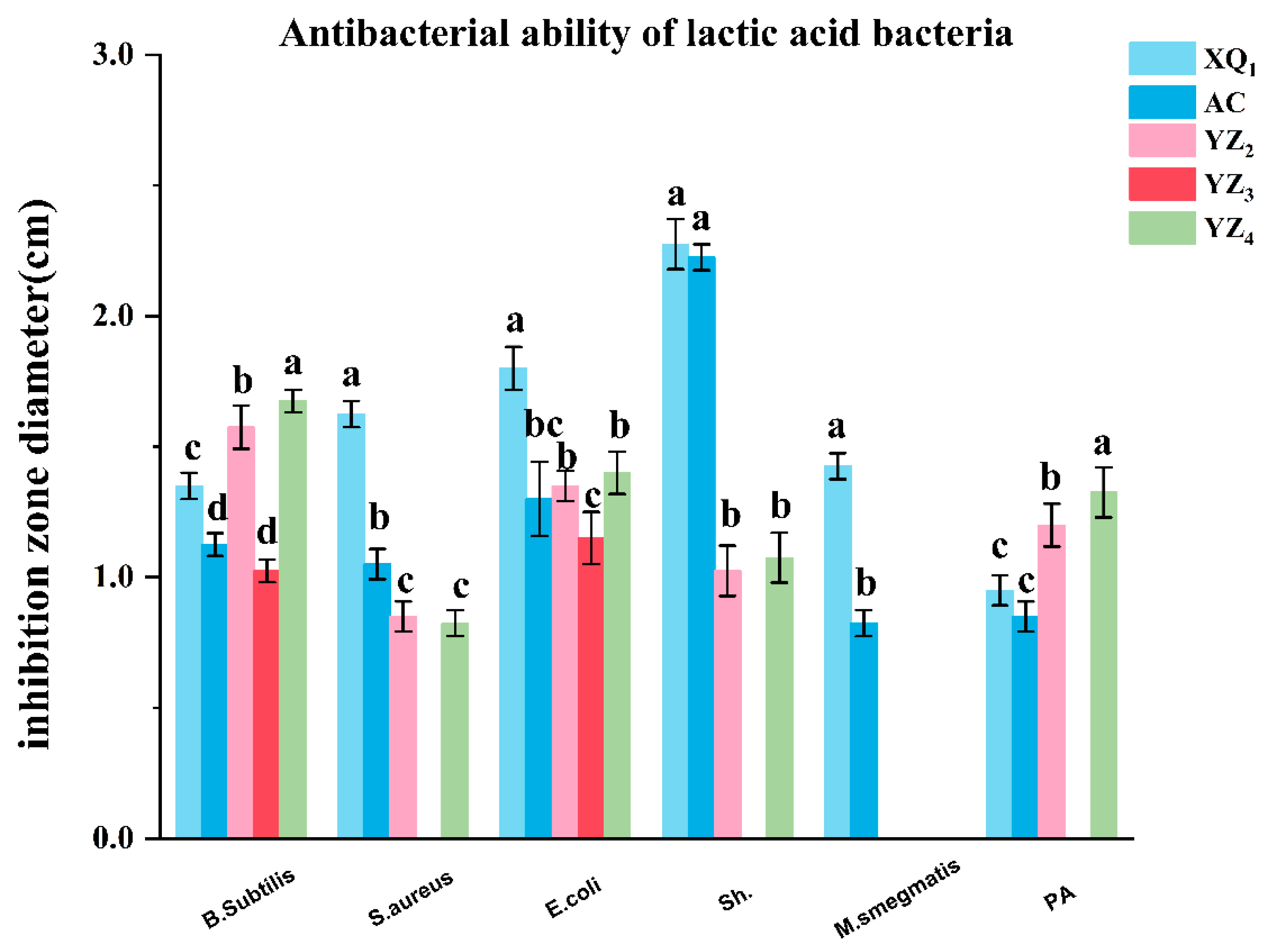
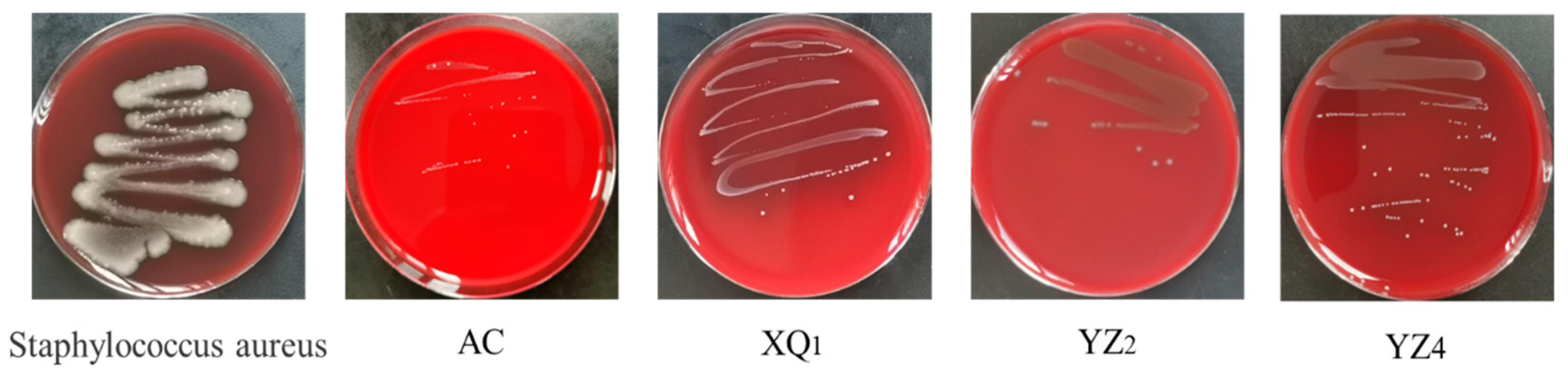
3.3.2. Antibacterial Activity
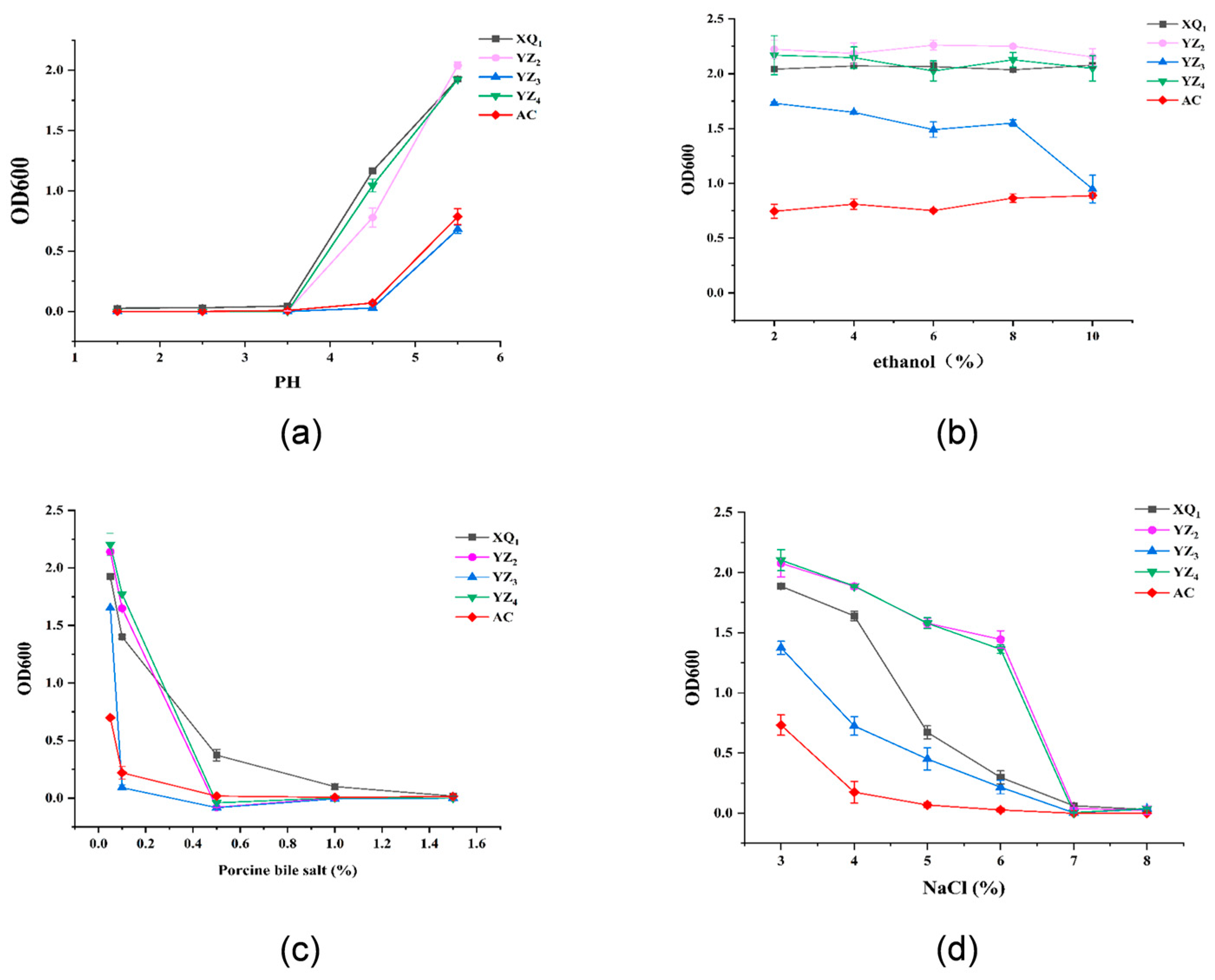
3.3.3. Tolerance
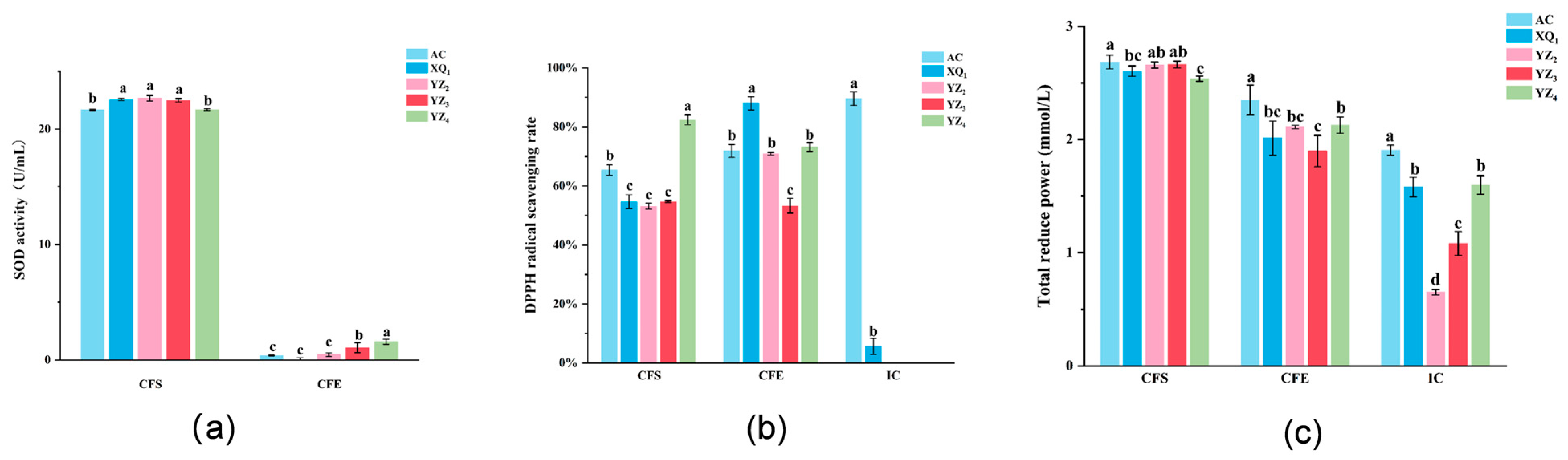
3.4. Antioxidant Activity of Strains
3.5. Safety Evaluation
3.5.1. Antibiotic Susceptibility
3.5.2. Hemolysis Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Ding, X.Y.; Yang, H.; Hu, J.W.; Jiang, F.; Chen, H.L. A study on heavy metals pollution in soil and fruits of Rosa roxburghii Tratt from the planting bases located in the Karst areas of Guizhou Province. Adv. Mater. Res. 2014, 1010, 88–95. [Google Scholar] [CrossRef]
- Hou, Z.; Zhao, L.; Wang, Y.; Liao, X. Effects of high pressure on activities and properties of superoxide dismutase from chestnut rose. Adv. Mater. Res. 2019, 294, 557–564. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Lu, X.; Peng, Q.; Xie, Y.; Yang, M. Research progress on main active components and pharmacological effects of Rosa roxburghii Tratt. Sci. Technol. Food Ind. 2020, 41, 328–335. [Google Scholar]
- Lu, M.; Zhang, H.; An, H. Chloroplast DNA-based genetic variation of Rosa roxburghii in Southwest China: Phylogeography and conservation implications. Hortic. Plant J. 2021, 7, 286–294. [Google Scholar] [CrossRef]
- Li, F.; Ding, Z.; Ke, W.; Xu, D.; Zhang, P.; Bai, J.; Mudassar, S.; Muhammad, I.; Guo, X. Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification. Bioresour. Technol. 2019, 282, 211–221. [Google Scholar] [CrossRef]
- Song, X.; Yang, Y.; Wang, C.; Zhu, W.; Zhou, C.; Wu, W. Rosa roxburghii tratt residue: A novel feed resource for cattle indicated by the non-deleterious performance and blood metabolites. Trop. Anim. Health Prod. 2024, 56, 340. [Google Scholar] [CrossRef]
- Carvalho, B.; Sales, G.; Schwan, R.; Ávila, C.L.S. Criteria for lactic acid bacteria screening to enhance silage quality. J. Appl. Microbiol. 2021, 130, 341–355. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current approaches on the roles of lactic acid bacteria in crop silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- Mejía-Avellaneda, L.F.; Suárez, H.; Jiménez, H.; Mesa, L. Challenges and opportunities for the production of lactic acid bacteria inoculants aimed for ensiling processes. Crit. Rev. Biotechnol. 2022, 42, 1028–1044. [Google Scholar] [CrossRef]
- Porto, M.C.W.; Kuniyoshi, T.M.; Azevedo, P.; Vitolo, M.; Oliveira, R.d.S. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Meruvu, H.; Harsa, S.T. Lactic acid bacteria: Isolation–characterization approaches and industrial applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 8337–8356. [Google Scholar] [CrossRef]
- Sifeeldein, A.; Wang, S.; Li, J.; Dong, Z.; Chen, L.; Kaka, N.A.; Shao, T. Phylogenetic identification of lactic acid bacteria isolates and their effects on the fermentation quality of sweet sorghum (Sorghum bicolor) silage. J. Appl. Microbiol. 2019, 126, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.M.; Saleh, A.M. Homofermentative Lactobacilli isolated from organic sources exhibit potential ability of lactic acid production. Front. Microbiol. 2023, 14, 1297036. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1. 4 and their antimicrobial application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef]
- Reuben, R.; Roy, P.; Sarkar, S.; Alam, A.R.U.; Jahid, I. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef]
- Nguyen-Sy, T.; Yew, G.Y.; Chew, K.W.; Nguyen, T.D.P.; Tran, T.N.T.; Le, T.D.H.; Vo, C.T.; Tran, H.K.P.; Mubashir, M.; Show, P.L. Potential cultivation of Lactobacillus pentosus from human breastmilk with rapid monitoring through the spectrophotometer method. Processes 2020, 8, 902. [Google Scholar] [CrossRef]
- Yehuala, G.A.; Shibeshi, N.T.; Kim, S.-H.; Park, M.-K. Characterization of autochthonous lactic acid bacteria isolated from a traditional Ethiopian beverage, Tella. Foods 2024, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L. Comprehensive evaluation of probiotic property, hypoglycemic ability and antioxidant activity of lactic acid bacteria. Foods 2022, 11, 1363. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Yang, Y.; Xiong, Z.; Lv, F.; Zhou, W.; Ai, L. Lactic acid bacteria with antioxidant activities alleviating oxidized oil induced hepatic injury in mice. Front. Microbiol. 2018, 9, 2684. [Google Scholar] [CrossRef]
- Banothu, V.; Neelagiri, C.; Adepally, U.; Lingam, J.; Bommareddy, K. Phytochemical screening and evaluation of in vitro antioxidant and antimicrobial activities of the indigenous medicinal plant Albizia odoratissima. Pharm. Biol. 2017, 55, 1155–1161. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Wu, S.; Chen, Y.; Chen, Z.; Zhou, Q.; Wei, F.; Li, P.; Gu, Q. Antioxidant properties and molecular mechanisms of Lactiplantibacillus plantarum ZJ316: A potential probiotic resource. Microorganisms 2023, 187, 115269. [Google Scholar] [CrossRef]
- Amelia, R.; Philip, K.; Pratama, Y.E.; Purwati, E. Characterization and probiotic potential of lactic acid bacteria isolated from dadiah sampled in West Sumatra. Food Sci. Technol. 2020, 41, 746–752. [Google Scholar] [CrossRef]
- Huligere, S.S.; Chandana Kumari, V.; Alqadi, T.; Kumar, S.; Cull, C.A.; Amachawadi, R.G.; Ramu, R. Isolation and characterization of lactic acid bacteria with potential probiotic activity and further investigation of their activity by α-amylase and α-glucosidase inhibitions of fermented batters. Front. Microbiol. 2023, 13, 1042263. [Google Scholar] [CrossRef]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Missotten, J.; Michiels, J.; Goris, J.; Herman, L.; Heyndrickx, M.; De Smet, S.; Dierick, N. Screening of two probiotic products for use in fermented liquid feed. Livest. Sci. 2007, 108, 232–235. [Google Scholar] [CrossRef]
- Xu, N.; Wang, J.; Chen, X.; Hu, K.; Wang, R. Screening, identification and application of good lactic acid bacteria in yogurt. Chin. J. Food Sci. 2019, 19, 98–107. [Google Scholar]
- Yao, Y.; Luo, J.; Zhang, P.; Wang, Y.; Lu, B.; Wu, G.; Zhang, J.; Luo, X.; Wang, L. Screening, Identification and Application of Lactic Acid Bacteria for Degrading Mycotoxin Isolated from the Rumen of Yaks. Microorganisms 2024, 12, 2260. [Google Scholar] [CrossRef]
- Kumar, M.; Tiwari, S.K.; Srivastava, S. Purification and Characterization of Enterocin LR/6, a Bacteriocin from Enterococcus faecium LR/6. Appl. Biochem. Biotechnol. 2009, 160, 40–49. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Si, H.; Liu, H.; Li, Z.; Nan, W.; Jin, C.; Sui, Y.; Li, G. Effect of Lactobacillus plantarum and Lactobacillus buchneri addition on fermentation, bacterial community and aerobic stability in lucerne silage. Anim. Prod. Sci. 2019, 59, 1528–1536. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M. Bacteriocin production by Lactobacillus pentosus ST712BZ isolated from boza. Braz. J. Microbiol. 2007, 38, 166–172. [Google Scholar] [CrossRef]
- Kang, S.; Long, J.; Park, M.S.; Ji, G.E.; Ju, Y.; Ku, S. Investigating human-derived lactic acid bacteria for alcohol resistance. Microb. Cell Factories 2024, 23, 118. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Wang, X.; Pan, Y.; Wang, M.; Xu, Y.; Gao, J.; Cui, H.; Li, C.; Chen, H. Molecular identification and probiotic potential characterization of lactic acid bacteria isolated from the pigs with superior immune responses. Front. Microbiol. 2024, 15, 1361860. [Google Scholar] [CrossRef]
- Kebouchi, M.; Galia, W.; Genay, M.; Soligot, C.; Lecomte, X.; Awussi, A.A.; Perrin, C.; Roux, E.; Dary-Mourot, A.; Le Roux, Y. Implication of sortase-dependent proteins of Streptococcus thermophilus in adhesion to human intestinal epithelial cell lines and bile salt tolerance. Appl. Microbiol. Biotechnol. 2016, 100, 3667–3679. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, X.; Hu, Y.-S.; Wu, Y.; Wang, Q.-Z.; Li, N.-N.; Guo, Q.-C.; Dong, X.-C. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- Chooruk, A.; Piwat, S.; Teanpaisan, R. Antioxidant activity of various oral Lactobacillus strains. J. Appl. Microbiol. 2017, 123, 271–279. [Google Scholar] [CrossRef]
- Sanzani, S.; Montemurro, C.; Di Rienzo, V.; Solfrizzo, M.; Ippolito, A. Genetic structure and natural variation associated with host of origin in Penicillium expansum strains causing blue mould. Int. J. Food Microbiol. 2013, 165, 111–120. [Google Scholar] [CrossRef]
- Li, K.; Gu, Q.; Yang, W.; Yu, X. In vitro screening and probiotic evaluation of anti-obesity and antioxidant lactic acid bacteria. Food Biosci. 2023, 54, 102844. [Google Scholar] [CrossRef]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef]
- Ghiasi, F.; Hashemi, S.M.B.; Abedi, E. Effective enhancement of food oxidative stability induced by Lactobacillus strains: In vitro activity. Food Control 2023, 153, 109912. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A.; Lee, H.-J. Antioxidant potential of exopolysaccharides from lactic acid bacteria: A comprehensive review. Int. J. Biol. Macromol. 2024, 281, 135536. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Tuo, Y.; Zhang, H.; Li, Y.; Xu, C.; Mu, G.; Jiang, S. Reducing antigenicity of β-lactoglobulin, probiotic properties and safety evaluation of Lactobacillus plantarum AHQ-14 and Lactobacillus bulgaricus BD0390. Food Biosci. 2021, 42, 101137. [Google Scholar] [CrossRef]
- Mayrhofer, S.; Van Hoek, A.H.; Mair, C.; Huys, G.; Aarts, H.J.; Kneifel, W.; Domig, K.J. Antibiotic susceptibility of members of the Lactobacillus acidophilus group using broth microdilution and molecular identification of their resistance determinants. Int. J. Food Microbiol. 2010, 144, 81–87. [Google Scholar] [CrossRef]
- Lakhlifi, T.; El Oirdi, S.; Maroui, I.; Zouhair, R.; Belhaj, A. Probiotic properties and safety aspect of three antifungal lactic acid bacteria strains isolated from wheat and camel milk. Biologia 2023, 78, 1129–1139. [Google Scholar] [CrossRef]
- Horie, M.; Ruengsomwong, S.; Ohmiya, Y. Analysis of lactic acid bacteria species in Miang, a post-fermented tea in Thailand, and their potential use as probiotics. Front. Microbiol. 2024, 15, 1450158. [Google Scholar] [CrossRef]
- Cai, T.; Wu, H.; Qin, J.; Qiao, J.; Yang, Y.; Wu, Y.; Qiao, D.; Xu, H.; Cao, Y. In vitro evaluation by PCA and AHP of potential antidiabetic properties of lactic acid bacteria isolated from traditional fermented food. LWT 2019, 115, 108455. [Google Scholar] [CrossRef]
- Nami, Y.; Bakhshayesh, R.V.; Manafi, M.; Hejazi, M.A. Hypocholesterolaemic activity of a novel autochthonous potential probiotic Lactobacillus plantarum YS5 isolated from yogurt. LWT 2019, 111, 876–882. [Google Scholar] [CrossRef]
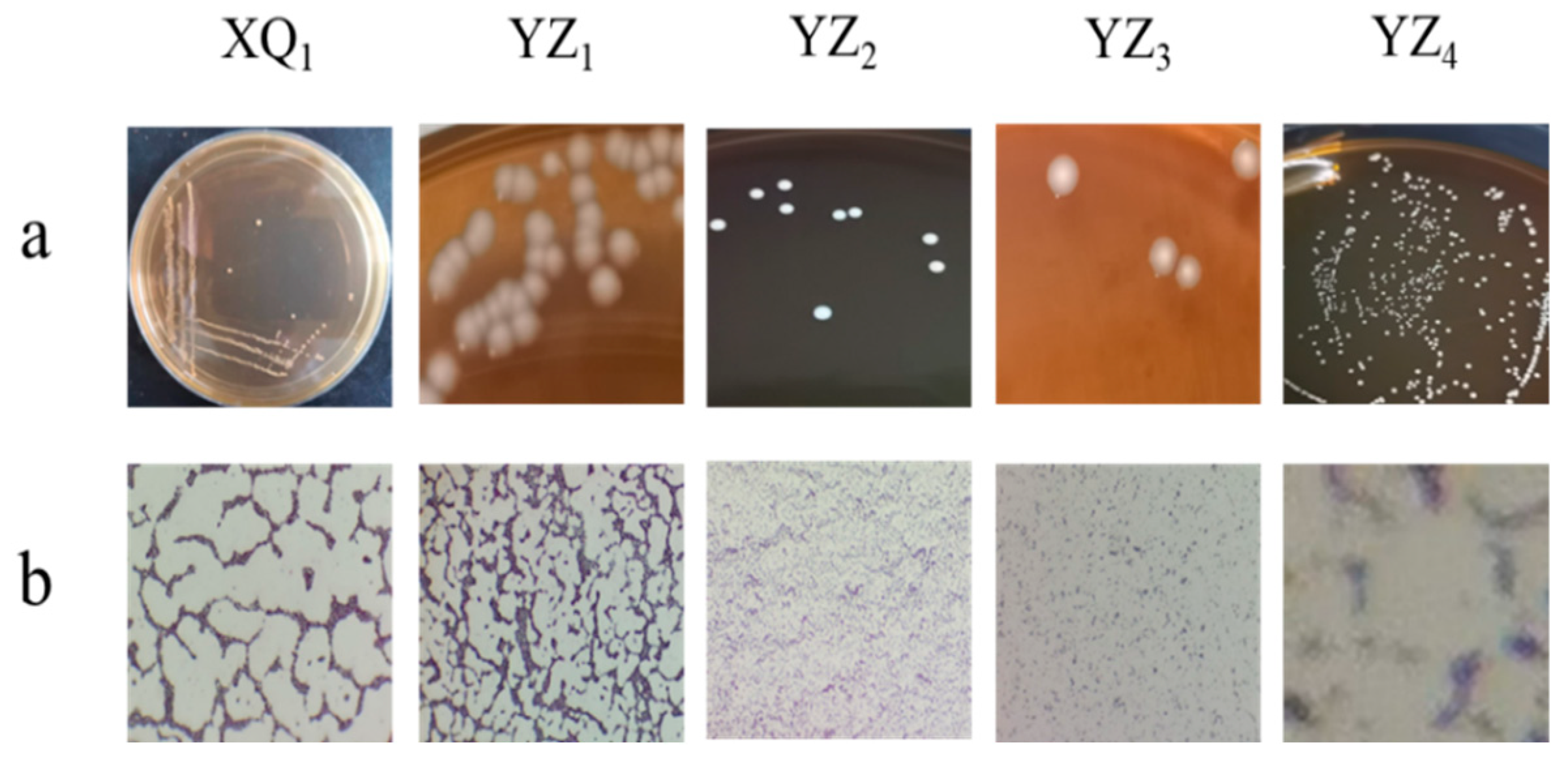
| Colony Morphology | Cell Morphology | ||||
|---|---|---|---|---|---|
| Shape | Texture | Color | Morphology | Gram Staining | |
| XQ1 | Circular, raised colonies | Smooth and fine-textured | Milky white | Rod shaped | Gram-positive bacteria |
| YZ1 | Circular, raised colonies | Thick and smooth | White with slight yellow | Rod shaped | Gram-positive bacteria |
| YZ2 | Circular, raised colonies | Smooth and fine-textured | Milky white | Rod shaped | Gram-positive bacteria |
| YZ3 | Irregular, serrated edges with depressions | Thick and wrinkled | White with slight yellow | Rod shaped | Gram-positive bacteria |
| YZ4 | Circular, raised colonies | Smooth and fine-textured | Milky white | Rod shaped | Gram-positive bacteria |
| XQ1 | YZ1 | YZ2 | YZ3 | YZ4 | |
|---|---|---|---|---|---|
| Glucose | + | + | + | + | + |
| Fructose | + | + | + | + | + |
| Maltose | + | + | + | + | + |
| Lactose | + | − | + | + | + |
| Sucrose | + | + | − | + | + |
| Mannitol | − | + | + | − | − |
| Salicin | + | + | + | + | + |
| Cellobiose | + | + | + | + | + |
| Esculin | + | + | + | + | + |
| Sorbitol | + | − | + | + | + |
| Raffinose | − | − | + | − | + |
| Inulin | − | + | − | − | + |
| Produce acid | + | + | + | + | + |
| Glucose produce gas | − | − | − | + | − |
| Catalase | − | + | − | − | − |
| Motility | − | + | − | − | − |
| Starch Hydrolysis | − | − | − | − | − |
| Sample | Description | Total Score | Per. Ident | Accession |
|---|---|---|---|---|
| XQ1 | Lactobacillus plantarum strain DMR17 | 2654 | 100.00% | MT229370.1 |
| YZ1 | Bacillus licheniformis strain BCRC 11702 | 2590 | 99.93% | NR116023.1 |
| YZ2 | Lactiplantibacillus plantarum strain HBUAS59638 | 2466 | 100.00% | ON125449.1 |
| YZ3 | Weissella cibaria strain II-I-59 | 2436 | 99.97% | NR036924.1 |
| YZ4 | Lactiplantibacillus plantarum strain JCM 1149 | 2521 | 99.93% | NR115605.1 |
| MIC (mg/L) | |||||
|---|---|---|---|---|---|
| Antibiotic | Breakpoint (mg/L) | AC | XQ1 | YZ2 | YZ4 |
| Clindamycin | 4 | 0.5 | >128 | 1 | 4 |
| Ampicillin | 2 | 2 | <0.125 | <0.125 | <0.125 |
| Erythromycin | 1 | 1 | 1 | 1 | 1 |
| Tetracyclin | 32 | 16 | 16 | 16 | 16 |
| Gentamicin | 16 | 8 | >128 | >128 | >128 |
| Kanamycin | 64 | >128 | >128 | >128 | >128 |
| Chloramphenicol | 8 | >128 | >128 | >128 | >128 |
| Vancomycin | n.r. | >128 | >128 | >128 | >128 |
| Streptomycin | n.r. | 64 | >128 | >128 | >128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Zhang, Y.; Yue, N.; Yu, K.; Zhou, L.; Ge, L.; Chen, F.; Yang, J.; Li, Q.; Deng, T.; et al. Isolation of Lactic Acid Bacteria from Naturally Ensiled Rosa roxburghii Tratt Pomace and Evaluation of Their Ensiling Potential and Antioxidant Properties. Foods 2025, 14, 1329. https://doi.org/10.3390/foods14081329
Pan X, Zhang Y, Yue N, Yu K, Zhou L, Ge L, Chen F, Yang J, Li Q, Deng T, et al. Isolation of Lactic Acid Bacteria from Naturally Ensiled Rosa roxburghii Tratt Pomace and Evaluation of Their Ensiling Potential and Antioxidant Properties. Foods. 2025; 14(8):1329. https://doi.org/10.3390/foods14081329
Chicago/Turabian StylePan, Xiong, Yafei Zhang, Ningbo Yue, Ke Yu, Lang Zhou, Lijuan Ge, Faju Chen, Juan Yang, Qiji Li, Tingfei Deng, and et al. 2025. "Isolation of Lactic Acid Bacteria from Naturally Ensiled Rosa roxburghii Tratt Pomace and Evaluation of Their Ensiling Potential and Antioxidant Properties" Foods 14, no. 8: 1329. https://doi.org/10.3390/foods14081329
APA StylePan, X., Zhang, Y., Yue, N., Yu, K., Zhou, L., Ge, L., Chen, F., Yang, J., Li, Q., Deng, T., & Yang, X. (2025). Isolation of Lactic Acid Bacteria from Naturally Ensiled Rosa roxburghii Tratt Pomace and Evaluation of Their Ensiling Potential and Antioxidant Properties. Foods, 14(8), 1329. https://doi.org/10.3390/foods14081329






