Preparation of Hydrophobic Purple Sweet Potato-Based Intelligent Packaging Films by Stearic Acid Coating and Heat Pressing Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
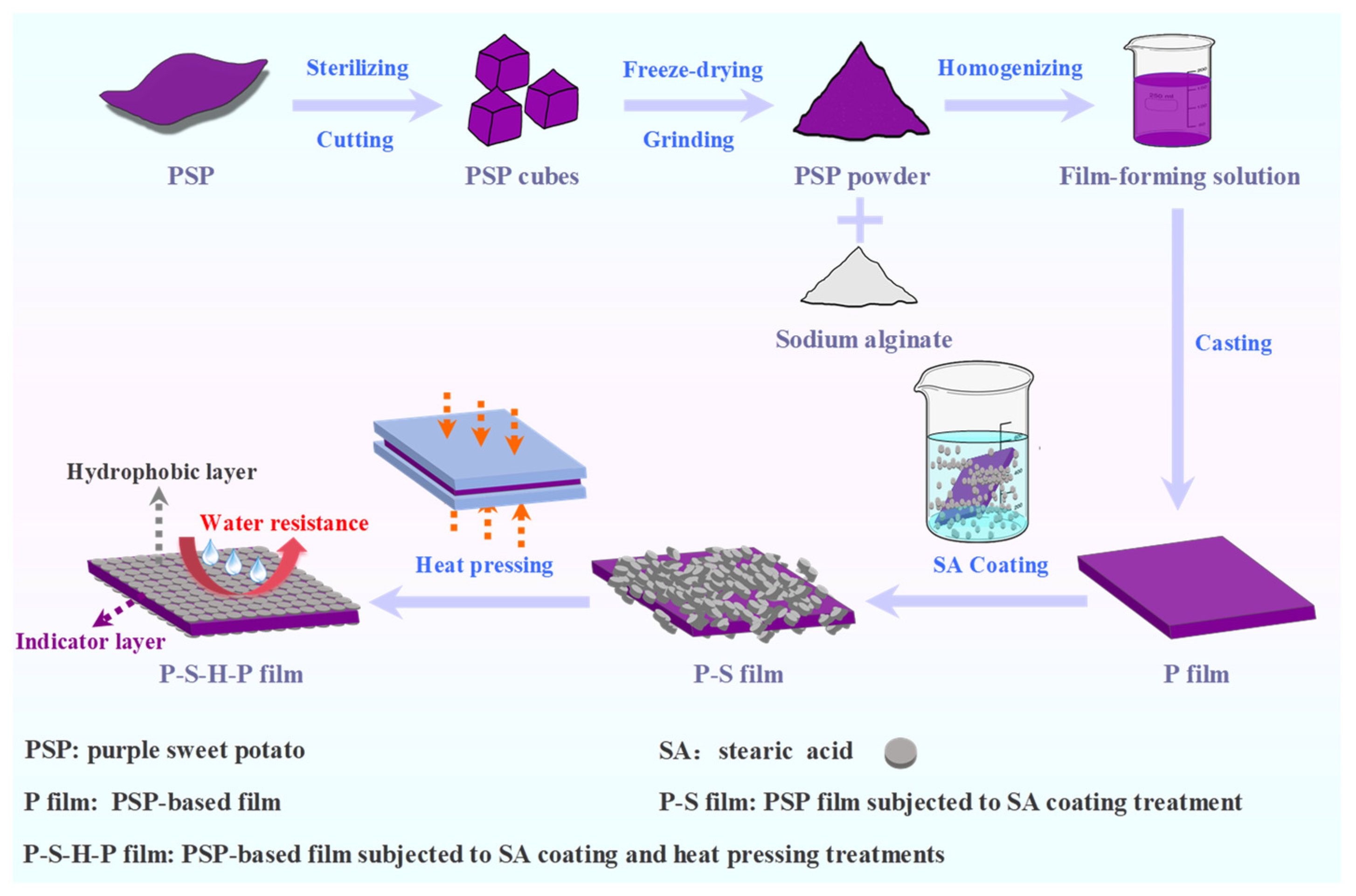
2.2. Preparation of the Thermally Treated PSP
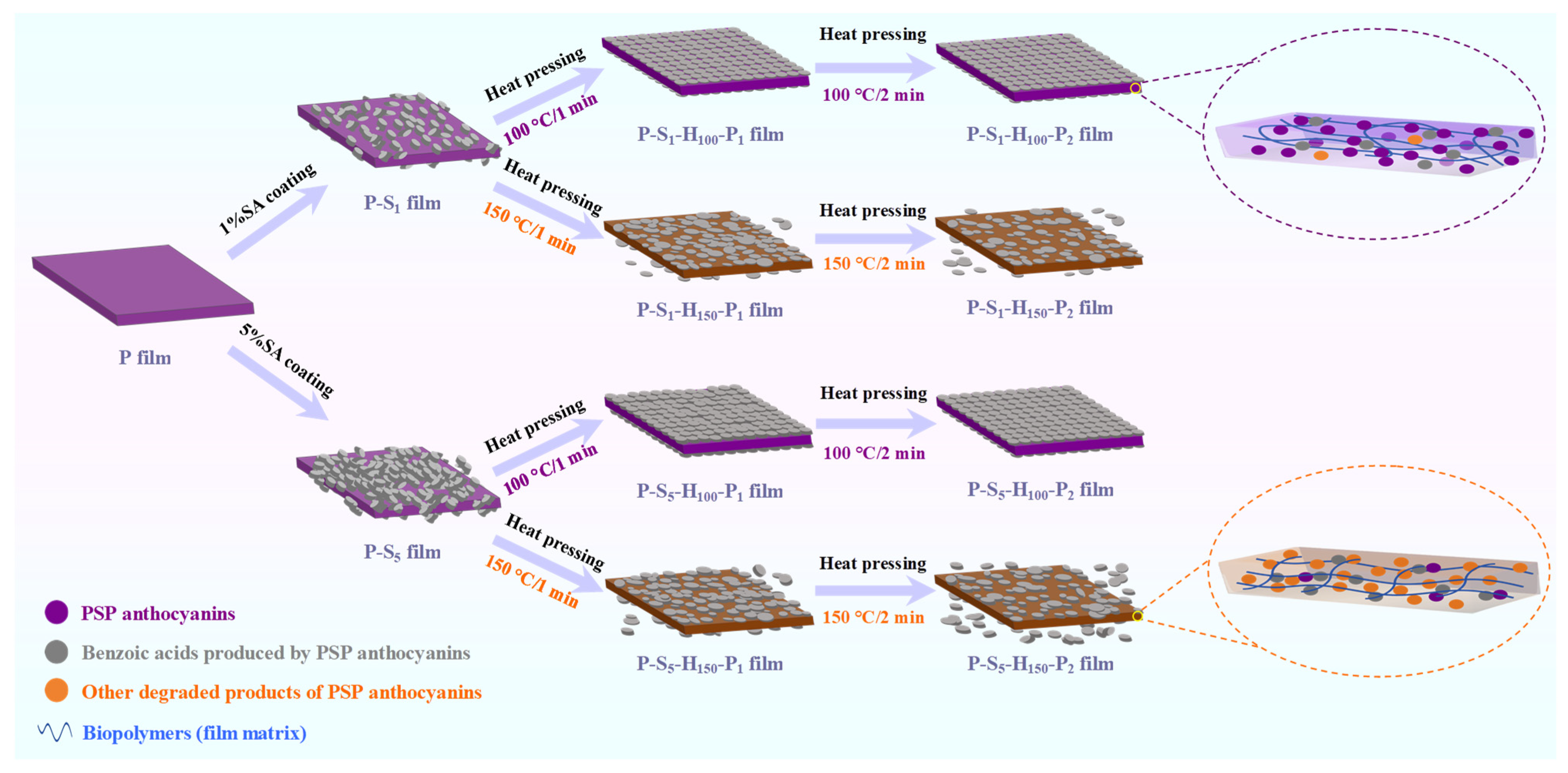
2.3. Preparation of Surface-Hydrophobized PSP-Based Intelligent Packaging Films
2.4. Structural Characterization of the Films
2.4.1. Scanning Electron Microscopical Observation
2.4.2. Infrared Spectroscopic Analysis
2.4.3. X-Ray Diffraction Analysis
2.5. Physical Property Measurements of the Films
2.5.1. Color
2.5.2. Transparency
2.5.3. Thermal Property
2.5.4. Mechanical Property
2.5.5. Moisture Content (MC)
2.5.6. Water Vapor Blocking Performance
2.5.7. Surface Wettability
2.5.8. Stability in Water
2.5.9. Biodegradability
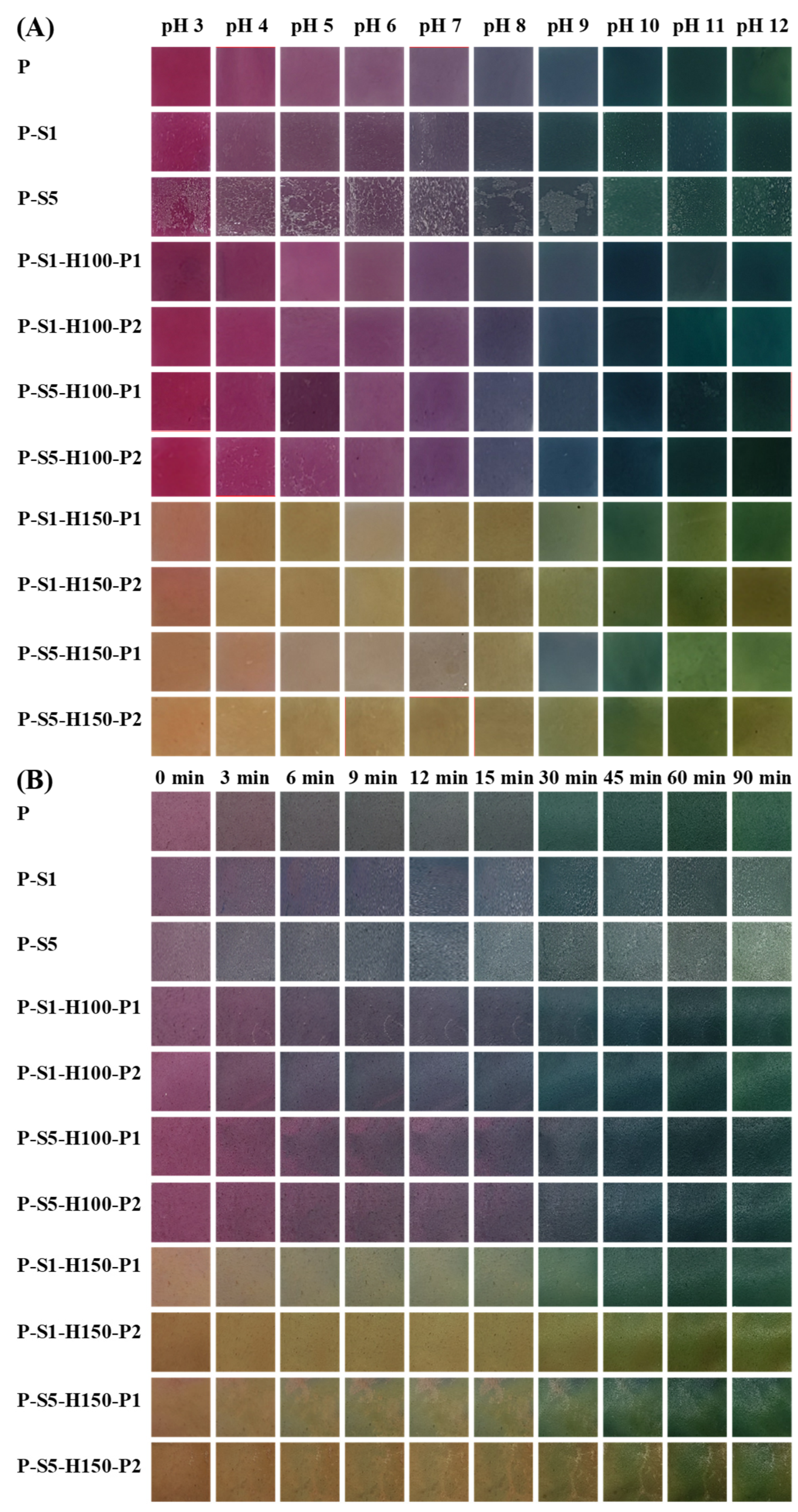
2.6. Measurement of the Color Changeability of the Films
2.7. Evalution of the Shrimp Freshness Monitoring Ability of the Films
2.8. Statistical Analysis
3. Results and Discussion
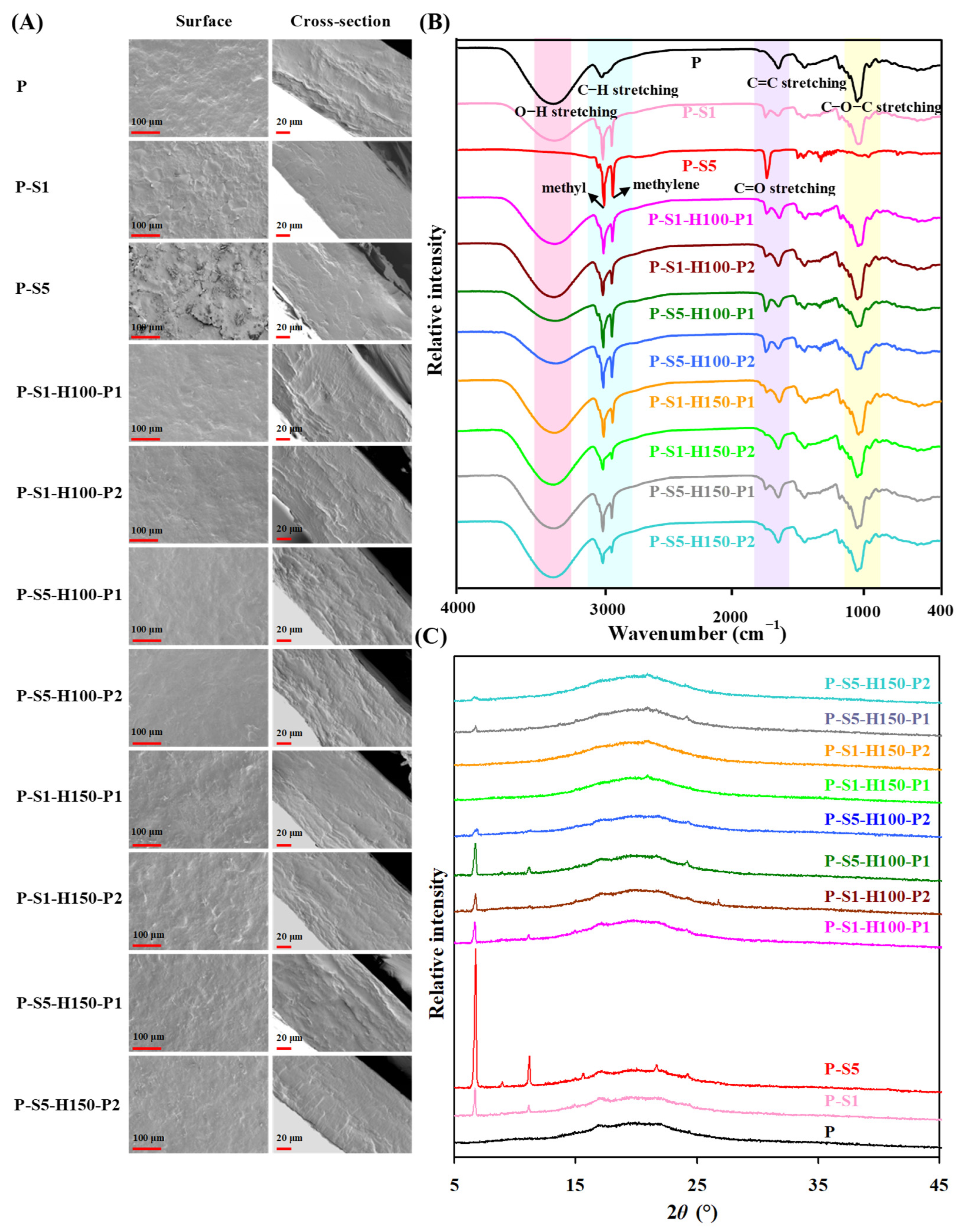
3.1. Microstructures of the Films
3.2. Infrared Spectra of the Films
3.3. Crystalline Characteristics of the Films
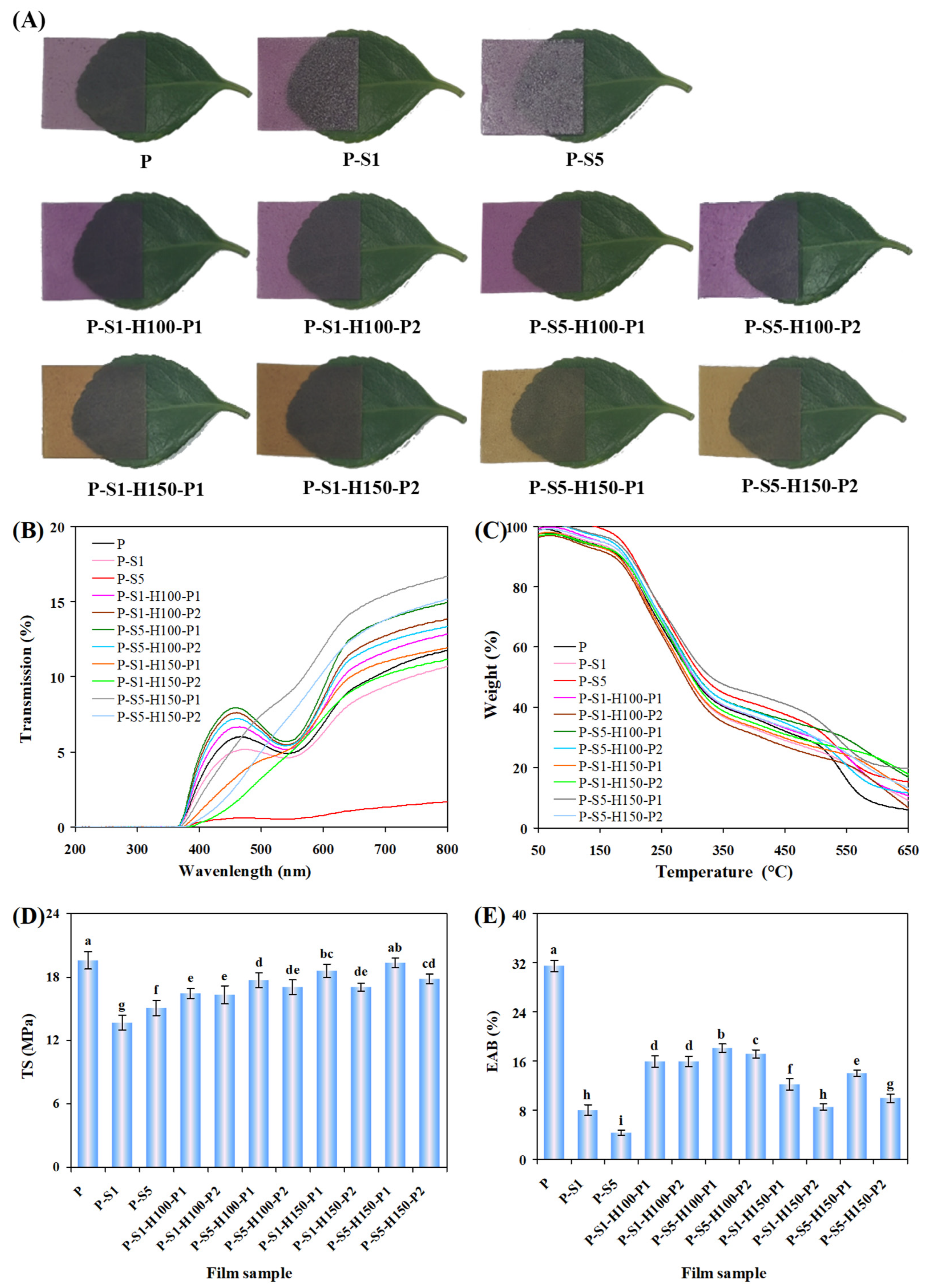
3.4. Color and Transparency of the Films
3.5. Thermal Property of the Films
3.6. Mechanical Property of the Films
3.7. MC of the Films
3.8. WVP of the Films
3.9. WCA of the Films
3.10. Stability of the Films in Water
3.11. Biodegradability of the Films
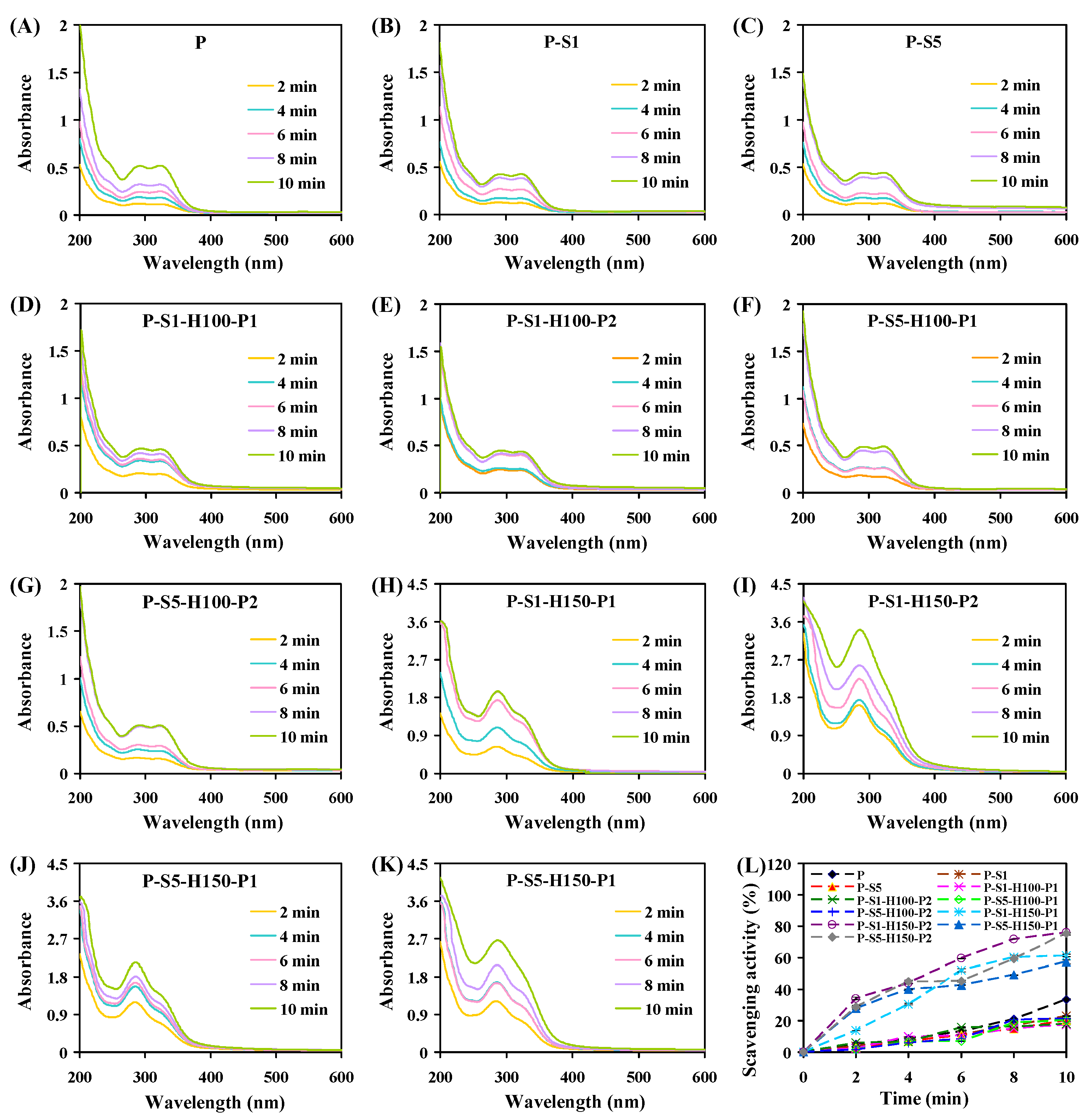
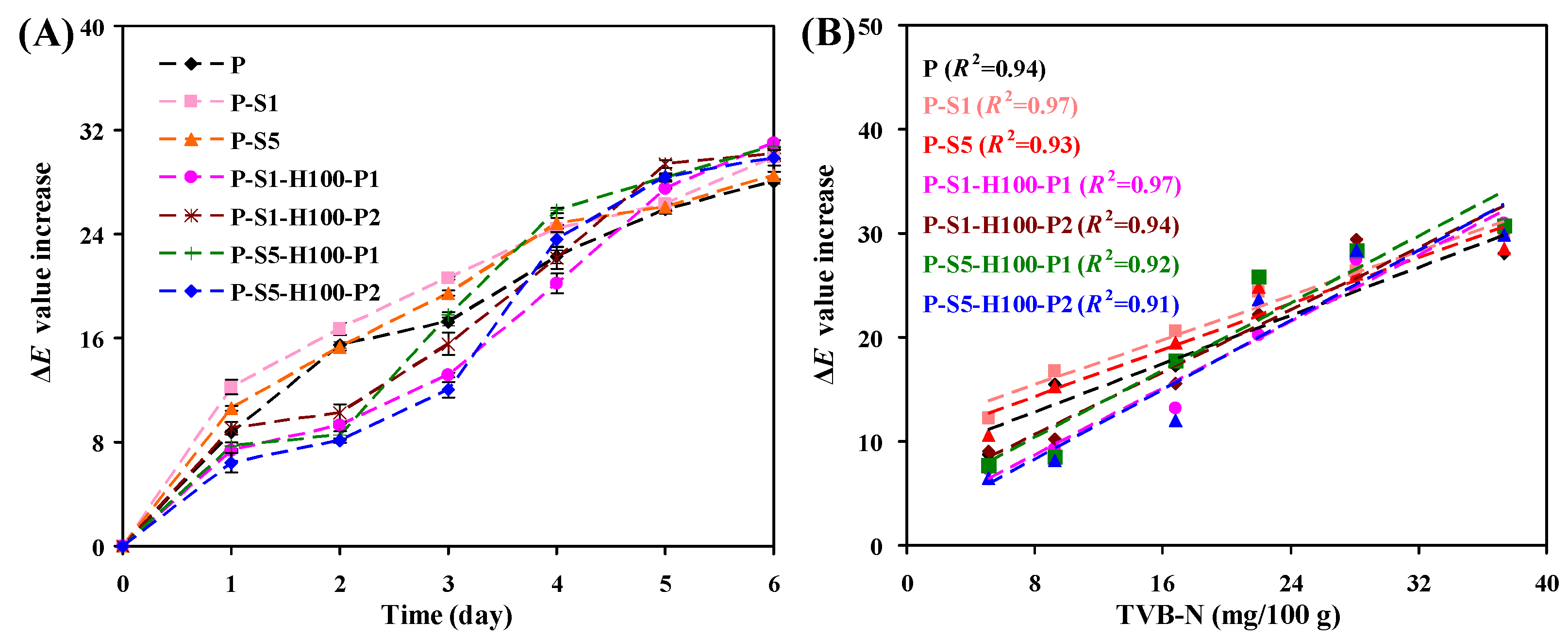
3.12. Color Changeability of the Films
3.13. The Mechanism of Action of SA Coating and Heat Pressing Treatments
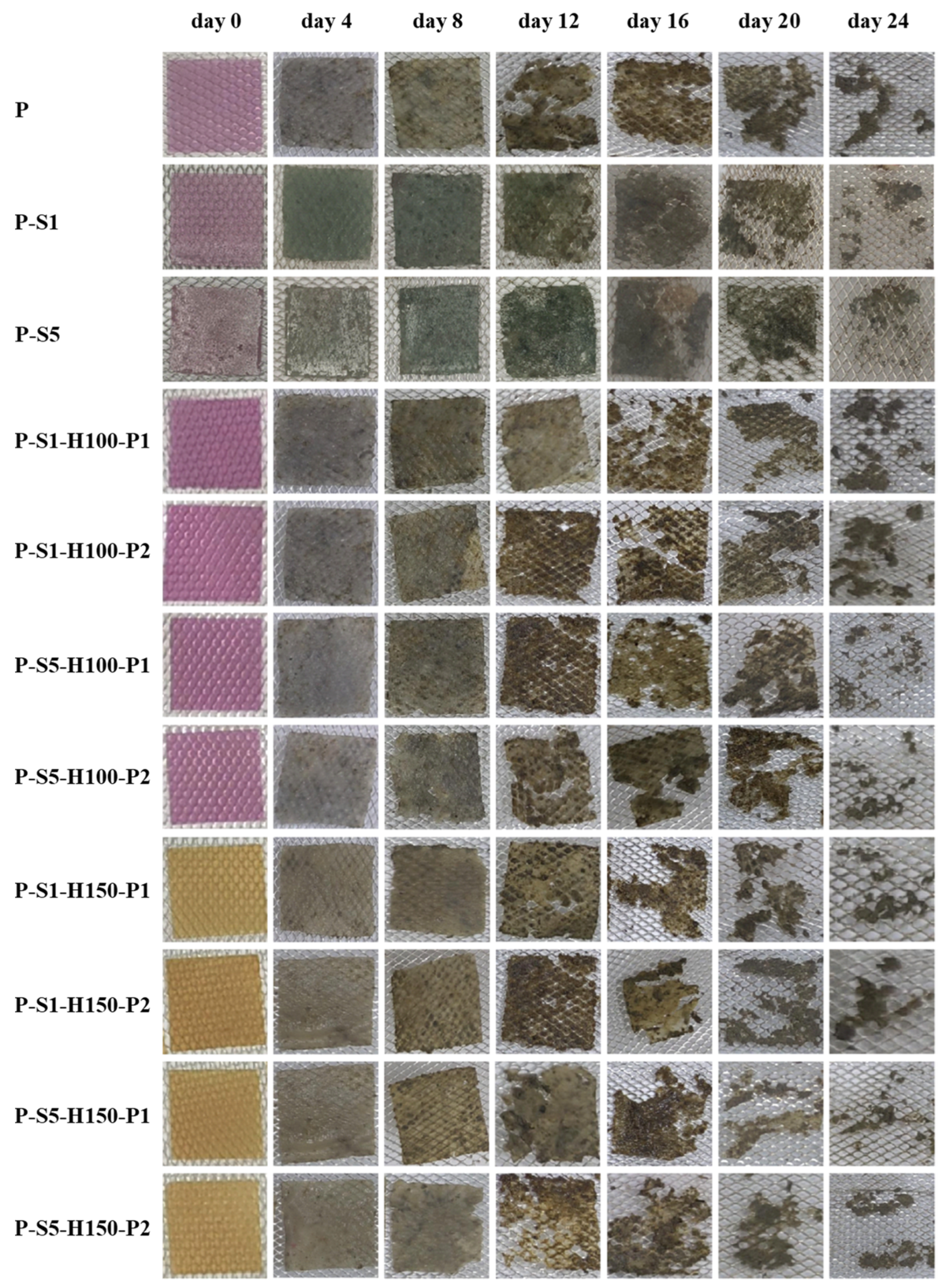
3.14. Shrimp Freshness Monitoring Ability of the Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, S.; Strenger, M.; Maier-Nöth, A.; Schmid, M. Food packaging and sustainability-consumer perception vs. correlated scientific facts: A review. J. Clean. Prod. 2021, 298, 126733. [Google Scholar]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocolloid. 2022, 125, 107419. [Google Scholar]
- Chiu, I.; Yang, T. Biopolymer-based intelligent packaging integrated with natural colourimetric sensors for food safety and sustainability. Anal. Sci. Adv. 2024, 5, e202300065. [Google Scholar]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. 2021, 61, 2297–2325. [Google Scholar]
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-based pH-sensitive smart packaging films for monitoring food freshness. J. Agric. Food Res. 2022, 9, 100340. [Google Scholar]
- Zhang, J.; Huang, X.; Shi, J.; Liu, L.; Zhang, X.; Zou, X.; Zhai, X.; Zhang, D.; Li, Y.; Shen, T. A visual bi-layer indicator based on roselle anthocyanins with high hydrophobic property for monitoring griskin freshness. Food Chem. 2021, 355, 129573. [Google Scholar]
- Wang, X.; Zhai, X.; Zou, X.; Li, Z.; Shi, J.; Yang, Z.; Sun, Y.; Arslan, M.; Chen, Z.; Xiao, J. Novel hydrophobic colorimetric films based on ethylcellulose/castor oil/anthocyanins for pork freshness monitoring. LWT 2022, 164, 113631. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.; Sánchez, M.Á.A.; Labidi, J. Halochromic and antioxidant capacity of smart films of chitosan/chitin nanocrystals with curcuma oil and anthocyanins. Food Hydrocolloid. 2022, 123, 107119. [Google Scholar]
- Hashim, S.B.; Tahir, H.E.; Lui, L.; Zhang, J.; Zhai, X.; Mahdi, A.A.; Ibrahim, N.A.; Mahunu, G.K.; Hassan, M.M.; Zou, X.; et al. Smart films of carbohydrate-based/sunflower wax/purple Chinese cabbage anthocyanins: A biomarker of chicken freshness. Food Chem. 2023, 399, 133824. [Google Scholar] [CrossRef]
- Cui, C.; Gao, L.; Dai, L.; Ji, N.; Qin, Y.; Shi, R.; Qiao, Y.; Xiong, L.; Sun, Q. Hydrophobic biopolymer-based films: Strategies, properties, and food applications. Food Eng. Rev. 2023, 15, 360–379. [Google Scholar]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Khan, T.S. Advancements in the biopolymer films for food packaging applications: A short review. Biotechnol. Sustain. Mater. 2024, 1, 2. [Google Scholar]
- Shi, S.; Xu, X.; Ren, Y.; Zhang, H.; Du, X.; Li, H.; Xia, X. Beeswax coating improves the hydrophobicity of sodium alginate/anthocyanin/cellulose nanocrystal indicator film. Food Hydrocolloid. 2023, 144, 108930. [Google Scholar]
- Wang, F.; Ma, R.; Tian, Y. Facile fabrication of thermostable and colorimetric starch-based waterproof coating with edible organic materials. Food Chem. 2022, 382, 132269. [Google Scholar]
- Yun, D.; Li, C.; Wang, Z.; Xu, F.; Chen, D.; Liu, J. Preparation of cost-effective and hydrophobic freshness indicating labels based on passion fruit peel powder and stearic acid. Food Biosci. 2023, 53, 102758. [Google Scholar]
- He, M.; Xu, M.; Zhang, L. Controllable stearic acid crystal induced high hydrophobicity on cellulose film surface. ACS Appl. Mater. Interfaces 2013, 5, 585–591. [Google Scholar]
- Balasubramaniam, S.L.; Patel, A.S.; Nayak, B. Surface modification of cellulose nanofiber film with fatty acids for developing renewable hydrophobic food packaging. Food Packag. Shelf Life 2020, 26, 100587. [Google Scholar]
- Li, C.; Sun, J.; Yun, D.; Wang, Z.; Tang, C.; Liu, J. A new method to prepare color-changeable smart packaging films based on the cooked purple sweet potato. Food Hydrocolloid. 2023, 137, 108397. [Google Scholar]
- Yun, D.; Li, C.; Sun, J.; Xu, F.; Tang, C.; Liu, J. A comparative study on the structure, physical property and halochromic ability of shrimp freshness indicators produced from nine varieties of steamed purple sweet potato. Food Chem. 2024, 449, 139222. [Google Scholar]
- Zong, Z.; Liu, M.; Chen, H.; Farag, M.A.; Wu, W.; Fang, X.; Niu, B.; Gao, H. Preparation and characterization of a novel intelligent starch/gelatin binary film containing purple sweet potato anthocyanins for Flammulina velutipes mushroom freshness monitoring. Food Chem. 2023, 405, 134839. [Google Scholar] [PubMed]
- Bu, N.; Wang, L.; Zhang, D.; Xiao, H.; Liu, X.; Chen, X.; Ma, C.; Mu, R. Highly hydrophobic gelatin nanocomposite film assisted by nano-ZnO/(3-aminopropyl) triethoxysilane/stearic acid coating for liquid food packaging. ACS Appl. Mater. Interfaces 2023, 15, 51713–51726. [Google Scholar]
- Yu, M.; Yang, L.; Yan, L.; Wang, T.; Wang, Y.; Qin, Y.; Xiong, L.; Shi, R.; Sun, Q. ZnO nanoparticles coated and stearic acid modified superhydrophobic chitosan film for self-cleaning and oil-water separation. Int. J. Biol. Macromol. 2023, 231, 123293. [Google Scholar]
- Tan, Y.; Liu, X.; Cheng, Z.; Zhan, Q.; Zhao, L. Preparation and characterization of bio-Based freshness indicator labels loaded with natural pigments with high stability and sensitivity. Foods 2024, 13, 4049. [Google Scholar] [CrossRef]
- Yue, Y.; Cheng, X.; Liu, H.; Zang, M.; Zhao, B.; Zhao, X.; Wang, L. Gellan gum and polyvinyl alcohol based triple-layer films enriched with Alhagi sparsifolia flower extract: Preparation, characterization, and application of dried shrimp preservation. Foods 2023, 12, 3979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, N.; Peng, H.; Yang, X.; Lin, D. The development of highly pH-sensitive bacterial cellulose nanofibers/gelatin-based intelligent films loaded with anthocyanin/curcumin for the fresh-keeping and freshness detection of fresh pork. Foods 2023, 12, 3719. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Chen, M.M.; Lu, Y.S.; Li, B.H.; Wu, Y.; Yang, S.B.; Liu, B.; Zhang, Y. Development of a chitosan and whey protein-based, biodegradable, colorimetric/fluorescent dual-channel monitoring label for real-time sensing of shrimp freshness. Int. J. Biol. Macromol. 2024, 262, 130203. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, D.; Zhou, R.; Zhu, R.; Fei, P.; Zhu, Z.Z.; Cheng, S.; Ding, W.P. Hydrophobic interface starch nanofibrous film for food packaging: From bioinspired design to self-cleaning action. J. Agric. Food Chem. 2021, 69, 5067–5075. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiao, X.; Zhang, Y.; Bai, H.; Xu, H.; Zhang, Z.; Lin, Y.; Yu, L.; Cao, Y. How APTMS acts as a bridge to enhance the compatibility of the interface between the hydrophilic poly (vinyl alcohol) film and the hydrophobic stearic acid coating. ACS Appl. Mater. Interfaces 2023, 15, 45322–45335. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yan, W.; Du, J.; Li, W.; Xu, T.; Ye, W.B. Thermal performances of stearic acid/sepiolite composite form-stable phase change materials with improved thermal conductivity for thermal energy storage. J. Therm. Anal. Calorim. 2021, 143, 3317–3329. [Google Scholar] [CrossRef]
- Wu, B.; Fu, W.; Kong, B.; Hu, K.; Zhou, C.; Lei, J. Preparation and characterization of stearic acid/polyurethane composites as dual phase change material for thermal energy storage. J. Them. Anal. 2018, 132, 907–917. [Google Scholar] [CrossRef]
- Ye, Q.; Han, Y.; Zhang, J.; Zhang, W.; Xia, C.; Li, J. Bio-based films with improved water resistance derived from soy protein isolate and stearic acid via bioconjugation. J. Clean. Prod. 2019, 214, 125–131. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Zhao, G.; Ye, F. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes: A review. Trends Food Sci. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Capello, C.; Trevisol, T.C.; Pelicioli, J.; Terrazas, M.B.; Monteiro, A.R.; Valencia, G.A. Preparation and characterization of colorimetric indicator films based on chitosan/polyvinyl alcohol and anthocyanins from agri-food wastes. J. Polym. Environ. 2021, 29, 1616–1629. [Google Scholar] [CrossRef]
- Huang, H.; Mao, L.; Wang, W.; Li, Z.; Qin, C. A facile strategy to fabricate antibacterial hydrophobic, high-barrier, cellulose papersheets for food packaging. Int. J. Biol. Macromol. 2023, 236, 123630. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, V.K.; Pham, T.T.H.; Pham, T.T.; Nguyen, T.D. Effects of surface modification with stearic acid on the dispersion of some inorganic fillers in PE matrix. J. Compos. Sci. 2021, 5, 270. [Google Scholar] [CrossRef]
- Long, J.; Zhang, W.; Zhao, M.; Ruan, C.Q. The reduce of water vapor permeability of polysaccharide-based films in food packaging: A comprehensive review. Carbohydr. Polym. 2023, 321, 121267. [Google Scholar] [CrossRef]
- Wei, X.; Pang, J.; Zhang, C.; Yu, C.; Chen, H.; Xie, B. Structure and properties of moisture-resistant konjac glucomannan films coated with shellac/stearic acid coating. Carbohydr. Polym. 2015, 118, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chang, C.; Zhang, L. Surface engineering of cellulose film with myristic acid for high strength, self-cleaning and biodegradable packaging materials. Carbohydr. Polym. 2021, 269, 118315. [Google Scholar] [CrossRef]
- Pirayesh, H.; Park, B.D.; Khanjanzadeh, H.; Park, H.J.; Cho, Y.J. Cellulosic material-based colorimetric films and hydrogels as food freshness indicators. Cellulose 2023, 30, 2791–2825. [Google Scholar] [CrossRef]


| Films | L* | a* | b* | ΔE |
|---|---|---|---|---|
| P | 60.47 ± 0.31 b | 18.01 ± 0.45 e | −0.65 ± 0.03 e | 37.62 ± 0.45 i |
| P-S1 | 56.06 ± 0.12 d | 19.71 ± 0.15 d | −3.20 ± 0.14 f | 42.15 ± 0.17 h |
| P-S5 | 53.37 ± 0.12 g | 19.84 ± 0.25 d | −2.93 ± 0.01 f | 63.36 ± 0.14 c |
| P-S1-H100-P1 | 52.41 ± 0.32 h | 32.05 ± 0.31 a | −9.72 ± 0.15 j | 52.22 ± 0.42 e |
| P-S1-H100-P2 | 56.66 ± 0.29 c | 28.27 ± 0.15 c | −6.01 ± 0.05 g | 46.30 ± 0.24 g |
| P-S5-H100-P1 | 54.95 ± 0.03 e | 30.00 ± 0.08 b | −8.40 ± 0.07 i | 48.85 ± 0.02 f |
| P-S5-H100-P2 | 54.10 ± 0.47 f | 29.98 ± 0.86 b | −7.15 ± 0.72 h | 49.43 ± 0.93 f |
| P-S1-H150-P1 | 60.46 ± 0.15 b | 12.56 ± 0.03 g | 44.48 ± 0.05 c | 60.15 ± 0.05 d |
| P-S1-H150-P2 | 55.01 ± 0.29 e | 16.67 ± 0.41 f | 51.37 ± 0.32 a | 69.72 ± 0.51 a |
| P-S5-H150-P1 | 64.42 ± 0.23 a | 11.13 ± 0.78 h | 36.91 ± 0.55 d | 51.57 ± 0.51 e |
| P-S5-H150-P2 | 55.74 ± 0.07 d | 16.53 ± 0.17 f | 49.47 ± 0.15 b | 67.66 ± 0.04 b |
| Films | Stage I | Stage II | Stage III | Tm (°C) | Weight Residue at 650 °C (%) | |||
|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | Weight Loss (%) | Temperature Range (°C) | Weight Loss (%) | Temperature Range (°C) | Weight Loss (%) | |||
| P | 50–140 | 5.96 | 140–391 | 57.05 | 391–650 | 31.06 | 231 | 5.93 |
| P-S1 | 50–151 | 6.07 | 151–389 | 60.32 | 389–650 | 24.57 | 219 | 9.04 |
| P-S5 | 50–150 | 2.47 | 150–393 | 55.94 | 393–650 | 26.34 | 222 | 15.25 |
| P-S1-H100-P1 | 50–141 | 4.37 | 141–392 | 58.23 | 392–650 | 26.81 | 235 | 10.59 |
| P-S1-H100-P2 | 50–143 | 7.60 | 143–394 | 61.03 | 394–650 | 24.80 | 233 | 6.56 |
| P-S5-H100-P1 | 50–143 | 6.54 | 143–395 | 54.35 | 395–650 | 22.37 | 274 | 16.74 |
| P-S5-H100-P2 | 50–142 | 3.06 | 142–396 | 57.48 | 396–650 | 27.86 | 230 | 11.61 |
| P-S1-H150-P1 | 50–142 | 6.48 | 142–399 | 60.09 | 399–650 | 21.39 | 232 | 12.04 |
| P-S1-H150-P2 | 50–144 | 6.30 | 144–396 | 58.71 | 396–650 | 17.24 | 270 | 17.74 |
| P-S5-H150-P1 | 50–141 | 2.56 | 141–395 | 52.78 | 395–650 | 24.94 | 235 | 19.72 |
| P-S5-H150-P2 | 50–142 | 4.59 | 142–393 | 57.98 | 393–650 | 24.34 | 238 | 13.10 |
| Time (day) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| TVB-N level (mg/100 g) | 4.11 ± 0.46 g | 5.12 ± 0.46 f | 9.23 ± 0.50 e | 16.78 ± 1.13 d | 22.02 ± 0.76 c | 28.01 ± 0.47 b | 37.33 ± 0.79 a |
| P |  |  |  |  |  |  |  |
| P-S1 |  |  |  |  |  |  |  |
| P-S5 |  |  |  |  |  |  |  |
| P-S1-H100-P1 |  |  |  |  |  |  |  |
| P-S1-H100-P2 |  |  |  |  |  |  |  |
| P-S5-H100-P1 |  |  |  |  |  |  |  |
| P-S5-H100-P2 |  |  |  |  |  |  |  |
| P-S1-H150-P1 |  |  |  |  |  |  |  |
| P-S1-H150-P2 |  |  |  |  |  |  |  |
| P-S5-H150-P1 |  |  |  |  |  |  |  |
| P-S5-H150-P2 |  |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, F.; Huang, X.; Sun, J.; Kan, J.; Liu, J. Preparation of Hydrophobic Purple Sweet Potato-Based Intelligent Packaging Films by Stearic Acid Coating and Heat Pressing Treatments. Foods 2025, 14, 1276. https://doi.org/10.3390/foods14071276
Liu X, Xu F, Huang X, Sun J, Kan J, Liu J. Preparation of Hydrophobic Purple Sweet Potato-Based Intelligent Packaging Films by Stearic Acid Coating and Heat Pressing Treatments. Foods. 2025; 14(7):1276. https://doi.org/10.3390/foods14071276
Chicago/Turabian StyleLiu, Xuanzhuo, Fengfeng Xu, Xiaoqian Huang, Jian Sun, Juan Kan, and Jun Liu. 2025. "Preparation of Hydrophobic Purple Sweet Potato-Based Intelligent Packaging Films by Stearic Acid Coating and Heat Pressing Treatments" Foods 14, no. 7: 1276. https://doi.org/10.3390/foods14071276
APA StyleLiu, X., Xu, F., Huang, X., Sun, J., Kan, J., & Liu, J. (2025). Preparation of Hydrophobic Purple Sweet Potato-Based Intelligent Packaging Films by Stearic Acid Coating and Heat Pressing Treatments. Foods, 14(7), 1276. https://doi.org/10.3390/foods14071276







