Abstract
Global population growth raises concerns about the availability of safe and nutritious food, along with its environmental and social impacts. In this context, plant-based foods have emerged as a promising solution, offering sustainable and affordable alternatives. Baru almonds (Dipteryx alata Vogel), a native Brazilian species, represent a viable and eco-friendly protein source with significant potential for food applications. This review discusses the nutritional composition, protein extraction methods and techno-functional properties of baru almonds, highlighting both advantages and limitations for food application. Baru proteins exhibit a high protein content (23–30%, w/w), a balanced essential amino acid profile, and valuable functional properties, including emulsifying capacity, foam stability, and moderate water- and oil-holding capacities. However, despite their potential, the lack of research on the gelation properties of baru proteins restricts their application in structured plant-based food formulations, where protein gelation is crucial for texture, water retention, and overall product stability. Further research is needed to evaluate their gel-forming ability and allergenic potential. Additionally, this review explores emerging protein extraction techniques that could improve protein quality and functionality, expanding their applicability in the food industry. By promoting biodiversity conservation and regional development, baru almonds contribute to the growing demand for sustainable protein sources.
1. Introduction
The demand for plant-based proteins has surged in recent years, driven by population growth and the need for sustainable, nutritious food alternatives that minimize reliance on finite natural resources associated with livestock production [1,2]. Scientific interest in vegetable proteins has increased significantly, with a notable rise in publications from 2001 to 2024 (Figure 1), highlighting their growing relevance across multidisciplinary fields like food science, nutrition, and agronomy.
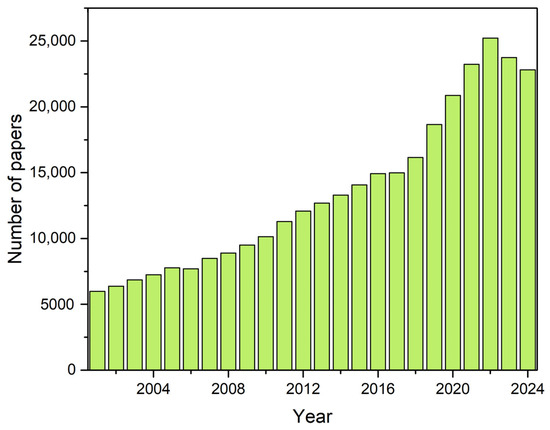
Figure 1.
Number of scientific publications reported by Web of Science containing the terms of the topic “plant protein”, “plant-based protein” and “vegetable protein”, from 2001 to 2024.
Many studies have highlighted a prevailing trend toward replacing animal proteins with plant-based proteins, such as soy, wheat, gluten, and pea [3,4,5,6,7]. However, while this shift is evident, it primarily relies on a limited number of conventionally cultivated monoculture crops, which pose significant environmental and social challenges, including soil degradation, biodiversity loss, and the concentration of land and resources [8]. As a result, the long-term sustainability of these protein sources is questionable. The environmental impact of extensive reliance on conventional plant proteins could be comparable to that of meat production. A study assessing the environmental footprint of various burger formulations, incorporating both animal- and plant-based proteins, found that poultry protein had the lowest environmental impact, followed by soybean, pork, lentil, and chickpea protein, based on parameters such as global warming potential, water footprint, energy demand, and ecotoxicity [9].
Furthermore, a lack of dietary diversity may negatively impact health. The global food supply heavily relies on a narrow spectrum of options, with a small number of plant and animal species contributing to 75% of our food intake [10]. This limited food biodiversity can lead to nutritional imbalances, such as unbalanced amino acid profile, reduced protein digestibility, and the excessive presence of antinutritional factors. Moreover, it poses risk of allergic reactions such as those associated with soy, a prevalent component of vegan diets and a recognized allergen by the Food and Drug Administration (FDA) [11]. Generally, plant-based proteins show inferior technological properties compared to animal proteins [12,13], necessitating the blending of multiple proteins in various formulations.
To address these challenges, exploring diverse and sustainable protein sources from global biomes is critical. Unconventional species like baru almonds offer promising nutritional and environmental benefits while supporting regional development and reducing deforestation [14]. Baru almonds reviews have highlighted the health benefits associated with baru consumption [15,16]. However, the specific properties of baru proteins remain relatively unexplored. This review aims to provide a comprehensive understanding of baru almond proteins, particularly concerning their extraction methods, nutritional value, and techno-functional properties for potential use as ingredients in food formulations. Furthermore, the opportunities and challenges involved in incorporating baru proteins into future foods will also be discussed.
2. Baru (Dipteryx alata): Biome, Characteristics and Almond Composition
The Cerrado, Brazil’s second-largest ecosystem, is among the world’s most biodiverse regions. However, approximately 50% of its area is occupied by agriculture, primarily monoculture, impacting 80% of its native vegetation and risking irreversible degradation by 2030 [17,18]. Sustainable use of native species like baru (Dipteryx alata Vogel), offers a solution to preserve biodiversity, reduce deforestation and minimize the environmental impact of long-distance ingredient transport.
Baru almonds, derived from the fruit of the Dipteryx alata tree (Figure 2), are sustainably harvested by rural communities, providing income while minimizing environmental impact. Valued for their nutritional content and peanut-like flavor, baru almonds have a growing global market, projected to rise from USD 5.1 million in 2022 to USD 47 million by 2032 [19].
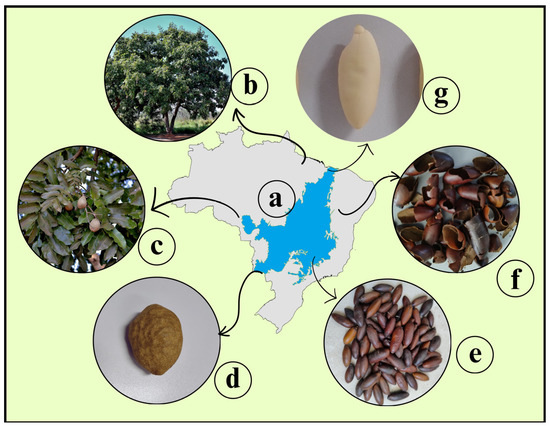
Figure 2.
Map of Brazil with the Cerrado region highlighted (a), where baru tree is found (b), baru fruit (c and d), baru almonds with skin (e), its skin (f) and the baru almond without skin (g).
Nutritionally, baru almonds are rich in protein (23–30%, w/w), fat (40–45.80%, w/w), dietary fiber (6.10–12.50%, w/w), and minerals such as calcium, iron, and zinc [20,21]. Their total protein content exceeds that of almonds (21%, w/w), pistachio (20%, w/w), and hazelnut (14.5%, w/w) [22]. Additionally, raw baru almonds are abundant in bioactive compounds like carotenoids (11.40 µg.100 g−1) and tocopherols (11.61 µg.100 g−1), contributing to their high antioxidant capacity. However, antinutritional factors like phytates and tannins can reduce nutrient bioavailability. Heat treatment effectively mitigates these compounds, enhancing their nutritional value [23].
Similar to soybean, pea, sunflower, rapeseed, hemp and pumpkin seeds, the predominant protein fraction in baru almonds is globulin (61.7%), followed by albumin, glutelin, and prolamin, highlighting its potential for diverse food applications [22,24]. The application of baru almonds as food ingredients aligns with global efforts to promote underutilized crops for sustainable and nutritious food solutions.
3. Nutritional Proprieties of Baru Proteins
The quality of proteins is determined by their essential amino acids (EAAs) content, digestibility, bioavailability, purity, and the impact of extraction and processing methods. Table 1 compares the amino acid profile of baru almond proteins with other high-protein sources. Baru almond flour contains 25.81 g/100 g of protein, lower than soybean flour (41.85 g/100 g), and animal-derived powders like eggs (48.56 g/100 g) and casein (81.57 g/100 g), but higher than other vegetable sources like pea flour (21.50 g/100 g), and Moringa oleifera leaves (moringa) (23.60 g/100 g), making it valuable source of protein, particularly in vegetarian and plant-based diets.

Table 1.
Essential amino acid profile (mg.g−1 of protein), total protein content (g.100 g−1) and in vitro protein digestibility (%) of high-protein sources.
The concentration of EAAs in proteins from animal sources such as dehydrated beef, casein powder, and egg powder meets the dietary recommendations of the FAO/WHO/UNU reference pattern [25]. In contrast, plant-based proteins often contain limiting amino acids. For example, soybean protein is deficient in methionine + cysteine (18.65 mg/g), making it necessary to combine it with other protein sources rich in these amino acids. Conversely, Moringa oleifera flour contains an excess of methionine + cysteine (71.40 mg/g) but is limited in histidine, isoleucine, and leucine. Given these differences, combining different plant protein sources, such as soybean and Moringa oleifera flour, could enhance amino acid balance and improve protein quality in food formulations.
However, baru almond flour demonstrates a robust EAA profile. Baru almond flour contains significant amounts of methionine + cysteine (22.00 mg/g of protein), histidine (23.4 mg/g of protein), isoleucine (37.5 mg/g of protein), and leucine (77.8 mg/g of protein), which indicates a well-balanced composition that aligns closely with the FAO/WHO/UNU dietary recommendations. When compared to the FAO reference values, baru almond flour meets or exceeds all these standards [25,26]. For example, compared to soybean flour—the most widely used plant-based protein in the food industry—baru almond flour presents higher methionine + cysteine (22.08 mg/g vs. 18.65 mg/g) and valine (51.8 mg/g vs. 48.16 mg/g) content. However, it falls short in other EAA, which can be attributed to the higher overall protein content in soybean flour. Despite this, the well-balanced and complete EAA profile of baru almond flour stands out, highlighting its potential as a nutritious alternative protein source.
Regarding pea flour, both baru almond and pea flour do not present limiting amino acids and show similar methionine + cysteine. Nevertheless, baru almond flour is superior in leucine (77.8 mg/g vs. 70.00 mg/g), phenylalanine + tyrosine (87.33 mg/g vs. 65.00 mg/g), threonine (44.90 mg/g vs. 37.00 mg/g), and valine (51.8 mg/g vs. 39.00 mg/g). When compared to another unconventional protein source, Moringa oleifera flour, baru almond flour shows no limiting amino acids, whereas Moringa oleifera leaves present limiting amounts of histidine (14.40 mg/g), isoleucine (28.80 mg/g), and leucine (56.60 mg/g). However, baru almond flour has significantly lower methionine + cysteine (22.08 mg/g vs. 71.40 mg/g) and lysine (48.4 mg/g vs. 58.20 mg/g) content. Over again, baru almond amino acid profile stands out for being a well-equilibrated source of EAA, as promising as moringa, but it does not present the dark green color and astringent flavor, which can affect sensory properties of foods [32].
Baru almond flour demonstrates high in vitro protein digestibility (IPD: 86.0%), comparable to animal-based proteins and superior to many plant-based options, including soybean and moringa flours. Its digestibility enhances the delivery of essential amino acids, making it suitable for diverse applications, including breads, snacks, vegan supplements, and protein bars. As global demand for plant-based proteins grows, baru almond flour emerges as a sustainable and innovative option that supports nutrition and biodiversity.
4. Extraction of Baru Almond Proteins
Baru almond protein extraction plays a crucial role in determining its functional properties, beginning with oil removal, typically performed via mechanical pressing, producing defatted baru almond flour (DBF), a protein-rich byproduct [33,34]. Combining mechanical pressing with solvent extraction improves oil recovery and protein yield while minimizing oxidation [35]. Safer solvents like ethanol and supercritical CO2 are preferred over traditional hexane for enhanced safety and efficiency [36].
Baru protein concentrate (BPC) and isolate (BPI) are obtained via alkaline extraction. This method is also applied to other plant sources, including soy, almonds, and peanuts. DBF is solubilized in an alkaline medium (pH~10) to enhance protein solubility through electrostatic repulsion, followed by centrifugation to collect the protein-rich supernatant for BPC production. Further acidification of supernatant to the isoelectric point (pI) (pH~4.8), causes protein aggregation, resulting in the BPI [37].
This process yields BPC and BPI with a high protein content (55.03 g/100 g and 84.11–88.4 g/100 g, respectively), comparable to or exceeding other protein isolates sources like soy (87.6 g/100 g) and casein (83.0 g/100 g), highlighting the feasibility and competitiveness of baru protein extraction for use in food formulation [37,38]. Despite its high efficiency, simplicity, and cost-effectiveness, the alkaline extraction-acid precipitation method has some drawbacks. It can lead to protein denaturation and the formation of insoluble aggregates, negatively impacting digestibility and techno-functional properties [39,40,41,42]. Additionally, the alkaline extraction generates large amounts of acid and alkali wastewater as environmental pollutants and may form toxic species, such as lysinoalanine, having a significant environmental footprint [41].
The Osborne method offers a milder alternative that allows the sequential separation of different protein fractions without the formation of toxic species, also, unlike alkali protein extraction, preserves the functionality of albumins and globulins, the main protein fractions in baru almonds. This sequential extraction is based on solubility, using water (pH 7.0, 1:30 w/v) for albumins, followed by 0.5 mol/L NaCl (pH 7.0, 1:30 w/v) for globulins, 700 g/L ethanol for prolamins, and 0.1 mol/L NaOH for glutelins. Stirring (60 min) and centrifugation (32,000× g, 40 min) were applied at each step, with dialysis (10 kDa membranes, 24–36 h) where applicable. This method recovers 80% of total baru almond proteins, with globulins (61.7%) as the predominant fraction, followed by albumins (14.0%), glutelins (3.3%), and prolamins [22]. Despite its effectiveness, the Osborne method is time-consuming and requires precise handling, limiting its industrial scalability [43].
Innovative techniques such as enzyme-assisted (EA) extraction reduce harsh chemical usage while improve protein yield and techno-functional properties by enhancing emulsification, solubility, and water- and oil-holding capacities, as observed in chickpea protein properties [44,45]. It is particularly suitable for heat-sensitive ingredients, but its commercial application is limited by high enzyme costs, sensitivity, and the potential formation of bitter byproducts [46].
Ultrasound-assisted extraction (US) is a sustainable alternative due to low energy consumption, maintenance costs, and processing time. When combined with conventional techniques, it increased protein yields from Spirulina (32.48% to 76.83%, 200 W, 26 kHz), while enhancing solubility and digestibility [47,48]. Despite its advantages, US protein extraction faces challenges such as heat generation, free radical formation, and limited scalability [49].
Pulsed electric field (PEF) is an eco-friendly and cost-effective protein extraction technique, that enables by-products recovery from food waste. Combining PEF with alkaline extraction (2.3 kV for 25 min) improved rice bran protein yield by 22.8% and enhanced functional properties, including oil-holding capacity and emulsifying (20.29–22.64%, 3.3–12.0%, respectively) [46]. However, high initial equipment costs and process optimization remain challenges for large-scale applications [50].
Deep eutectic solvents (DES), formed from a hydrogen bond acceptor (HBA) and donor (HBD), are non-toxic, recyclable, and cost-effective, making them suitable for protein extraction. For instance, a DES made from choline chloride (HBA) and levulinic acid (HBD), in a 6:1 molar ratio, extracted 39.16 mg/g of protein from bamboo shoots, outperforming conventional methods (23.88 mg/g) [51]. Despite their potential, further research is needed to assess their compatibility, biodegradability, and recyclability for broader food industry applications.
Integrating emerging extraction methods can enhance protein yield and functionality, making baru almonds proteins valuable ingredients for diverse food applications. Nevertheless, selecting an appropriate extraction approach requires balancing efficiency, cost, and sustainability to minimize environmental impact while maximizing protein recovery. Hybrid strategies, combining conventional and green technologies, may offer an optimal compromise for scalable and eco-friendly industrial applications.
5. Baru Almonds Techno-Functional Properties
Proteins play a crucial role in food product development, influencing sensory characteristics and techno-functional properties. Baru almond proteins offer a promising plant-based alternative, making essential to evaluate their functionality and competitiveness with other protein sources. The next sections will compare the techno-functional properties of baru almonds with animal and plant proteins.
5.1. Solubility
Protein solubility is a foundational property influencing applications like emulsification, foaming, and gelation. Baru proteins follow a U-shaped solubility curve relative to pH, similar to other plant-based proteins like soybean, pea, and moringa (Figure 3). At alkaline pH (8–10), baru proteins show high solubility (approximately 83%), surpassing pea (80%), and moringa (58%) proteins but slightly below soy, whey, egg, and sodium caseinate proteins, which exceed 88%. Above their pI, proteins exhibit a net negative charge, increasing mutual repulsion and solubility.
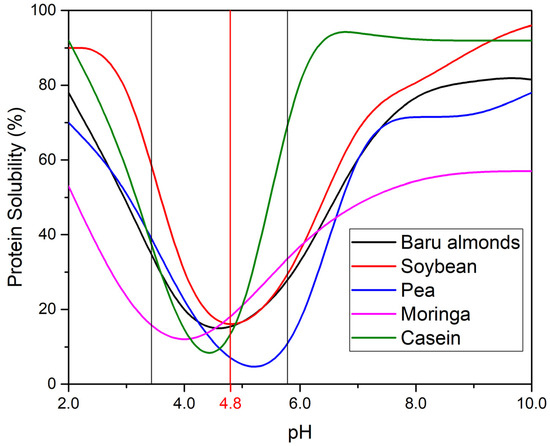
Figure 3.
Solubility profile of proteins from baru almond [37], soybean [52], Moringa oleifera leaves [53], pea [54] and sodium caseinate [55], with their isoelectric point (pI) region indicated by vertical black lines. The pI of baru almond proteins (pI = 4.8) is specifically marked with a vertical red line. Adapted with permission from [37], 2016, John Wiley and Sons; ref. [52] 1976, American Chemical Society, ref. [54], 2013, Elsevier, ref. [55], 2009, Cambridge University and ref. [53], 2020, Creative Commons license.
At acidic pH (3–5), near to their pI, baru proteins experience a sharp decrease in solubility, similar to soy protein isolate and sodium caseinate, which form precipitates near their pI. This reduced solubility limits their use in low-pH products like beverages and acidic dressings, where high solubility is essential for stability.
Several strategies have been proposed to improve the solubility of plant proteins in acidic conditions. Enzymatic hydrolysis, which generates peptides with enhanced solubility [56], and complexation with highly soluble proteins, such as whey proteins, have both been shown to enhance protein solubilization and resistance to aggregation [57]. Additionally, modifications such as the use of sodium alginate under microwave radiation [58] and the combination of pulsed electric field with pH shifting have demonstrated improvements in protein solubility [59]. Future research could explore the use of stabilizers, such as gum Arabic [60], in combination with baru protein isolate to improve protein stability under acidic conditions, potentially broadening their application in various food matrices by preventing precipitation and increasing solubility at acidic pH.
Despite their limitations at acidic pH, the solubility profile and mild flavor of baru proteins position them as a promising alternative to soy in neutral pH applications, such as plant-based beverages. With plant proteins representing only 18% of protein-rich beverages, the underrepresentation in this market highlights a significant opportunity for innovative product development using baru proteins [61].
The solubility of baru proteins across different pHs plays a crucial role in their incorporation into aqueous systems and in enhancing their functional properties, such as emulsification. This functionality is essential in stabilizing food systems, including emulsified products like non-dairy creams and protein-enriched drinks, further expanding the potential uses of baru proteins in the food industry.
5.2. Emulsifying Activity and Stability
Emulsifying capacity (EC) measures the efficiency of proteins emulsification and it is calculated as the ratio of emulsion layer height to the total mixture height after mechanical emulsification. Emulsifying kinetic stability (ES) evaluates the percentage of emulsion that remains stable over time, determined by the ratio of initial to remaining emulsion volume after stress tests like centrifugation, heating, or storage [62]. Table 2 presents the emulsifying properties of baru proteins, offering a comparison with other high-protein sources.

Table 2.
Techno-functional properties of baru almonds and other high proteins sources.
The DBF showed EC and ES (51 and 50%, respectively) slightly smaller than BPC (55 and 51%, respectively) and BPI (55 and 54%, respectively). Despite the higher protein content of BPI (84.11 g.100 g−1) compared to DBF (49.00 g.100 g−1) and BPC (57.65 g.100 g−1), there is no significant improvement in EC and ES, suggesting that these properties depend more on the quality than the quantity of soluble proteins [37,62,72]. The emulsifying properties of baru almond proteins are largely attributed to their high globulin content, typical of legume seeds like soybeans. Globulins are amphipathic, with both hydrophobic and hydrophilic regions, allowing them to adsorb to the oil–water interface and reduce interfacial tension. This enables them to stabilize oil-in-water emulsions effectively. The molecular structure of globulins, which facilitates their ability to form stable layers around oil droplets, makes them efficient emulsifiers in various food applications [73,74].
Compared to other proteins, BPI offers competitive emulsifying properties. It surpasses sodium caseinate (30%) and Moringa oleifera protein isolate (20%) in EC, demonstrating its potential as a robust plant-based emulsifier. Furthermore, BPI’s EC is comparable to that of soy (48.2%) and pea protein isolates (57.1%), both widely used plant-based emulsifiers in foods like cereals, bakery, and meat products. However, BPI EC is lower than whey protein isolate (73.4%), which is known for its superior solubility and interfacial activity. Whey, soy, and egg proteins are often associated with allergenic reactions, making it essential to evaluate the allergenicity of baru proteins to ensure they meet low-allergenicity standards.
At neutral pH, baru proteins display adequate EC and emulsifying stability (ES), making them suitable for plant-based beverages. Defatted baru flour (DBF) has shown potential as a wheat flour substitute, with 25% DBF cookies achieving sensory acceptance comparable to wheat-based counterparts and enhancing nutritional value [75]. Baru almonds have also been utilized in innovative products, such as protein-enriched cereal bars and vegan “Dulce de leche”, both receiving high sensory scores (higher than 7) for appearance, flavor, and texture [14,76].
The EC and ES of baru almond proteins, particularly in the forms of defatted flour, protein concentrate, and isolate, highlight their potential as versatile emulsifying agents in various food applications. Also, baru almond proteins may offer advantages as a lower-allergen alternative to traditional emulsifiers, pending further studies on allergenicity. These findings suggest that baru almond proteins could serve as valuable ingredients in developing plant-based food products and clean-label formulations, expanding their potential applications in the food industry.
5.3. Foam Capacity and Stability
Foam capacity (FC) and foam stability (FS) are essential properties in aerated food products, influencing texture and structure in products like breads, cakes, meringues, and ice creams. FC refers to the volume of foam that proteins generate, while FS describes the protein’s ability to stabilize the foam against gravitational and mechanical stress.
FC and FS values for baru proteins and other proteins are presented in Table 2. BPI demonstrates an FC of 58.30%, surpassing soybean protein isolate (SPI, 25.00%) and Moringa oleifera protein isolate (13.00%). Conversely, higher SPI FC values reported in other studies (82.00% to 250.00%) highlight the influence of blending speed and time, suggesting similar adjustments could enhance the FC of BPI [64,77,78].
The FC and FS of BPC (95.00% and 23.00%, respectively) and DBF (69.00% and 35.00%, respectively) differ from BPI (58.30% and 96%, respectively), reflecting the impact of extraction processes on protein structure. In BPI, globulins are the main protein fraction, forming viscoelastic films that enhance foam stability [79,80]. Although its FC is lower than whey (320.00%), pea (300.00%), and sodium caseinate (290.00%), its superior FS (96.00% vs. 35–58.00%) makes it ideal for stable foam applications like whipped toppings and vegan meringues.
Future research could explore how various processing techniques influence the foaming properties of plant proteins, potentially enhancing their functionality in food applications. For instance, high-intensity ultrasound has been shown to modify the structure of soy protein isolates, leading to improved foaming capacity by reducing particle size and altering interfacial properties [78]. Similarly, heat treatments can affect the surface hydrophobicity and solubility of soy protein concentrates, thereby enhancing their foaming properties [81]. Additionally, the glycation reaction, when combined with ultrasound treatment, has been reported to improve the foaming properties of brewer’s spent grain protein [82]. Investigating these and other processing methods, such as enzymatic hydrolysis and pH modification, could provide valuable insights into optimizing the foaming characteristics of plant proteins for diverse food applications.
5.4. Gelation
Gelation (GE) is a key techno-functional property for developing structured plant-based foods, such as meat, egg, yogurt, and cheese analogs. It typically occurs through thermal denaturation, where protein molecules unfold, aggregate, and form a protein network.
While extensive studies have focused on the emulsifying and foaming capacities of baru proteins, their gelation properties remain largely unexplored, representing a critical knowledge gap. Considering insights from other plant-based proteins, as soy proteins (12%) and pea proteins (14%), they require higher concentrations compared to animal proteins like ovalbumin (1%) to achieve gelation [67,83,84]. These variations highlight how protein type and molecular composition influence gelation behavior. Smaller globular proteins, such as those predominant in pea protein, require higher concentrations to form a gel. Since globulins are the major protein fraction in baru almond, further research is needed to determine the least gelation concentration of its protein concentrates and isolates, as well as the gelation behavior of individual protein fractions. Additionally, studies on their denaturation temperature and its impact on gel strength are essential to better understand their functionality [22,83,85].
Given the unique protein profile of baru almonds, investigating the gelation properties of baru proteins under various conditions may further expand their application in different food matrices, offering functional and textural benefits that align with the growing demand for plant-based, texture-optimized foods. Future research should explore the effects of protein concentration and thermal treatments on the gelation behavior of baru proteins. Studies on almond proteins have shown that heating protein solutions (3.6% protein (w/v), 85–95 °C, 30 min) can induce gel formation with high water-holding capacity and gel strength comparable to dairy gels [86]. Additionally, enzymatic hydrolysis has been reported to modify the gelation properties of almond proteins (8% protein (w/v), 85 °C, 30 min), where limited hydrolysis (degree of hydrolysis less than 10%) resulted in gels with improved water-holding capacity and texture [87]. Investigating similar conditions for baru proteins, including varying protein concentrations, thermal treatments, and enzymatic modifications, could provide valuable insights into optimizing their gelation properties for diverse food applications.
5.5. Water- and Oil-Holding Capacity
Water-holding capacity (WHC) measures a protein’s ability to retain water. In applications such as soups, baked goods, meat analogues, sausages, and custards, high WHC helps maintain moisture and product stability. For example, in meat analogues, plant proteins with high WHC contribute to replicating the texture and juiciness of traditional meat. In emulsified meat products, such as sausages and mortadella, WHC influences gel formation, ensuring product integrity and preventing phase separation. On the other hand, oil-holding capacity (OHC) indicates a protein’s ability to retain oil, enhancing flavor, mouthfeel, and structure in lipid-rich foods. In cheeses and sauces, high OHC helps maintain a creamy and homogeneous texture. Additionally, OHC plays a crucial role in restructured meats and meat substitutes, where oil retention improves texture and enhances the sensory profile of the final product [88,89,90,91,92].
Interestingly, studies in the literature have shown that the WHC of baru proteins decreases as protein concentration increases, with DBF exhibiting the highest WHC value (225.98%, 49.00 g·100 g−1), followed by BPC (193.84%, 57.65 g·100 g−1) and BPI (118.30%, 88.40 g·100 g−1) [37,62]. This decline in WHC for BPI is likely associated with conformational changes induced during the acid precipitation step during protein extraction, leading to partial protein aggregation and reduced structural flexibility [58]. Additionally, this step further alters protein conformation, decreasing the availability of hydrophilic sites and contributing to WHC reduction [93]. Similarly, OHC shows a decreasing trend, with DBF exhibiting the highest value (199.80%) compared to BPI (155.50%), likely due to the loss of low-molecular-weight protein fractions, such as albumins and globulins, during the extraction process [37,62].
Baru proteins have a distinct amino acid profile that contributes to their functional properties. Hydrophilic amino acids like lysine and glutamine enhance WHC, while hydrophobic residues such as leucine and phenylalanine improve OHC [26,94,95]. Compared to other isolates, BPI’s WHC is lower than pea (300–430%), soybean (310.90%), and sodium caseinate (300%), but higher than whey protein isolate. This balance between moisture retention and solubility supports its use in formulations requiring enhanced texture and stability.
BPI demonstrates a higher OHC (155.50%) than soybean protein isolate (60.00%), enhancing its suitability for fat-rich foods, but it falls short of whey protein isolate (320.00%) and sodium caseinate (280.00%) [37,66,69]. The superior OHC of caseins are attributed to their flexible structures, which expose more hydrophobic sites compared to the globular proteins in baru almonds. These differences highlight BPI’s potential for specific lipid-rich food applications, despite structural limitations.
Furthermore, OHC of BPI (155.50%) is comparable to the lower range of pea (110–250.00%), and Moringa oleifera leaves (160–355.00%) protein isolates, likely due to the similar globulin composition of pea and baru proteins. However, the specific proteins in baru almonds remain unidentified, warranting further research to better understand and optimize BPI’s structural properties for targeted industrial applications.
These intermediate WHC and OHC values position baru proteins as a promising alternative for food formulations requiring moderate water and oil retention, such as meat substitutes, sauces, and bakery products. Moreover, the balanced amino acid profile and compatibility with other protein sources highlight their potential as a functional ingredient.
6. Conclusions
Baru almond proteins hold significant potential as a sustainable and versatile ingredient for the food industry. Their balanced nutritional profile, which meets all FAO essential amino acid standards and their functional properties make them ideal for incorporation into diverse food products, including vegan and plant-based formulations. Advanced extraction methods can optimize their usability and efficiency, supporting industrial scalability. Furthermore, the utilization of baru almonds promotes Cerrado conservation and regional socio-economic development, aligning with global sustainability goals.
However, despite their promising functional characteristics, the gelation properties of baru proteins remain underexplored. A deeper understanding of their gel-forming behavior is crucial for expanding their applications in food systems, particularly in texturized plant-based products. Continued research on optimizing their functionality, including gelation mechanisms and allergenic potential, is essential to fully harness their applications in innovative food formulations.
Author Contributions
N.M.R.M., data curation, formal analysis, investigation, methodology, validation, and writing—original draft. E.A.H., formal analysis, methodology, validation, visualization, and writing—review and editing. J.d.P.R., formal analysis, methodology, validation, visualization, and writing—review and editing. M.C.T.R.V., methodology, resources, validation, visualization, and writing—review and editing. A.C.d.S.P., conceptualization, funding acquisition, resources, validation, visualization, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 311788/2021-1; 406919/2021-6; 403724/2023-6), Fundação de Apoio à Pesquisa de Minas Gerais (FAPEMIG: APQ-02024-21; APQ-00823-22; APQ-03953-22; BPD-00305-22; RED-00186-22), and Financiadora de Estudos e Projetos (FINEP 01.23.0632.00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BPC | Baru protein concentrate |
| BPI | Baru protein isolate |
| DBF | Defatted baru almond flour |
| DES | Deep eutectic solvents |
| EA | Enzyme-assisted extraction |
| EAAs | Essential amino acids |
| EC | Emulsifying capacity |
| ES | Emulsifying kinetic stability |
| FC | Foam capacity |
| FS | Foam stability |
| GE | Gelation |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| IPD | In vitro protein digestibility |
| OHC | Oil-holding capacity |
| PEF | Pulsed electric field |
| pI | Isoelectric point |
| SPI | Soybean protein isolate |
| US | Ultrasound-assisted extraction |
| WHC | Water-holding capacity |
References
- Mohammad, Z.H.; Ahmad, F.; Ibrahim, S.A. Biotechnology Approaches to Food Security: Risks and Solutions. In Microbial Biotechnology in the Food Industry; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–13. [Google Scholar]
- Wang, J.; Azam, W. Natural Resource Scarcity, Fossil Fuel Energy Consumption, and Total Greenhouse Gas Emissions in Top Emitting Countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Messina, M.; Duncan, A.M.; Glenn, A.J.; Mariotti, F. Perspective: Plant-Based Meat Alternatives Can Help Facilitate and Maintain a Lower Animal to Plant Protein Intake Ratio. Adv. Nutr. 2023, 14, 392–405. [Google Scholar] [CrossRef]
- Webb, D.; Dogan, H.; Li, Y.; Alavi, S. Use of Legume Flours and Fiber for Tailoring Structure and Texture of Pea Protein-based Extruded Meat Alternatives. J. Food Sci. 2023, 88, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.; Dogan, H.; Li, Y.; Alavi, S. Physico-Chemical Properties and Texturization of Pea, Wheat and Soy Proteins Using Extrusion and Their Application in Plant-Based Meat. Foods 2023, 12, 1586. [Google Scholar] [CrossRef]
- Webb, D.; Li, Y.; Alavi, S. Chemical and Physicochemical Features of Common Plant Proteins and Their Extrudates for Use in Plant-Based Meat. Trends Food Sci. Technol. 2023, 131, 129–138. [Google Scholar]
- Zhang, X.; Zhao, Y.; Zhang, T.; Zhang, Y.; Jiang, L.; Sui, X. Potential of Hydrolyzed Wheat Protein in Soy-Based Meat Analogues: Rheological, Textural and Functional Properties. Food Chem. 2023, 20, 100921. [Google Scholar] [CrossRef]
- Khatri, P.; Kumar, P.; Shakya, K.S.; Kirlas, M.C.; Tiwari, K.K. Understanding the Intertwined Nature of Rising Multiple Risks in Modern Agriculture and Food System. Environ. Dev. Sustain. 2023, 26, 24107–24150. [Google Scholar] [CrossRef]
- Deprá, M.C.; Dias, R.R.; Sartori, R.B.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. Nexus on Animal Proteins and the Climate Change: The Plant-Based Proteins Are Part of the Solution? Food Bioprod. Process. 2022, 133, 119–131. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on Future Protein Production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef]
- Sharma, G.M.; Ma, Y.; Luccioli, S. Recalls Associated with Food Allergens and Gluten in FDA-Regulated Foods from Fiscal Years 2013 to 2019. J. Food Prot. 2023, 86, 100069. [Google Scholar] [CrossRef]
- Boukid, F.; Hassoun, A.; Zouari, A.; Tülbek, M.; Mefleh, M.; Aït-Kaddour, A.; Castellari, M. Fermentation for Designing Innovative Plant-Based Meat and Dairy Alternatives. Foods 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Lima, D.S.; Egea, M.B.; Cabassa, I.D.C.C.; de Almeida, A.B.; de Sousa, T.L.; de Lima, T.M.; Loss, R.A.; Volp, A.C.P.; de Vasconcelos, L.G.; Dall’Oglio, E.L.; et al. Technological Quality and Sensory Acceptability of Nutritive Bars Produced with Brazil Nut and Baru Almond Coproducts. Lwt 2021, 137, 110467. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Fernandes, D.C.; Naves, M.M.V. Baru (Dipteryx alata Vog.) Fruit as an Option of Nut and Pulp with Advantageous Nutritional and Functional Properties: A Comprehensive Review. NFS J. 2021, 24, 26–36. [Google Scholar] [CrossRef]
- Lima, M.d.C.; do Nascimento, H.M.A.; da Silva, J.Y.P.; de Brito Alves, J.L.; de Souza, E.L. Evidence for the Beneficial Effects of Brazilian Native Fruits and Their By-Products on Human Intestinal Microbiota and Repercussions on Non-Communicable Chronic Diseases—A Review. Foods 2023, 12, 3491. [Google Scholar] [CrossRef]
- WWF-Brazil Conversion in the Brazilian Cerrado Increases by 25% in 2022 and Registers the Highest Rate in 7 Years. Available online: https://www.wwf.org.br/?84401/Deforestation-in-the-Brazilian-Cerrado-increases-by-25-in-2022-and-registers-the-highest-rate-in-seven-years (accessed on 27 March 2025).
- Silva, B.d.O.; Moitinho, M.R.; Panosso, A.R.; Oliveira, D.M.d.S.; Montanari, R.; Moraes, M.L.T.d.; Milori, D.M.B.P.; Bicalho, E.d.S.; La Scala, N. Implications of Converting Native Forest Areas to Agricultural Systems on the Dynamics of CO2 Emission and Carbon Stock in a Cerrado Soil, Brazil. J. Environ. Manag. 2024, 358, 120796. [Google Scholar] [CrossRef]
- Fact MR Baru Nuts Market. Available online: https://www.factmr.com/report/1362/baru-nuts-market (accessed on 27 March 2025).
- Oliveira-Alves, S.C.; Pereira, R.S.; Pereira, A.B.; Ferreira, A.; Mecha, E.; Silva, A.B.; Serra, A.T.; Bronze, M.R. Identification of Functional Compounds in Baru (Dipteryx alata Vog.) Nuts: Nutritional Value, Volatile and Phenolic Composition, Antioxidant Activity and Antiproliferative Effect. Food Res. Int. 2020, 131, 109026. [Google Scholar] [CrossRef]
- Souza, R.G.M.; Gomes, A.C.; de Castro, I.A.; Mota, J.F. A Baru Almond–Enriched Diet Reduces Abdominal Adiposity and Improves High-Density Lipoprotein Concentrations: A Randomized, Placebo-Controlled Trial. Nutrition 2018, 55–56, 154–160. [Google Scholar] [CrossRef]
- da Cruz, K.S.; da Silva, M.A.; de Freitas, O.D.; Neves, V.A. Partial Characterization of Proteins from Baru (Dipteryx alata Vog) Seeds. J. Sci. Food Agric. 2011, 91, 2006–2012. [Google Scholar] [CrossRef]
- Siqueira, A.P.S.; Pacheco, M.T.B.; Naves, M.M.V. Nutritional Quality and Bioactive Compounds of Partially Defatted Baru Almond Flour. Food Sci. Technol. 2015, 35, 127–132. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar Singh, S.; Singha, P. A Comprehensive Review on the Recent Trends in Extractions, Pretreatments and Modifications of Plant-Based Proteins. Food Res. Int. 2024, 190, 114575. [Google Scholar] [CrossRef] [PubMed]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; FAO: Auckland, New Zealand, 2011. [Google Scholar]
- Fernandes, D.C.; Freitas, J.B.; Czeder, L.P.; Naves, M.M.V. Nutritional Composition and Protein Value of the Baru (Dipteryx alata Vog.) Almond from the Brazilian Savanna. J. Sci. Food Agric. 2010, 90, 1650–1655. [Google Scholar] [CrossRef]
- Clatterbuck, K.L.; Kehrberg, N.L.; Marable, N.L. Solubility and in Vitro Digestibility of Soy Flours, Concentrates and Isolates. J. Food Sci. 1980, 45, 931–935. [Google Scholar] [CrossRef]
- Pires, C.V.; Oliveira, M.G.d.A.; Rosa, J.C.; Costa, N.M.B. Qualidade Nutricional e Escore Químico de Aminoácidos. Ciênc. Tecnol. Aliment. 2006, 26, 179–187. [Google Scholar]
- Pedersen, B.; Eggum, B.O. Prediction of Protein Digestibility by an in Vitro Enzymatic PH-stat Procedure. Z. Tierphysiol. Tierernahr. Futtermittelkd. 1983, 49, 265–277. [Google Scholar] [CrossRef]
- Benhammouche, T.; Melo, A.; Martins, Z.; Faria, M.A.; Pinho, S.C.M.; Ferreira, I.M.L.P.V.O.; Zaidi, F. Nutritional Quality of Protein Concentrates from Moringa oleifera Leaves and in Vitro Digestibility. Food Chem. 2021, 348, 128858. [Google Scholar] [CrossRef]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate Composition and Anti-Nutritional Factors of Fava-Bean (Vicia faba), Green-Pea and Yellow-Pea (Pisum sativum) Flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D. Potentiality of Moringa oleifera as a Nutritive Ingredient in Different Food Matrices. Plant Foods Hum. Nutr. 2023, 78, 25–37. [Google Scholar] [CrossRef]
- Aracava, K.K.; Capellini, M.C.; Gonçalves, D.; Soares, I.D.; Margoto, C.M.; Rodrigues, C.E.C. Valorization of the Baru (Dipteryx alata Vog.) Processing Chain: Technological Properties of Defatted Nut Flour and Oil Solubility in Ethanol and Isopropanol. Food Chem. 2022, 383, 132587. [Google Scholar] [CrossRef]
- Lima, D.C.; Noguera, N.H.; del Pilar Flores Granados, A.; Rodrigues, R.A.F. Hydrocolloids and Partially Defatted Cake on Encapsulation of Baru Oil (Dipteryx alata Vogel): A Study on Emulsion, Particle, and Oxidative Stability. Food Bioproc Tech. 2023, 16, 2598–2610. [Google Scholar] [CrossRef]
- Choudhry, R.; Yasmin, A.; Aslam, M.A.; Imran, A.; Ahmad, R.S.; Saeed, F.; Islam, F.; Zahoor, T.; Shah, M.A.; Rasool, A. Extraction of Protein from Apricot Kernel Oil Press Cake through Innovative Techniques and the Formulation of Supplemented Yogurt. Food Sci. Nutr. 2023, 11, 6085–6095. [Google Scholar] [CrossRef] [PubMed]
- Chañi-Paucar, L.O.; Osorio-Tobón, J.F.; Johner, J.C.F.; Meireles, M.A.A. A Comparative and Economic Study of the Extraction of Oil from Baru (Dipteryx alata) Seeds by Supercritical CO2 with and without Mechanical Pressing. Heliyon 2021, 7, e05971. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Â.A.; Favaro, S.P.; Miranda, C.H.B.; Neves, V.A. Preparation and Characterization of Baru (Dipteryx alata Vog) Nut Protein Isolate and Comparison of Its Physico-Chemical Properties with Commercial Animal and Plant Protein Isolates. J. Sci. Food Agric. 2016, 97, 151–157. [Google Scholar] [CrossRef]
- Guimarães, R.d.C.A.; Favaro, S.P.; de Souza, A.D.V.; Soares, C.M.; Nunes, Â.A.; de Oliveira, L.C.S.; Honer, M.R. Thermal Properties of Defatted Meal, Concentrate, and Protein Isolate of Baru Nuts (Dipteryx alata Vog.). Food Sci. Technol. 2012, 32, 52–55. [Google Scholar] [CrossRef]
- Yang, J.; Kornet, R.; Ntone, E.; Meijers, M.G.J.; van den Hoek, I.A.F.; Sagis, L.M.C.; Venema, P.; Meinders, M.B.J.; Berton-Carabin, C.C.; Nikiforidis, C.V. Plant Protein Aggregates Induced by Extraction and Fractionation Processes: Impact on Techno-Functional Properties. Food Hydrocoll. 2024, 155, 110223. [Google Scholar] [CrossRef]
- Hernández-Álvarez, A.J.; Mondor, M.; Nosworthy, M.G. Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Springer International Publishing: Cham, Switzerland, 2023; ISBN 9783031169687. [Google Scholar]
- Gao, K.; Rao, J.; Chen, B. Plant Protein Solubility: A Challenge or Insurmountable Obstacle. Adv. Colloid. Interface Sci. 2024, 324, 103074. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced Alkaline Extraction Techniques for Isolating and Modifying Plant-Based Proteins. Food Hydrocoll. 2023, 145, 109132. [Google Scholar] [CrossRef]
- Das, R.S.; Zhu, X.; Hannon, S.; Mullins, E.; Alves, S.; Garcia-Vaquero, M.; Tiwari, B.K. Exploring Osborne Fractionation and Laboratory/Pilot Scale Technologies (Conventional Extraction, Ultrasound-Assisted Extraction, High-Pressure Processing and Hydrodynamic Cavitation) for Protein Extraction from Faba Bean (Vicia faba L.). Innov. Food Sci. Emerg. Technol. 2023, 89, 103487. [Google Scholar] [CrossRef]
- Tirgar, M.; Silcock, P.; Carne, A.; Birch, E.J. Effect of Extraction Method on Functional Properties of Flaxseed Protein Concentrates. Food Chem. 2017, 215, 417–424. [Google Scholar] [CrossRef]
- Perović, M.N.; Pajin, B.S.; Antov, M.G. The Effect of Enzymatic Pretreatment of Chickpea on Functional Properties and Antioxidant Activity of Alkaline Protein Isolate. Food Chem. 2022, 374, 131809. [Google Scholar] [CrossRef]
- Thongkong, S.; Klangpetch, W.; Unban, K.; Tangjaidee, P.; Phimolsiripol, Y.; Rachtanapun, P.; Jantanasakulwong, K.; Schönlechner, R.; Thipchai, P.; Phongthai, S. Impacts of Electroextraction Using the Pulsed Electric Field on Properties of Rice Bran Protein. Foods 2023, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Purdi, T.S.; Setiowati, A.D.; Ningrum, A. Ultrasound-Assisted Extraction of Spirulina Platensis Protein: Physicochemical Characteristic and Techno-Functional Properties. J. Food Meas. Charact. 2023, 17, 5474–5486. [Google Scholar] [CrossRef]
- Sert, D.; Rohm, H.; Struck, S. Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality. Foods 2022, 11, 4029. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Structure, Gelation Mechanism of Plant Proteins versus Dairy Proteins and Evolving Modification Strategies. Trends Food Sci. Technol. 2024, 147, 104464. [Google Scholar] [CrossRef]
- Ramaswamy, R.; Bala Krishnan, S. Pulsed Electric Field Treatment in Extracting Proteins from Legumes: A Review. Processes 2024, 12, 2667. [Google Scholar] [CrossRef]
- Lin, Z.; Jiao, G.; Zhang, J.; Celli, G.B.; Brooks, M.S.-L. Optimization of Protein Extraction from Bamboo Shoots and Processing Wastes Using Deep Eutectic Solvents in a Biorefinery Approach. Biomass Convers. Biorefin. 2021, 11, 2763–2774. [Google Scholar] [CrossRef]
- Franzen, K.L.; Kinsella, J.E. Functional Properties of Succinylated and Acetylated Soy Protein. J. Agric. Food Chem. 1976, 24, 788–795. [Google Scholar] [CrossRef]
- Roger, R.A.; Rawdkuen, S. Properties of Moringa oleifera Leaf Protein from Alkaline-acid Extraction. Food Appl. Biosci. J. 2020, 8, 43–67. [Google Scholar]
- Liang, H.N.; Tang, C.H. PH-Dependent Emulsifying Properties of Pea [Pisum sativum (L.)] Proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Foley, J.; O’Connell, C. Comparative Emulsifying Properties of Sodium Caseinate and Whey Protein Isolate in 18% Oil in Aqueous Systems. J. Dairy. Res. 1990, 57, 377–391. [Google Scholar] [CrossRef]
- Qi, X.; Luo, Y.; Fei, W.; Shen, M.; Chen, Y.; Yu, Q.; Xie, J. Effects of Enzyme Hydrolysis-Assisted Fibrillation Treatment on the Solubility, Emulsifying Properties and Antioxidant Activity of Rice Protein. Int. J. Biol. Macromol. 2024, 279, 135378. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H. Mechanism of the Structural Interaction between Whey and Lentil Proteins in the Unique Creation of a Protein Structure. J. Food Sci. 2021, 86, 5282–5294. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, T.; Song, T.; Chen, C.; Venkitasamy, C.; Pan, Z.; Zhang, H. Solubility, Structural Properties, and Immunomodulatory Activities of Rice Dreg Protein Modified with Sodium Alginate under Microwave Heating. Food Sci. Nutr. 2019, 7, 2556–2564. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.H.; Wen, Q.H.; He, F.; Xu, F.Y.; Chen, B.R.; Zeng, X.A. Combination of Pulsed Electric Field and PH Shifting Improves the Solubility, Emulsifying, Foaming of Commercial Soy Protein Isolate. Food Hydrocoll. 2023, 134, 108049. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The Structural Modification of Pea Protein Concentrate with Gum Arabic by Controlled Maillard Reaction Enhances Its Functional Properties and Flavor Attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Ahern, N.; Arendt, E.K.; Sahin, A.W. Protein Soft Drinks: A Retail Market Analysis and Selected Product Characterization. Beverages 2023, 9, 73. [Google Scholar] [CrossRef]
- Guimarães, R.d.C.A.; Favaro, S.P.; Viana, A.C.A.; Braga Neto, J.A.; Neves, V.A.; Honer, M.R. Study of the Proteins in the Defatted Flour and Protein Concentrate of Baru Nuts (Dipteryx alata Vog). Food Sci. Technol. 2012, 32, 464–470. [Google Scholar] [CrossRef]
- Bocarando-Guzmán, M.D.; Luna-Suárez, S.; Hernández-Cázares, A.S.; Herrera-Corredor, J.A.; Hidalgo-Contreras, J.V.; Ríos-Corripio, M.A. Comparison of the Physicochemical and Functional Properties of Flour and Protein Isolate from Moringa (Moringa oleifera Lam.) Leaves. Int. J. Food Prop. 2022, 25, 733–747. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Plant Protein versus Dairy Proteins: A PH-Dependency Investigation on Their Structure and Functional Properties. Foods 2023, 12, 368. [Google Scholar] [CrossRef]
- Furtado, G.d.F.; Mantovani, R.A.; Consoli, L.; Hubinger, M.D.; da Cunha, R.L. Structural and Emulsifying Properties of Sodium Caseinate and Lactoferrin Influenced by Ultrasound Process. Food Hydrocoll. 2017, 63, 178–188. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Chawla, P.; Vakkalagadda, R.; Sharma, A. Succinylation of Sodium Caseinate and Its Effect on Physicochemical and Functional Properties of Protein. LWT-Food Sci. Technol. 2015, 64, 1270–1277. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the Functional Properties of Pea, Chickpea and Lentil Protein Concentrates Processed Using Ultrafiltration and Isoelectric Precipitation Techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Yamauchi, K.; Shimizu, M.; Kamiya, T. Emulsifying Properties of Whey Protein. J. Food Sci. 1980, 45, 1237–1242. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Ma, Y.; He, Y.; Fu, R.; Qayum, A.; Jiang, Z.; Wang, L. Low Temperature Extrusion Promotes Transglutaminase Cross-Linking of Whey Protein Isolate and Enhances Its Emulsifying Properties and Water Holding Capacity. Food Hydrocoll. 2022, 125, 107410. [Google Scholar] [CrossRef]
- Jiang, S.; Hussain, M.A.; Cheng, J.; Jiang, Z.; Geng, H.; Sun, Y.; Sun, C.; Hou, J. Effect of Heat Treatment on Physicochemical and Emulsifying Properties of Polymerized Whey Protein Concentrate and Polymerized Whey Protein Isolate. Lwt 2018, 98, 134–140. [Google Scholar] [CrossRef]
- Chobert, J.-M.; Catherine Bertrand-Harb, J.; Nicolas, M.-G. Solubility and Emulsifying Properties of Caseins and Whey Proteins Modified Enzymatically by Trypsin. J. Agric. Food Chem. 1988, 36, 883–892. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y. Composition, Structure and Functional Properties of Protein Concentrates and Isolates Produced from Walnut (Juglans regia L.). Int. J. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying Properties of Soy Proteins: A Critical Review with Emphasis on the Role of Conformational Flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Ma, J.; Pan, C.; He, R.; Chen, W.; Pei, J.; Zhong, Q.; Chen, H.; Chen, W. Physicochemical Properties and Oil-Water Interfacial Behavior of Subcritical Water-Treated Coconut (Cocos nucifera L.) Globulins. Food Hydrocoll. 2024, 152, 109897. [Google Scholar] [CrossRef]
- Pineli, L.d.L.d.O.; de Carvalho, M.V.; de Aguiar, L.A.; de Oliveira, G.T.; Celestino, S.M.C.; Botelho, R.B.A.; Chiarello, M.D. Use of Baru (Brazilian Almond) Waste from Physical Extraction of Oil Toproduce Flour and Cookies. LWT-Food Sci. Technol. 2015, 60, 50–55. [Google Scholar] [CrossRef]
- de Jesus, E.P.; Techi Diniz, L.G.; Alves, V.; da Silva, Y.P.; Schmitz, A.C.; Quast, L.B.; dos Passos Francisco, C.T.; Tormen, L.; Bertan, L.C. From Nut to Dulce de Leche: Development of a Vegan Alternative–Physicochemical Characterization, Microbiological Evaluation and Sensory Analysis. Food Humanit. 2023, 1, 581–588. [Google Scholar] [CrossRef]
- Zeng, M.; Adhikari, B.; He, Z.; Qin, F.; Huang, X.; Chen, J. Improving the Foaming Properties of Soy Protein Isolate Through Partial Enzymatic Hydrolysis. Dry. Technol. 2013, 31, 1545–1552. [Google Scholar] [CrossRef]
- Morales, R.; Martínez, K.D.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Modification of Foaming Properties of Soy Protein Isolate by High Ultrasound Intensity: Particle Size Effect. Ultrason. Sonochem 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Shen, P.; Ha, S.M.L.; Peng, J.; Landman, J.; Sagis, L.M.C. Role of Pulse Globulins and Albumins in Air-Water Interface and Foam Stabilization. Food Hydrocoll. 2025, 160, 110792. [Google Scholar] [CrossRef]
- Shen, P.; Peng, J.; Sagis, L.M.C.; Landman, J. Molecular, Interfacial and Foaming Properties of Pulse Proteins. Food Hydrocoll. 2024, 156, 110313. [Google Scholar] [CrossRef]
- Shao, Y.; Lin, K.; Kao, Y. Modification of Foaming Properties of Commercial Soy Protein Isolates and Concentrates by Heat Treatments. J. Food Qual. 2016, 39, 695–706. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Li, L.; Zong, X.; Coldea, T.E.; Yang, H.; Zhao, H. Enhancing the Foaming Properties of Brewer’s Spent Grain Protein by Ultrasound Treatment and Glycation Reaction. Food Funct. 2023, 14, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.K.; Grossmann, L.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional and Physical Properties of Commercial Pulse Proteins Compared to Soy Derived Protein. Future Foods 2022, 6, 100155. [Google Scholar] [CrossRef]
- Nakamura, R.; Sugiyama, H.; Sato, Y. Factors Contributing to the Heat-Induced Aggregation of Ovalbumin. Agric. Biol. Chem. 1978, 42, 819–824. [Google Scholar] [CrossRef]
- Gravel, A.; Dubois-Laurin, F.; Turgeon, S.L.; Doyen, A. The Role of the 7S/11S Globulin Ratio in the Gelling Properties of Mixed β-Lactoglobulin/Pea Proteins Systems. Food Hydrocoll. 2024, 156, 110273. [Google Scholar] [CrossRef]
- Devnani, B.; Ong, L.; Kentish, S.; Gras, S. Heat Induced Denaturation, Aggregation and Gelation of Almond Proteins in Skim and Full Fat Almond Milk. Food Chem. 2020, 325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bhandari, B.; Gaiani, C.; Prakash, S. Altering Almond Protein Function through Partial Enzymatic Hydrolysis for Creating Gel Structures in Acidic Environment. Curr. Res. Food Sci. 2022, 5, 653–664. [Google Scholar] [CrossRef]
- Tarahi, M.; Abdolalizadeh, L.; Hedayati, S. Mung Bean Protein Isolate: Extraction, Structure, Physicochemical Properties, Modifications, and Food Applications. Food Chem. 2024, 444, 138626. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.A.; Shan, T.P.; Devi, M.; Pindi, W.; Akanda, J.H.; Mamat, H.; Yin, F.H. Effect of Seaweed Powder on the Quality of the Pineapple-Chili Sauce. Adv. Soc. Sci. Educ. Humanit. Res. 2020, 406, 34–39. [Google Scholar]
- Langendörfer, L.J.; Wüst, A.; Hensel, O.; Diakité, M. Functional Properties of Legume Protein and Their Application in the Development of a Plant-Based Hollandaise Sauce. Chem. Eng. Trans. 2023, 102, 1–6. [Google Scholar] [CrossRef]
- Han, Z.; Liu, S.; Cao, J.; Yue, X.; Shao, J.-H. A Review of Oil and Water Retention in Emulsified Meat Products: The Mechanisms of Gelation and Emulsification, the Application of Multi-Layer Hydrogels. Crit. Rev. Food Sci. Nutr. 2024, 64, 8308–8324. [Google Scholar] [CrossRef]
- Wang, W.; Jia, R.; Hui, Y.; Zhang, F.; Zhang, L.; Liu, Y.; Song, Y.; Wang, B. Utilization of Two Plant Polysaccharides to Improve Fresh Goat Milk Cheese: Texture, Rheological Properties, and Microstructure Characterization. J. Dairy. Sci. 2023, 106, 3900–3917. [Google Scholar] [CrossRef]
- Jeong, M.S.; Cho, S.J. Effect of PH-Shifting on the Water Holding Capacity and Gelation Properties of Mung Bean Protein Isolate. Food Res. Int. 2024, 177. [Google Scholar] [CrossRef]
- Mitaku, S.; Hirokawa, T.; Tsuji, T. Amphiphilicity Index of Polar Amino Acids as an Aid in the Characterization of Amino Acid Preference at Membrane-Water Interfaces. Bioinformatics 2002, 18, 608–616. [Google Scholar] [CrossRef]
- Biswas, K.M.; DeVido, D.R.; Dorsey, J.G. Evaluation of Methods for Measuring Amino Acid Hydrophobicities and Interactions. J. Chromatogr. A 2003, 1000, 637–655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).