UPLC-MS/MS Analysis of Hydroxyanthracene Derivatives in Botanical Food Products and Supplements: Surveillance of the Italian Market
Abstract
1. Introduction
1.1. Hydroxyanthracene Derivatives: Botanical Sources, Pharmacological Properties, and Applications
1.2. Safety Concerns and Regulatory Aspects
1.3. Analytical Approaches: State-of-the-Art and Objectives
2. Materials and Methods
2.1. Sampling
2.2. Standards and Reagents
2.3. Sample and Quality Control Preparations
2.4. Instruments and Analytical Conditions
2.5. Validation Design
3. Results and Discussion
3.1. Method Development and Optimization
3.2. Performance Evaluation
3.3. Quantification
3.4. Method Application: Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Safety of Hydroxyanthracene Derivatives for Use in Food. EFSA J. 2018, 16, e05090. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific Opinion on Additional Scientific Data Related to the Safety of Preparations of Rheum palmatum L., Rheum officinale Baill. and Their Hybrids, Rhamnus Purshiana D.C., Rhamnus Frangula L. and Cassia Senna L., Submitted Pursuant to Article 8(4) of Regulation (EC) No 1925/2006. EFSA J. 2024, 22, e8766. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.H. Naturally Occurring Quinones, 1st ed.; Springer Nature Dordrecht: Dordrecht, The Netherlands, 1987; ISBN 978-0-412-26730-7. [Google Scholar]
- Evans, W.C. Chapter 21: Phenols and phenolic glycosides. In Trease and Evans Pharmacognosy, 16th ed.; Saunders Elsevier: London, UK, 2009; pp. 235–249. ISBN 978-0-7020-2934-9. [Google Scholar]
- Puri, B.; Hall, A. Quinones. In Phytochemical Dictionary—A Handbook of Bioactive Compounds from Plants; Routledge: London, UK, 1998; p. 23. ISBN 978-0-429-20460-9. [Google Scholar]
- Fanali, S.; Aturki, Z.; D’Orazio, G.; Rocco, A.; Ferranti, A.; Mercolini, L.; Raggi, M.A. Analysis of Aloe-Based Phytotherapeutic Products by Using Nano-LC-MS. J. Sep. Sci. 2010, 33, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Akao, T.; Kobashi, K.; Namba, T. Cleavages of the O- and C-Glucosyl Bonds of Anthrone and 10,10’-Bianthrone Derivatives by Human Intestinal Bacteria. Pharmacology 2008, 47, 125–133. [Google Scholar] [CrossRef]
- Kim, S.; Pressman, P.; Clemens, R.; Moore, A.; Hamilton, R.; Hayes, A.W. The Absence of Genotoxicity of Aloe Vera Beverages: A Review of the Literature. Food Chem. Toxicol. 2023, 174, 113628. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Brilli, V.; Menniti-Ippolito, F.; Ippoliti, I.; Potenza, S.; Renda, F.; Mazzanti, G.; Vitalone, A.; et al. Adverse Events Related to Herbal Dietary Supplements and Over-the-Counter Medications Containing Laxatives: A 10-Year Update from the Italian Phytovigilance and Pharmacovigilance Systems. Ann. dell’Istituto Super. Sanità 2022, 58, 131–138. [Google Scholar] [CrossRef]
- Thakur, M. Rhubarb (Rheum sp.): A Rare and Endangered Medicinal Plant of the Himalayas. In Advances in Medicinal and Aromatic Plants; Apple Academic Press: New York, NY, USA, 2024; ISBN 978-1-03-268690-5. [Google Scholar]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Marra, M.; Conforti, F.; Lupi, F.R.; Gabriele, D.; Borges, F.; Sinicropi, M.S. Aloe Vera―An Extensive Review Focused on Recent Studies. Foods 2024, 13, 2155. [Google Scholar] [CrossRef]
- Baldi, A.; Sommella, E.; Campiglia, P.; Daglia, M. Aloe Gel-Base Food Products: Chemical, Toxicological, and Regulatory Aspects. Regul. Toxicol. Pharmacol. 2021, 119, 104818. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Soković, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe Vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef]
- Hu, J.; Lloyd, M.; Hobbs, C.; Cox, P.; Burke, K.; Pearce, G.; Streicker, M.A.; Gao, Q.; Frankos, V. Absence of Genotoxicity of Purified Aloe Vera Whole Leaf Dry Juice as Assessed by an in Vitro Mouse Lymphoma Tk Assay and an in Vivo Comet Assay in Male F344 Rats. Toxicol. Rep. 2021, 8, 511–519. [Google Scholar] [CrossRef]
- Ţebrencu, C.E.; Creţu, R.M.; Mitroi, G.R.; Iacob, E.; Ionescu, E. Phytochemical Evaluation and HPTLC Investigation of Bark and Extracts of Rhamnus Frangula Linn. Phytochem. Rev. 2015, 14, 613–621. [Google Scholar] [CrossRef]
- Jothy, S.L.; Torey, A.; Darah, I.; Choong, Y.S.; Saravanan, D.; Chen, Y.; Latha, L.Y.; Deivanai, S.; Sasidharan, S. Cassia Spectabilis (DC) Irwin et Barn: A Promising Traditional Herb in Health Improvement. Molecules 2012, 17, 10292–10305. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Ji, H.; Zhou, X.; Wei, X.; Chen, Y.; Fu, Y.; Ma, Y.; Han, Q.; Sun, Y.; Gao, Y.; et al. Pharmacology, Toxicology, and Metabolism of Sennoside A, A Medicinal Plant-Derived Natural Compound. Front. Pharmacol. 2021, 12, 714586. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the Substantiation of a Health Claim Related to Hydroxyanthracene Derivatives and Improvement of Bowel Function Pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3412. [CrossRef]
- European Commission. Commission Regulation (EU) 2021/468 of 18 March 2021 Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Botanical Species Containing Hydroxyanthracene Derivatives; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Galli, C.L.; Cinelli, S.; Ciliutti, P.; Melzi, G.; Marinovich, M. Aloe-Emodin, a Hydroxyanthracene Derivative, Is Not Genotoxic in an in Vivo Comet Test. Regul. Toxicol. Pharmacol. 2021, 124, 104967. [Google Scholar] [CrossRef]
- Galli, C.L.; Cinelli, S.; Ciliutti, P.; Melzi, G.; Marinovich, M. Lack of in Vivo Genotoxic Effect of Dried Whole Aloe Ferox Juice. Toxicol. Rep. 2021, 8, 1471–1474. [Google Scholar] [CrossRef]
- Nesslany, F.; Simar-Meintières, S.; Ficheux, H.; Marzin, D. Aloe-Emodin-Induced DNA Fragmentation in the Mouse in Vivo Comet Assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 678, 13–19. [Google Scholar] [CrossRef]
- Melzi, G.; Galli, C.L.; Ciliutti, P.; Marabottini, C.; Marinovich, M. Lack of Genotoxicity of Rhubarb (Rhizome) in the Ames and Micronucleus in Vitro Tests. Toxicol. Rep. 2022, 9, 1574–1579. [Google Scholar] [CrossRef]
- Tinti, L.; Cicaloni, V.; Nezi, P.; Isoldi, G.; Etiope, P.; Barlozzini, B.; Pecorari, R.; Salvini, L. Hydroxyanthracene Derivates Citotoxicity: A Differential Evaluation between Single Molecule and Whole Plant Extract. Front. Plant. Sci. 2023, 14, 1166075. [Google Scholar] [CrossRef]
- European Commission PAFF Committees. Summary Report: Sante.Ddg2.g.5(2020)7913268; B.02 Exchange of views and possible opinion of the Committee on a draft Commission Regulation (EU) amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards botanical species containing hydroxyanthracene derivatives; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Malysheva, S.V.; Guillaume, B.; Vanhee, C.; Masquelier, J. Determination of 16 Hydroxyanthracene Derivatives in Food Supplements Using LC-MS/MS: Method Development and Application. Toxins 2024, 16, 505. [Google Scholar] [CrossRef]
- Loschi, F.; Faggian, M.; Sut, S.; Ferrarese, I.; Maccari, E.; Peron, G.; Dall’acqua, S. Development of an LC–DAD–MS-Based Method for the Analysis of Hydroxyanthracene Derivatives in Food Supplements and Plant Materials. Molecules 2022, 27, 1932. [Google Scholar] [CrossRef] [PubMed]
- Fernand, V.E.; Dinh, D.T.; Washington, S.J.; Fakayode, S.O.; Losso, J.N.; van Ravenswaay, R.O.; Warner, I.M. Determination of Pharmacologically Active Compounds in Root Extracts of Cassia Alata L. by Use of High Performance Liquid Chromatography. Talanta 2008, 74, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Genovese, S.; Tammaro, F.; Menghini, L.; Carlucci, G.; Epifano, F.; Locatelli, M. Comparison of Three Different Extraction Methods and HPLC Determination of the Anthraquinones Aloe-Emodine, Emodine, Rheine, Chrysophanol and Physcione in the Bark of Rhamnus alpinus L. (Rhamnaceae). Phytochem. Anal. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Genovese, S.; Carlucci, G.; Kremer, D.; Randic, M.; Epifano, F. Development and Application of High-Performance Liquid Chromatography for the Study of Two New Oxyprenylated Anthraquinones Produced by Rhamnus Species. J. Chromatogr. A 2012, 1225, 113–120. [Google Scholar] [CrossRef]
- Aichner, D.; Ganzera, M. Analysis of Anthraquinones in Rhubarb (Rheum palmatum and Rheum officinale) by Supercritical Fluid Chromatography. Talanta 2015, 144, 1239–1244. [Google Scholar] [CrossRef]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of Phenolic Compounds in Rhubarbs Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef]
- Ni, Y.; Song, R.; Kokot, S. Analysis of HPLC Fingerprints: Discrimination of Raw and Processed Rhubarb Samples with the Aid of Chemometrics. Anal. Methods 2012, 4, 171–176. [Google Scholar] [CrossRef]
- Tang, W.; Wan, M.; Zhu, Z.; Chen, G.; Huang, X. Simultaneous Determination of Eight Major Bioactive Compounds in Dachengqi Tang (DT) by High-Performance Liquid Chromatography. Chin. Med. 2008, 3, 5. [Google Scholar] [CrossRef][Green Version]
- Cao, G.; Chen, X.; Wu, X.; Li, Q.; Zhang, H. Rapid Identif Ication and Comparative Analysis of Chemical Constituents in Herbal Medicine Fufang Decoction by Ultra-High-Pressure Liquid Chromatography Coupled with a Hybrid Linear Ion Trap–High-Resolution Mass Spectrometry. Biomed. Chromatogr. 2015, 29, 698–708. [Google Scholar] [CrossRef]
- Di Minno, A.; Morone, M.V.; Ullah, H.; Sommella, E.; Buccato, D.G.; De Lellis, L.F.; Campiglia, P.; De Filippis, A.; Galdiero, M.; Daglia, M. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)-Based Quantification of Hydroxyanthracene Derivatives in Aloe vera (L.) Burm. f. Gel Commercial Beverages and Preliminary Safety Evaluation through in Vitro Genotoxicity Studies. Food Saf. Health 2024, 2, 489–496. [Google Scholar] [CrossRef]
- Zonta, F.; Bogoni, P.; Masotti, P.; Micali, G. High-Performance Liquid Chromatographic Profiles of Aloe Constituents and Determination of Aloin in Beverages, with Reference to the EEC Regulation for Flavouring Substances. J. Chromatogr. A 1995, 718, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O.; Schmitt, M.; Dekant, W.; Stopper, H.; Schlatter, J.; Schreier, P.; Lutz, W.K. Occurrence of Emodin, Chrysophanol and Physcion in Vegetables, Herbs and Liquors. Genotoxicity and Anti-Genotoxicity of the Anthraquinones and of the Whole Plants. Food Chem. Toxicol. 1999, 37, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Petrić, J.; Šarkanj, B.; Mujić, I.; Mujić, A.; Sulyok, M.; Krska, R.; Šubarić, D.; Jokić, S. Effect of Pretreatments on Mycotoxin Profiles and Levels in Dried Figs. Arch. Ind. Hyg. Toxicol. 2019, 69, 328–333. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, Y.; Sun, A.; Gao, B.; Sun, C.; Xiong, J. Validation of a Rapid and Simple High-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry Method for Simultaneous Analysis of 15 Key Chemicals in Slimming Foods and Herbal Products. J. Chromatogr. Sci. 2018, 56, 912–919. [Google Scholar] [CrossRef]
- Wang, P.G.; Zhou, W.; Wamer, W.; Krynitsky, A.; Rader, J. Simultaneous Determination of Aloin A and Aloe Emodin in Products Containing Aloe Vera by Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry. Anal. Methods 2012, 4, 3612–3619. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Gul, W.; Avula, B.; Khan, I.A. Determination of the Anthraquinones Aloe-Emodin and Aloin-A by Liquid Chromatography with Mass Spectrometric and Diode Array Detection. J. AOAC Int. 2007, 90, 28–42. [Google Scholar] [CrossRef]
- ChemDraw—Revvity Signals Software. Software Version 23.1.1. Available online: https://revvitysignals.com/products/research/chemdraw (accessed on 29 January 2025).
- Sibhat, G.; Kahsay, G.; Van Schepdael, A.; Adams, E. Fast and Easily Applicable LC-UV Method for Analysis of Bioactive Anthrones from Aloe Leaf Latex. J. Pharm. Biomed. Anal. 2021, 195, 113834. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2023/2783 of 14 December 2023 Laying Down the Methods of Sampling and Analysis for the Control of the Levels of Plant Toxins in Food and Repealing Regulation (EU) 2015/705; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- 4WUR EURL-MP-Guidance Doc_003 (Version 1.1) Guidance Document on Performance Criteria (Draft 17 September 2021). Available online: https://www.wur.nl/en/research-results/research-institutes/food-safety-research/reference-laboratory/european-union-reference-laboratory/eurl-mycotoxins-plant-toxins/library-eurl-mp.htm (accessed on 17 January 2025).
- Ding, W.; Wu, X.; Zhong, J.; Wan, J. Effects of Temperature, PH and Light on the Stability of Aloin A and Characterisation of Its Major Degradation Products. Int. J. Food Sci. Technol. 2014, 49, 1773–1779. [Google Scholar] [CrossRef]
- Migues, V.H.; Mauricio, J.; Gomes, A.F.; David, J.P. Determination of Anthraquinones in Rhamnus Purshiana Using High-Performance Liquid Chromatography Coupled to Diode Array Detector and Simple Ultraviolet Spectroscopic Analysis. J. Sep. Sci. 2022, 45, 2478–2487. [Google Scholar] [CrossRef]
- Zwerger, M.; Deisl, A.; Hammerle, F.; Ganzera, M. Determination of Anthraquinones in Frangula Alnus by Supercritical Fluid Chromatography. J. Chromatogr. A 2024, 1737, 465432. [Google Scholar] [CrossRef]
- Mulder, P. EURL-MP-Report_002 Inventory Analytical Methods Hydroxyanthracene Derivatives. Available online: https://www.wur.nl/en/research-results/research-institutes/food-safety-research/reference-laboratory/european-union-reference-laboratory/eurl-mycotoxins-plant-toxins/library-eurl-mp.htm (accessed on 24 January 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- European Commission Health and Food Safety Directorate-General (DG SANTE). Guidance Document on Identification of Mycotoxins in Food and Feed. SANTE/12089/2016; European Commission DG SANTE: Bruxelles, Belgium; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Taoheed, A.; Tolulope, A.; Saidu, A.; Odewumi, O.; Sunday, R.M.; Usman, M. Phytochemical Properties, Proximate and Mineral Composition of Curcuma Longa Linn. and Zingiber Officinale Rosc.: A Comparative Study. J. Sci. Res. Rep. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Annapurna, A.S.; Abhirami, D.; Umesh, T.G. Comparative Study of Phytochemicals and Bioactivities of the Leaf Extracts of Curcuma Amada and Curcuma Karnatakensis. S. Afr. J. Bot. 2021, 142, 441–450. [Google Scholar] [CrossRef]
- Oghenejobo, M. Antibacterial Evaluation, Phytochemical Screening and Ascorbic Acid Assay of Turmeric (Curcuma longa). MOJ Bioequivalence Bioavailab. 2017, 4, 00063. [Google Scholar] [CrossRef]
- Kumari, P.; Kumari, C.; Singh, P.S. Phytochemical Screening of Selected Medicinal Plants for Secondary Metabolites. Int. J. Life-Sci. Sci. Res. 2017, 3, 1151–1157. [Google Scholar] [CrossRef]
- Wang, G.; Wang, G.; Liu, J.; Yu, B.; Wang, F.; Liu, J. Studies on the chemical constituents of Kalimeris indica. Zhong Yao Cai—J. Chin. Med. Mater. 2010, 33, 551–554. [Google Scholar]
- Abdallah, M.F.; Krska, R.; Sulyok, M. Occurrence of Ochratoxins, Fumonisin B2, Aflatoxins (B1 and B2), and Other Secondary Fungal Metabolites in Dried Date Palm Fruits from Egypt: A Mini-Survey. J. Food Sci. 2018, 83, 559–564. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Mariscal-Domínguez, M.F.; Cruz-Flores, P.; Campas-Baypoli, O.N.; Cantú-Soto, E.U.; Sanches-Silva, A. An HPLC Procedure for the Quantification of Aloin in Latex and Gel from Aloe Barbadensis Leaves. J. Chromatogr. Sci. 2017, 55, 251–257. [Google Scholar] [CrossRef]
- Girreser, U.; Ugolini, T.; Çiçek, S.S. Quality Control of Aloe vera (Aloe barbadensis) and Aloe ferox Using Band-Selective Quantitative Heteronuclear Single Quantum Correlation Spectroscopy (Bs-QHSQC). Talanta 2019, 205, 120109. [Google Scholar] [CrossRef]

| Genus | Botanical Species | Common Names | Family | Anthraquinones | Parts Used |
|---|---|---|---|---|---|
| Aloe L. | Aloe Vera L. or Aloe barbadensis Miller, Aloe ferox Miller Aloe africana Mill. | Barbados aloe, cape aloe, African aloe | Aloaceae | Aloe-emodin, aloin A And aloin B | Leaf, leaf gel |
| Rheum L. | Rheum palmatum L., Rheum officinale Baillon | Chinese rhubarb | Polygonaceae | Emodin, palmidin C, rhein, sennoside A, sennoside B | Root and rhizome |
| Cassia L. | Cassia fistula L., Cassia angustifolia M. Vahl or Senna alexandrina Mill. or Cassia senna L. | Purging cassia, Alexandrian senna | Fabaceae/Leguminosae | Chrysophanol, physcion, rhein, sennoside B | Leaf and fruit |
| Senna Mill. | Senna occidentalis L. or Cassia occidentalis L. | Septicweed | Aloe-emodin, emodin, emodin anthrone and physcion | Bark, leaf and seed | |
| Frangula Mill., Rhamnus L. | Frangula alnus Mill., or Rhamnus frangula L. | Frangula | Rhamnaceae | Emodin anthrone, glucofrangulin A, glucofrangulin B and palmidin C | Bark |
| Rhamnus purshiana D.C. | Cascara buckthorn, sacred bark | Cascarosides, aloe-emodin and emodin | Bark |
| Analyte | Molecular Formula | Chemical Structure 1 | CAS Number |
|---|---|---|---|
| Aloin A | C21H22O9 |  | 1415-73-2 |
| Aloin B | C21H22O9 | 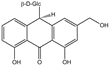 | 28371-16-6 |
| Emodin | C15H10O5 | 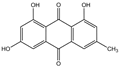 | 518-82-1 |
| Aloe-emodin | C15H10O5 | 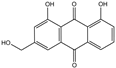 | 481-72-1 |
| Danthron (1,8-Dihydroxyanthraquinone) | C14H8O4 | 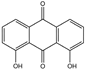 | 117-10-2 |
| Danthron-D4 (1,8-Dihydroxyanthraquinone-D4) | C14H4D4O4 | 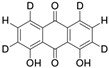 | Not assigned |
| MS/MS Parameters | Conditions |
|---|---|
| Ionization mode | ESI+/ESI− |
| Capillary voltage | 2.0 kV (ESI+)/2.0 kV (ESI−) |
| Cone voltage | +40 V/−40 V |
| Source temperature | 150 °C |
| Desolvation temperature | 600 °C |
| Desolvation gas flow | (N2) = 1000 L/h |
| Collision gas flow | (Ar) = 0.17 mL/min |
| HADs | Retention Time (min) | Precursor Ion (m/z) | Product Ion (m/z) Q/q 1 | CE | Ionization Mode |
|---|---|---|---|---|---|
| Aloin B | 2.80 | 417 [M−H]− | 279/279 | 36/20 | ESI (−) |
| Aloin A | 2.86 | 417 [M−H]− | 279/279 | 36/20 | ESI (−) |
| Aloe-emodin | 3.71 | 269 [M−H]− | 211/240 | 20/20 | ESI (−) |
| Emodin | 4.10 | 269 [M−H]− | 197/225 | 20/20 | ESI (−) |
| Danthron | 4.25 | 241 [M+H]+ | 121/139 | 25/35 | ESI (+) |
| Danthron-D4 | 4.25 | 245 [M+H]+ | 143 | 40 | ESI (+) |
| Sample No | Sample Type | Aloin A | Aloin B | Sum: Aloin A + B | Aloe Emodin | Emodin | Danthron |
|---|---|---|---|---|---|---|---|
| 7 | Aloe beverage | 0.5 | 0.5 | 1.0 | <LOQ 1 | <LOQ | <LOQ |
| 18 | Solid food supplement | 29.5 | 29.9 | 59.4 | <LOQ | 19.3 | <LOQ |
| 28 | Solid food supplement | 742.3 | 385.9 | 1128.2 | 23.1 | 222.6 | <LOQ |
| 29 | Solid food supplement | 881.2 | 471.7 | 1352.9 | 27.5 | 259.7 | <LOQ |
| 30 | Solid food supplement | 28.2 | 23.2 | 51.4 | 0.9 | 8.2 | <LOQ |
| 32 | Solid food supplement | <LOQ | <LOQ | <LOQ | 213.4 | 43.1 | <LOQ |
| 33 | Solid food supplement | <LOQ | <LOQ | <LOQ | 0.8 | 36.4 | <LOQ |
| 34 | Solid food supplement | <LOQ | <LOQ | <LOQ | <LOQ | 1.0 | <LOQ |
| 35 | Solid food supplement | <LOQ | <LOQ | <LOQ | 3.0 | <LOQ | <LOQ |
| 36 | Solid food supplement | 66.4 | 35.4 | 101.8 | 5.9 | 51.4 | <LOQ |
| 38 | Herbal infusion | <LOQ | <LOQ | <LOQ | 23.5 | 104.4 | <LOQ |
| 39 | Herbal infusion | <LOQ | <LOQ | <LOQ | <LOQ | 2.0 | <LOQ |
| 41 | Herbal infusion | <LOQ | <LOQ | <LOQ | 12.5 | 4.1 | <LOQ |
| 43 | Herbal infusion | <LOQ | <LOQ | <LOQ | 24.2 | 6.3 | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peloso, M.; Capriotti, A.; Accurso, D.; Butovskaya, E.; Fedrizzi, G.; Caprai, E. UPLC-MS/MS Analysis of Hydroxyanthracene Derivatives in Botanical Food Products and Supplements: Surveillance of the Italian Market. Foods 2025, 14, 1229. https://doi.org/10.3390/foods14071229
Peloso M, Capriotti A, Accurso D, Butovskaya E, Fedrizzi G, Caprai E. UPLC-MS/MS Analysis of Hydroxyanthracene Derivatives in Botanical Food Products and Supplements: Surveillance of the Italian Market. Foods. 2025; 14(7):1229. https://doi.org/10.3390/foods14071229
Chicago/Turabian StylePeloso, Mariantonietta, Alessandro Capriotti, Damiano Accurso, Elena Butovskaya, Giorgio Fedrizzi, and Elisabetta Caprai. 2025. "UPLC-MS/MS Analysis of Hydroxyanthracene Derivatives in Botanical Food Products and Supplements: Surveillance of the Italian Market" Foods 14, no. 7: 1229. https://doi.org/10.3390/foods14071229
APA StylePeloso, M., Capriotti, A., Accurso, D., Butovskaya, E., Fedrizzi, G., & Caprai, E. (2025). UPLC-MS/MS Analysis of Hydroxyanthracene Derivatives in Botanical Food Products and Supplements: Surveillance of the Italian Market. Foods, 14(7), 1229. https://doi.org/10.3390/foods14071229





