Exogenous Melatonin Application Enhances Pepper (Capsicum annuum L.) Fruit Quality via Activation of the Phenylpropanoid Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Determination of Total Phenolics and Total Flavonoids Contents
2.3. Determination of Phenolic Compounds Contents
2.4. Determination of Endogenous MT Content
2.5. Determination of Enzyme Activities Involved in the Phenylpropanoid Metabolism Pathway
2.6. RNA Extraction, cDNA Synthesis, and qRT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of Exogenous MT on the Contents of Total Phenolics and Total Flavonoids in Pepper Fruits
3.2. Effect of Exogenous MT on the Contents of Phenolic Compounds in Pepper Fruits
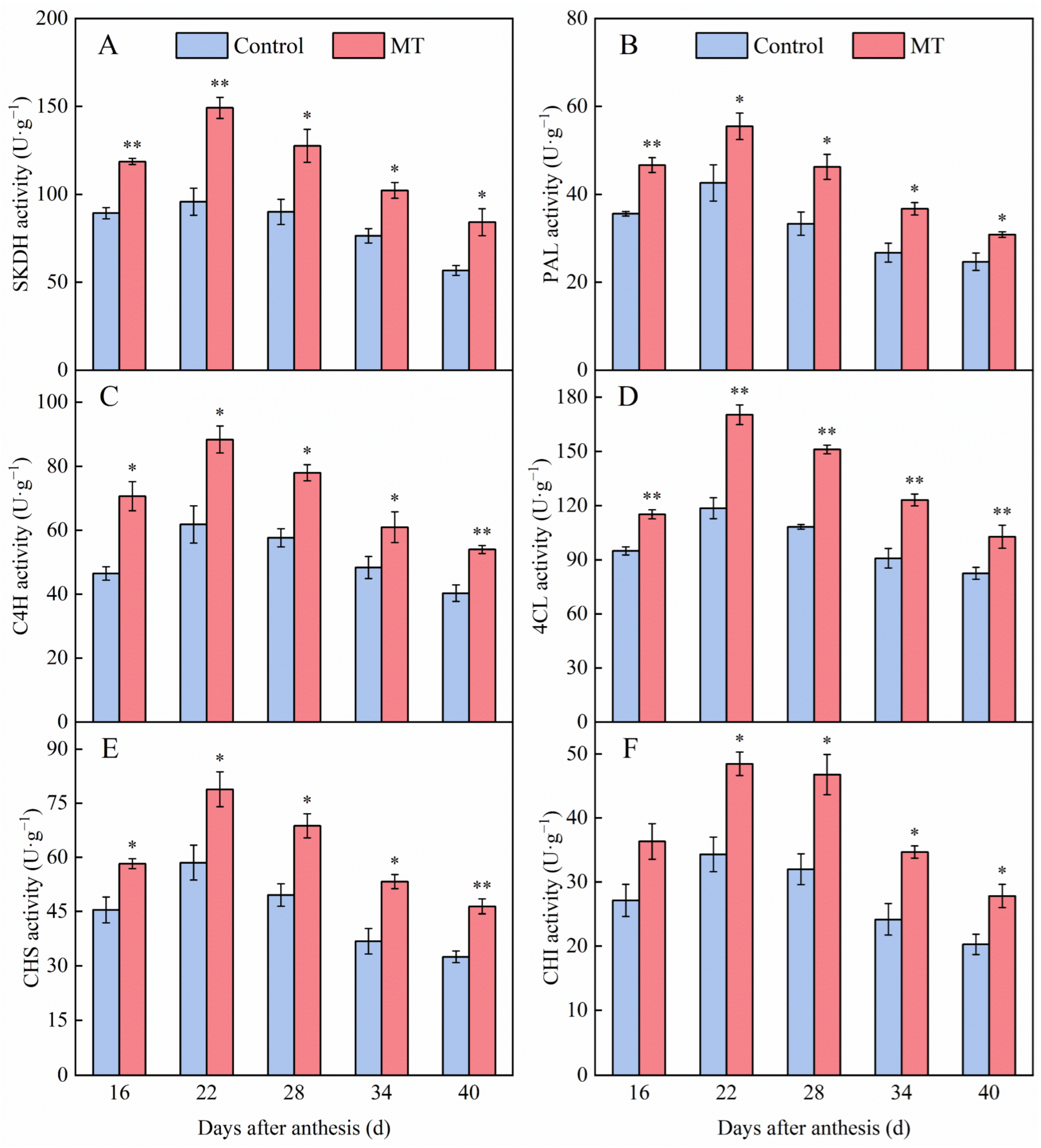
3.3. Effect of Exogenous MT on Enzyme Activities Involved in the Phenylpropane Metabolism in Pepper Fruits
3.4. Effect of Exogenous MT on Genes Expression Involved in the Phenylpropane Metabolism in Pepper Fruits
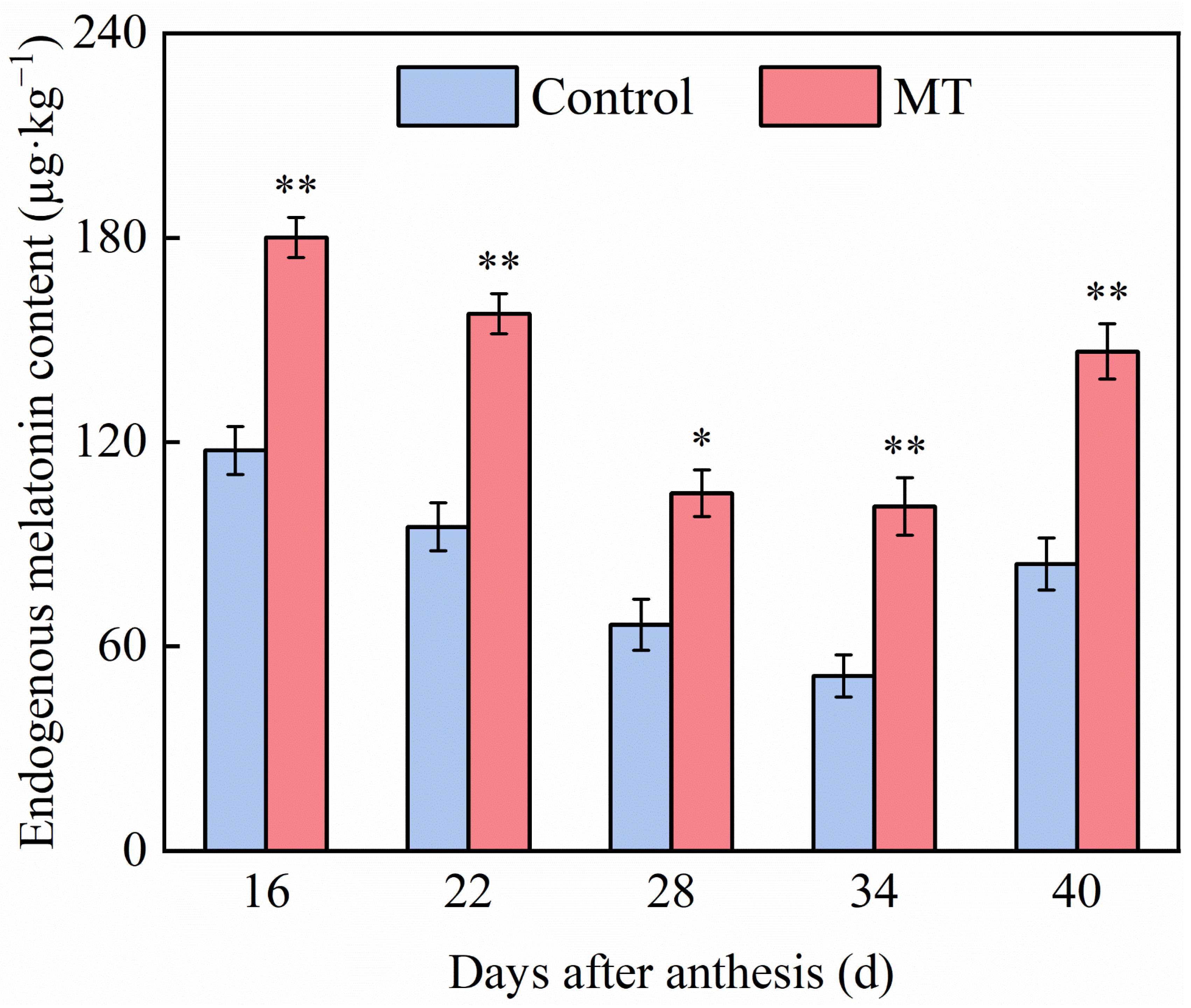
3.5. Effect of Exogenous MT on the Content of Exogenous MT in Pepper Fruits
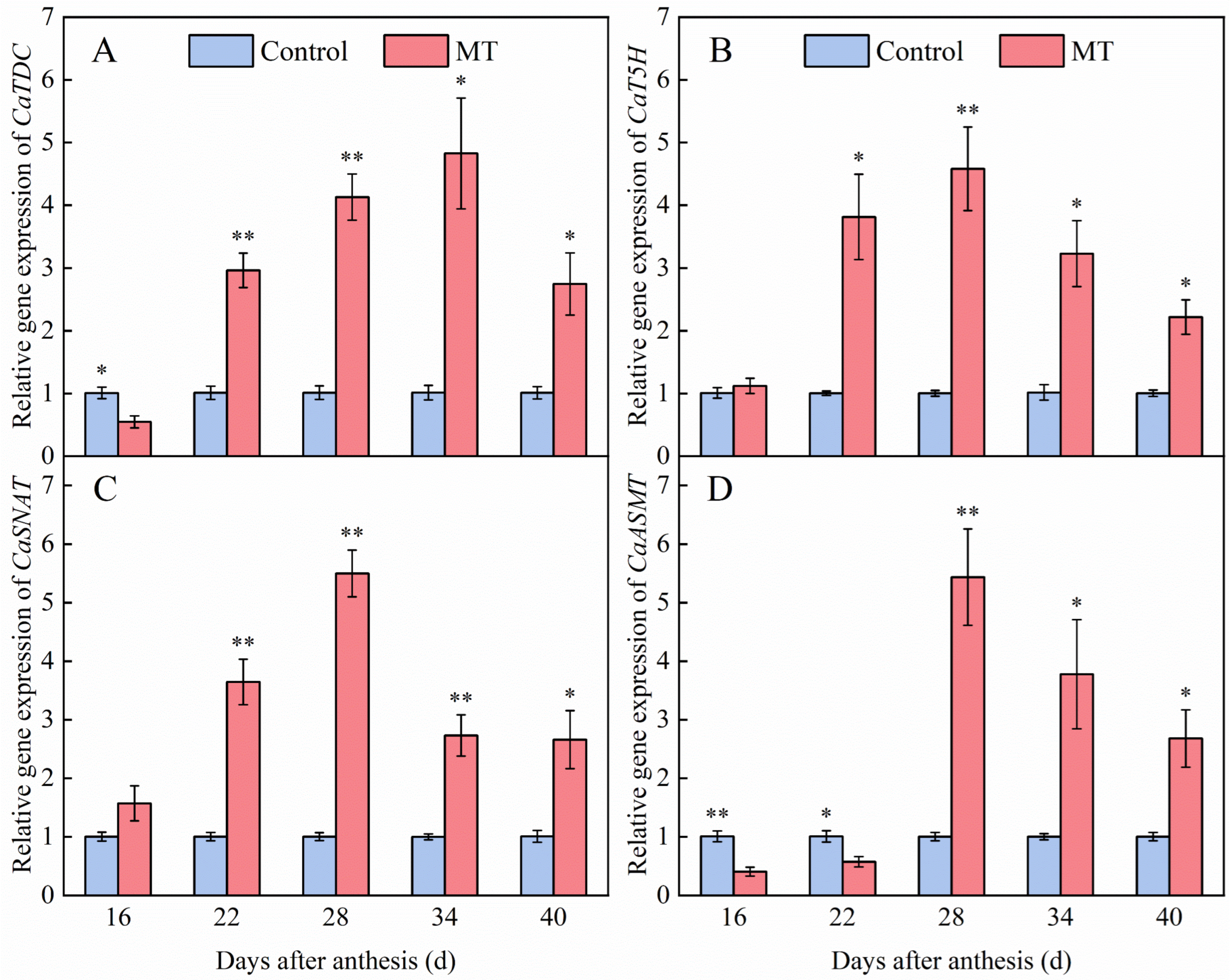
3.6. Effect of Exogenous MT on Genes Expression Involved in the Endogenous MT Synthesis Pathway in Pepper Fruits
3.7. Correlation Analysis
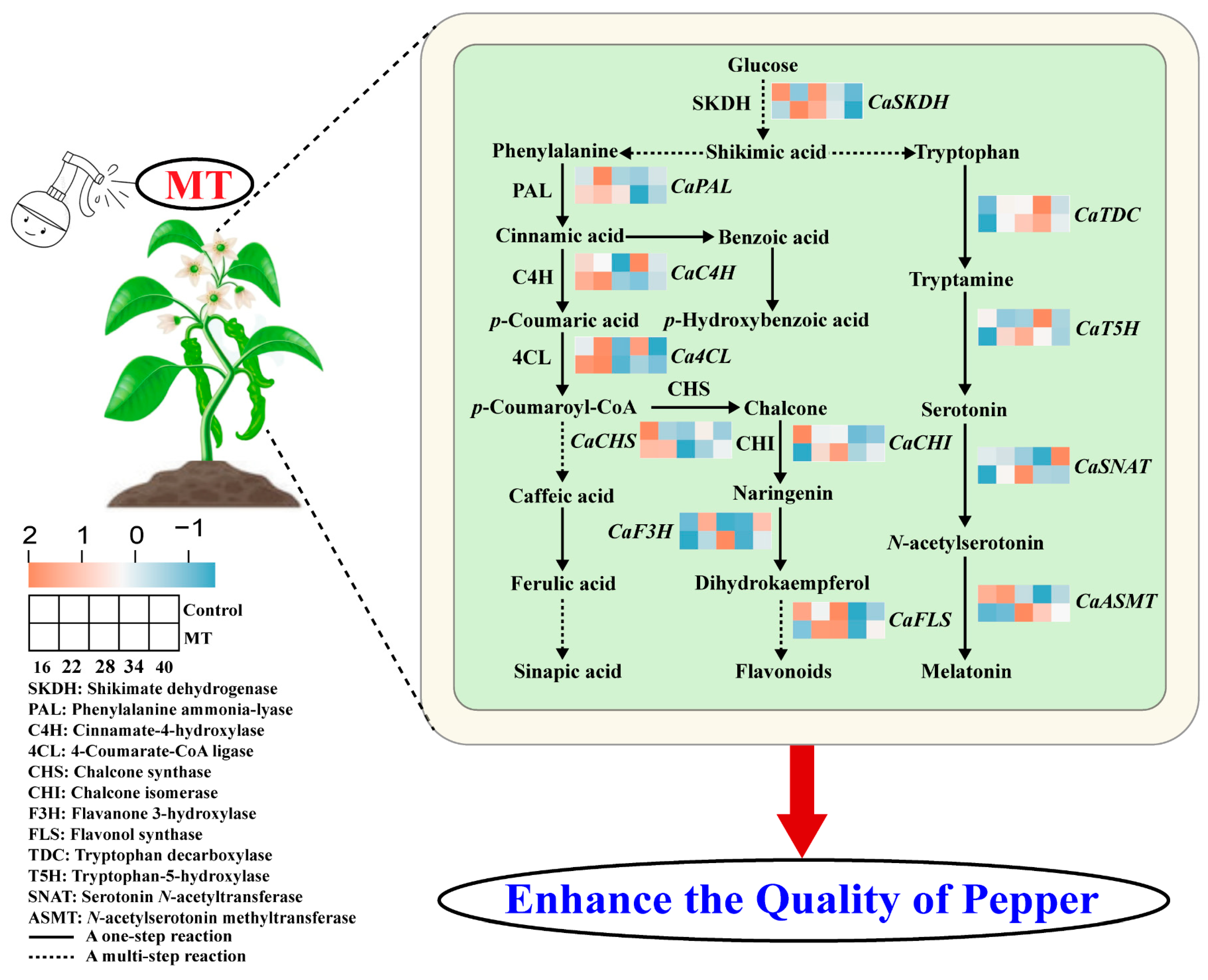
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Wang, X.; Sun, J.; Wang, X.; Zhu, Z.; Ayhan, D.H.; Yi, S.; Yan, M.; Zhang, L.; Meng, T.; et al. Two telo-mere-to-telomere gapless genomes reveal insights into Capsicum evolution and capsaicinoid biosynthesis. Nat. Commun. 2024, 15, 4295. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, T.; Gómez-García, M.d.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An an-cient Latin-American crop with outstanding bioactive compounds and nutraceutical potential—A review. Compr. Rev. Food. Sci. Food Saf. 2020, 9, 2972–2993. [Google Scholar] [CrossRef]
- Bassey, E.J.; Cheng, J.H.; Sun, D.W. Novel nonthermal and thermal pretreatments for enhancing drying performance and improving quality of fruits and vegetables. Trends Food Sci. Technol. 2021, 112, 137–148. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, L.; Yang, Y.; Xie, M.; Tang, Y.; Lai, Y.; Sun, B.; Huang, Z.; Wang, J.; Li, H. CaMYB80 enhances the cold tolerance of pepper by directly targeting CaPOA1. Hortic. Res. 2024, 11, uhae219. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, T.; Deng, Z.; Gamboa, G.G.; Ge, Q.; Xu, P.; Zhang, Q.; Zhang, J.; Meng, J.; Reíter, R.J.; et al. Plant-derived melatonin from food: A gift of nature. Food Funct. 2021, 12, 2829–2849. [Google Scholar] [CrossRef]
- Zeng, H.; Bai, Y.; Wei, Y.; Reíter, R.J.; Shi, H. Phytomelatonin as a central molecule in plant disease resistance. J. Exp. Bot. 2022, 73, 5874–5885. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, M.; Li, H.; Liu, Y.; Fan, S.; Zhang, N.; Guo, Y.D. Strategies and prospects for melatonin to alleviate abiotic stress in horticultural plants. Hortic. Plant J. 2023, 10, 601–614. [Google Scholar] [CrossRef]
- Wang, Q.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reíter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2011, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Tanou, G.; Sarrou, E.; Karagiannis, E.; Ganopoulos, I.; Martens, S.; Molassiotis, A. Pre- and Post-harvest Melatonin Application Boosted Phenolic Compounds Accumulation and Altered Respiratory Characters in Sweet Cherry Fruit. Front. Nutr. 2021, 8, 695061. [Google Scholar] [CrossRef]
- Marak, K.A.; Mir, H.; Singh, P.K.; Siddiqui, M.W.; Ranjan, T.; Singh, D.R.; Siddiqui, M.H.; Irfan, M. Exogenous melatonin delays oxidative browning and improves postharvest quality of litchi fruits. Sci. Hortic. 2023, 322, 112408. [Google Scholar] [CrossRef]
- Rahmanzadeh-Ishkeh, S.; Shirzad, H.; Tofighi, Z.; Fattahi, M.R.; Ghosta, Y. Exogenous melatonin prolongs raspberry postharvest life quality by increasing some antioxidant and enzyme activity and phytochemical contents. Sci. Rep. 2024, 14, 11508. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Fan, Z.; Zeng, Z.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.; Tao, N.; Lakshmanan, P.; Chen, J.; et al. Exogenous melatonin maintains leaf quality of postharvest Chinese flowering cabbage by modulating respiratory metabolism and energy status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Bai, Z.; Ni, J.; Tang, J.; Sun, D.; Yan, Z.; Zhang, J.; Niu, L.; Zhang, Y. Bioactive components, antioxidant and antimicrobial activities of Paeonia rockii fruit during development. Food Chem. 2021, 343, 128444. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, D.; Liu, C.; Chen, C.; Tian, M.; Jiang, A. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Jin, N.; Li, J.; Wang, S.; Meng, X.; Ma, X.; He, X.; Zhang, G.; Luo, S.; Lyu, J.; Yu, J. A Comprehensive Evaluation of Effects on Water-Level Deficits on Tomato Polyphenol Composition, Nutritional Quality and Antioxidant Capacity. Antioxidants 2022, 11, 1585. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hao, W.; Yan, L.; Zhang, H.; Zhang, J.; Liu, C.; Lu, Z. Postharvest melatonin treatment enhanced antioxidant activity and promoted GABA biosynthesis in yellow-flesh peach. Food Chem. 2023, 419, 136088. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, R.C.; Zhu, W.; Shi, Y.; Ni, S.; Wu, C.; Li, T. Impact of dielectric barrier discharge cold plasma on the quality and phenolic metabolism in blueberries based on metabonomic analysis. Postharvest Biol. Technol. 2023, 197, 112208. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, A.; Feng, K.; Gu, S.; Xu, D.; Hu, W. Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biol. Technol. 2019, 157, 110958. [Google Scholar] [CrossRef]
- Ji, N.; Wang, J.; Li, Y.; Li, M.; Jin, P.; Zheng, Y. Involvement of PpWRKY70 in the methyl jasmonate primed disease resistance against Rhizopus stolonifer of peaches via activating phenylpropanoid pathway. Postharvest Biol. Technol. 2021, 174, 111466. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.E.; Baghdadi, A.; Ahmad, I.A. Changes in Phytochemical Synthesis, Chalcone Synthase Activity and Pharmaceutical Qualities of Sabah Snake Grass (Clinacanthus nutans L.) in Relation to Plant Age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sang, W.; Xu, R.; Cao, J. Alteration of flesh color and enhancement of bioactive substances via the stimulation of anthocyanin biosynthesis in ‘Friar’ plum fruit by low temperature and the removal. Food Chem. 2020, 310, 125862. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Reichelt, M.; Yoshida, K.; Gershenzon, J.; Constabel, C.P. Two R2R3-MYB proteins are broad repressors of flavonoid and phenylpropanoid metabolism in poplar. Plant J. 2018, 96, 949–965. [Google Scholar] [CrossRef]
- Vighi, I.L.; Crizel, R.L.; Perin, E.C.; Rombaldi, C.V.; Galli, V. Crosstalk During Fruit Ripening and Stress Response Among Abscisic Acid, Calcium-Dependent Protein Kinase and Phenylpropanoid. Crit. Rev. Plant Sci. 2019, 38, 99–116. [Google Scholar] [CrossRef]
- Curiel, J.A.; de Las Rivas, B.; Rodríguez, H.; Esteban-Torres, M.; Reverón, I.; Santamaría, L.; Landete, J.M.; Plaza-Vinuesa, L.; Sánchez-Arroyo, A.; Jiménez, N.; et al. Food phenolics and Lactiplantibacillus plantarum. Int. J. Food Microbiol. 2024, 412, 110555. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Shang, F.; Liu, R.; Wu, W.; Han, Y.; Fang, X.; Chen, H.; Gao, H. Effects of melatonin on the components, quality and anti-oxidant activities of blueberry fruits. LWT 2021, 147, 111582. [Google Scholar] [CrossRef]
- Yan, R.; Xu, Q.; Dong, J.; Kebbeh, M.; Shuling, S.; Chen, H.; Zheng, X. Effects of exogenous melatonin on ripening and decay incidence in plums (Prunus salicina L. cv. Taoxingli) during storage at room temperature. Sci. Hortic. 2022, 292, 110655. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous Melatonin Treatment Induces Disease Resistance against Botrytis cinerea on Post-Harvest Grapes by Activating Defence Responses. Foods 2022, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Mudrić, S.Ž.; Gašić, U.M.; Dramićanin, A.M.; Ćirić, I.; Opsenica, D.M.; Djordjević, J.P.; Momirović, N.; Tešić, Ž.L. The polyphenolics and carbohydrates as indicators of botanical and geographical origin of Serbian autochthonous clones of red spice paprika. Food Chem. 2017, 217, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, R.; Zhu, F. Physicochemical interactions of maize starch with ferulic acid. Food Chem. 2016, 199, 372–379. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- de Sá Mendes, N.; de Andrade Gonçalves, É.C.B. The role of bioactive components found in peppers. Trends Food Sci. Technol. 2020, 99, 229–243. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods. 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Carvalho, A.V.; de AMattietto, R.; de Oliveira Rios, A.; de Almeida Maciel, R.; Moresco, K.S.; de Souza Oliveira, T.C. Bioactive compounds and antioxidant activity of pepper (Capsicum sp.) genotypes. J. Food Sci. Technol. 2015, 52, 7457–7464. [Google Scholar] [CrossRef]
- Grebenstein, N.; Frank, J. Rapid baseline-separation of all eight tocopherols and tocotrienols by reversed-phase liquid-chromatography with a solid-core pentafluorophenyl column and their sensitive quantification in plasma and liver. J. Chromatogr. A 2012, 1243, 39–46. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Sergio, C.S.A.; Santos, P.D.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum). Ultrason. Sonochem. 2016, 31, 284–294. [Google Scholar] [CrossRef]
- Wang, Z.; Pu, H.; Shan, S.; Zhang, P.; Li, J.; Song, H.; Xu, X. Melatonin enhanced chilling tolerance and alleviated peel browning of banana fruit under low temperature storage. Postharvest Biol. Technol. 2021, 179, 111571. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Shang, Y.; Wang, X.; Du, G.; Lyu, D. Preharvest application of melatonin induces anthocyanin accumulation and related gene upregulation in red pear (Pyrus ussuriensis). J. Integr. Agric. 2021, 20, 2126–2137. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Ban, Z.; Jiang, N.; Yang, M.; Li, L. Role of exogenous melatonin involved in phenolic metabolism of Zizyphus jujuba fruit. Food Chem. 2021, 341, 128268. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Wang, J.; Tang, Z.; Yu, J.; Wu, Y.; Liu, Z.; Wang, J.; Wang, G.; Qiang, T. Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites. Foods 2022, 11, 4097. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Liu, F.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; de Oliveira, M.V.V.; Kleven, B.; Maéda, H. The entry reaction of the plant shikimate pathway is subjected to highly complex metabolite-mediated regulation. Plant Cell 2021, 33, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Zhu, Y.; Zong, Y.; Liang, W.; Ackah, S.; Chai, X.; Li, Y.; Bi, Y.; Prusky, D. β-Aminobutyric acid treatment accelerates the deposition of suberin polyphenolic and lignin at wound sites of potato tubers during healing. Postharvest Biol. Technol. 2021, 179, 111566. [Google Scholar] [CrossRef]
- Niu, Y.; Ye, L.; Wang, Y.; Shi, Y.; Liu, Y.; Luo, A. Transcriptome analysis reveals salicylic acid treatment mitigates chilling injury in kiwifruit by enhancing phenolic synthesis and regulating phytohormone signaling pathways. Postharvest Biol. Technol. 2023, 205, 112483. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Wang, H.; Shu, C.; Chen, L.; Cao, J.; Jiang, W. The combination treatment of chlorogenic acid and sodium alginate coating could accelerate the wound healing of pear fruit by promoting the metabolic pathway of phenylpropane. Food Chem. 2023, 414, 135689. [Google Scholar] [CrossRef]
- Ni, R.; Niu, M.; Fu, J.; Tan, H.; Zhu, T.; Zhang, J.; Lou, H.; Zhang, P.; Li, J.X.; Cheng, A.X. Molecular and structural characterization of a promiscuous chalcone synthase from the fern species Stenoloma chusanum. J. Integr. Plant Biol. 2022, 64, 1935–1951. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Liu, N.; Yan, Z.; Tan, S.; Xu, Y.; Luo, Z. Postharvest MeJA maintains the shelf quality of kiwifruit after cold storage by regulating antioxidant capacity and activating the disease resistance. Postharvest Biol. Technol. 2024, 211, 112827. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Yu, G.; Wang, Q.; Zeng, Q.; Jiang, Z.; Gao, L. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chem. 2019, 286, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Woodward, A.; Yamoah, E.A. Methyl jasmonate advances fruit ripening, colour development, and improves antioxidant quality of ‘Yoho’ and ‘Jiro’ persimmon. Food Chem. 2024, 459, 140360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, T.; Liu, G.; Hu, M.; Yun, Z.; Duan, X.; Cai, K.; Jiang, G. Inhibition of downy blight and enhancement of resistance in litchi fruit by postharvest application of melatonin. Food Chem. 2021, 347, 129009. [Google Scholar] [CrossRef]
- Sharafi, Y.; Jannatizadeh, A.; Fard, J.R.; Aghdam, M.S. Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci. Hortic. 2021, 288, 110304. [Google Scholar] [CrossRef]
- Mansouri, S.; Sarikhani, H.; Sayyari, M.; Aghdam, M.S. Melatonin accelerates strawberry fruit ripening by triggering GAMYB gene expression and promoting ABA accumulation. Sci. Hortic. 2021, 281, 109919. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Arnao, M.B. Phytomelatonin: From Intracellular Signaling to Global Horticulture Market. J. Pineal Res. 2024, 76, e12990. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Pang, L.; Chen, L.; Jiang, Y.; Zhang, C.; Liang, F.; Duan, L. Role of exogenous melatonin in quality maintenance of sweet cherry: Elaboration in links between phenolic and amino acid metabolism. Food Biosci. 2023, 56, 103223. [Google Scholar] [CrossRef]






| Wavelength | 240 nm | 280 nm | 322 nm |
|---|---|---|---|
| Phenolic compounds | Rutin | Benzoic acid | Gentianic acid |
| Protocatechuic acid | Ferulic acid | Sinapic acid | |
| Quercetin | Cinnamic acid | Cynarin | |
| p-Hydroxybenzoic acid | p-Coumaric acid | Kaempferol | |
| Chlorogenic acid | Gallic acid | Caffeic acid |
| Gene Name | Gene ID | 5′-3′ of the Forward Primer | 5′-3′ of the Reverse Primer |
|---|---|---|---|
| CaSKDH | XM_016721816 | GCAGAGGTTGGAGCTACAGTTGTG | CAGGTGCTAATCCGTCGGTGAAC |
| CaPAL | XM_016699298 | GGCGTCGATGGTCCTGTTTGAG | GTGTGTCAGATGGTCCGTGAACTC |
| CaC4H | NM_001325053 | CGGTGAGCATTGGAGGAAGATGAG | CCTCAACAACACTAGCCACCTCAG |
| Ca4CL | NM_001324830 | AGGGACTACAGGGCTACCAAAAGG | AAAGGCAAGCAACACATCAACACG |
| CaCHS | NM_001325005 | ATGGTGACCGTGGAGGAGGTTC | GAAGGAGTCGCCGTGCCAATG |
| CaCHI | XM_047399431 | CCGGCTGGGTCATTGACGATTAG | TCTTTGCGGCAGGTGAAACTCC |
| CaF3H | XM_016722868 | TCATGCTTCCACCTGGCTCTG | GGAGAATAACTCTTCCATCACCTGTC |
| CaFLS | XM_016716195 | GCCTAAGAACCCTCCTTCCTACAG | CCAATCCAAGCCCAAGTGACAAG |
| CaTDC | XM_016725244 | GTGGATTACAAAGACTGGCAAATAGG | TAATGTGACTCTGAAGATTGGCTACG |
| CaT5H | XM_016717872 | CTCAACTCGCCGAACTCATACTC | GTAATATCAGAGCAACCGAAGGAGAG |
| CaSNAT | XM_016697799 | CCTCCATCACCTCAATTCCATCTTC | GCAAACGCTACAGGGCTTCC |
| CaASMT | XM_047403946 | TGGTGGTGATGGAACAACATTAAGG | TGCAACAGAAGCAACATGAGGTAG |
| CaACTIN | XM_016720449 | CTGCTGTCCATCTGCTCTCTGTTG | GTGGTCTCGTATTTGGCCCTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Han, K.; Ma, W.; Zhang, J.; Xie, J. Exogenous Melatonin Application Enhances Pepper (Capsicum annuum L.) Fruit Quality via Activation of the Phenylpropanoid Metabolism. Foods 2025, 14, 1247. https://doi.org/10.3390/foods14071247
Gao F, Han K, Ma W, Zhang J, Xie J. Exogenous Melatonin Application Enhances Pepper (Capsicum annuum L.) Fruit Quality via Activation of the Phenylpropanoid Metabolism. Foods. 2025; 14(7):1247. https://doi.org/10.3390/foods14071247
Chicago/Turabian StyleGao, Feibiao, Kangning Han, Weilan Ma, Jing Zhang, and Jianming Xie. 2025. "Exogenous Melatonin Application Enhances Pepper (Capsicum annuum L.) Fruit Quality via Activation of the Phenylpropanoid Metabolism" Foods 14, no. 7: 1247. https://doi.org/10.3390/foods14071247
APA StyleGao, F., Han, K., Ma, W., Zhang, J., & Xie, J. (2025). Exogenous Melatonin Application Enhances Pepper (Capsicum annuum L.) Fruit Quality via Activation of the Phenylpropanoid Metabolism. Foods, 14(7), 1247. https://doi.org/10.3390/foods14071247






