Changes in Quality and Metabolites of Pickled Purple Radish During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Determination of the Hunter Color, Hardness, and pH
2.3. Determination of Total Phenolic (TPC) and Total Flavonoid (TFC) Contents
2.4. Determination of Total Anthocyanin Content (TAC)
2.5. Determination of Free Sugar Content Using Gas Chromatography–Mass Spectrometry (GC–MS)
2.6. Analysis of Non-Volatile Metabolites by Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-QToF-MS)
2.7. Determination of the ABTS+ Radical-Scavenging Activity and Ferric Reducing Antioxidant Power (FRAP)
2.8. Statistical Analysis
3. Results and Discussion
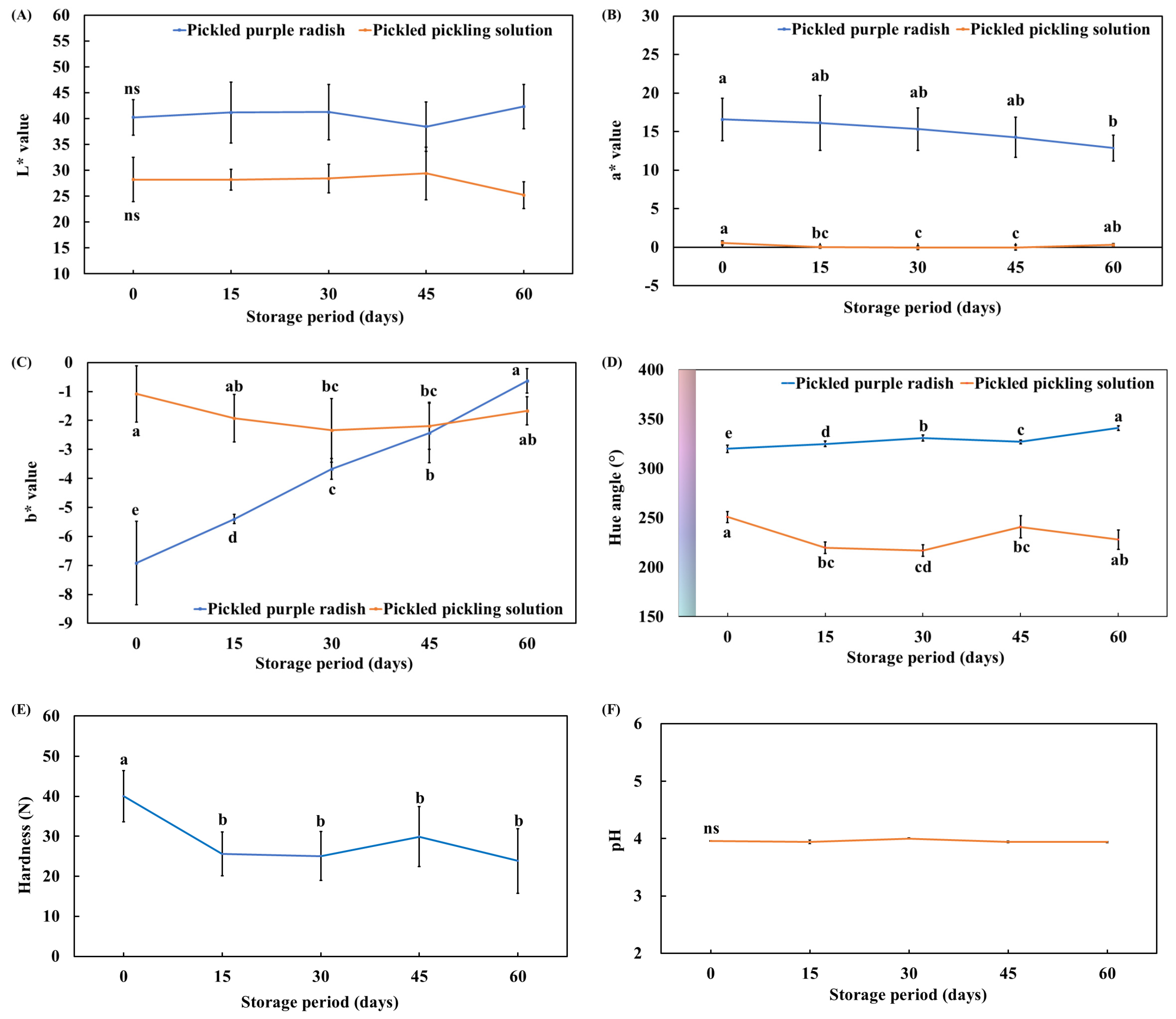
3.1. Changes in the Physicochemical Properties (Color, Hardness, and pH) of Pickled Purple Radish During Storage
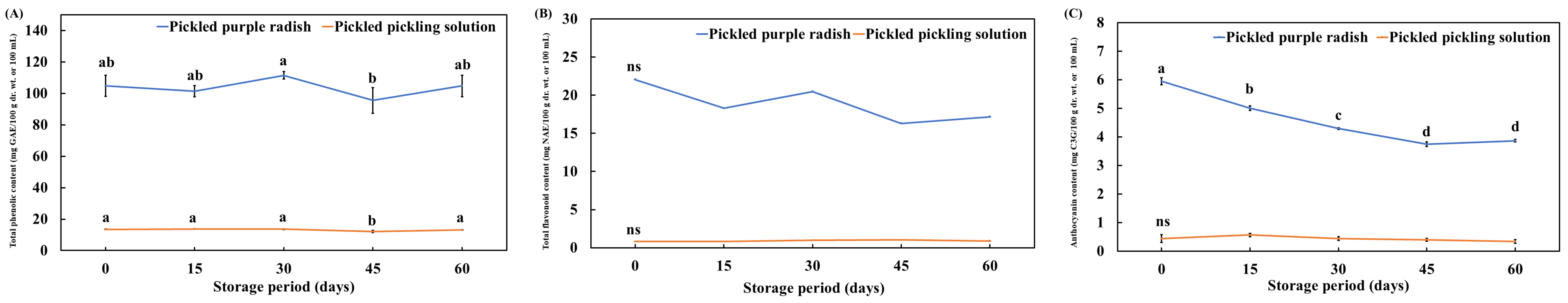
3.2. Changes in the TPC, TFC, and TAC of Pickled Purple Radish During Storage
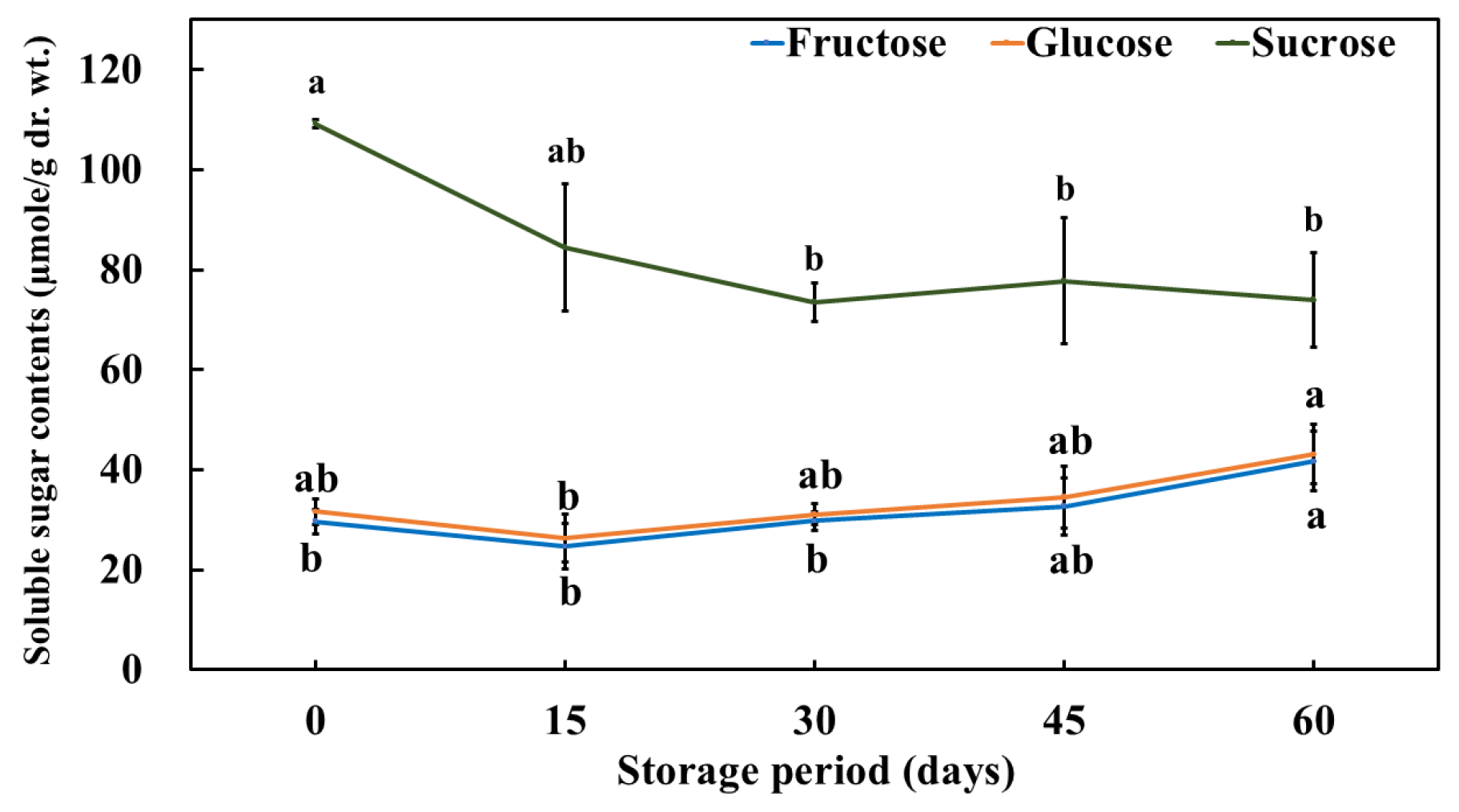
3.3. Change in the Free Sugar (Fructose, Glucose, and Sucrose) Content of Pickled Purple Radish During Storage
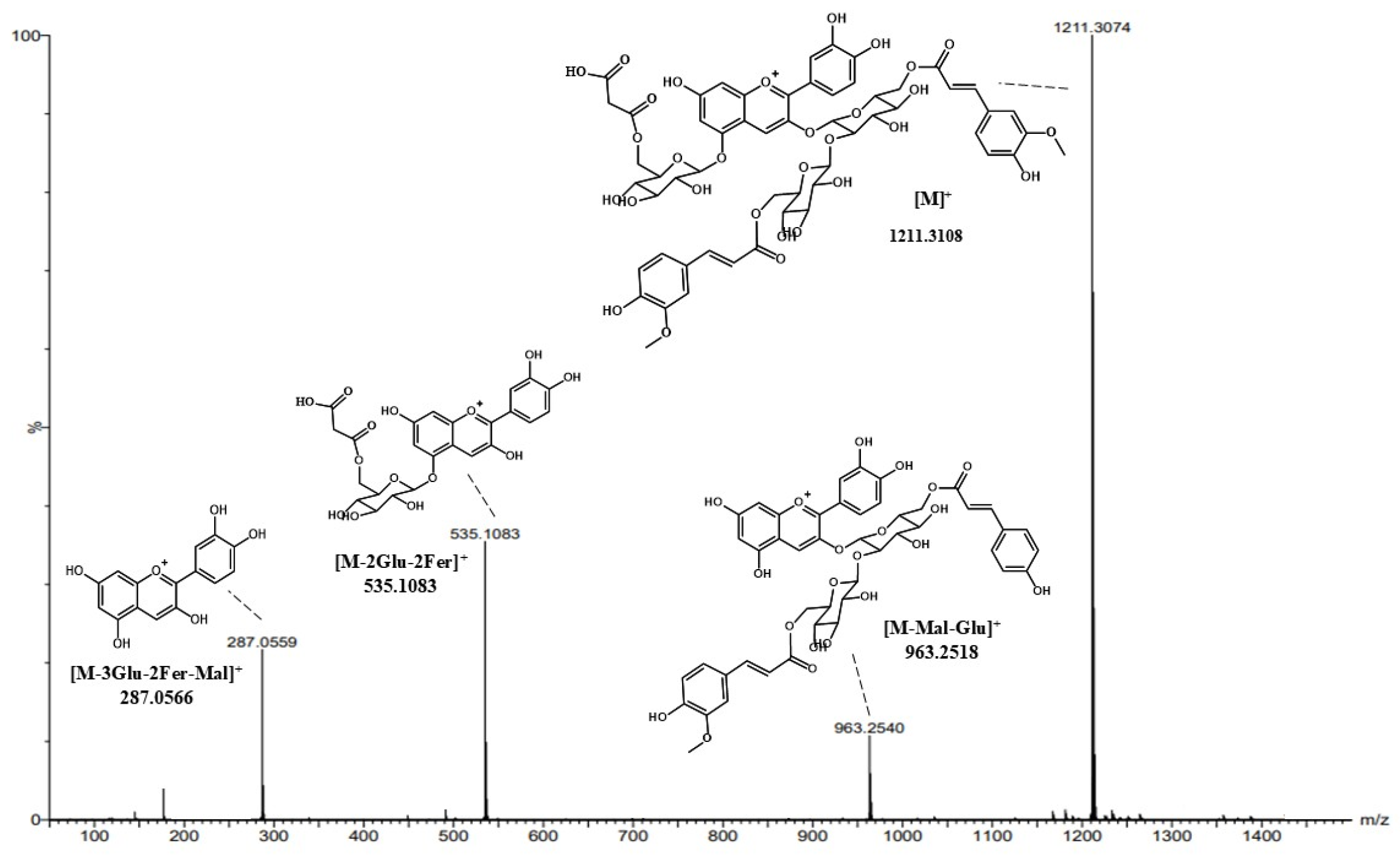
3.4. Metabolites of Pickled Purple Radish During Storage
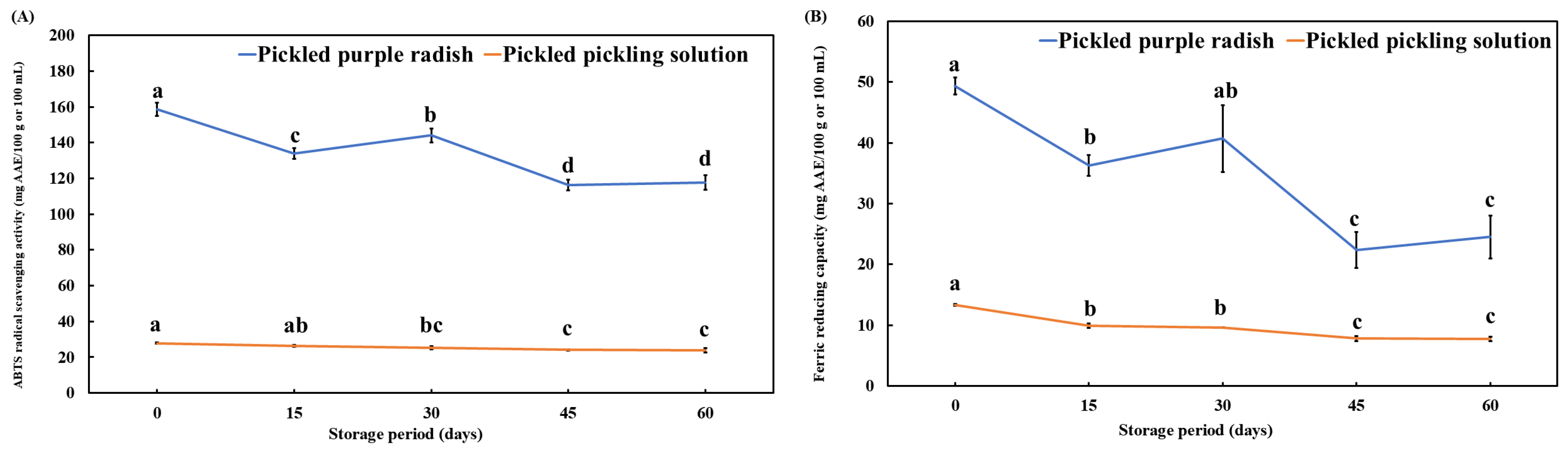
3.5. Changes in the ABTS+ Radical-Scavenging Activity and FRAP of Pickled Purple Radish During Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC-QToF-ESI-MS | Liquid chromatography quadrupole time-of-flight mass spectrometry |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| TAC | Total anthocyanin content |
| MSTFA | N-methyl-N-(trimethylsilyl)trifluoroacetamide |
| TMCS | Trifluoroacetamide |
| FRAP | Ferric reducing antioxidant power |
| AAE | Ascorbic acid equivalent |
| HPLC | High pressure liquid chromatography |
| BPI | Base peak intensity |
| LysoPC | Lysophosphatidylcholine |
| LysoPE | Lysophosphatidylethanolamine |
| Cy | Cyanidin |
| Glu | Glucose |
| Mal | Malonic acid |
| Fer | Ferulic acid |
| Soph | Sophoroside |
| Caf | Caffeic acid |
| Cou | p-Coumaric acid |
| Sin | Sinapinic acid |
References
- Rho, J.O.; Kim, Y.O.; Lee, Y.S. Quality characteristics of pickled color radish and sensory evaluation by elementary, middle, high and university students. J. East Asian Soc. Diet. Life 2013, 23, 569–576. [Google Scholar]
- Jeong, E.-J.; Lee, N.K.; Yum, E.J.; Nam, K.; Oh, J.; Kim, Y.-S.; Park, J.-Y.; Kim, S.-J.; Jeong, Y.-S. Effect of calcium chloride on the texture of pickled radish wrap. Korean J. Food Preserv. 2015, 22, 452–457. [Google Scholar] [CrossRef]
- Kim, B.; Hur, O.; Lee, J.-E.; Assefa, A.D.; Ko, H.-C.; Chung, Y.-J.; Rhee, J.-H.; Hahn, B.-S. Characterization of phenotypic traits and evaluation of glucosinolate contents in radish germplasms (Raphanus sativus L.). Korean J. Plant Resour. 2021, 34, 575–599. [Google Scholar]
- Zaki, H.; Takahata, Y.; Yokoi, S. Analysis of the morphological and anatomical characteristics of roots in three radish (Raphanus sativus L.) cultivars that differ in root shape. J. Hortic. Sci. Biotechnol. 2012, 87, 172–178. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar]
- Iwata, H.; Niikura, S.; Matsuura, S.; Takano, Y.; Ukai, Y. Diallel analysis of root shape of Japanese radish (Raphanus sativus L.) based on elliptic Fourier descriptors. Breed. Sci. 2000, 50, 73–80. [Google Scholar] [CrossRef]
- Jing, P.; Ruan, S.-Y.; Dong, Y.; Zhang, X.-G.; Yue, J.; Kan, J.-Q.; Slavin, M.; Yu, L. Optimization of purification conditions of radish (Raphanus sativus L.) anthocyanin-rich extracts using chitosan. LWT—Food Sci. Technol. 2011, 44, 2097–2103. [Google Scholar] [CrossRef]
- Koley, T.K.; Khan, Z.; Oulkar, D.; Singh, B.K.; Maurya, A.; Singh, B.; Banerjee, K. High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab. J. Chem. 2020, 13, 1355–1366. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Joo, S.-Y.; Park, J.-D.; Choi, Y.-S.; Sung, J.-M. Quality characteristics and antioxidant activity of red radish (Bordeaux and watermelon radish) tea with use of different processing methods. Korean J. Food Nutr. 2017, 30, 908–915. [Google Scholar] [CrossRef]
- Yan, C.; Huang, Y.; Zhang, S.; Cui, L.; Jiao, Z.; Peng, Z.; Luo, X.; Liu, Y.; Qiu, Z. Dynamic profiling of intact glucosinolates in radish by combining UHPLC-HRMS/MS and UHPLC-QqQ-MS/MS. Front. Plant Sci. 2023, 14, 1216682. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Lim, S.; Chae, W.B.; Park, J.E.; Park, H.R.; Lee, E.J.; Huh, J.H. Root Glucosinolate Profiles for Screening of Radish (Raphanus sativus L.) Genetic Resources. Front. Plant Sci. 2016, 64, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; He, Z.; Zhang, J. Identification and analysis of major flavor compounds in radish taproots by widely targeted metabolomics. Front. Nutr. 2022, 9, 889407. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.-J.; Ruan, S.-Y.; Xie, Z.-H.; Dong, Y.; Yu, L.L. Anthocyanin and glucosinolate occurrences in the roots of Chinese red radish (Raphanus sativus L.), and their stability to heat and pH. Food Chem. 2012, 133, 1569–1576. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Kim, K.-B.; An, D.-G.; Hwang, S.-Y.; Nam, J.-S.; Choi, S.-K. Quality characteristics of radish pickle added with different amounts of white wine. Culin. Sci. Hosp. Res. 2015, 21, 72–85. [Google Scholar]
- Bhusal, S.; Shrestha, R.; Upadhya, N. Preparation of chicken meat pickle and its storage stability studies at room temperature. Gold. Gate J. Food Sci. Technol. 2017, 3, 59–62. [Google Scholar]
- Di, C.; Jia, W. Metabolomics as a critical tool for deeper understanding of pickled foods: From biomarker discovery to nutrition function. Trends Food Sci. Technol. 2024, 147, 104456. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.; Soladoye, O.P. Towards innovative food processing of flavonoid compounds: Insights into stability and bioactivity. LWT 2021, 150, 111968. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. Effect of cooking on the contents of glucosinolates and their degradation products in selected Brassica vegetables. J. Funct. Foods 2016, 23, 412–422. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.-Y.; Deng, Q.; Li, G.; Su, G.; Liu, J.; David Wang, H.-M. Extraction and characterization of phenolic compounds with antioxidant and antimicrobial activities from pickled radish. Food Chem. Toxicol. 2020, 136, 111050. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choi, Y.-J.; Lee, M.J.; Seo, H.-Y.; Yun, Y.-R.; Min, S.G.; Lee, H.J.; Lee, J.H.; Kang, S.R.; Kim, H.J.; et al. Quality characteristics of radish pickle with natural preservatives. J. Korean Soc. Food Cult. 2020, 35, 577–581. [Google Scholar] [CrossRef]
- Park, B.-H.; Cho, H.-S.; Oh, B.-Y. Physicochemical characteristics of kimchi treated with chitosan during fermentation. Korean J. Hum. Ecol. 2002, 5, 85–93. [Google Scholar]
- Liu, W.; Zhang, L.; Karrar, E.; Wu, D.; Chen, C.; Zhang, Z.; Li, J. A cooperative combination of non-targeted metabolomics and electronic tongue evaluation reveals the dynamic changes in metabolites and sensory quality of radish during pickling. Food Chem. 2024, 446, 138886. [Google Scholar]
- Malien Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra-and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef]
- Wrolstad, R. Anthocyanin pigments—Bioactivity and coloring properties. J. Food Sci. 2004, 69, C419–C425. [Google Scholar] [CrossRef]
- Daravingas, G.; Cain, R. Thermal degradation of black raspberry anthocyanin pigments in model systems. J. Food Sci. 1968, 33, 138–142. [Google Scholar] [CrossRef]
- Ando, Y.; Hagiwara, S.; Nabetani, H. Thermal inactivation kinetics of pectin methylesterase and the impact of thermal treatment on the texture, electrical impedance characteristics and cell wall structure of Japanese radish (Raphanus sativus L.). J. Food Eng. 2017, 199, 9–18. [Google Scholar]
- Afsharnia, F. Optimization of in vitro and in vivo antifungal effects of trehalose coating included Artemisia sieberi essential oil on mulberry (Morus alba var. nigra) fruits using the hybrid RSM-GRA method. Food Sci. Biotechnol. 2023, 32, 921–935. [Google Scholar] [CrossRef]
- Chae, S.-H.; Lee, S.-H.; Moon, J.-H.; Cho, J.-Y. Comparison of metabolites and antioxidative activity in leaves and roots of purple radish cultivars. Food Sci. Preserv. 2024, 31, 985–998. [Google Scholar]
- Chae, S.H.; Lee, O.N.; Park, H.Y.; Ku, K.M. Seasonal effects of glucosinolate and sugar content determine the pungency of small-type (Altari) radishes (Raphanus sativus L.). Plants 2022, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wi, G.; Moon, J.; Cho, J. Changes in LC-MS-based untargeted non-volatile metabolites of infused black tea according to different storage temperature and period. J. Korean Tea Soc. 2024, 30, 58–71. [Google Scholar]
- Sanches, P.H.; Oliveira, D.C.d.; dos Reis, I.G.; Fernandes, A.M.; Silva, A.A.; Eberlin, M.N.; Carvalho, P.O.; Duarte, G.H.; Porcari, A.M. Fitting structure-data files (.SDF) libraries to Progenesis QI identification searches. J. Braz. Chem. Soc. 2023, 34, 1013–1019. [Google Scholar]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar]
- Chae, S.-H.; Lee, Y.-S.; Kim, J.-H.; Han, T.-H.; Ku, K.-M. Metabolite and elastase activity changes in beach rose (Rosa rugosa) fruit and seeds at various stages of ripeness. Plants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Lee, M.-K.; Yang, H.-J.; Kim, S.-K.; Park, S.-H.; Moon, S.-W. Determination of suitable kohlrabi (Brassica oleracea var. gongylodes) cultivars for pickle preparation. Prev. Nutr. Food Sci. 2010, 15, 152–158. [Google Scholar]
- Hye Jeong, L.; Jong Gyu, K. The changes of components and texture out of carrot and radish pickles during the storage. Korean J. Food Nutr. 2000, 13, 563–569. [Google Scholar]
- Ji, F.D.; Ji, B.P.; Li, B.; Lu, F. Effect of fermentation on nitrate, nitrite and organic acid contents in traditional pickled Chinese cabbage. J. Food Process. Preserv. 2009, 33, 175–186. [Google Scholar]
- Singh, B.; Koley, T.; Karmakar, P.; Tripathi, A.; Singh, B.; Singh, M. Pigmented radish (Raphanus sativus): Genetic variability, heritability and inter-relationships of total phenolics, anthocyanins and antioxidant activity. Indian J. Agric. Sci. 2017, 87, 1600–1606. [Google Scholar]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [PubMed]
- Tsukui, A.; Murakami, T.; Hayashi, K. Stability of Perilla Anthocyanin Pigment in Waste from the Manufacture of Pickled Japanese Apricot. Food Preserv. Sci. 2005, 31, 103–109. [Google Scholar]
- Li, X.; Liu, G.; Tu, Y.; Li, J.; Yan, S. Ferulic acid pretreatment alleviates the decrease in hardness of cooked chinese radish (Raphanus sativus L. var. longipinnatus Bailey). Food Chem. 2019, 278, 502–508. [Google Scholar] [CrossRef]
- Park, B.-H.; Jeon, E.-R.; Kim, S.-D.; Cho, H.-S. Changes in the quality characteristics of lotus root pickle with beet extract during storage. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1124–1129. [Google Scholar] [CrossRef]
- Ozer, M.H.; Akbudak, B.; Uylaser, V.; Tamer, E. The effect of controlled atmosphere storage on pickle production from pickling cucumbers cv.‘Troy’. Eur. Food Res. Technol. 2006, 222, 118–129. [Google Scholar]
- Tomita, S.; Watanabe, J.; Kuribayashi, T.; Tanaka, S.; Kawahara, T. Metabolomic evaluation of different starter culture effects on water-soluble and volatile compound profiles in nozawana pickle fermentation. Food Chem. Mol. Sci. 2021, 2, 100019. [Google Scholar]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Chen, X.; Guo, S.; Xiang, W.; Liu, L.; Du, H. Characterization of the microbial communities and their correlations with chemical profiles in assorted vegetable Sichuan pickles. Food Control 2020, 113, 107174. [Google Scholar]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus L.): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods. 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Liu, Z.; Zhu, X.; Zhai, L.; Xu, L.; Yu, R.; Gong, Y.; Liu, L. De novo transcriptome sequencing of radish (Raphanus sativus L.) and analysis of major genes involved in glucosinolate metabolism. BMC Genom. 2013, 14, 836. [Google Scholar] [CrossRef]
- Kim, J.K.; Baskar, T.B.; Park, S.U. Total phenolic and flavonoid contents and antioxidant activities of two Raphanus sativus L. cultivars (Cherry belle and Valentine). Biosci. Biotechnol. Res. Asia 2016, 13, 31–36. [Google Scholar] [CrossRef]
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, S151–S183. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.A.; Abdalla, I.G.; Alfawaz, M.A.; Mohammed, M.A.; Al Maiman, S.A.; Osman, M.A.; Yagoub, A.E.A.; Hassan, A.B. Effects of extraction solvents on the total phenolic content, total flavonoid content, and antioxidant activity in the aerial part of root vegetables. Agriculture 2022, 12, 1820. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar]
- Baltaciotglu, C.; Velioglu, S.; Karacabey, E. Changes in total phenolic and flavonoid contents of rowanberry fruit during postharvest storage. J. Food Qual. 2011, 34, 278–283. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Bąkowska, A.; Kucharska, A.Z.; Oszmiański, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin–polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Chişbora, C.; Cimpoiu, C. Degradation kinetics of anthocyanins from European cranberrybush (Viburnum opulus L.) fruit extracts. Effects of temperature, pH and storage solvent. Molecules 2012, 17, 11655–11666. [Google Scholar] [CrossRef]
- Song, H.-N.; Ji, S.-A.; Park, H.-R.; Kim, H.-H.; Hogstrand, C. Impact of various factors on color stability of fresh blueberry juice during storage. Prev. Nutr. Food Sci. 2018, 23, 46. [Google Scholar] [CrossRef]
- Amr, A.; Al-Tamimi, E. Stability of the crude extracts of Ranunculus asiaticus anthocyanins and their use as food colourants. Int. J. Food Sci. Technol. 2007, 42, 985–991. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Shang, H.; Meng, X. Effect of methyl jasmonate on the anthocyanin content and antioxidant activity of blueberries during cold storage. J. Sci. Food Agric. 2015, 95, 337–343. [Google Scholar] [PubMed]
- Clemens, R.A.; Jones, J.M.; Kern, M.; Lee, S.Y.; Mayhew, E.J.; Slavin, J.L.; Zivanovic, S. Functionality of sugars in foods and health. Compr. Rev. Food Sci. Food Saf. 2016, 15, 433–470. [Google Scholar] [PubMed]
- Zhou, D.; Chen, S.; Xu, R.; Tu, S.; Tu, K. Interactions among chilling tolerance, sucrose degradation and organic acid metabolism in UV-C-irradiated peach fruit during postharvest cold storage. Acta Physiol. Plant. 2019, 41, 79. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, G.; Wang, D.; Hu, R.; Li, H.; Liu, S.; Zhang, Q.; Ming, J.; Chi, Y. Effects of dry-salting and brine-pickling processes on the physicochemical properties, nonvolatile flavour profiles and bacterial community during the fermentation of Chinese salted radishes. LWT 2022, 157, 113084. [Google Scholar]
- Hayashi, T.; Aoki, S. Effect of irradiation on the carbohydrate metabolism responsible for sucrose accumulation in potatoes. J. Agric. Food Chem. 1985, 33, 14–17. [Google Scholar] [CrossRef]
- Hodges, D.M.; Munro, K.D.; Forney, C.F.; McRae, K.B. Glucosinolate and free sugar content in cauliflower (Brassica oleracea var. botrytis cv. Freemont) during controlled-atmosphere storage. Postharvest Biol. Technol. 2006, 40, 123–132. [Google Scholar]
- Kang, J.N.; Kim, J.S.; Lee, S.M.; Won, S.Y.; Seo, M.S.; Kwon, S.-J. Analysis of phenotypic characteristics and sucrose metabolism in the roots of Raphanus sativus L. Front. Plant Sci. 2021, 12, 716782. [Google Scholar] [CrossRef]
- Lewthwaite, S.; Sutton, K.; Triggs, C. Free sugar composition of sweetpotato cultivars after storage. N. Z. J. Crop Hortic. Sci. 1997, 25, 33–41. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural sweeteners: The relevance of food naturalness for consumers, food security aspects, sustainability and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 6285. [Google Scholar] [CrossRef]
- Godshall, M.A.; Eggleston, G.; Thompson, J.; Kochergin, V. Sugar. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 1–84. [Google Scholar]
- Lin, L.-Z.; Sun, J.; Chen, P.; Harnly, J.A. LC-PDA-ESI/MSn identification of new anthocyanins in purple Bordeaux radish (Raphanus sativus L. variety). J. Agric. Food Chem. 2011, 59, 6616–6627. [Google Scholar]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in food. Glucosinolates Ref. Ser. Phytochem. 2017, 87–132. [Google Scholar]
- Petropoulos, S.; Di Gioia, F.; Ntatsi, G. Vegetable organosulfur compounds and their health promoting effects. Curr. Pharm. Des. 2017, 23, 2850–2875. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kwon, M.S.; Hwang, H.; Choi, H.-S.; Lee, W.; Choi, S.-P.; Jo, H.; Hong, S.W. A review of the health benefits of kimchi functional compounds and metabolites. Microbiol. Biotechnol. Lett. 2023, 51, 353–373. [Google Scholar] [CrossRef]
- Ishida, M.; Nagata, M.; Ohara, T.; Kakizaki, T.; Hatakeyama, K.; Nishio, T. Small variation of glucosinolate composition in Japanese cultivars of radish (Raphanus sativus L.) requires simple quantitative analysis for breeding of glucosinolate component. Breed. Sci. 2012, 62, 63–70. [Google Scholar] [CrossRef]
- Kim, S.-J.; Uddin, M.R.; Park, S.U. Glucosinolate accumulation in three important radish (Raphanus sativus) cultivars. Aust. J. Crop Sci. 2013, 7, 1843–1847. [Google Scholar]
- Iqbal, S.; Ali, U.; Fadlalla, T.; Li, Q.; Liu, H.; Lu, S.; Guo, L. Genome wide characterization of phospholipase A & C families and pattern of lysolipids and diacylglycerol changes under abiotic stresses in Brassica napus L. Plant Physiol. Biochem. 2020, 147, 101–112. [Google Scholar]
- Kobayashi, W.; Kobayashi, T.; Takahashi, A.; Kumakura, K.; Ayabe, S.; Matsuoka, H. Branched-chain amino acid synthesis and glucosinolate–myrosinase system during takuan-zuke processing of radish root. J. Food Biochem. 2021, 45, e13983. [Google Scholar] [CrossRef]
- Ahmed, S.; Al-Rehaily, A.J.; Alam, P.; Alqahtani, A.S.; Hidayatullah, S.; Rehman, M.T.; Mothana, R.A.; Abbas, S.S.; Khan, M.; Khalid, J.M. Antidiabetic, antioxidant, molecular docking and HPTLC analysis of miquelianin isolated from Euphorbia schimperi C. Presl. Saudi Pharm. J. 2019, 27, 655–663. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Wu, C.; Fan, G.; Li, T.; Dong, C. Retardation of postharvest softening of blueberry fruit by methyl jasmonate is correlated with altered cell wall modification and energy metabolism. Sci. Hortic. 2021, 276, 109752. [Google Scholar] [CrossRef]
- Šamec, D.; Piljac-Žegarac, J. Postharvest stability of antioxidant compounds in hawthorn and cornelian cherries at room and refrigerator temperatures—Comparison with blackberries, white and red grapes. Sci. Hortic. 2011, 131, 15–21. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Šamec, D. Antioxidant stability of small fruits in postharvest storage at room and refrigerator temperatures. Food Res. Int. 2011, 44, 345–350. [Google Scholar] [CrossRef]
- Aalim, H.; Shishir, M.R.I.; Yosri, N.; Arslan, M.; Tahir, H.E.; Hashim, S.B.; Karim, N.; Zhai, X.; Li, Z.; Zhou, C. Systematic review of the digestive fate of rice phenolic compounds: Insights into bioavailability, influencing factors, encapsulation strategies, and health implications. Trends Food Sci. Technol. 2024, 156, 104833. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Tan, Q.; Qiu, X.; Mei, S. Comparative transcriptome analysis reveals key genes associated with pigmentation in radish (Raphanus sativus L.) skin and flesh. Sci. Rep. 2021, 11, 11434. [Google Scholar]
- Abdullah, Z.L.; Mohammed, R.K. The study of the antibacterial effect of anthocyanin pigment extracted from red cabbage (Brassica oleracea var. capitata f. rubra) and red radish peels (Raphanus sativus. var. sativus). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; p. 052089. [Google Scholar]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar]

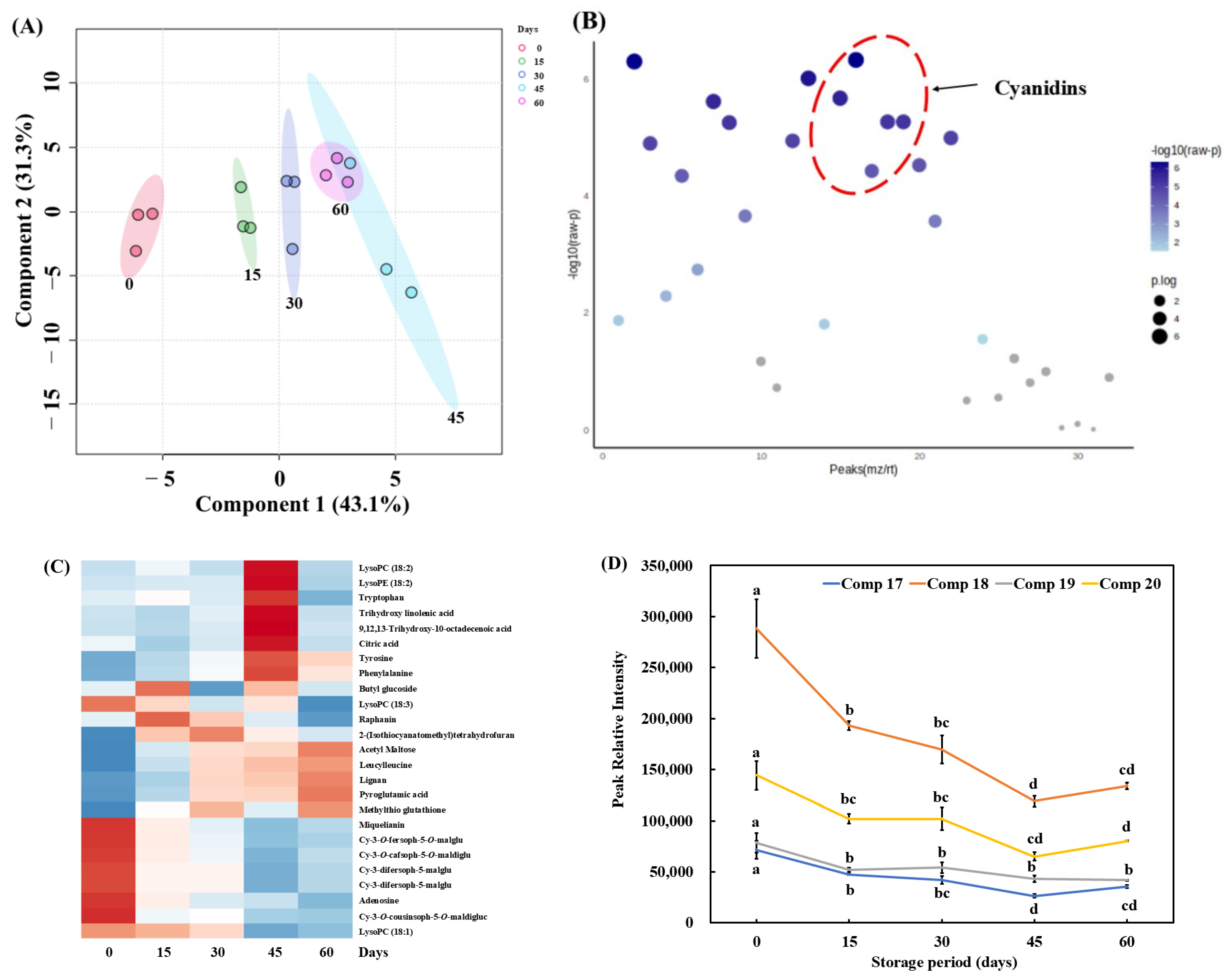
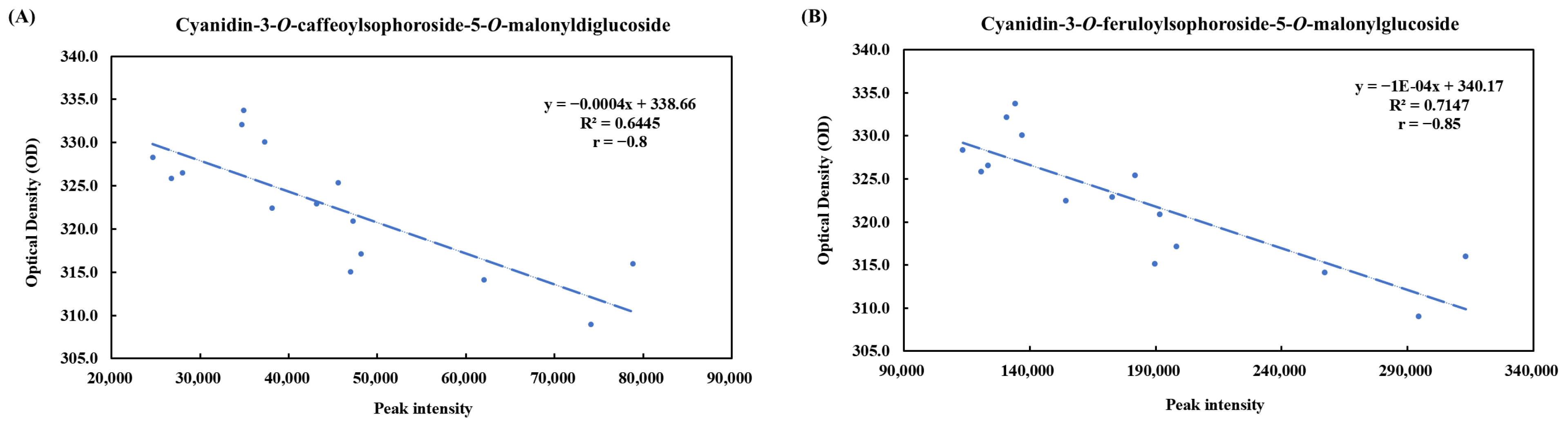
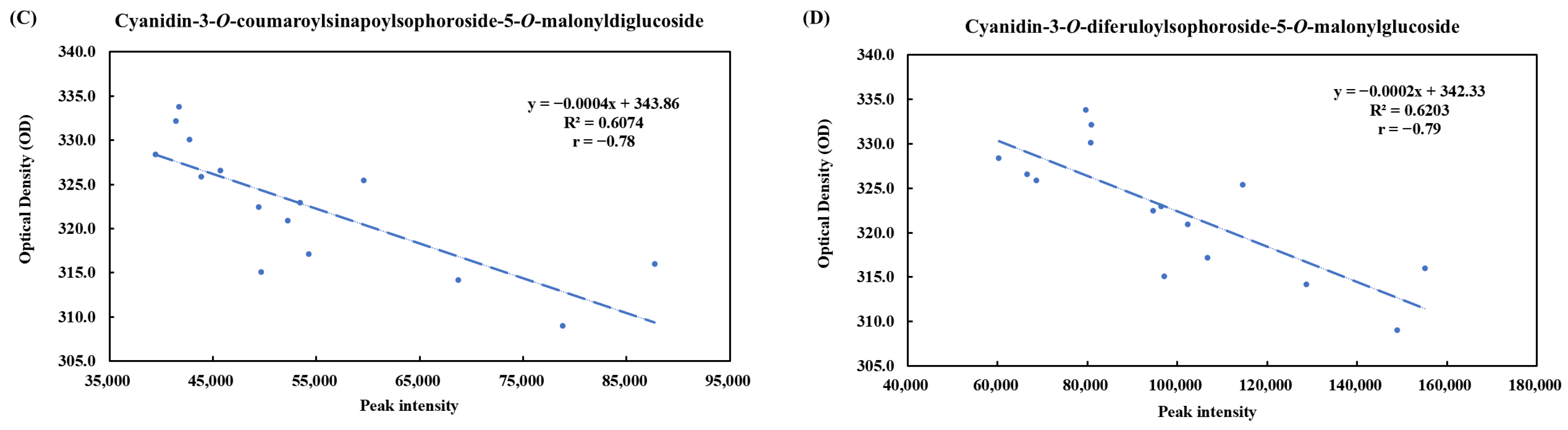
| No. | tR (min) | Fragment Ions (m/z) | Mode of Ionization | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | Molecular Formula | Predicted Compounds | |
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| 1 | 1.02 | 68.9973 | 111.0090, 87.0100 | [M-H]− | 191.0212 | 191.0200 | 4.7 | C6H8O7 | Citric acid |
| 2 | 1.13 | 84.0458 | 128.0311 | [M+H]+ | 130.0547 | 130.0513 | 4.2 | C5H7NO3 | Pyroglutamic acid |
| 3 | 1.39 | 141.9600 | 117.0195, 73.5326 | [M-H]− | 117.0188 | 117.0195 | 5.0 | C4H6O4 | Succinic acid |
| 4 | 1.42 | 165.0558 | 163.0397, 118.0229 | [M+H]+ | 182.0791 | 182.0826 | 4.9 | C9H11NO3 | Tyrosine |
| 5 | 1.57 | 86.0979 | - | [M+H]+ | 132.1024 | 132.1033 | 5.1 | C6H13NO2 | Isoleucine |
| 6 | 1.65 | 205.0718, 61.0081 | 59.6404 | [M+H]+ | 385.1368 | 385.1022 | 1.5 | C14H24O12 | Acetyl maltose |
| 7 | 1.90 | 136.0632 | 134.0462 | [M+H]+ | 268.1051 | 268.1050 | 1.5 | C10H13N5O4 | Adenosine |
| 8 | 2.68 | 126.9580 | - | [M+H]+ | 144.0483 | 144.0493 | 4.5 | C6H9NOS | 2-(Isothiocyanatomethyl)tetrahydrofuran |
| 9 | 3.09 | 120.0822 | 103.6744 | [M+H]+ | 166.0868 | 166.0876 | 4.8 | C9H11NO2 | Phenylalanine |
| 10 | 4.90 | 308.8376 | - | [M+H]+ | 354.0715 | 354.0798 | 1.1 | C11H20N3O6S2 | Methylthio glutathione |
| 11 | 6.81 | 188.0718 | 159.8946 | [M+H]+ | 205.0972 | 205.0981 | 3.2 | C11H12N2O2 | Tryptophan |
| 12 | 7.67 | 235.9700 | - | [M+Na]+ | 259.1329 | 259.1165 | 2.7 | C10H20O6 | Butyl hexose |
| 13 | 9.50 | 235.9700 | [M+Na]+ | 259.1329 | 259.1165 | 2.7 | C10H20O6 | Butyl hexose | |
| 14 | 10.93 | - | 151.9011 | [M-H]− | 477.0697 | 477.0643 | −5.4 | C21H18O13 | Miquelianin |
| 15 | 11.39 | 86.0975 | - | [M+H]+ | 245.1884 | 245.1870 | 2.0 | C12H24N2O3 | Leucylleucine |
| 16 | 11.95 | 112.0230 | [M+H]+ | 176.0204 | 176.0219 | 4.5 | C6H9NOS2 | Raphanin | |
| 17 | 12.02 | 773.1924, 287.0566 | - | [M]+ | 1021.2461 | 1021.2479 | 1.8 | C45H49O27 | Cyanidin-3-O-caffeoylsophoroside-5-O-malonyldiglucoside |
| 18 | 12.32 | 787.3702, 287.1151 | - | [M]+ | 1035.2618 | 1035.2627 | 0.9 | C46H51O27 | Cyanidin-3-O-feruloylsophoroside-5-O-malonylglucoside |
| 19 | 12.44 | 963.2558, 697.1616 | 1371.35 | [M]+ | 1373.3617 | 1373.3645 | 1.9 | C62H69O35 | Cyanidin-3-O-coumaroylsinapoylsophoroside-5-O-malonyldiglucoside |
| 20 | 12.55 | 1035.2664, 963.2518 | - | [M]+ | 1211.3093 | 1211.3108 | 1.4 | C56H59O30 | Cyanidin-3-O-diferuloylsophoroside-5-O-malonylglucoside |
| 21 | 12.73 | 1035.2664, 963.2518 | - | [M]+ | 1211.3093 | 1211.3108 | 1.4 | C56H59O30 | Cyanidin-3-O-diferuloylsophoroside-5-O-malonylglucoside isomer 1 |
| 22 | 12.96 | - | 160.0445 | [M-H]− | 693.2036 | 693.2034 | −4.6 | C32H38O17 | Lignan |
| 23 | 14.01 | 279.0948, 81.5212 | 211.1340 | [M-H]− | 327.2162 | 327.2179 | 2.4 | C18H34O5 | Trihydroxylinolenic acid |
| 24 | 14.28 | 169.9780, 97.9925 | 265.1494 | [M-H]− | 329.2328 | 329.2334 | 1.8 | C18H34O5 | 9,12,13-Trihydroxy-10-octadecenoic acid |
| 25 | 16.31 | 335.2590 | 542.2498, 277.2166 | [M+H]+ | 476.2652 | 476.2780 | 0.6 | C23H42NO7P | LysoPE(18:3) |
| 26 | 16.47 | 540.3062, 335.2580 | 552.2858, 277.2175 | [M+H]+ | 518.3228 | 518.3246 | −0.2 | C26H48NO7P | LysoPC(18:3) |
| 27 | 16.73 | 540.3063, 335.2588 | 552.2856, 277.2162 | [M+H]+ | 518.3228 | 518.3246 | −0.2 | C26H48NO7P | LysoPC(18:3) |
| 28 | 17.10 | 423.2740, 337.2740 | 279.2337 | [M+H]+ | 478.2881 | 478.2930 | −0.8 | C23H44NO7P | LysoPE(18:2) |
| 29 | 17.32 | 502.3282, 337.2740 | 554.3004 | [M+H]+ | 520.3048 | 520.3397 | −1.2 | C26H50NO7P | LysoPC(18:2) |
| 30 | 17.63 | 502.3281, 337.2718 | 554.2996 | [M+H]+ | 520.3048 | 520.3397 | −1.2 | C26H50NO7P | LysoPC(18:2) |
| 31 | 17.98 | 436.2665, 313.2737 | 255.9257 | [M+H]+ | 454.2812 | 454.2928 | −1.3 | C21H44NO7P | LysoPE(16:0) |
| 32 | 18.15 | 339.2892, 223.9893 | 281.2467 | [M+H]+ | 480.3247 | 480.3084 | −1.2 | C23H46NO7P | LysoPE(18:1) |
| 33 | 18.28 | 459.2485, 104.1074 | 480.3099 | [M+H]+ | 496.3356 | 496.3398 | −1.0 | C24H50NO7P | LysoPC(16:0) |
| 34 | 18.45 | 504.3404, 184.9869 | 566.3459, 281.2491 | [M+H]+ | 522.3558 | 522.3554 | −1.1 | C26H52NO7P | LysoPC(18:1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, S.-H.; Lee, S.-H.; Kim, S.-H.; Song, S.-H.; Moon, J.-H.; Kim, H.-W.; Cho, J.-Y. Changes in Quality and Metabolites of Pickled Purple Radish During Storage. Foods 2025, 14, 1259. https://doi.org/10.3390/foods14071259
Chae S-H, Lee S-H, Kim S-H, Song S-H, Moon J-H, Kim H-W, Cho J-Y. Changes in Quality and Metabolites of Pickled Purple Radish During Storage. Foods. 2025; 14(7):1259. https://doi.org/10.3390/foods14071259
Chicago/Turabian StyleChae, Seung-Hun, Sang-Hyeon Lee, Seung-Hwan Kim, Si-Hun Song, Jae-Hak Moon, Heon-Woong Kim, and Jeong-Yong Cho. 2025. "Changes in Quality and Metabolites of Pickled Purple Radish During Storage" Foods 14, no. 7: 1259. https://doi.org/10.3390/foods14071259
APA StyleChae, S.-H., Lee, S.-H., Kim, S.-H., Song, S.-H., Moon, J.-H., Kim, H.-W., & Cho, J.-Y. (2025). Changes in Quality and Metabolites of Pickled Purple Radish During Storage. Foods, 14(7), 1259. https://doi.org/10.3390/foods14071259






