Exploring the Nutraceutical Potential of a Food–Medicine Compound for Metabolic-Associated Fatty Liver Disease via Lipidomics and Network Pharmacology
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Crude Plant Extracts
2.2. Network Pharmacology Analysis of Potential Bioactive Compounds
2.3. UPLC-QTOF-MS/MS Conditions
2.4. MAFLD In Vitro Cell Model
2.5. Cell Viability Analysis by CCK-8 Assays
2.6. Observation of Intracellular Lipid Droplets by Oil Red O Staining
2.7. Determination of Intracellular Lipid Related Indicators
2.8. Lipidomics Analysis
2.9. Statistical Analysis
3. Results
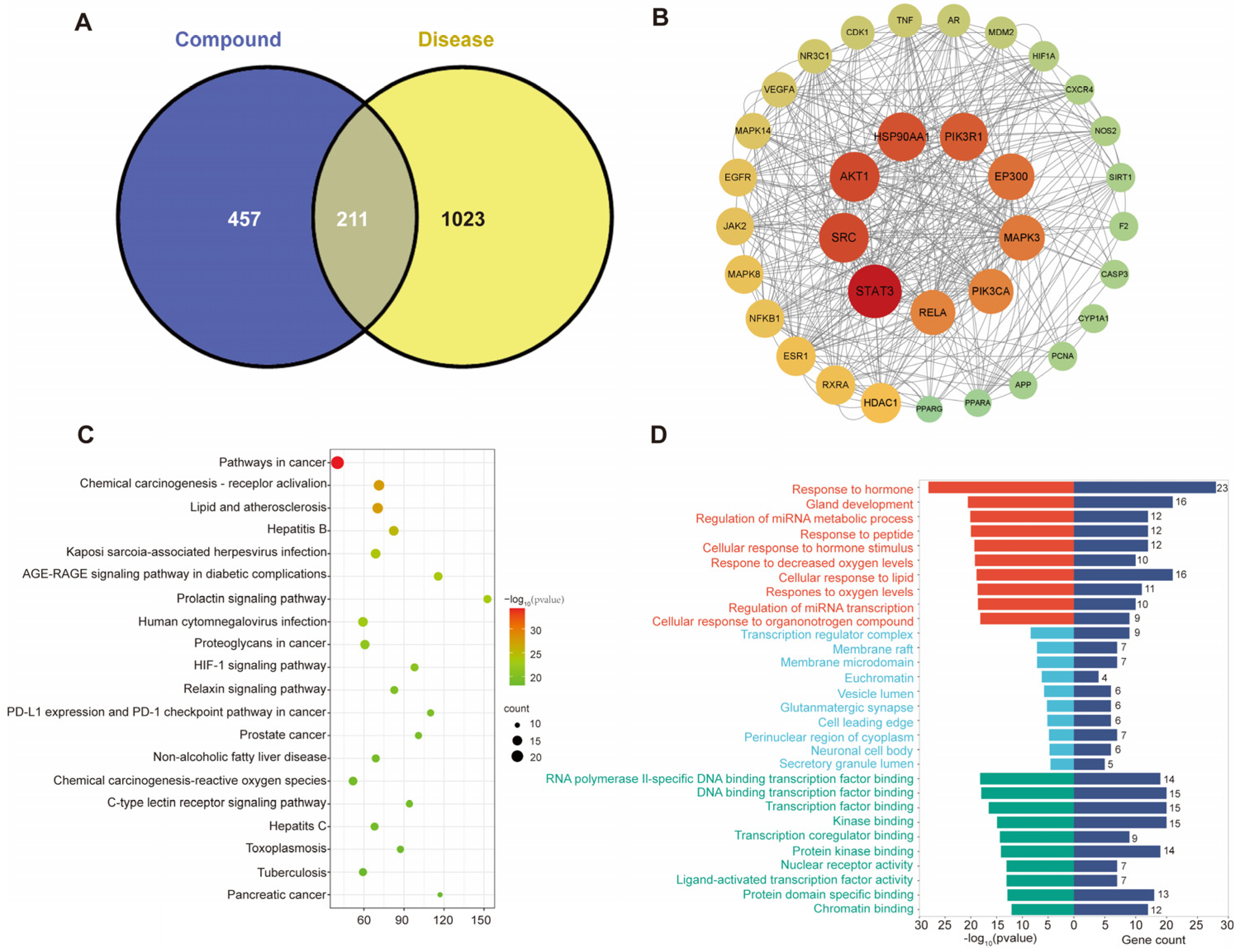
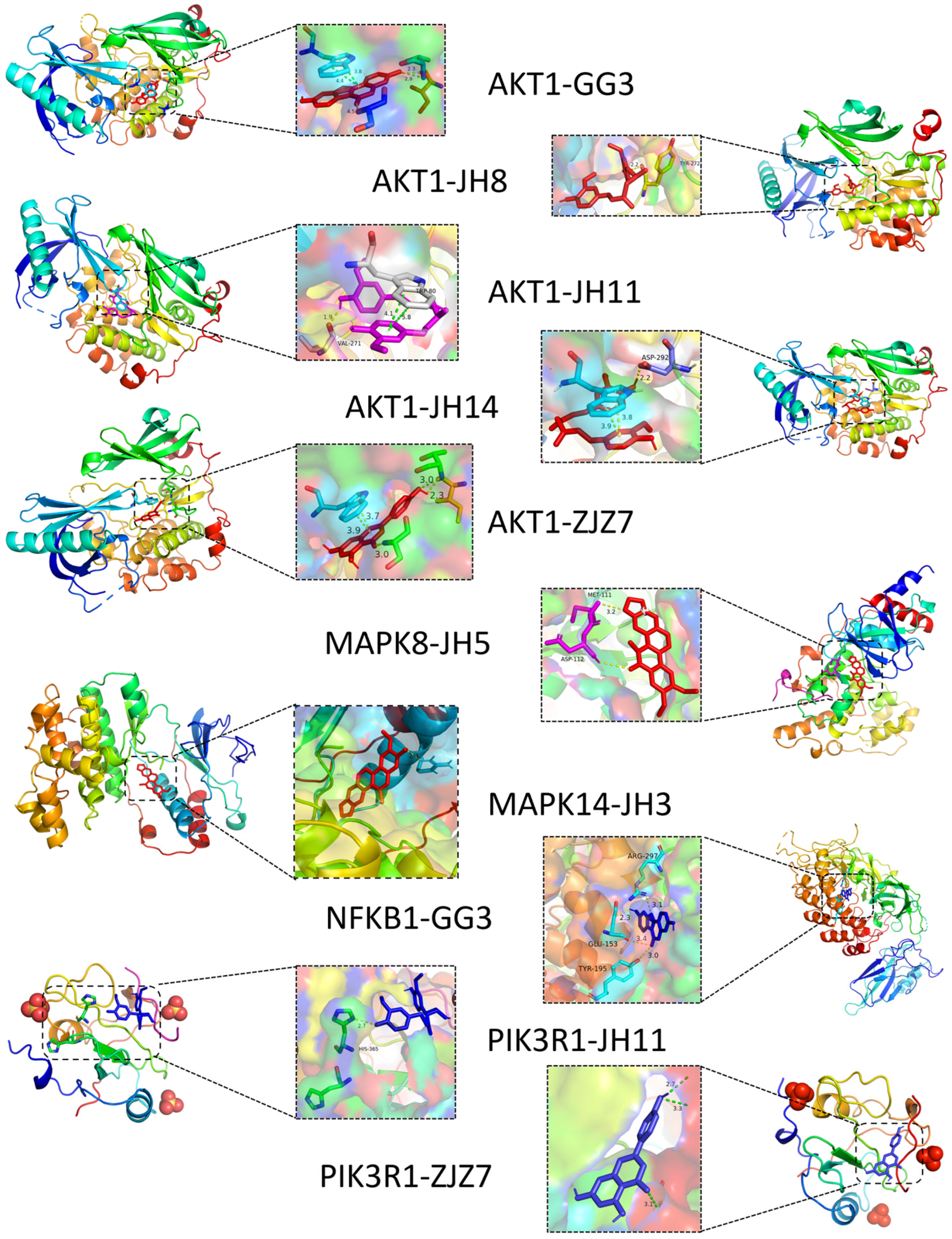
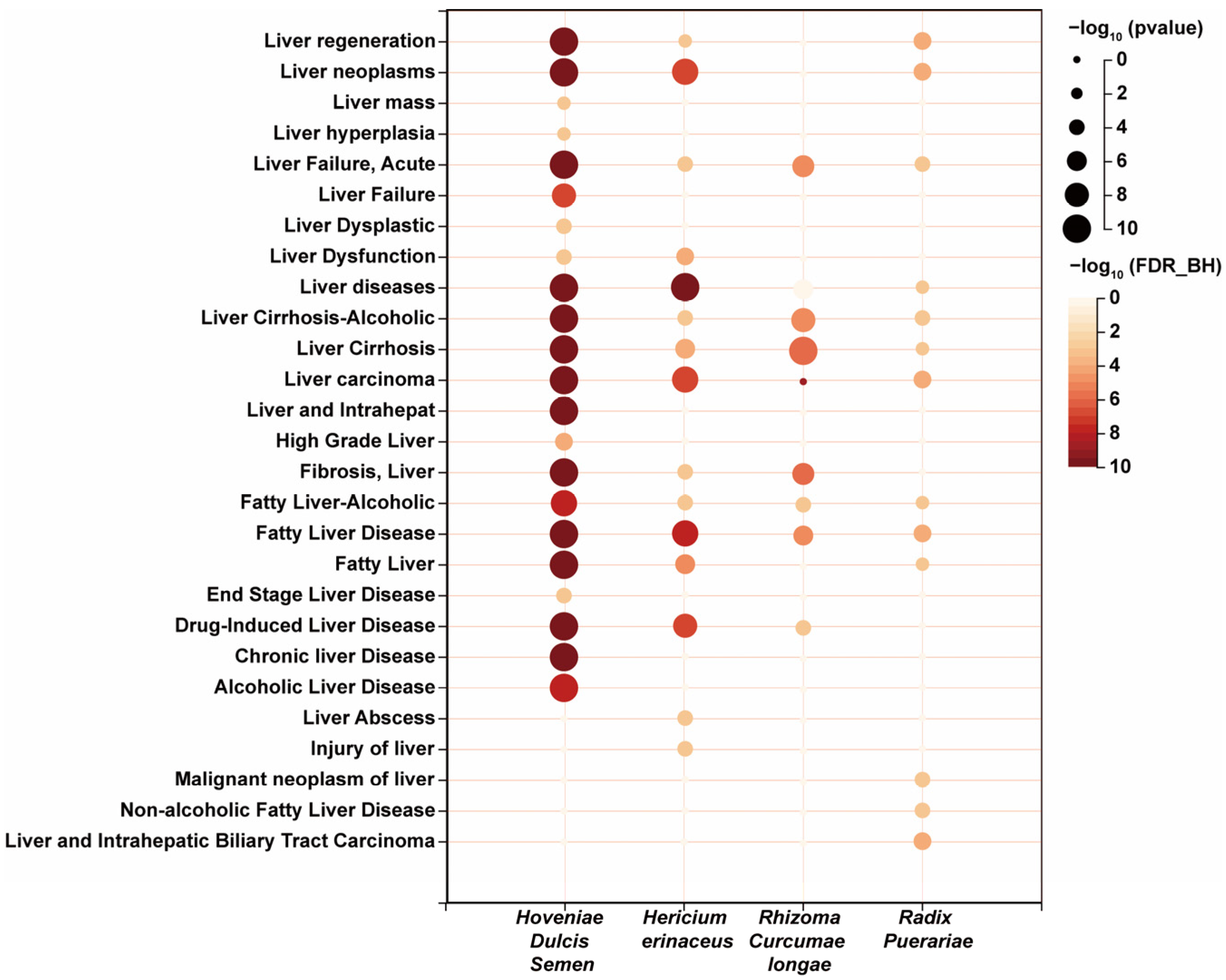
3.1. Prediction of Potential Active Ingredients and Key Targets for Repairing Liver Injury Based on Network Pharmacology
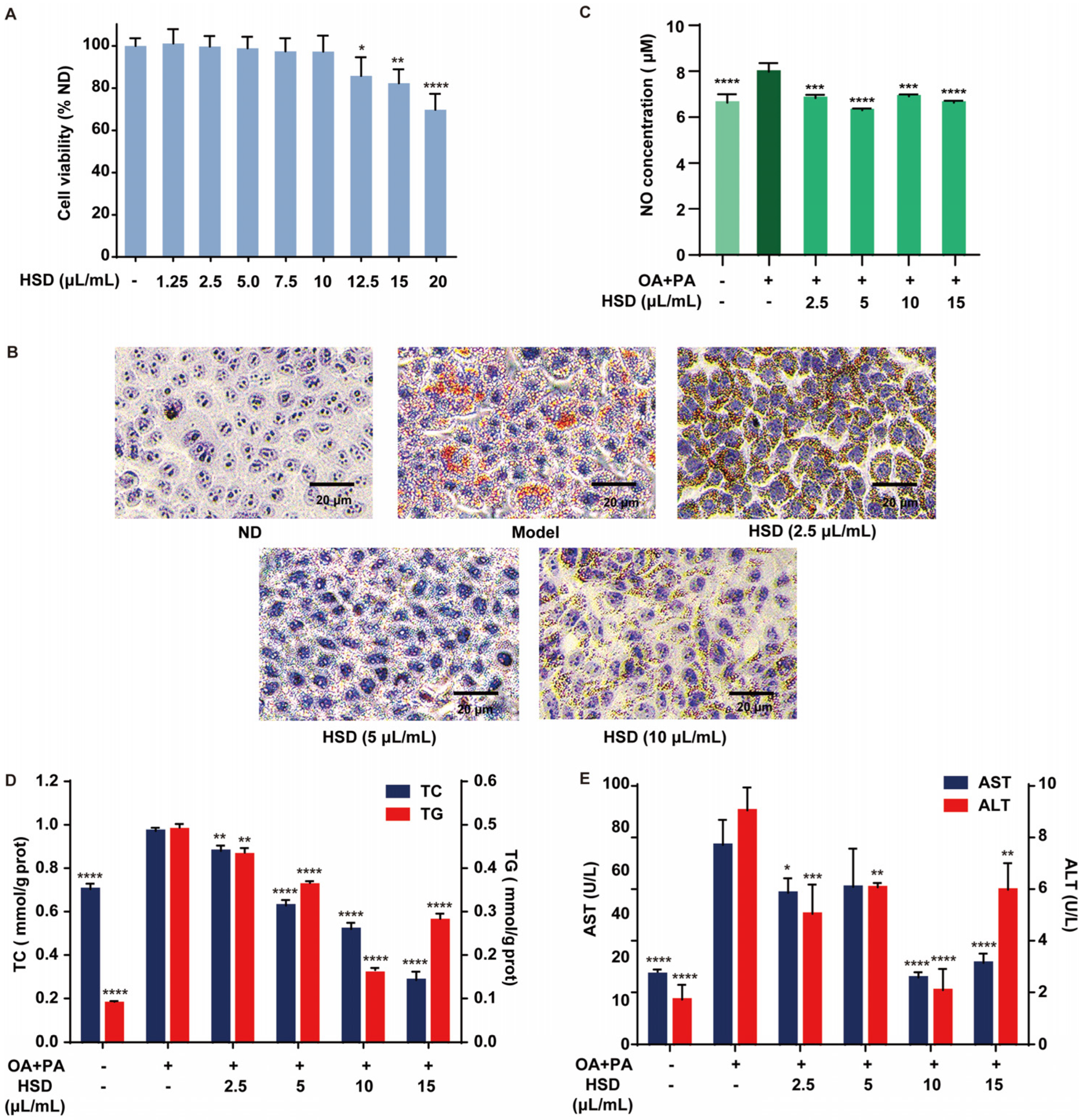
3.2. Improvement of Fatty Acid-Induced Lipid Accumulation in HepG2 Cells by HSD
3.3. Alleviation in Fatty Acid-Induced Liver Injury in HepG2 Cells by HSD
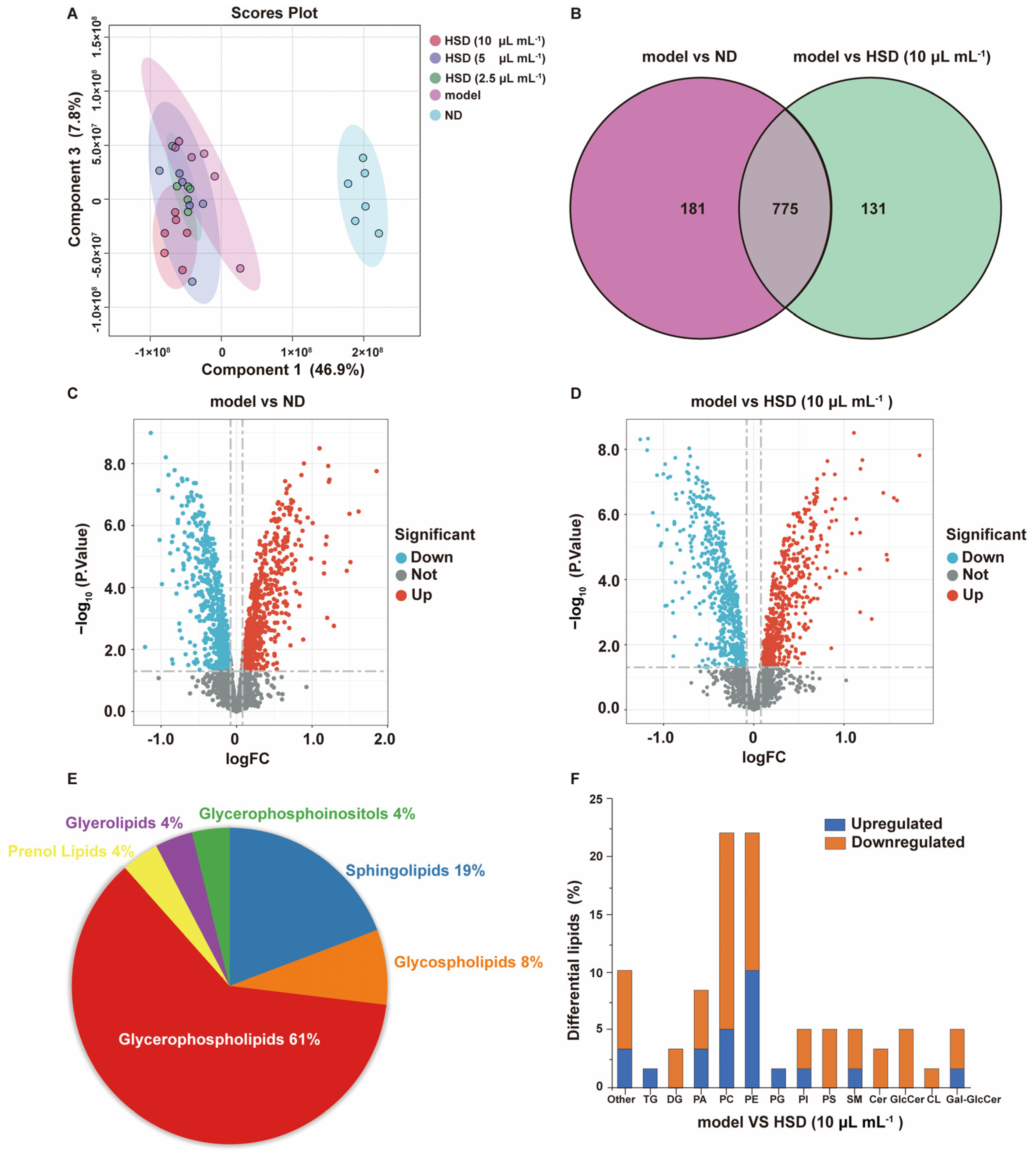
3.4. Analysis of the Effect of HSD on Lipid Composition in Fatty Acid-Induced HepG2 Cells Based on Lipidomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAFLD | Metabolic-associated fatty liver disease |
| FMH | Food and Medicine Homology |
| HSD | HepaSynergy Decoction |
| PPI | Protein–Protein Interaction |
| TG | Triglyceride |
| TC | Total Cholesterol |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| OA | Oleic Acid |
| PA | Palmitic Acid |
| AKT1 | Protein Kinase B |
| PIK3R1 | Phosphoinositide-3-Kinase Regulatory Subunit 1 |
| MAPK8 | Mitogen-Activated Protein Kinase 8 |
| MAPK14 | Mitogen-Activated Protein Kinase 14 |
| NFKB1 | Nuclear Factor Kappa B Subunit 1 |
| IL-1β | Interleukin 1 Beta |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor Necrosis Factor Alpha |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible Nitric Oxide Synthase |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| DG | Diglyceride |
| Cer | Ceramide |
| GlcCer | Glucosylceramide |
| CL | Cardiolipin |
| GRAS | Generally recognized as safe |
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Dai, X.; Feng, J.; Chen, Y.; Huang, S.; Shi, X.; Liu, X.; Sun, Y. Traditional Chinese Medicine in nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Chin. Med. 2021, 16, 68. [Google Scholar] [CrossRef]
- Gong, P.; Long, H.; Guo, Y.; Wang, Z.; Yao, W.; Wang, J.; Yang, W.; Li, N.; Xie, J.; Chen, F. Chinese herbal medicines: The modulator of nonalcoholic fatty liver disease targeting oxidative stress. J. Ethnopharmacol. 2024, 318, 116927. [Google Scholar] [CrossRef]
- Leng, Y.R.; Zhang, M.H.; Luo, J.G.; Zhang, H. Pathogenesis of NASH and Promising Natural Products. Chin. J. Nat. Med. 2021, 19, 12–27. [Google Scholar] [CrossRef]
- Wei, W.; Liu, L.; Liu, X.; Tao, Y.; Zhao, X.; Gong, J.; Wang, Y.; Liu, S. Exploring the Therapeutic Effects of Black Ginseng on Non-Alcoholic Fatty Liver Disease by Using Network Pharmacology and Molecular Docking. Chem. Biodivers. 2022, 19, e202200719. [Google Scholar] [CrossRef]
- Qu, J.; Chen, Q.; Wei, T.; Dou, N.; Shang, D.; Yuan, D. Systematic characterization of Puerariae Flos metabolites in vivo and assessment of its protective mechanisms against alcoholic liver injury in a rat model. Front. Pharmacol. 2022, 13, 915535. [Google Scholar] [CrossRef]
- Bao, Y.; Han, X.; Liu, D.; Tan, Z.; Deng, Y. Gut microbiota: The key to the treatment of metabolic syndrome in traditional Chinese medicine—A case study of diabetes and nonalcoholic fatty liver disease. Front. Immunol. 2022, 13, 1072376. [Google Scholar] [CrossRef]
- Li, M.; Cheng, D.; Peng, C.; Huang, Y.; Geng, J.; Huang, G.; Wang, T.; Xu, A. Therapeutic mechanisms of the medicine and food homology formula Xiao-Ke-Yin on glucolipid metabolic dysfunction revealed by transcriptomics, metabolomics and microbiomics in mice. Chin. Med. 2023, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Chen, X.; Ren, R.; Li, L.; Zhang, B.; Wang, Q.; Meng, Y.; Qiu, Z.; Wang, G.; Zheng, G.; et al. Integration of network pharmacology, lipidomics, and transcriptomics analysis to reveal the mechanisms underlying the amelioration of AKT-induced nonalcoholic fatty liver disease by total flavonoids in vine tea. Food Funct. 2024, 15, 5158–5174. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.-M.; Yi, J.; Tang, Y.; Chen, B.-W.; Long, H.-P.; Liu, Y.-F.; Ou-yang, Y.; Zhang, W.-J.; Tang, R.-M.; Liu, B.-Y. A UPLC-Q-TOF/MS and network pharmacology method to explore the mechanism of Anhua fuzhuan tea intervention in nonalcoholic fatty liver disease. Food Funct. 2023, 14, 3686–3700. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, P.; Chen, W.; Bai, Y. Mechanisms of Gynostemma pentaphyllum against non-alcoholic fibre liver disease based on network pharmacology and molecular docking. J. Cell. Mol. Med. 2022, 26, 3760–3771. [Google Scholar] [CrossRef]
- Tian, D.; Yang, Y.; Yu, M.; Han, Z.-Z.; Wei, M.; Zhang, H.-W.; Jia, H.-M.; Zou, Z.-M. Anti-inflammatory chemical constituents of Flos Chrysanthemi Indici determined by UPLC-MS/MS integrated with network pharmacology. Food Funct. 2020, 11, 6340–6351. [Google Scholar] [CrossRef]
- Fang, S.; Dong, L.; Liu, L.; Guo, J.; Zhao, L.; Zhang, J.; Bu, D.; Liu, X.; Huo, P.; Cao, W.; et al. HERB: A high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2020, 49, D1197–D1206. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Li, J.; Zhou, F.; Xiao, K.; Liu, Y.; Zhang, M.; Wang, S.; Yang, S. Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 302, 115876. [Google Scholar] [CrossRef]
- Amberger, J.S.; Hamosh, A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1–2. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2014, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, J.; Yang, W.; Hou, J.; Yang, M.; Da, J.; Wang, Y.; Jiang, B.; Liu, X.; Wu, W.; et al. HPLC/qTOF-MS-oriented characteristic components data set and chemometric analysis for the holistic quality control of complex TCM preparations: Niuhuang Shangqing pill as an example. J. Pharmaceut. Biomed. 2014, 89, 130–141. [Google Scholar] [CrossRef]
- Lee, M.R.; Park, K.I.; Ma, J.Y. Leonurus japonicus Houtt Attenuates Nonalcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Cells and Mice Fed a High-Fat Diet. Nutrients 2017, 10, 20. [Google Scholar] [CrossRef]
- Lu, Z. Lipid Metabolic Effects and Mechanisms of Main Components of Hugan Qingzhi Tablet on Hepatic Steatosis HepG2 Cell Model. Master’s Thesis, Southern Medical University, Guangzhou, China, 2015. [Google Scholar]
- Lee, S.; Son, B.; Jeon, J.; Park, G.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Song, J.-Y.; Youn, B. Decreased Hepatic Lactotransferrin Induces Hepatic Steatosis in Chronic Non-Alcoholic Fatty Liver Disease Model. Cell. Physiol. Biochem. 2018, 47, 2233–2249. [Google Scholar] [CrossRef]
- Hao, M.; Yao, Z.; Zhao, M.; Chen, Z.; Wang, P.; Sang, X.; Yang, Q.; Wang, K.; Han, X.; Cao, G. Active ingredients screening and pharmacological mechanism research of curcumae rhizoma-sparganii rhizoma herb pair ameliorates liver fibrosis based on network pharmacology. J. Ethnopharmacol. 2023, 305, 116111. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.-C.; Zhou, R.-J.; Zhao, X.; Liu, M.; Ye, H.; Xie, M.-L. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression. Chem.-Biol. Interact. 2017, 275, 171–177. [Google Scholar] [CrossRef]
- Fu, X.; Tan, Y.; Shi, M.; Zeng, C.; Qin, S. Multi-Index Comprehensive Assessment Optimized Critical Flavonoids Extraction from Semen Hoveniae and Their In Vitro Digestive Behavior Evaluation. Foods 2023, 12, 773. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Zhu, T.; Luo, Y.-y.; Kang, X.-j.; Pang, M.-x.; Li, H.-z.; Xu, H.; Gu, C.; Pan, S.-h.; et al. Semen hoveniae extract ameliorates alcohol-induced chronic liver damage in rats via modulation of the abnormalities of gut-liver axis. Phytomedicine 2019, 52, 40–50. [Google Scholar] [CrossRef]
- Lv, P.; Zhu, F.; Lv, M.; Bao, J.; Lv, L.; Xu, X.; Tao, Y. Comparative analysis of the liver-protective effects of raw and stir-fried semen of in rats via gas chromatography–mass spectrometry–based serum metabolomic profiling and chemometrics. Biomed. Chromatogr. 2023, 37, e5578. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Xia, T.; Bi, Y.; Liu, B.; Fu, J.; Zhu, R. Molecular mechanisms and therapeutic implications of dihydromyricetin in liver disease. Biomed. Pharmacother. 2021, 142, 111927. [Google Scholar] [CrossRef]
- Silva, J.; Yu, X.; Moradian, R.; Folk, C.; Spatz, M.H.; Kim, P.; Bhatti, A.A.; Davies, D.L.; Liang, J. Dihydromyricetin Protects the Liver via Changes in Lipid Metabolism and Enhanced Ethanol Metabolism. Alcohol. Clin. Exp. Res. 2020, 44, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Ding, H.; Hu, S.; Wu, X.; Ma, A.; Ma, Y. Lutein Prevents Liver Injury and Intestinal Barrier Dysfunction in Rats Subjected to Chronic Alcohol Intake. Nutrients 2023, 15, 1229. [Google Scholar] [CrossRef]
- Guo, C.; Xue, G.; Pan, B.; Zhao, M.; Chen, S.; Gao, J.; Chen, T.; Qiu, L. Myricetin Ameliorates Ethanol-Induced Lipid Accumulation in Liver Cells by Reducing Fatty Acid Biosynthesis. Mol. Nutr. Food Res. 2019, 63, 1801393. [Google Scholar] [CrossRef]
- He, Y.-X.; Liu, M.-N.; Wang, Y.-Y.; Wu, H.; Wei, M.; Xue, J.-Y.; Zou, Y.; Zhou, X.; Chen, H.; Li, Z. Hovenia dulcis: A Chinese medicine that plays an essential role in alcohol-associated liver disease. Front. Pharmacol. 2024, 15, 1337633. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Z.; Lv, Y.; Ge, C.; Luo, X.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Hericium erinaceus polysaccharides ameliorate nonalcoholic fatty liver disease via gut microbiota and tryptophan metabolism regulation in an aged laying hen model. Int. J. Biol. Macromol. 2024, 273, 132735. [Google Scholar] [CrossRef]
- Niu, B.; Zhang, L.; Chen, B.; Liu, X.; Yang, F.; Ren, Y.; Xiang, H.; Wang, P.; Li, J. Extraction, purification, structural characteristics, biological activities, modifications, and applications from Hericium erinaceus polysaccharides: A review. Int. J. Biol. Macromol. 2025, 291, 138932. [Google Scholar] [CrossRef]
- Hao, L.; Xie, Y.; Wu, G.; Cheng, A.; Liu, X.; Zheng, R.; Huo, H.; Zhang, J. Protective Effect of Hericium erinaceus on Alcohol Induced Hepatotoxicity in Mice. Evid.-Based Complement. Altern. Med. eCAM 2015, 2015, 418023. [Google Scholar] [CrossRef]
- Wu, L.; Lv, Y.; Ge, C.; Luo, X.; Hu, Z.; Huang, W.; Zhan, S.; Shen, X.; Yu, D.; Liu, B. Polysaccharide from Hericium erinaceus improved laying performance of aged hens by promoting yolk precursor synthesis and follicle development via liver-blood-ovary axis. Poult. Sci. 2024, 103, 103810. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, X.; Gao, T.; Zhu, Z.; Qing, Y.; Liao, W.; Lin, W. Herb pairs containing Curcumae Rhizoma (Ezhu): A review of bio-active constituents, compatibility effects and t-copula function analysis. J. Ethnopharmacol. 2024, 319, 117199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liao, W.; Zhu, Z.; Chen, J.; Yang, Q.; Zheng, Y.; Zhang, X.; Limsila, B.; Lu, M.; Fu, S.; et al. Essential oil from the raw and vinegar-processed Rhizoma Curcumae ameliorate CCl4-induced liver fibrosis: Integrating network pharmacology and molecular mechanism evaluation. Food Funct. 2021, 12, 4199–4220. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, M.; Fang, H.; Su, Y.; Zhang, L.; Zhou, H. Puerarin Prevents Acute Liver Injury via Inhibiting Inflammatory Responses and ZEB2 Expression. Front. Pharmacol. 2021, 12, 727916. [Google Scholar] [CrossRef]
- Wang, J.F.; Guo, Y.X.; Niu, J.Z.; Liu, J.; Wang, L.Q.; Li, P.H. Effects of Radix Puerariae flavones on liver lipid metabolism in ovariectomized rats. World J. Gastroenterol. WJG 2004, 10, 1967–1970. [Google Scholar] [CrossRef]
- Bormon, R.; Srivastava, E.; Ali, R.; Singh, P.; Kumar, A.; Verma, S. Anti-proliferative, -migratory and -clonogenic effects of long-lasting nitric oxide release in HepG2 cells. Chem. Commun. 2024, 60, 3527–3530. [Google Scholar] [CrossRef]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef]
- Xu, C.; Huang, S.; Lin, Y.; Huang, Y.; Guo, F. Effect of Camellia Oil on Lipid Metabolism of HepG2 Cells Induced by Free Fatty Acids. Sci. Technol. Food Ind. 2023, 44, 375–384. [Google Scholar] [CrossRef]
- Gao, J.; Ma, L.; Yin, J.; Liu, G.; Ma, J.; Xia, S.; Gong, S.; Han, Q.; Li, T.; Chen, Y.; et al. Camellia (Camellia oleifera bel.) seed oil reprograms gut microbiota and alleviates lipid accumulation in high fat-fed mice through the mTOR pathway. Food Funct. 2022, 13, 4977–4992. [Google Scholar] [CrossRef]
- Jadhao, S.B.; Yang, R.Z.; Lin, Q.; Hu, H.; Anania, F.A.; Shuldiner, A.R.; Gong, D.W. Murine alanine aminotransferase: cDNA cloning, functional expression, and differential gene regulation in mouse fatty liver. Hepatology 2004, 39, 1297–1302. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, A.J.; Govaere, O.; Geng, D.; Ratziu, V.; Allison, M.; Bousier, J.; Petta, S.; de Oliviera, C.; Bugianesi, E.; Schattenberg, J.M.; et al. Metabolic signatures across the full spectrum of non-alcoholic fatty liver disease. JHEP Rep. 2022, 4, 100477. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; You, L.; Guan, P.W.; Fang, C.N.; Qin, W.S.; Liu, X.Y.; Xu, G.W. Risk analysis of serum chemical residues for metabolic associated fatty liver disease based on exposome-lipidome wide association study. Se Pu 2024, 42, 164–175. [Google Scholar] [CrossRef]
- Peng, K.Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, Y.; Wang, J.; Huang, Y.; Yang, R.; Zhang, Y.; Ma, Y.; Wen, Y.; Luo, G.; Zhang, S.; et al. Hepatic sphingomyelin phosphodiesterase 3 promotes steatohepatitis by disrupting membrane sphingolipid metabolism. Cell Metab. 2025, 37, 1–18. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Petrov, D.; Jucan, A.E.; Lacatusu, C.M.; Floria, M.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets. Diagnostics 2021, 11, 2053. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Gordillo, R.; Gancheva, S.; Strassburger, K.; Herder, C.; Esposito, I.; Schlensak, M.; Scherer, P.E.; Roden, M. Role of ceramide-to-dihydroceramide ratios for insulin resistance and non-alcoholic fatty liver disease in humans. BMJ Open Diabetes Res. Care 2020, 8, e001860. [Google Scholar] [CrossRef]
- Jiang, M.; Li, C.; Liu, Q.; Wang, A.; Lei, M. Inhibiting Ceramide Synthesis Attenuates Hepatic Steatosis and Fibrosis in Rats With Non-alcoholic Fatty Liver Disease. Front. Endocrinol. 2019, 10, 665. [Google Scholar] [CrossRef]
- Summers, S.A. Ceramides: Nutrient Signals that Drive Hepatosteatosis. J. Lipid Atheroscler. 2020, 9, 50–65. [Google Scholar] [CrossRef]
- Durand, M.; Coué, M.; Croyal, M.; Moyon, T.; Tesse, A.; Atger, F.; Ouguerram, K.; Jacobi, D. Changes in Key Mitochondrial Lipids Accompany Mitochondrial Dysfunction and Oxidative Stress in NAFLD. Oxidative Med. Cell. Longev. 2021, 2021, 9986299. [Google Scholar] [CrossRef]
- Li, Q.; Liu, W.; Feng, Y.; Hou, H.; Zhang, Z.; Yu, Q.; Zhou, Y.; Luo, Q.; Luo, Y.; Ouyang, H.; et al. Radix Puerariae thomsonii polysaccharide (RPP) improves inflammation and lipid peroxidation in alcohol and high-fat diet mice by regulating gut microbiota. Int. J. Biol. Macromol. 2022, 209, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wu, J.; Zhao, X.; Li, Z.; Yu, J.; Shao, T.; Hou, X.; Zhou, L.; Wang, C.; Wang, G.; et al. Structural elucidation of an active polysaccharide from Radix Puerariae lobatae and its protection against acute alcoholic liver disease. Carbohydr. Polym. 2024, 325, 121565. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, W.; Zhang, H.; Chen, C.; Liu, R.; Hou, H.; Luo, Q.; Yu, Q.; Ouyang, H.; Feng, Y.; et al. α-D-1,3-glucan from Radix Puerariae thomsonii improves NAFLD by regulating the intestinal flora and metabolites. Carbohydr. Polym. 2023, 299, 120197. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhu, W.; Li, Z.; Ling, S. Effect of juice and fermented vinegar from Hovenia dulcis peduncles on chronically alcohol-induced liver damage in mice. Food Funct. 2012, 3, 628–634. [Google Scholar] [CrossRef]
- Chilakala, R.; Moon, H.J.; Kim, K.; Yang, S.; Cheong, S.H. Anti-obesity effects of Camellia (Camellia oleifera Abel) oil treatment on high-fat diet-induced obesity in C57BL/6J mice. Phys. Act. Nutr. 2023, 27, 50–61. [Google Scholar] [CrossRef]
| Pathway Name | Total | Hits | Expect | p Value | Holm P | FDR |
|---|---|---|---|---|---|---|
| Glycerophospholipids | 40,000 | 5.0000 | 16 | 2.57 × 10−6 | 1.22 × 10−3 | 6.12 × 10−4 |
| Sphingolipids | 629 | 0.0787 | 5 | 1.56 × 10−8 | 7.41 × 10−6 | 7.41 × 10−6 |
| Glycosphingolipids | 13,400 | 1.6800 | 2 | 0.507 | 1.0 | 1.0 |
| Glycerophosphoinositols | 3190 | 0.3990 | 1 | 0.331 | 1.0 | 1.0 |
| Prenol lipids | 3830 | 0.4780 | 1 | 0.383 | 1.0 | 1.0 |
| Glycerolipids | 42,900 | 5.3600 | 1 | 0.998 | 1.0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Cui, J.; Jiang, Y.; Zhang, J.; Jiang, J.; Zhang, Q.; Hu, Y. Exploring the Nutraceutical Potential of a Food–Medicine Compound for Metabolic-Associated Fatty Liver Disease via Lipidomics and Network Pharmacology. Foods 2025, 14, 1257. https://doi.org/10.3390/foods14071257
Deng Y, Cui J, Jiang Y, Zhang J, Jiang J, Zhang Q, Hu Y. Exploring the Nutraceutical Potential of a Food–Medicine Compound for Metabolic-Associated Fatty Liver Disease via Lipidomics and Network Pharmacology. Foods. 2025; 14(7):1257. https://doi.org/10.3390/foods14071257
Chicago/Turabian StyleDeng, Yuru, Jie Cui, Yuxuan Jiang, Jian Zhang, Jinchi Jiang, Quanbin Zhang, and Yonghong Hu. 2025. "Exploring the Nutraceutical Potential of a Food–Medicine Compound for Metabolic-Associated Fatty Liver Disease via Lipidomics and Network Pharmacology" Foods 14, no. 7: 1257. https://doi.org/10.3390/foods14071257
APA StyleDeng, Y., Cui, J., Jiang, Y., Zhang, J., Jiang, J., Zhang, Q., & Hu, Y. (2025). Exploring the Nutraceutical Potential of a Food–Medicine Compound for Metabolic-Associated Fatty Liver Disease via Lipidomics and Network Pharmacology. Foods, 14(7), 1257. https://doi.org/10.3390/foods14071257







