Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Strains
2.2. Preparation of Infant Formula Milk and Collection of Human Milk Samples
2.3. Ethical Aspects
2.4. Treatments
2.4.1. Holder Pasteurization (HoP)
2.4.2. Ultra-High-Pressure Homogenization (UHPH)
2.5. Microbiological Analysis of the Milk Samples and Lethality Determination
2.6. Determination of the Inactivation Kinetics and Effect of Treatment Variables
2.7. Immunological Components Quantification in HM Samples
2.7.1. Preparation of HM Samples for Immunoassays
2.7.2. sIgA, IgG, and IgM Immunoassays
2.8. Statistical Analysis
3. Results and Discussion
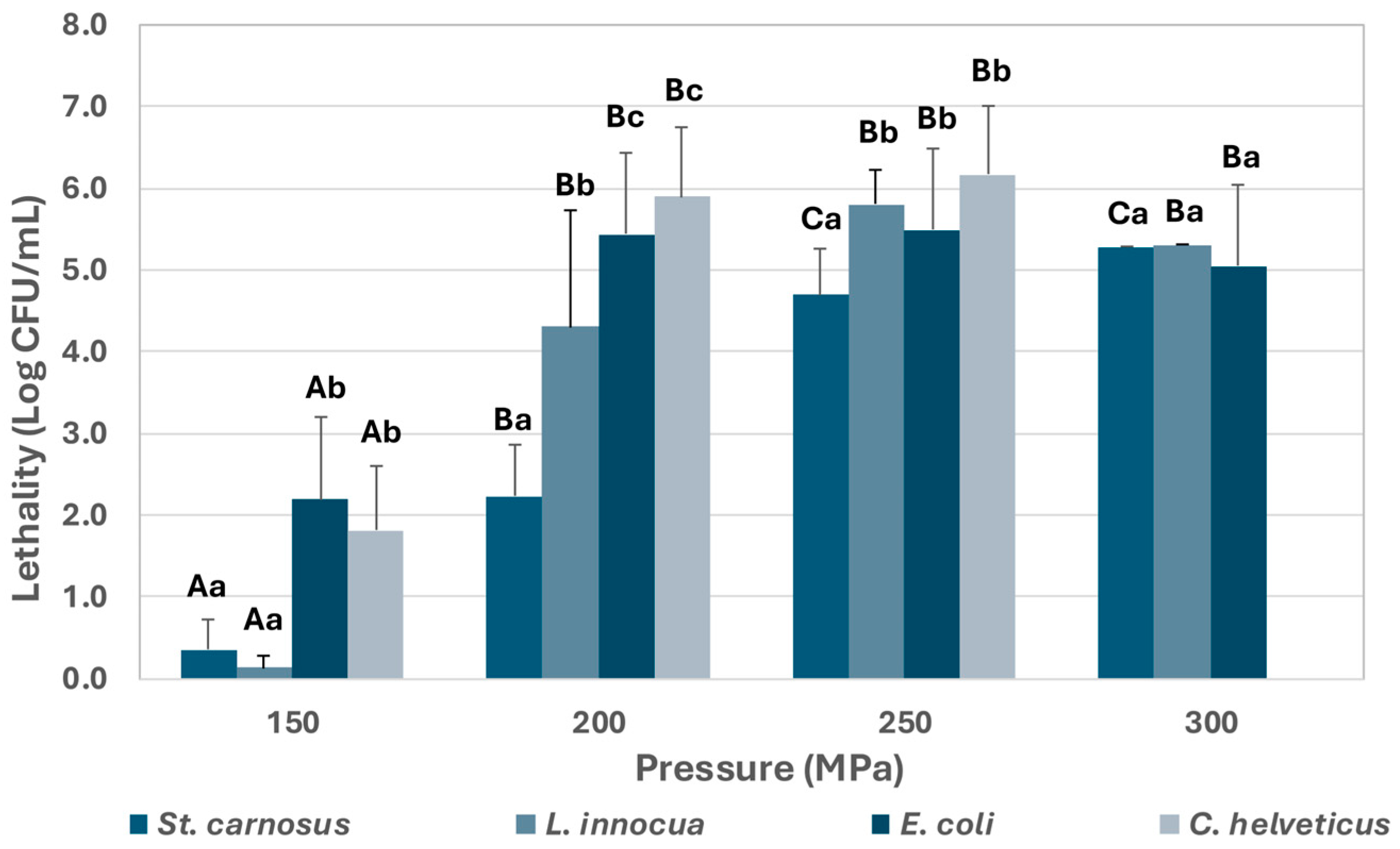
3.1. Effect of UHPH on Pathogen Strains Inoculated in Reconstituted Powdered Infant Formula and Human Milk
3.2. Mathematical Modeling of Treatments
3.3. Effect of UHPH on Immunoglobulin Isotype Concentrations in Human Milk
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarzynka, S.; Strom, K.; Barbarska, O.; Pawlikowska, E.; Minkiewicz-Zochniak, A.; Rosiak, E.; Oledzka, G.; Wesolowska, A. Combination of High-Pressure Processing and Freeze-Drying as the Most Effective Techniques in Maintaining Biological Values and Microbiological Safety of Donor Milk. Int. J. Env. Res. Public Health 2021, 18, 2147. [Google Scholar] [CrossRef]
- Calvo, J.; García Lara, N.R.; Gormaz, M.; Peña, M.; Martínez Lorenzo, M.J.; Ortiz Murillo, P.; Brull Sabaté, J.M.; Samaniego, C.M.; Gayà, A. Recomendaciones Para La Creación y El Funcionamiento de Los Bancos de Leche Materna En España. An. Pediatría 2018, 89, 65.e1–65.e6. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EU) 2024/1938 of the European Parliament and of the Council of 13 June 2024 on Standards of Quality and Safety for Substances of Human Origin Intended for Human Application and Repealing Directives 2002/98/EC and 2004/23/EC. Off. J. Eur. Union 2024, L series, 80. [Google Scholar]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Bernatowicz-Lojko, U.; Borszewska-Kornacka, M.K.; van Goudoever, J.B. Innovative Techniques of Processing Human Milk to Preserve Key Components. Nutrients 2019, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Vieco, D.; Rodríguez, J.M.; Espinosa-Martos, I.; Corzo, N.; Montilla, A.; García-Serrano, A.; Calvo, M.V.; Fontecha, J.; Serrano, J.; Fernández, L.; et al. High-Temperature Short-Time and Holder Pasteurization of Donor Milk: Impact on Milk Composition. Life 2021, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Moro, G.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The Effect of Holder Pasteurization on Nutrients and Biologically-Active Components in Donor Human Milk: A Review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef]
- Shini, V.S.; Udayarajan, C.T.; Nisha, P. A Comprehensive Review on Lactoferrin: A Natural Multifunctional Glycoprotein. Food Funct. 2022, 13, 11954–11972. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.E.; Billeaud, C.; Rachel, B.; Calvo, J.; Cavallarin, L.; Christen, L.; Escuder-Vieco, D.; Gaya, A.; Lembo, D.; Wesolowska, A.; et al. Processing of Donor Human Milk: Update and Recommendations From the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 49. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation. Food Eng. Rev. 2021, 13, 490–508. [Google Scholar] [CrossRef]
- Tobin, J.; Heffernan, S.P.; Mulvihill, D.M.; Huppertz, T.; Kelly, A.L. Applications of High-Pressure Homogenization and Microfluidization for Milk and Dairy Products. In Emerging Dairy Processing Technologies; Wiley: Hoboken, NJ, USA, 2015; pp. 93–114. [Google Scholar]
- Graikini, D.; Alvarez-Sabatel, S.; Puértolas, E.; Pérez, M.D.; Sánchez, L. Effect of Ultra-High Pressure Homogenization on the Antirotaviral Activity of Bovine Milk Whey. Innov. Food Sci. Emerg. Technol. 2024, 96, 103745. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparison of Ultra High Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical and Chemical Quality of Soymilk. LWT-Food Sci. Technol. 2012, 46, 42–48. [Google Scholar] [CrossRef]
- Zamora, A.; Guamis, B. Opportunities for Ultra-High-Pressure Homogenisation (UHPH) for the Food Industry. Food Eng. Rev. 2015, 7, 130–142. [Google Scholar] [CrossRef]
- Pinho, C.R.G.; Franchi, M.A.; Tribst, A.A.L.; Cristianinia, M. Effect of High Pressure Homogenization Process on Bacillus Stearothermophilus and Clostridium Sporogenes Spores in Skim Milk. Procedia Food Sci. 2011, 1, 869–873. [Google Scholar] [CrossRef][Green Version]
- Irazusta, A.; Rodríguez-Camejo, C.; Jorcin, S.; Puyol, A.; Fazio, L.; Arias, F.; Castro, M.; Hernández, A.; López-Pedemonte, T. High-Pressure Homogenization and High Hydrostatic Pressure Processing of Human Milk: Preservation of Immunological Components for Human Milk Banks. J. Dairy. Sci. 2020, 103, 5978–5991. [Google Scholar] [CrossRef] [PubMed]
- Briñez, W.J.; Roig-Sagués, A.X.; Hernández-Herrero, M.M.; Guamis-López, B. Inactivation of Two Strains of Escherichia Coli Inoculated into Whole and Skim Milk by Ultrahigh-Pressure Homogenisation. Lait 2006, 86, 241–249. [Google Scholar] [CrossRef]
- Briñez, W.J.; Roig-Sagués, A.X.; Herrero, M.M.H.; López, B.G. Inactivation of Listeria Innocua in Milk and Orange Juice by Ultrahigh-Pressure Homogenization. J. Food Prot. 2006, 69, 86–92. [Google Scholar] [CrossRef]
- Briñez, W.J.; Roig-Sagués, A.X.; Herrero, M.M.H.; López, B.G. Inactivation of Staphylococcus Spp. Strains in Whole Milk and Orange Juice Using Ultra High Pressure Homogenisation at Inlet Temperatures of 6 and 20 °C. Food Control. 2007, 18, 1282–1288. [Google Scholar] [CrossRef]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature Delivery Influences the Immunological Composition of Colostrum and Transitional and Mature Human Milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef]
- Rio-Aige, K.; Fernández-Bargalló, A.; Vegas-Lozano, E.; Miñarro-Alonso, A.; Castell, M.; Selma-Royo, M.; Martínez-Costa, C.; Rodríguez-Lagunas, M.J.; Collado, M.C.; Pérez-Cano, F.J. Breast Milk Immune Composition Varies during the Transition Stage of Lactation: Characterization of Immunotypes in the MAMI Cohort. Front. Nutr. 2023, 10, 1252815. [Google Scholar] [CrossRef]
- Datta, N.; Hayes, M.G.; Deeth, H.C.; Kelly, A.L. Significance of Frictional Heating for Effects of High Pressure Homogenisation on Milk. J. Dairy Res. 2005, 72, 393–399. [Google Scholar] [CrossRef]
- Hayes, M.G.; Kelly, A.L. High Pressure Homogenisation of Raw Whole Bovine Milk (a) Effects on Fat Globule Size and Other Properties. J. Dairy Res. 2003, 70, 297–305. [Google Scholar] [CrossRef]
- van Asselt, E.; Zwietering, M. A Systematic Approach to Determine Global Thermal Inactivation Parameters for Various Food Pathogens. Int. J. Food Microbiol. 2006, 107, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhou, B.; Zou, H.; Wang, Y.; Liao, X.; Hu, X.; Zhang, Y. High Pressure Homogenization Inactivation of Escherichia Coli and Staphylococcus Aureus in Phosphate Buffered Saline, Milk and Apple Juice. Lett. Appl. Microbiol. 2021, 73, 159–167. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Ferrari, G. Microbial Inactivation by High Pressure Homogenization: Effect of the Disruption Valve Geometry. J. Food Eng. 2013, 115, 362–370. [Google Scholar] [CrossRef]
- Moro, G.E.; Arslanoglu, S. Heat Treatment of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 165–166. [Google Scholar] [CrossRef]
- Morniroli, D.; Consales, A.; Crippa, B.L.; Vizzari, G.; Ceroni, F.; Cerasani, J.; Colombo, L.; Mosca, F.; Giannì, M.L. The Antiviral Properties of Human Milk: A Multitude of Defence Tools from Mother Nature. Nutrients 2021, 13, 694. [Google Scholar] [CrossRef]
- Conboy-Stephenson, R.; Ross, R.P.; Kelly, A.L.; Stanton, C. Donor Human Milk: The Influence of Processing Technologies on Its Nutritional and Microbial Composition. Front. Nutr. 2024, 11, 1468886. [Google Scholar] [CrossRef]
- Contador, R.; Delgado-Adámez, J.; Delgado, F.J.; Cava, R.; Ramírez, R. Effect of Thermal Pasteurisation or High Pressure Processing on Immunoglobulin and Leukocyte Contents of Human Milk. Int. Dairy. J. 2013, 32, 1–5. [Google Scholar] [CrossRef]
- Koenig, Á.; Diniz, E.M.d.A.; Barbosa, S.F.C.; Vaz, F.A.C. Immunologic Factors in Human Milk: The Effects of Gestational Age and Pasteurization. J. Hum. Lact. 2005, 21, 439–443. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Robert-Guroff, M. IgG Surfaces as an Important Component in Mucosal Protection. Nat. Med. 2000, 6, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]

| Matrix | UHPH (MPa) | Tv (°C) | Ti (°C) | Tf (°C) |
|---|---|---|---|---|
| Infant formula | 151.67 ± 3.66 | 60.20 ± 2.21 | 21.47 ± 0.86 | 18.24 ± 1.34 |
| 201.22 ± 2.21 | 76.89 ± 8.04 | 20.93 ± 1.54 | 18.13 ± 1.12 | |
| 251.41 ± 3.81 | 88.35 ± 8.37 | 20.94 ± 1.52 | 17.56 ± 1.17 | |
| 300.50 ± 2.12 | 121.00 ± 0.00 | 24.95 ± 0.07 | 17.60 ± 0.14 | |
| Human milk | 153.29 ± 2.81 | 60.86 ± 1.86 | 13.16 ± 0.08 | 18.21 ± 0.20 |
| 204.14 ± 2.48 | 75.43 ± 1.72 | 13.40 ± 0.00 | 18.41 ± 0.07 | |
| 251.44 ± 1.94 | 85.22 ± 0.44 | 13.32 ± 0.20 | 15.89 ± 1.27 |
| Microorganism | Z Value (°C) | D70°C (s) | D75°C (s) |
|---|---|---|---|
| Listeria monocytogenes | 7.0 | 5.23 | 1.01 |
| Staphylococcus aureus | 8.8 | 15.42 | 4.17 |
| Cronobacter sakazakii | 6.3 | 1.85 | 0.30 |
| Escherichia coli | 10.6 | 12.83 | 4.33 |
| Microorganism | Models | R2 adj | RMSE |
|---|---|---|---|
| St. carnosus | 0.992 | 0.225 | |
| 0.992 | 0.225 | ||
| 0.856 | 3.067 | ||
| L. innocua | 0.999 | 0.099 | |
| 0.999 | 0.099 | ||
| 0.946 | 3.472 | ||
| E. coli | 0.998 | 0.102 | |
| 0.998 | 0.102 | ||
| 0.986 | 3.117 | ||
| F. helveticus | 0.994 | 0.199 | |
| 0.994 | 0.199 | ||
| 0.980 | 3.367 |
| Raw HM | HoP | UHPH 200 | UHPH 250 | |
|---|---|---|---|---|
| sIgA (%) | 100.0 ± 3.0 A | 50.9 ± 2.6 B | 83.0 ± 3.6 A | 90.5 ± 7.4 A |
| IgG (%) | 100.0 ± 11.7 A | 54.9 ± 6.9 B | 107.9 ± 10.2 A | 98.9 ± 16.0 A |
| IgM (%) | 100.0 ± 0.62 A | 46.5 ± 3.2 B | 112.8 ± 5.5 A | 108.7 ± 5.1 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalali, K.; Pastor-Villaescusa, B.; Flores-Rojas, K.; Pleguezuelos, V.; Pérez-Cano, F.J.; Franch-Masferrer, À.; Trujillo-Mesa, A.J.; Hernández-Herrero, M.M.; Roig-Sagués, A.X. Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk. Foods 2025, 14, 1310. https://doi.org/10.3390/foods14081310
Jalali K, Pastor-Villaescusa B, Flores-Rojas K, Pleguezuelos V, Pérez-Cano FJ, Franch-Masferrer À, Trujillo-Mesa AJ, Hernández-Herrero MM, Roig-Sagués AX. Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk. Foods. 2025; 14(8):1310. https://doi.org/10.3390/foods14081310
Chicago/Turabian StyleJalali, Kimia, Belén Pastor-Villaescusa, Katherine Flores-Rojas, Vanessa Pleguezuelos, Francisco J. Pérez-Cano, Àngels Franch-Masferrer, Antonio J. Trujillo-Mesa, M. Manuela Hernández-Herrero, and Artur X. Roig-Sagués. 2025. "Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk" Foods 14, no. 8: 1310. https://doi.org/10.3390/foods14081310
APA StyleJalali, K., Pastor-Villaescusa, B., Flores-Rojas, K., Pleguezuelos, V., Pérez-Cano, F. J., Franch-Masferrer, À., Trujillo-Mesa, A. J., Hernández-Herrero, M. M., & Roig-Sagués, A. X. (2025). Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk. Foods, 14(8), 1310. https://doi.org/10.3390/foods14081310








