Evaluation of Commercially Available Products of Cannabis sativa L. Inflorescences to Identify Their Contents of Elemental and Phenolic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Material and Extraction Procedure
2.3. Analysis of Phenolic Compounds, Total Phenolics, and Other Analytes
2.4. Antioxidant Activity
2.5. Statistical Analysis
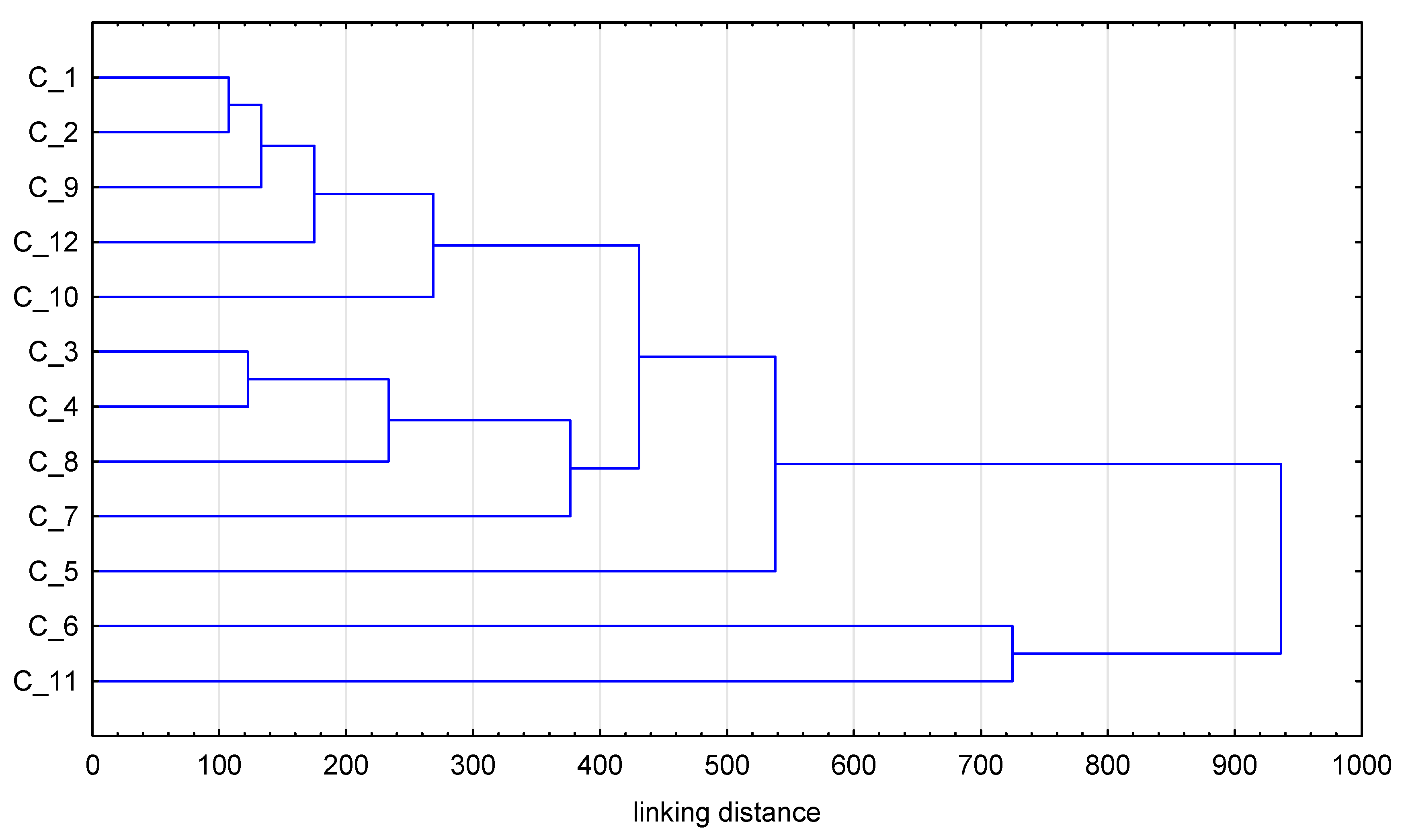
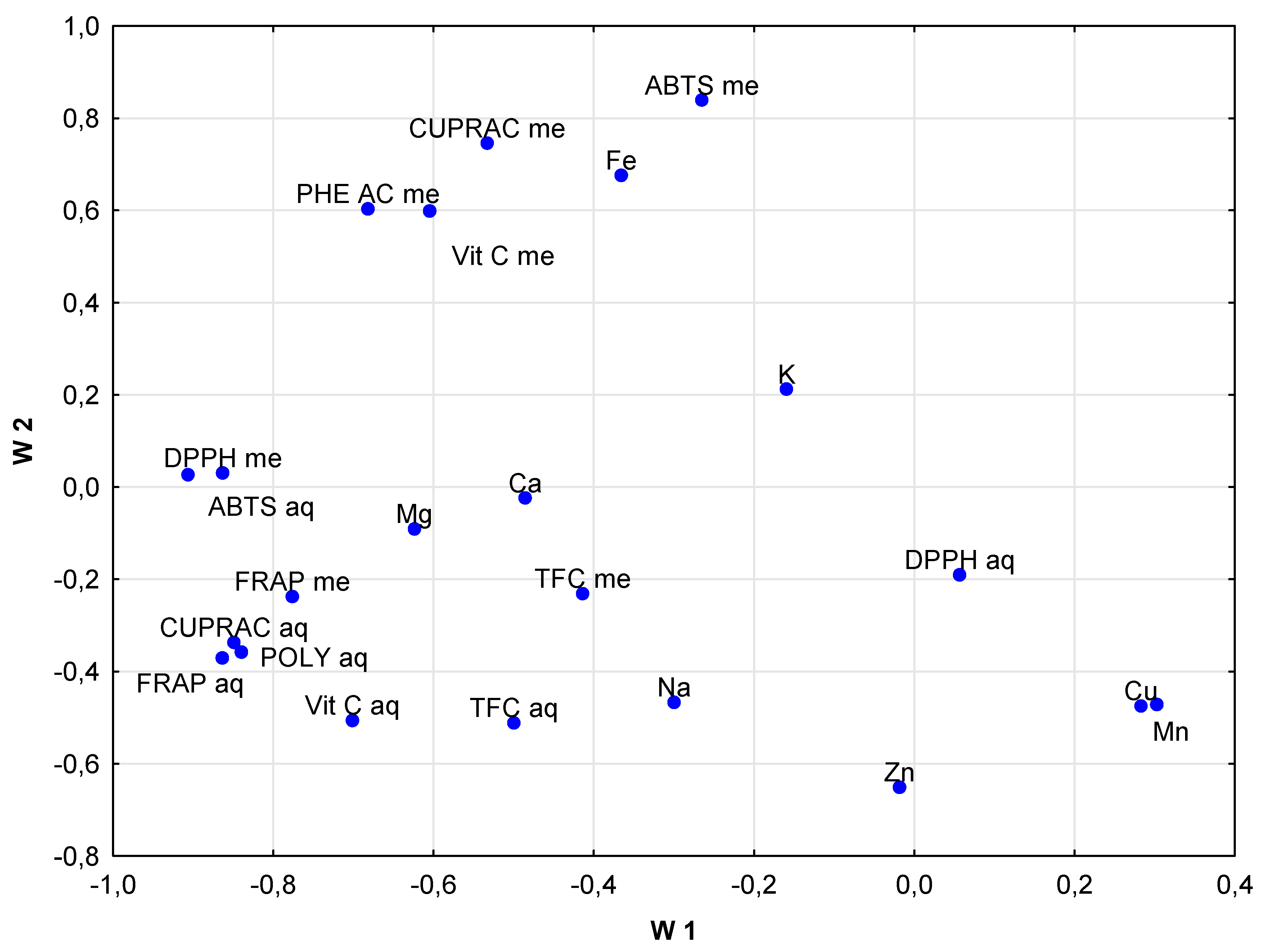
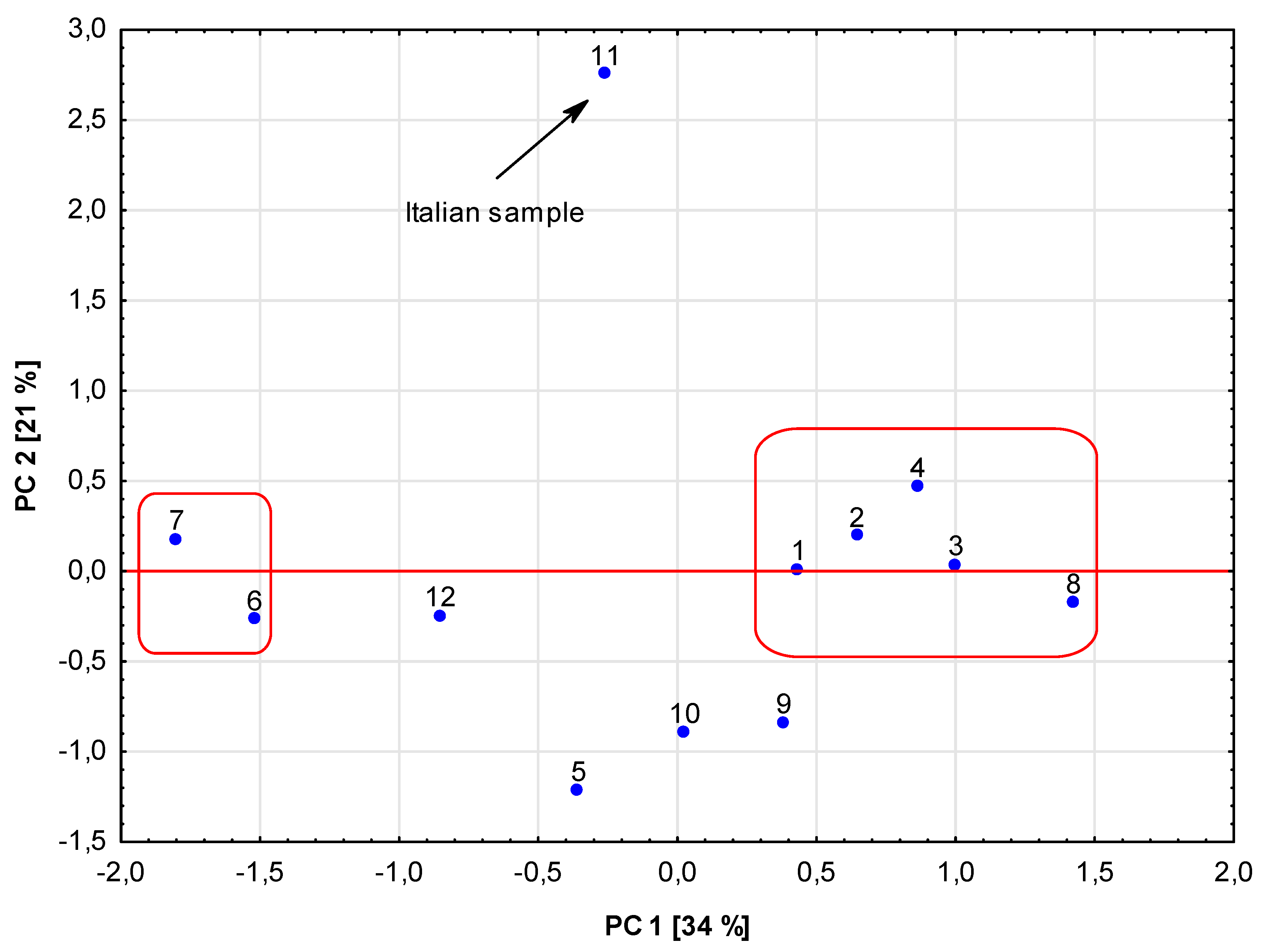
3. Results and Discussion
3.1. Elements
3.2. Phenolic Composition
3.3. Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High-Performance Liquid Chromatography |
| FAAS | Flame Atomic Absorption Spectrometry |
| FRAP | Ferric-Reducing Antioxidant Power |
| CUPRAC | Cupric-Reducing Antioxidant Capacity |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| ABTS | Radical-Scavenging Activity |
| DW | Dry Weight |
References
- Hanuš, L.O. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [PubMed]
- Procaccia, S.; Lewitus, G.M.; Lipson Feder, C.; Shapira, A.; Berman, P.; Meiri, D. Cannabis for Medical Use: Versatile Plant Rather Than a Single Drug. Front. Pharmacol. 2022, 13, 894960. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2017, 25, 1160–1185. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis sativa L. Cultivated at Three Sowing Densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. inflorescences using UHPLC-Q-Orbitrap HRMs. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Serventi, L.; Flores, G.A.; Cusumano, G.; Barbaro, D.; Tirillini, B.; Venanzoni, R.; Angelini, P.; Acquaviva, A.; Di Simone, S.C.; Orlando, G.; et al. Comparative Investigation of Antimicrobial and Antioxidant Effects of the Extracts from the Inflorescences and Leaves of the Cannabis sativa L. cv. strawberry. Antioxidants 2023, 12, 219. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Sip, S.; Szulc, P.; Walkowiak, J.; Cielecka-Piontek, J. The Antioxidant and Neuroprotective Potential of Leaves and Inflorescences Extracts of Selected Hemp Varieties Obtained with scCO2. Antioxidants 2023, 12, 1827. [Google Scholar] [CrossRef]
- Ahmed, M.; Ji, M.; Qin, P.; Gu, Z.; Liu, Y.; Sikandar, A.; Iqbal, M.F.; Javeed, A. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979. [Google Scholar] [CrossRef]
- Saxena, A.; Puranik, N. Phytochemistry and Antiperoxidative Potential of Cannabis sativa L. Leaves Methanol Extracts: An In Vitro Study. J. Drug Deliv. Therap. 2023, 13, 110–116. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Pinela, J.; Ćirić, A.; Calhelha, R.C.; Sokovič, M.; Ferreira, I.C.F.R.; Barros, L.; Torija-Isasa, E.; de Cortes Sanchez-Mata, M. Chemical composition and biological activities of whole and dehulled hemp (Cannabis sativa L.) seeds. Food Chem. 2022, 374, 131754. [Google Scholar] [CrossRef] [PubMed]
- Benkirane, C.; Ben Moumen, A.; Fauconnier, M.L.; Belhaj, K.; Abid, M.; Caid, H.S.; Elamrani, A.; Mansouri, F. Bioactive compounds from hemp (Cannabis sativa L.) seeds: Optimization of phenolic antioxidant extraction using simplex lattice mixture design and HPLC-DAD/ESI-MS2 analysis. RSC Adv. 2022, 12, 25764–25777. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Kornpointner, C.; Sainz Martinez, A.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, K.; Halbwirt, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots. Ind. Crops Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as antioxidant agents and their intervention abilities in antioxidant action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of phenolic compounds and terpenes from Cannabis sativa L. By-products: From conventional to intensified processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.G.; Komnou, A.A.; Vasilou, C. Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis Sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Milan, J.; Michalska, A.; Jurowski, K. The comprehensive review about elements accumulation in industrial hemp (Cannabis sativa L.). Food Chem. Toxicol. 2024, 184, 114344. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Kasiotis, K.M.; Nisianakis, P.; Machera, K. Macro and Trace Elements in Hemp (Cannabis sativa L.) Cultivated in Greece: Risk Assessment of Toxic Elements. Front. Chem. 2021, 9, 654308. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Konieczynski, P.; Orhan, I.E.; Abaci, N.; Viapiana, A. Chemical Composition, Antioxidant and Anti-Enzymatic Activity of Golden Root (Rhodiola rosea L.) Commercial Samples. Antioxidants 2022, 11, 919. [Google Scholar] [CrossRef]
- Konieczynski, P.; Zarkov, A.; Viapiana, A.; Chrubczynska, A.; Mpandzo, E.; Wesolowski, M. Studies on the chemical composition of plants used in traditional medicine in Congo. Open Chem. 2022, 20, 370–378. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessi, M.A. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Mendel, P.; Vyhnánek, T.; Braidot, E.; Filippi, A.; Trojan, V.; Bjelkova, M.; Vaverkova, M.D.; Adamcova, D.; Zloch, J.; Brtnicky, M.; et al. Fiber Quality of Hemp (Cannabis sativa L.) Grown in Soil Irrigated by Landfill Leachate Water. J. Nat. Fibers 2022, 19, 3288–3299. [Google Scholar] [CrossRef]
- Corrado, G.; Pannico, A.; Zarrelli, A.; Kyriacou, M.C.; De Pascale, S.; Rouphael, Y. Macro and trace element mineral composition of six hemp varieties grown as microgreens. J. Food Comp. Anal. 2022, 114, 104750. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Torija-Isasa, M.E.; de Cortes Sánchez-Mata, M. Mineral elements and related antinutrients, in whole and hulled hemp (Cannabis sativa L.) seeds. J. Food Comp. Anal. 2022, 109, 104516. [Google Scholar] [CrossRef]
- Bhatt, L.R.; Dawadi, P.; Syangtan, G.; Siddiqui, M.A.; Lama, B.; Nepal, K.; Joshi, D.R.; Bhatt, L.R. Nutritional value and antioxidant properties of Cannabis seeds from Makwanpur district of central Nepal. Sci. World 2022, 15, 103–112. [Google Scholar] [CrossRef]
- Aazza, S. Application of Multivariate Optimization for Phenolic Compounds and Antioxidants Extraction from Moroccan Cannabis sativa Waste. J. Chem. 2021, 2021, 9738656. [Google Scholar] [CrossRef]
- Cásedas, G.; Moliner, C.; Maggi, F.; Mazzara, E.; Lopez, V. Evaluation of two different Cannabis sativa L. extracts as antioxidant and neuroprotective agents. Front Pharmacol. 2022, 13, 1009868. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.W.; Poudineh, Z.; Shearer, M.; Taylor, N.; MacPherson, S.; Raghavan, V.; Orsat, V.; Lefsrud, M. Relationship between Total Antioxidant Capacity, Cannabinoids and Terpenoids in Hops and Cannabis. Plants 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
| No | Sample name on the Package | Morphological Part | Origin | Cultivar |
|---|---|---|---|---|
| 1 | Konopie z Mazur Hemp tea | Inflorescence 80%/leaves 10%/seeds 10% | Poland | Santica |
| 2 | Koyi Hemp tea | Leaves 50%/inflorescence 50% | unknown | unknown |
| 3 | Carun Young hemp leaves | Leaves 100% | Czech Republic | unknown |
| 4 | Konopie na Maksa Hemp tea | Leaves 50%/inflorescence 50% | unknown | unknown |
| 5 | Marysieńka Hemp tea | Leaves 25%/inflorescence 65% and seeds 10% | Poland | unknown |
| 6 | Konopie Hemp tea | Inflorescence 50%/leaves 50% | unknown | unknown |
| 7 | Herbatka Konopna Hemp tea | Inflorescence 100% | unknown | unknown |
| 8 | Herbatka Konopna Hemp tea | Inflorescence 100% | Poland | Finola |
| 9 | Cannabis sativa | Aerial parts 100% | Lithuania | Futura |
| 10 | Cannabis sativa | Aerial parts 100% | Poland | Fibror |
| 11 | Cannabis sativa | Aerial parts 100% | Italy | Enctalina |
| 12 | Cannabis sativa | Aerial parts 100% | Poland | Felina |
| No. | Fe | Mn | Zn | Cu | Na | Mg | Ca | K |
|---|---|---|---|---|---|---|---|---|
| 1 | 202.15 ± 2.61 e | 54.25 ± 0.18 d | 44.42 ± 0.25 e | 2.60 ± 0.07 ac | 135.0 ± 0.30 i | 5.6 ± 0.05 b | 54.2 ± 0.50 a | 29.9 ± 0.20 k |
| 2 | 172.84 ± 0.78 a | 88.24 ± 0.14 g | 39.57 ± 0.07 b | 4.55 ± 0.07 abc | 76.0 ± 0.90 e | 5.4 ± 0.04 c | 49.5 ± 0.51 g | 16.0 ± 0.14 g |
| 3 | 190.35 ± 1.13 d | 106.45 ± 0.18 h | 54.51 ± 0.25 c | 4.11 ± 0.07 abc | 113.3 ± 1.50 g | 5.4 ± 0.04 ac | 30.7 ± 0.33 e | 9.3 ± 0.08 c |
| 4 | 190.35 ± 2.45 a | 36.75 ± 0.24 a | 43.85 ± 0.40 b | 7.78 ± 0.12 b | 41.0 ± 0.10 c | 6.4 ± 0.05 d | 58.9 ± 0.55 cd | 16.4 ± 0.13 h |
| 5 | 176.87 ± 2.53 a | 167.15 ± 0.18 k | 98.84 ± 0.18 i | 5.72 ± 0.37 ab | 221.0 ± 0.50 a | 6.6 ± 0.06 ef | 71.1± 0.62 h | 18.1 ± 0.16 i |
| 6 | 723.03 ± 1.45 b | 55.32 ± 0.19 c | 55.32 ± 0.19 g | 7.13 ± 0.07 ab | 130.5 ± 0.50 h | 5.5 ± 0.05 ab | 36.3 ± 0.32 b | 10.7 ± 0.05 d |
| 7 | 180.34 ± 0.26 a | 37.57 ± 0.32 b | 25.52 ± 0.14 d | 4.86 ± 0.07 abc | 343.5 ± 0.40 k | 8.8 ± 0.07 i | 74.9± 0.67 i | 14.4 ± 0.10 e |
| 8 | 122.44 ± 1.30 c | 136.78 ± 0.21 j | 28.83 ± 0.07 a | 22.68 ± 0.07 d | 230.2 ± 0.20 j | 5.5 ± 0.04 ab | 37.2 ± 0.32 b | 15.0 ± 0.11 g |
| 9 | 138.19 ± 1.91 b | 128.48 ± 0.18 i | 84.51 ± 0.29 h | 22.29 ± 0.46 d | 87.6 ± 0.50 f | 6.7 ± 0.05 f | 44.0 ± 0.41 f | 3.7 ± 0.03 a |
| 10 | 252.64 ± 1.42 f | 204.18 ± 2.55 l | 59.93 ± 0.39 c | 32.97 ± 0.77 e | 220.5 ± 0.50 a | 6.5 ± 0.06 de | 55.9 ± 0.54 ac | 7.0 ± 0.04 b |
| 11 | 937.91 ± 5.85 a | 83.55 ± 0.28 f | 30.92 ± 0.20 a | 1.20 ± 0.07 c | 10.3 ± 0.60 b | 6.2 ± 0.06 g | 54.1 ± 0.49 a | 20.1 ± 0.10 j |
| 12 | 137.25 ± 0.43 b | 70.79 ± 0.14 e | 51.67 ± 0.18 f | 3.73 ± 0.03 abc | 55.5 ± 0.50 d | 8.5 ± 0.07 h | 61.0 ± 0.56 d | 39.0 ± 0.22 l |
| The mean n = 12 | 285.90 ± 1.84 | 97.50 ± 0.40 | 51.67 ± 0.22 | 10.00 ± 0.19 | 138.7 ± 0.54 | 6.4 ± 0.05 | 52.3 ± 0.49 | 16.6 ± 0.11 |
| TPC (mg GEA/g DW) | TFC (µg QE/g DW) | TPAC (µg CA/g DW) | AA (mg AA/g DW) | |
| Hydromethanolic extracts | ||||
| 1 | 6.19 ± 0.51 abc | 402.68 ± 10.46 cd | 1679.87 ± 273.39 a | 5.16 ± 0.74 a |
| 2 | 7.27 ± 1.30 abcd | 437.70 ± 51.16 de | 2167.29 ± 527.83 a | 7.29 ± 0.84 bc |
| 3 | 5.82 ± 0.21ab | 308.11 ± 33.69 abc | 1172.03 ± 55.91 a | 5.25 ± 0.10 ab |
| 4 | 5.83 ± 0.26 ab | 265.43 ± 32.50 ab | 1068.63 ± 169.50 a | 5.12 ± 0.52 a |
| 5 | 5.69 ± 0.72 ab | 207.37 ± 20.47 a | 1083.41 ± 134.43 a | 5.86 ± 0.57 abc |
| 6 | 10.13 ± 0.61 cd | 619.82 ± 18.88 f | 2270.04 ± 276.31 a | 7.61 ± 0.04 c |
| 7 | 11.38 ± 1.11 de | 294.63 ± 17.05 abc | 2382.53 ± 199.81 a | 10.88 ± 0.37 d |
| 8 | 3.87 ± 0.17 a | 230.20 ± 21.23 a | 1028.02 ± 43.40 a | 5.30 ± 0.52 ab |
| 9 | 6.89 ± 0.31 abc | 386.88 ± 22.48 d | 2333.72 ± 21.66 a | 7.77 ± 0.59 c |
| 10 | 9.23 ± 3.07 bcd | 432.42 ± 73.97 de | 1418.94 ± 136.42 a | 5.97 ± 1.92 abc |
| 11 | 14.32 ± 1.11 e | 255.27 ± 34.49 a | 4487.74 ± 905.66 b | 11.67 ± 0.45 d |
| 12 | 8.29 ± 0.82 bcd | 528.18 ± 20.14 ef | 4552.32 ± 749.64 b | 6.73 ± 0.40 c |
| TPC (mg GEA/g DW) | TFC (µg QE/g DW) | TPAC (µg CA/g DW) | AA (mg AA/g DW) | |
| Aqueous extracts | ||||
| 1 | 7.66 ± 0.15 c | 467.26 ± 5.46 d | 2014.18 ± 48.35 b | 12.52 ± 0.70 ab |
| 2 | 6.67 ± 0.12 bc | 404.03 ± 23.10 c | 2011.45 ± 10.55 b | 11.59 ± 0.31a |
| 3 | 5.80 ± 1.16 ab | 257.04 ± 7.17 ab | 1887.11 ± 26.39 b | 10.52 ± 0.44 a |
| 4 | 5.13 ± 0.26 a | 224.56 ± 10.08 a | 1219.47 ± 101.83 a | 10.78 ± 0.70 a |
| 5 | 14.28 ± 0.37 d | 687.44 ± 31.76 e | 3414.55 ± 107.49 c | 17.23 ± 0.28 c |
| 6 | 16.52 ± 0.09 e | 773.44 ± 24.31 f | 7734.72 ± 169.21 g | 14.84 ± 0.39 bc |
| 7 | 14.53 ± 0.32 d | 294.28 ± 2.36 b | 6720.06 ± 63.55 f | 17.23 ± 0.76 cd |
| 8 | 4.64 ± 0.29 a | 241.30 ± 0.37 ab | 1933.87 ± 51.39 b | 11.12 ± 0.87 a |
| 9 | 7.58 ± 0.37 c | 437.15 ± 4.44 cd | 4354.92 ± 78.61 e | 15.55 ± 1.18 cd |
| 10 | 7.57 ± 0.38 c | 430.15 ± 1.24 d | 3965.55 ± 79.22 d | 17.77 ± 0.66 d |
| 11 | 5.18 ± 0.20 a | 259.57 ± 9.21 a | 2129.14 ± 40.41 b | 12.17 ± 0.88 a |
| 12 | 18.26 ± 0.28 f | 455.65 ± 22.04 c | 4429.79 ± 87.47 e | 16.07 ± 0.20 cd |
| CUPRAC mg AA/g DW | ABTS mg TE/g DW | FRAP µmol Fe2+/g DW | DPPH mg TE/g DW | |
| No | Hydromethanolic extracts | |||
| 1 | 15.13 ± 2.01 abc | 64.69 ± 3.31 ab | 65.87 ± 3.95 ab | 3.06 ± 0.07 a |
| 2 | 15.56 ± 0.50 bc | 64.30 ± 2.62 ab | 60.80 ± 4.27 ab | 2.97 ± 0.05 a |
| 3 | 20.67 ± 0.74 cde | 60.97 ± 3.57 a | 60.04 ± 0.35 ab | 3.60 ± 0.04 a |
| 4 | 20.65 ± 1.97 cd | 56.99 ± 6.67 a | 61.80 ± 2.99 ab | 3.36 ± 0.05 a |
| 5 | 15.35 ± 2.29 bc | 68.28 ± 4.40 abc | 61.27 ± 0.02 ab | 3.01 ± 0.29 a |
| 6 | 25.84 ± 1.28 ef | 88.61 ± 9.56 c | 132.97 ± 9.51 d | 7.54 ± 0.27 b |
| 7 | 31.69 ± 3.16 f | 82.58 ± 4.69 bc | 111.90 ± 14.12 d | 7.85 ± 0.55 b |
| 8 | 10.42 ± 2.00 a | 65.22 ± 6.76 ab | 42.47 ± 1.52 a | 2.54 ± 0.23 a |
| 9 | 13.26 ± 0.86 ab | 60.61 ± 2.30 a | 82.07 ± 5.80 b | 3.58 ± 0.61 a |
| 10 | 24.72 ± 2.53 de | 63.49 ± 1.64 b | 126.85 ± 13.50 d | 2.96 ± 0.99 a |
| 11 | 46.50 ± 3.09 g | 192.82 ± 10.74 d | 67.05 ± 8.06 ab | 3.84 ± 0.31 a |
| 12 | 23.23 ± 1.78 de | 59.20 ± 3.00 a | 107.52 ± 10.78 cd | 7.34 ± 0.44 b |
| CUPRAC mg AA/g DW | ABTS mg TE/g DW | FRAP µmol Fe2+/g DW | DPPH mg TE/g DW | |
| No | Aqueous extracts | |||
| 1 | 20.38 ± 0.53 d | 23.32 ± 0.93 d | 139.43 ± 4.21 c | 0.84 ± 0.23 a |
| 2 | 16.29 ± 0.43 bc | 22.20 ± 0.89 cd | 117.25 ± 4.78 bc | 2.13 ± 0.18 d |
| 3 | 15.68 ± 0.58 b | 19.64 ± 0.52 bc | 121.64 ± 1.95 c | 3.24 ± 0.09 e |
| 4 | 10.49 ± 0.24 a | 23.94 ± 0.65 d | 90.45 ± 6.19 a | 1.38 ± 0.26 bc |
| 5 | 28.84 ± 1.13 e | 39.47 ± 0.82 f | 224.55 ± 13.27 e | 0.80 ± 0.05 a |
| 6 | 40.39 ± 0.54 g | 41.66 ± 0.22 f | 317.83 ± 10.18 g | 1.80 ± 0.11 a |
| 7 | 36.75 ± 1.35 f | 49.04 ± 1.34 g | 287.46 ± 5.36 f | 1.90 ± 0.11 d |
| 8 | 12.39 ± 0.17 a | 15.50 ± 0.77 a | 87.87 ± 7.32 a | 1.01 ± 0.05 ab |
| 9 | 18.40 ± 0.60 cd | 19.05 ± 0.48 b | 179.82 ± 6.66 d | 2.95 ± 0.13 e |
| 10 | 19.82 ± 0.58 d | 24.06 ± 0.38 d | 142.05 ± 3.85 c | 2.03 ± 0.12 d |
| 11 | 12.96 ± 0.79 a | 30.60 ± 0.64 e | 95.95 ± 3.96 a | 1.17 ± 0.14 ab |
| 12 | 20.15 ± 0.62 d | 28.11 ± 1.29 e | 181.41 ± 7.12 d | 1.83 ± 0.08 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wozniczka, K.; Viapiana, A.; Roszkowska, A.; Plenis, A.; Baczek, T.; Konieczynski, P. Evaluation of Commercially Available Products of Cannabis sativa L. Inflorescences to Identify Their Contents of Elemental and Phenolic Compounds. Foods 2025, 14, 1208. https://doi.org/10.3390/foods14071208
Wozniczka K, Viapiana A, Roszkowska A, Plenis A, Baczek T, Konieczynski P. Evaluation of Commercially Available Products of Cannabis sativa L. Inflorescences to Identify Their Contents of Elemental and Phenolic Compounds. Foods. 2025; 14(7):1208. https://doi.org/10.3390/foods14071208
Chicago/Turabian StyleWozniczka, Katarzyna, Agnieszka Viapiana, Anna Roszkowska, Alina Plenis, Tomasz Baczek, and Pawel Konieczynski. 2025. "Evaluation of Commercially Available Products of Cannabis sativa L. Inflorescences to Identify Their Contents of Elemental and Phenolic Compounds" Foods 14, no. 7: 1208. https://doi.org/10.3390/foods14071208
APA StyleWozniczka, K., Viapiana, A., Roszkowska, A., Plenis, A., Baczek, T., & Konieczynski, P. (2025). Evaluation of Commercially Available Products of Cannabis sativa L. Inflorescences to Identify Their Contents of Elemental and Phenolic Compounds. Foods, 14(7), 1208. https://doi.org/10.3390/foods14071208







