Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicrobial Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga Biomass
2.2. Extraction of Bioactive Compounds from Lobosphaera sp.
2.3. Identification of Phenolic Compounds in Lobosphaera sp. Extracts
2.4. Identification of Fatty Acids Profile in Lobosphaera sp. Extracts
2.5. Cellular Cytotoxicity Analysis of Lobosphaera sp. Extracts
2.6. Characterization of Bioactivity of Lobosphaera sp. Extracts
2.6.1. Total Phenolic Content (TPC)
2.6.2. Antioxidant Activity
2.7. Extraction of Polysaccharides from Dried Lobosphaera sp.
2.8. Extraction of Protein from Lobosphaera sp.
2.9. Film Production Using the “Casting” Method
2.10. Film Characterization
2.10.1. Determination of Physical Properties of the Film
- Thickness
- Color
- Water vapor permeability
- Solubility
- Tensile strength (TS) and elongation at break (EAB)
2.10.2. Determination of the Film Antioxidant Activity (ABTS, DPPH)
2.10.3. Determination of the Film Antimicrobial Activity
2.11. Film Application on Strawberry
2.11.1. Moisture Loss
2.11.2. Microbiological Analyses
2.12. Statistical Analysis
3. Results
3.1. Yield of Extraction of Bioactive Compounds, Polysaccharides, and Proteins from Freeze-Dried Lobosphaera sp.
3.2. Phenolic Compounds in Lobosphaera sp. Bioactive-Rich Extract
3.3. Fatty Acids in Lobosphaera sp. Bioactive-Rich Extract
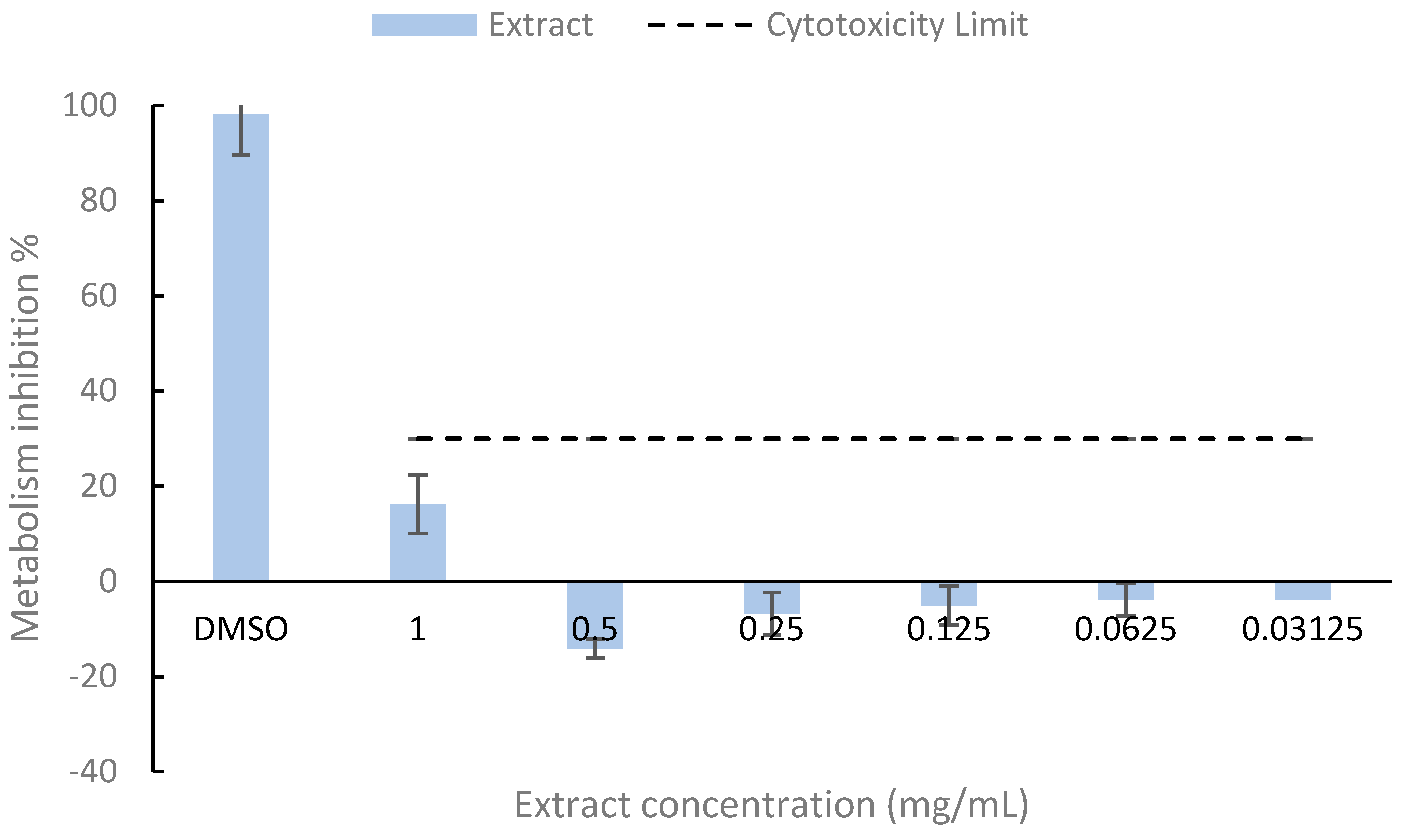
3.4. Cytotoxicity of Lobosphaera sp. Bioactive-Rich Extract
3.5. Total Phenolic Content and Antioxidant Activity of Lobosphaera Bioactive-Rich Extract
3.6. Produced Films
3.7. Film Properties
3.7.1. Physical Properties
- Thickness and water vapor permeability (WVP)
- Color Parameters
- Solubility characteristics
- Mechanical properties
3.7.2. Antioxidant Activity of the Films
3.7.3. Antimicrobial Activity of the Films
3.8. Film Application Results
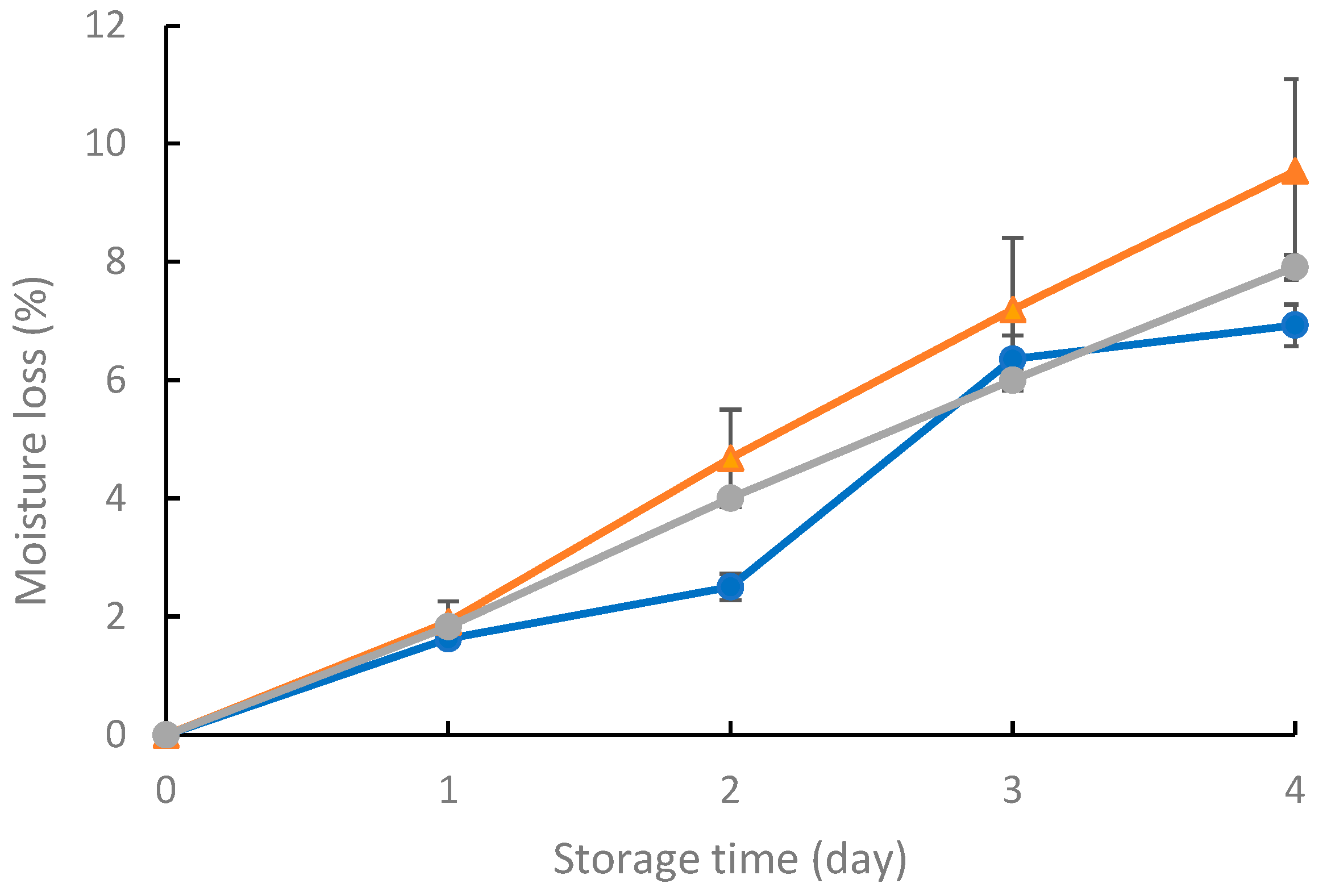
3.8.1. Strawberry Moisture Loss
3.8.2. Mesophilic Aerobic Bacteria, Molds, and Yeasts Counts in Strawberry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Lim, S.R.; Jeong, D.G.; Kim, J.H. Characterization of an Oleaginous Unicellular Green Microalga, Lobosphaera incisa (Reisigl, 1964) Strain K-1, Isolated from a Tidal Flat in the Yellow Sea, Republic of Korea. Front. Microbiol. 2018, 9, 2159. [Google Scholar] [CrossRef] [PubMed]
- Iskandarov, U.; Sitnik, S.; Shtaida, N.; Didi-Cohen, S.; Leu, S.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Cloning and Characterization of a GPAT-like Gene from the Microalga Lobosphaera incisa (Trebouxiophyceae): Overexpression in Chlamydomonas Reinhardtii Enhances TAG Production. J. Appl. Phycol. 2016, 28, 907–919. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional High-Value Products from Microalgae: A Review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef] [PubMed]
- Novichkova, E.; Chumin, K.; Eretz-Kdosha, N.; Boussiba, S.; Gopas, J.; Cohen, G.; Khozin-Goldberg, I. DGLA from the Microalga Lobosphaera Incsa P127 Modulates Inflammatory Response, Inhibits iNOS Expression and Alleviates NO Secretion in Raw264.7 Murine Macrophages. Nutrients 2020, 12, 2892. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wong, C.Y.; Koh, R.Y.; Lim, C.L.; Kok, Y.Y.; Chye, S.M. Natural Bioactive Compounds from Macroalgae and Microalgae for the Treatment of Alzheimer’s Disease: A Review. Yale J. Biol. Med. 2024, 97, 205–224. [Google Scholar] [CrossRef]
- Lazado, C.C.; Nayak, S.; Khozin-Goldberg, I.; Zilberg, D. The Gut Mucosal Barrier of Zebrafish (Danio Rerio) Responds to the Time-Restricted Delivery of Lobosphaera Incisa-Enriched Diets. Fish Shellfish. Immunol. 2019, 89, 368–377. [Google Scholar] [CrossRef]
- Sagaram, U.S.; Gaikwad, M.S.; Nandru, R.; Dasgupta, S. Microalgae as Feed Ingredients: Recent Developments on Their Role in Immunomodulation and Gut Microbiota of Aquaculture Species. FEMS Microbiol. Lett. 2021, 368, fnab071. [Google Scholar] [CrossRef]
- Carissimi, M.; Flôres, S.H.; Rech, R. Effect of Microalgae Addition on Active Biodegradable Starch Film. Algal Res. 2018, 32, 201–209. [Google Scholar] [CrossRef]
- Morales-Jiménez, M.; Gouveia, L.; Yáñez-Fernández, J.; Castro-Muñoz, R.; Barragán-Huerta, B.E. Production, Preparation and Characterization of Microalgae-Based Biopolymer as a Potential Bioactive Film. Coatings 2020, 10, 120. [Google Scholar] [CrossRef]
- Agustini, N.W.S.; Kusmiati, K.; Admirasari, R.; Nurcahyanto, D.A.; Hidhayati, N.; Apriastini, M.; Afiati, F.; Priadi, D.; Fitriani, B.M.; Adalina, Y.; et al. Characterization of Corn-Starch Edible Film with the Addition of Microalgae Extract Chlorella Vulgaris as an Antioxidant Applied to Dodol (Glutinous-Rice Cake) Products. Case Stud. Chem. Environ. Eng. 2023, 8, 100511. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Ribeiro, T.B.; Lopes, A.I.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules 2024, 29, 1935. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, A.A.; Campos, D.A.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.; Pintado, M. Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract. Sustainability 2020, 12, 5556. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.; Morais, P.; Miranda, A.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Novel Avocado Oil-Functionalized Yogurt with Anti-Obesity Potential: Technological and Nutraceutical Perspectives. Food Biosci. 2022, 50, 101983. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.C.; Rodríguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Bigels as Delivery Systems of Bioactive Fatty Acids Present in Functional Edible Oils: Coconut, Avocado, and Pomegranate. Gels 2023, 9, 349. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Martins, V.F.R.; Coelho, M.; Machado, M.; Costa, E.; Gomes, A.M.; Poças, F.; Sperotto, R.A.; Rosa-Martinez, E.; Vasconcelos, M.; Pintado, M.E.; et al. Integrated Valorization of Fucus spiralis Alga: Polysaccharides and Bioactives for Edible Films and Residues as Biostimulants. Foods 2024, 13, 2938. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Edible Films with Protein and Bioactive Compounds from Arthrospira sp. Biol. Life Sci. Forum 2024, 40, 6. [Google Scholar]
- Commission Internationale de L’Eclairage (C.I.E.). Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; C.I.E.: Paris, France, 1978; TC 1. [Google Scholar]
- Commission Regulation (EU). No. 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- D 882-891; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. Annual book of ASTM standards. American Society for Testing & Materials: Philadelphia, PA, USA, 1991.
- Lopes, A.I.; Melo, A.; Caleja, C.; Pereira, E.; Finimundy, T.C.; Afonso, T.B.; Silva, S.; Ivanov, M.; Soković, M.; Tavaria, F.K.; et al. Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings 2023, 13, 1487. [Google Scholar] [CrossRef]
- Moreira, M.R.; Roura, S.I.; Ponce, A. Effectiveness of chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. Food Sci. Technol. 2011, 44, 2335–2341. [Google Scholar] [CrossRef]
- Monteiro, M.; Santos, R.A.; Iglesias, P.; Couto, A.; Serra, C.R.; Gouvinhas, I.; Barros, A.; Oliva-Teles, A.; Enes, P.; Díaz-Rosales, P. Effect of Extraction Method and Solvent System on the Phenolic Content and Antioxidant Activity of Selected Macro- and Microalgae Extracts. J. Appl. Phycol. 2020, 32, 349–362. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Bart, N.; De Paepe, D.; Bart, G.J.E.; De Cooman, L. Detection of Flavonoids in Microalae from Different Evolutionary Lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; Brabanter, J.D.; Cooman, L.D. Antioxidant Potential of Microalgae in Relation to Their Phenolic and Carotenoid Content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Sozmen, A.B.; Canbay, E.; Sozman, E.Y.; Ovez, B. The Effect of Temperature and Light IntensityDuring Cultivation of Chlorella Miniata on Antioxidant, Antiinflamatry Potentials and Phenolic Compound Acomulation. Biocat. Agric. Biotech. 2018, 14, 366–374. [Google Scholar] [CrossRef]
- Safafar, H.; Wagenen, J.; Moller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds, Tocopherols, Contribute to the Antioxidant Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Parkes, R.; McGee, D.; McDonnell, A.; Gilliespie, E.; Touzet, N. Rapid Secreening of Phenolic in Extracts of Photosyntetic Organisms Separated using a C18 Monolithic Column Based HPLC-UV Method. J. Chrom. B 2022, 1213, 123521. [Google Scholar] [CrossRef]
- Andriopoulos, V.; Gkioni, M.D.; Koutra, E.; Mastropetros, S.G.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Total Phenolic Content, Biomass Composition, and Antioxidant activity of Selected Marine Microalga Species with Potential in Aquaculture Feed. Antioxidants 2022, 11, 1320. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Guéguen, E. Influence of Carbon Sources on the Phenolic Compound Productionby Euglena gracilis Using an Untargeted Metabolomic Approach. Biomolecules 2022, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Zorin, B.; Grundman, O.; Khozin-Goldberg, I.; Leu, S.; Shapira, M. Development of a Nuclear Transformation System for Oleaginous Green Alga Lobosphaera (Parietochloris) incisa and Genetic Complementation of a Mutant Strain, Deficient in Arachidonic Acid Biosynthesis Development of a Nuclear Transformation System for Oleaginous Green Alga Lobosphaera (Parietochloris) incisa and Genetic Complementation of a Mutant Strain, Deficient in Arachidonic Acid Biosynthesis. PLoS ONE 2014, 9, e105223. [Google Scholar]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, J.; Li, A. Cytotoxicity and Intestinal Permeability of Phycotoxins Assessed by the Human Caco-2 Cell Model. Ecotoxicol. Environ. Saf. 2023, 249, 114447. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, S.; Feng, G.; Wu, L.; Feng, Y.; Guo, T.; Yang, Y.; Wu, H.; Zeng, M. Microalgae Aqueous Extracts Exert Intestinal Protective Effects in Caco-2 Cells and Dextran Sodium Sulphate-Induced Mouse Colitis. Food Funct. 2020, 11, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, P.S.; Coimbra, R.S.T.; Caetano, N.S. Exploring the Antioxidant Potential of Lobosphaera Sp. and Odontella Sp. Biomasses under Different Extraction and Preservation Conditions. In Proceedings of the Seagriculture EU 2023 12th International Seaweed Conference, Trondheim, Norway, 21–22 June 2023. [Google Scholar]
- Torres, P.; Osaki, S.; Silveira, E.; dos Santos, D.Y.A.C.; Chow, F. Comprehensive evaluation of Folin-Ciocalteu Assay for Total Phenolic Quantification in Algae (Chlorophyta, Phaeophyceae and Rhodophyta). Algal Res. 2024, 80, 103503. [Google Scholar] [CrossRef]
- Andriopoulos, V.; Kornaros, M. Microalgal Phenolics: Systematic Review with a Focus on Methodological Assessment and Meta-Analysis. Mar. Drugs 2024, 22, 460. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Chatzikonstantinou, A.V.; Mataragas, M.; Kondyli, E.; Stamatis, H.; Bosnea, L. Evaluation of the Antioxidant and Physicochemical Properties of Microalgae/Whey Protein-Based Edible Films. Biol. Life Sci. Forum 2021, 6, 97. [Google Scholar] [CrossRef]
- Silva, G.A.; de Farias Neves, F.; Tribuzi, G. Development and Characterization of Edible Films Based on a Mixture of the Seaweeds Ulva lactuca and Kappaphycus Alvarezii. J. Appl. Phycol. 2024, 36, 2325–2341. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharjee, S.K.; Mudenur, C.; Ghosh, T.; Goud, V.V.; Katiyar, V. Development of Antioxidant-Rich Edible Active Films and Coatings Incorporated with de-Oiled Ethanolic Green Algae Extract: A Candidate for Prolonging the Shelf Life of Fresh Produce. RSC Adv. 2022, 12, 13295–13313. [Google Scholar] [CrossRef]
- Zinina, O.; Merenkova, S.; Galimov, D. Development of biodegradable alginate-based films with bioactive properties and optimal structural characteristics with incorporation of protein hydrolysates. Sustainability 2023, 15, 15086. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and Characterization of Active Films Based on Chitosan Incorporated Tea Polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef]
- Benlloch-Tinoco, M.; Gentile, P.; Taylor, L.; Girón-Hernandez, J. Alginate edible films as delivery systems for green tea polyphenols. Food Hydrocoll. 2025, 158, 110518. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.M.; Chang, W.S.; Kim, Y.K. Enhancing Microalgae Content in Biocomposites through a Mechanical Grinding Method. Polymers 2023, 15, 4557. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Yun, J.H.; Kim, H.S.; Hong, J.S.; Ahn, K.H. Dispersion of unfractionated microalgae in various polymers and its influence on rheological and mechanical properties. Korea Aust. Rheol. J. 2023, 35, 19–29. [Google Scholar] [CrossRef]
- Kia, E.M.; Ghasempour, Z.; Alizadeh, M. Fabrication of an Eco-Friendly Antioxidant Biocomposite: Zedo Gum/Sodium Caseinate Film by Incorporating Microalga (Spirulina platensis). J. Appl. Polym. Sci. 2017, 135, 46024. [Google Scholar]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Antimicrobial activities of microalgae: An invited review. In Book Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Barcelona, Spain, 2011; Volume 1, pp. 1272–1280. [Google Scholar]
- Borreby, C.; Hvidtfeldt, T.A.; Jespersen, M.G.; dos Santos, P.T.; Houborg, S.D.; Lillebæk, E.M.S.; Kemp, M.; Kallipolitis, B.H. Long-chain unsaturated free fatty acids reduce the host cell invasion of Listeria monocytogenes outbreak strains. Front. Cell. Infect. Microbiol. 2025, 15, 1542165. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Johnson, E.A. Inhibition of Listeria monocytogenes by fatty acids and monoglycerides. Appl. Environ. Microbiol. 1992, 58, 624–629. [Google Scholar] [CrossRef]
- Denton, M.; Dealler, S.F.; Birkenhead, D.; Lacey, R.W. Inhibition of Listeria by unsaturated fatty acids. J. Nut. Med. 1991, 2, 383–386. [Google Scholar]
- Zhou, T.; Wang, H.; Han, Q.; Song, Z.; Yu, D.; Li, G.; Liu, W.; Dong, C.; Ge, S.; Chen, X. Fabrication and characterization of an alginate-based film incorporated with cinnamaldehyde for fruit preservation. Int. J. Bio. Macromol. 2024, 274, 133398. [Google Scholar] [CrossRef]
- Martins, V.; Lopes, A.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Edible coatings with polysaccharides and bioactive compounds from Exhausted olive oil pomace to extend the shelf life of strawberry. In Proceedings of the International Conference on Sustainable Foods—Achieving the Sustainable Development Goals (ICSF), Bragança, Portugal, 24–25 July 2024. [Google Scholar]
- Shirai, M.A.; Baú, T.R.; Zanela, J.; Pimentel, T.C. Microalgae as an innovative active ingredient for edible films and coatings for food applications. Algal Res. 2025, 86, 103959. [Google Scholar] [CrossRef]
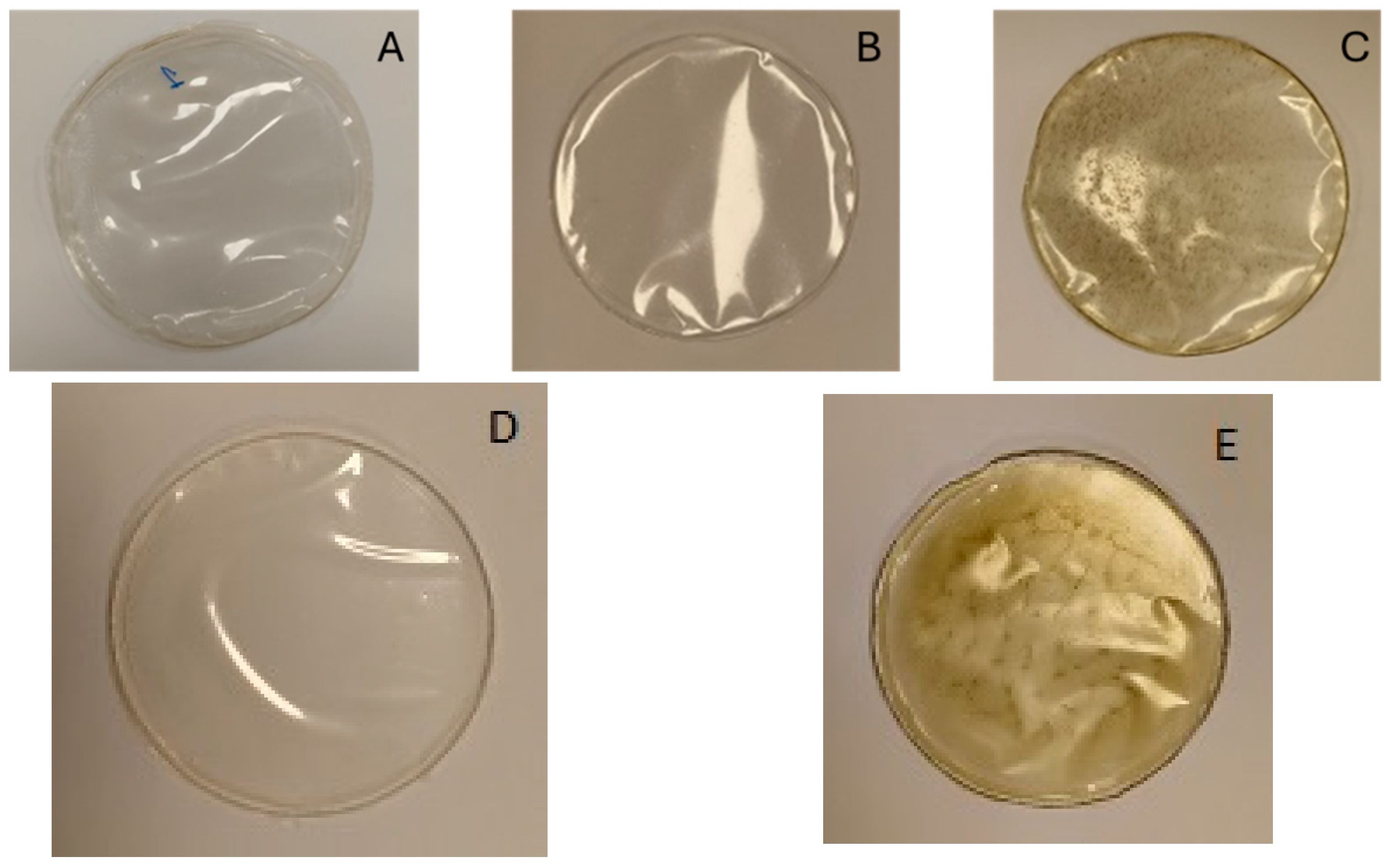

| Film Formulation | Code |
|---|---|
| Alginate (2% w/v) | A |
| Alginate (2% w/v) + PS-rich extract (0.5% w/v) | B |
| Alginate (2% w/v) + PS-rich extract (0.5% w/v) + bioactive-rich extract (0.25% w/v) | C |
| Alginate (2% w/v) + P-rich extract (0.5% w/v) | D |
| Alginate (2% w/v) + P-rich extract (0.5% w/v) + bioactive-rich extract (0.25% w/v) | E |
| Fatty Acids | Chain Length | Quantity (mg/g) |
|---|---|---|
| Butyric acid | C4 | 0.195 ± 0.005 |
| Caproic acid | C6 | 0.066 ± 0.003 |
| Caprylic acid | C8 | 0.160 ± 0.013 |
| Capric acid | C10 | 0.118 ± 0.012 |
| Lauric acid | C12 | 0.456 ± 0.086 |
| Myristic acid | C14 | 0.495 ± 0.150 |
| Myristoleic acid | C14:1 | 0.674 ± 0.104 |
| Pentadecylic acid | C15 | 7.884 ± 2.349 |
| cis-10-Pentadecenoic Acid | C15:1 c10 | 0.725 ± 0.156 |
| Palmitic acid | C16 | 443.900 ± 129.518 |
| Palmitoleic acid | C16:1 c9 | 0.645 ± 0.006 |
| Margaric acid | C17 | 8.258 ± 8.060 |
| cis-10-Heptadecenoic acid | C17:1 c10 | 0.880 ± 0.321 |
| Stearic acid | C18 | 12.772 ± 0.190 |
| Elaidic acid | C18:1 t9 | 0.202 ± 0.042 |
| Oleic acid | C18:1c9 | 200.459 ± 50.139 |
| Linolelaidic acid (ω-6) | C18:2 t6 | 5.180 ± 1.190 |
| Linoleic acid (ω-6) | C18:2 c6 | 110.241 ±110.119 |
| Arachidic acid | C20 | 1.717 ± 0.421 |
| γ-linolenic acid (ω-6) | γ C18:3 | 187.497 ± 50.972 |
| Gondoic acid | C20:1 | 3.410 ± 1.241 |
| α-linolenic acid (ω-3) | α C18:3 | 5.048 ± 1.014 |
| Heneicosylic acid | C21 | 1.218 ± 0.166 |
| Eicosadienic acid | C22:2 c11 c14 | 1.314 ± 0.272 |
| Behenic acid | C22 | 2.507 ± 0.388 |
| Eicosadienoic acid (ω-6) | C20:3 c8 c11 c14 | 3.258 ± 1.279 |
| Erucic acid | C22:1 c13 | 6.567 ± 2.094 |
| Eicosapentaenoic acid (ω-3) | C20:5 | 2.354 ± 0.074 |
| Tricosylic acid | C23 | 4.091 ± 1.294 |
| cis-13,16-Docosadienoic acid | C22:2 | 0.807 ± 0.001 |
| Lignoceric acid | C24 | 1.033 ± 0.326 |
| Nervoic acid | C24:1 | 2.086 ± 0.219 |
| Docosahexaenoic acid | C22:6 | 2.826 ±0.994 |
| Σ fat acids | 1019.0456 ± 363.151 | |
| Σ SFA | 485.414 ± 143.042 | |
| Σ MUFA | 215.648 ± 54.226 | |
| Σ PUFA | 318.526 ± 165.953 | |
| Σ Mufa + Σ PUFA | 534.174 ± 220.179 | |
| Σ PUFA ω-3 | 10.228 ±2.114 | |
| Σ PUFA ω-6 | 306.176 ±163.561 | |
| AI | 0.890 ± 0.123 | |
| TI | 1.649 ± 0.206 | |
| HH | 3.750 ± 0.003 | |
| HPI | 1.151 ± 0.156 |
| TPC | ABTS | DPPH | ORAC |
|---|---|---|---|
| (mg GAE/100 mg DW) | (µmol TE/100 mg DW) | ||
| 1.07 ± 0.05 | 2.44 ± 0.27 | 1.67 ± 0.15 | 11.90 ± 1.22 |
| Film | Thickness (mm) | WVPR (g·m−2·day−1) | WVP (g·mm·m−2·day−1·kPa−1) |
|---|---|---|---|
| A | 0.046 ± 0.003 d | 575.99 ± 15.50 bc | 23.45 ± 1.47 bc |
| B | 0.060 ± 0.003 c | 660.93 ± 16.49 c | 26.67 ± 0.26 c |
| C | 0.068 ± 0.007 bc | 483.09 ± 39.20 b | 20.99 ± 2.17 abc |
| D | 0.075 ± 0.003 ab | 470.61 ± 17.87 b | 19.21 ± 2.80 ab |
| E | 0.084 ± 0.003 a | 271.10 ± 19.84 a | 12.27 ± 0.98 a |
| Film | L* | a* | b* | Hue (°) | Chroma |
|---|---|---|---|---|---|
| A | 51.79 ± 3.77 c | 2.30 ± 0.15 b | 0.93 ± 0.11d | 21.85 ± 1.32 d | 2.48 ± 0.18 d |
| B | 59.15 ± 9.03 bc | 2.24 ± 0.45 b | 7.72 ± 0.92 c | 73.32 ± 2.49 b | 7.78 ± 0.97 c |
| C | 70.43 ± 8.64 ab | 0.34 ± 0.05 c | 12.98 ± 1.73 b | 88.46 ± 0.42 a | 12.98 ± 1.72 b |
| D | 85.71 ± 6.02 a | 3.7 ± 0.33 a | 4.95 ± 0.47 c | 53.19 ± 0.71 c | 6.18 ± 0.57 c |
| E | 54.06 ± 7.80 c | −0.95 ± 0.27 d | 17.47 ± 2.66 a | −86.96 ± 0.61 e | 17.49 ± 2.67 a |
| Film | H2O |
Acetic Acid
3% |
EtOH
10% |
EtOH
20% |
EtOH
50% |
|---|---|---|---|---|---|
| A | 100 | 16.9 ± 1.6 b | 100 | 100 | 100 |
| B | 100 | 13.6 ± 1.5 b | 100 | 100 | 100 |
| C | 100 | 17.3 ± 1.8 b | 100 | 100 | 100 |
| D | 100 | 35.2 ± 6.8 a | 100 | 100 | 100 |
| E | 100 | 32.0 ± 2.5 a | 100 | 100 | 100 |
| Film | Elongation at Break (%) | Tensile Strength (MPa) |
|---|---|---|
| A | 4.97 ± 1.99 a | 31.19 ± 6.63 a |
| C | 5.23 ± 1.92 a | 32.00 ± 5.65 a |
| E | 4.08 ± 0.84 a | 28.77 ± 6.33 a |
| Film | ABTS | DPPH |
|---|---|---|
| (µM TE/mg Film) | ||
| A | 120.15 ± 6.81 c | 85.97 ± 3.19 c |
| B | 260.67 ± 31.94 b | 111.34 ± 16.63 bc |
| C | 394.61 ± 35.69 a | 153.15 ± 20.07 b |
| D | 369.62 ± 73.71 a | 115.39 ± 13.81 bc |
| E | 451.06 ± 14.68 a | 212.81 ± 39.12 a |
| Film | Mesophilic Aerobic Bacteria (log (CFU/g Strawberry)) | Molds and Yeasts (log (CFU/g Strawberry)) |
|---|---|---|
| A | 2.29 ± 2.14 b | 2.33 ± 0.24 b |
| C | 1,77 ± 1.81 ab | 1.11 ± 0.40 a |
| E | 1.34 ± 1.05 a | 0.63 ± 0.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, V.F.R.; Lopes, A.I.; Machado, M.; Costa, E.M.; Ribeiro, T.B.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicrobial Activities. Foods 2025, 14, 1327. https://doi.org/10.3390/foods14081327
Martins VFR, Lopes AI, Machado M, Costa EM, Ribeiro TB, Poças F, Pintado M, Morais RMSC, Morais AMMB. Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicrobial Activities. Foods. 2025; 14(8):1327. https://doi.org/10.3390/foods14081327
Chicago/Turabian StyleMartins, Valter F. R., Ana I. Lopes, Manuela Machado, Eduardo M. Costa, Tânia B. Ribeiro, Fátima Poças, Manuela Pintado, Rui M. S. C. Morais, and Alcina M. M. B. Morais. 2025. "Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicrobial Activities" Foods 14, no. 8: 1327. https://doi.org/10.3390/foods14081327
APA StyleMartins, V. F. R., Lopes, A. I., Machado, M., Costa, E. M., Ribeiro, T. B., Poças, F., Pintado, M., Morais, R. M. S. C., & Morais, A. M. M. B. (2025). Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicrobial Activities. Foods, 14(8), 1327. https://doi.org/10.3390/foods14081327












