Sustainable Meat Alternatives: Incorporation of Tenebrio molitor and Alphitobius diaperinus Powders into Pork-Based Hybrid Hams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hybrid Hams Preparation
2.2. Color Measurement
2.3. Nutritional Composition
2.4. Total Phenolic Compounds and Antioxidant Activity Determination
2.5. Texture Profile Analysis (TPA)
2.6. Rheology Measurement
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
| Amino Acid (g/100 g) | T. molitor Powder | A. diaperinus Powder | T. molitor */** | A. diaperinus ***/**** |
|---|---|---|---|---|
| Asp + Asn | 8.32 ± 0.16 a | 7.54 ± 0.10 b | nd/nd | nd/5.42 |
| Ser | 2.62 ± 0.11 a | 2.35 ± 0.14 a | 2.17/1.39–1.73 | 4.76/2.84 |
| Glu + Gln | 9.01 ± 0.21 a | 9.01 ± 0.02 a | nd/nd | nd/7.74 |
| His | 3.76 ± 0.19 b | 5.39 ± 0.32 a | 2.41/1.49–1.81 | 7.38/2.40 |
| Gly | 1.20 ± 0.01 b | 2.35 ± 0.10 a | 2.53/2.41–2.66 | 4.62/2.81 |
| Arg | 6.36 ± 0.09 a | 5.95 ± 0.37 a | 2.75/nd | 6.07/3.63 |
| Thr | 0.69 ± 0.02 b | 1.27 ± 0.08 a | 2.17/1.27–1.45 | 4.45/2.58 |
| Ala | 2.96 ± 0.10 a | 2.66 ± 0.02 b | 5.55/3.35–3.74 | 6.62/4.43 |
| Pro | 2.64 ± 0.11 a | 2.75 ± 0.13 a | 3.79/2.67–2.98 | 6.68/3.94 |
| Val | 2.52 ± 0.15 b | 2.87 ± 0.01 a | 3.21/2.25–3.14 | 6.14/3.61 |
| Tyr | 88.61 ± 1.22 b | 115.54 ± 1.76 a | nd/3.25–3.88 | 7.89/4.57 |
| Trp | 2.33 ± 0.02 b | 2.98 ± 0.00 a | nd/nd | nd/1.22 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ververis, E.; Niforou, A.; Poulsen, M.; Pires, S.M.; Federighi, M.; Samoli, E.; Naska, A.; Boué, G. Substituting Red Meat with Insects in Burgers: Estimating the Public Health Impact Using Risk-Benefit Assessment. Food Chem. Toxicol. 2024, 189, 114764. [Google Scholar] [CrossRef] [PubMed]
- Etter, B.; Michel, F.; Siegrist, M. Which Are the Most Promising Protein Sources for Meat Alternatives? Food Qual. Prefer. 2024, 119, 105226. [Google Scholar] [CrossRef]
- Osasona, A.I.; Olaofe, O. Nutritional and Functional Properties of Cirina forda Larva from Ado-Ekiti, Nigeria Olorunfemi Olaofe Nutritional and Functional Properties of Cirina forda Larva from Ado-Ekiti, Nigeria. Afr. J. Food Sci. 2010, 4, 775–777. [Google Scholar]
- Omotoso, O.T. Nutritional Quality, Functional Properties and Anti-Nutrient Compositions of the Larva of Cirina forda (Westwood) (Lepidoptera: Saturniidae). J. Zhejiang Univ. Sci. B 2006, 7, 51–55. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and Characterisation of Protein Fractions from Five Insect Species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-Treated Mealworm Larvae and Silkworm Pupae as a Novel Protein Ingredient in Emulsion Sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Qian, L.; Deng, P.; Chen, F.; Cao, Y.; Sun, H.; Liao, H. The Exploration and Utilization of Functional Substances in Edible Insects: A Review. Food Prod. Process Nutr. 2022, 4, 11. [Google Scholar] [CrossRef]
- Tang, C.; Yang, D.; Liao, H.; Sun, H.; Liu, C.; Wei, L.; Li, F. Edible Insects as a Food Source: A Review. Food Prod. Process Nutr. 2019, 1, 8. [Google Scholar] [CrossRef]
- Regulation (EU) 2021/882. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/882/oj/eng (accessed on 23 January 2025).
- Regulation (EU) 2023/58. Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/58/oj (accessed on 23 January 2025).
- Regulation (UE) 2022/188. Available online: https://eur-lex.europa.eu/eli/reg_impl/2022/188/oj (accessed on 11 March 2025).
- Regulation (EU) 2021/1975. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/1975/oj (accessed on 11 March 2025).
- Regulation (EU) 2025/89. Available online: https://eur-lex.europa.eu/eli/reg_impl/2025/89/oj (accessed on 11 March 2025).
- Azzollini, D.; Wibisaphira, T.; Lakemond, C.M.M.; Fogliano, V. Toward the Design of Insect-Based Meat Analogue: The Role of Calcium and Temperature in Coagulation Behavior of Alphitobius diaperinus Proteins. LWT 2019, 100, 75–82. [Google Scholar] [CrossRef]
- Maciejewska, M.; Dąbrowska, A.; Cano-Lamadrid, M. Sustainable Protein Sources: Functional Analysis of Tenebrio molitor Hydrolysates and Attitudes of Consumers in Poland and Spain Toward Insect-Based Foods. Foods 2025, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor Larva-Based Ingredients for the Food Industry: A Review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser Mealworm (Alphitobius diaperinus) Powder as a Novel Baking Ingredient for Manufacturing High-Protein, Mineral-Dense Snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional Value of Insects and Ways to Manipulate Their Composition. J. Insects Food Feed. 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.-H. Role of Temperature on Growth and Metabolic Rate in the Tenebrionid Beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.; Borges, S.; Pintado, M. Enzymatic Hydrolysis of Insect Alphitobius diaperinus towards the Development of Bioactive Peptide Hydrolysates. Food Funct. 2020, 11, 3539–3548. [Google Scholar] [CrossRef]
- Gkinali, A.-A.; Matsakidou, A.; Paraskevopoulou, A. Characterization of Tenebrio molitor Larvae Protein Preparations Obtained by Different Extraction Approaches. Foods 2022, 11, 3852. [Google Scholar] [CrossRef]
- Sun, H.; Necochea Velazco, O.; Lakemond, C.; Dekker, M.; Cadesky, L.; Mishyna, M. Differences in Moisture Sorption Characteristics and Browning of Lesser Mealworm (Alphitobius diaperinus) Ingredients. LWT 2021, 142, 110989. [Google Scholar] [CrossRef]
- Vieira, M.R.; Simões, S.; Carrera-Sánchez, C.; Raymundo, A. Development of a Clean Label Mayonnaise Using Fruit Flour. Foods 2023, 12, 2111. [Google Scholar] [CrossRef] [PubMed]
- Castellar, M.; Obón, J.; Fernández-López, J. The Isolation and Properties of a Concentrated Red-purple Betacyanin Food Colourant from Opuntia stricta Fruits. J. Sci. Food Agric. 2006, 86, 122–128. [Google Scholar] [CrossRef]
- Matheus, J.; Alegria, M.J.; Nunes, M.C.; Raymundo, A. Algae-Boosted Chickpea Hummus: Improving Nutrition and Texture with Seaweeds and Microalgae. Foods 2024, 13, 2178. [Google Scholar] [CrossRef]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-Protein Conversion Factors for Edible Insects on the Swiss Market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Viegas, Â.; Alegria, M.J.; Raymundo, A. Sustainable Jam with Apple Pomace: Gelling, Rheology, and Composition Analysis. Gels 2024, 10, 580. [Google Scholar] [CrossRef]
- AACC. Moisture—Air-Oven Methods; AACC International Method 44-15.02; AACC International: Eagan, MN, USA, 2009. [Google Scholar]
- NP 1615:2002; Portuguese Norm—Meat and Meat Products. Determination of Total Ash. Reference Method. Portuguese Institute of Quality: Costa da Caparica, Portugal, 2002.
- Beltrão Martins, R.; Gouvinhas, I.; Nunes, M.C.; Alcides Peres, J.; Raymundo, A.; Barros, A.I.R.N.A. Acorn Flour as a Source of Bioactive Compounds in Gluten-Free Bread. Molecules 2020, 25, 3568. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.; Oppolzer, D.; Ramos, A.; Ferreira, L.; Rosa, E.A.; Rodrigues, M.; Domínguez-Perles, R.; Barros, A.I. Evaluating the Freezing Impact on the Proximate Composition of Immature Cowpea (Vigna unguiculata L.) Pods: Classical versus Spectroscopic Approaches. J. Sci. Food Agric. 2017, 97, 4295–4305. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant Capacity and Total Phenolics of Cyphostemma Digitatum before and after Processing: Use of Different Assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Carrasco-Sandoval, J.; Falcó, I.; Sánchez, G.; Fabra, M.J.; López-Rubio, A.; Rodriguez, A.; Henríquez-Aedo, K.; Aranda, M. Multivariable Optimization of Ultrasound-Assisted Extraction for the Determination of Phenolic and Antioxidants Compounds from Arrayan (Luma apiculata (DC.) Burret) Leaves by Microplate-Based Methods and Mass Spectrometry. J. Appl. Res. Med. Aromat. Plants 2022, 28, 100356. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput Micro Plate Assays for Screening Flavonoid Content and DPPH-scavenging Activity in Sorghum Bran and Flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Carrera Sanchez, C.; Santos, A.J.; Figueira, D.; Prista, C.; Raymundo, A. Impact of Grass Pea Sweet Miso Incorporation in Vegan Emulsions: Rheological, Nutritional and Bioactive Properties. Foods 2023, 12, 1362. [Google Scholar] [CrossRef]
- Raymundo, A. Emulsões Alimentares. In A Química e a Reologia no Processamento de Alimentos; Piaget Institute: Lisbon, Portugal, 2004; pp. 96–116. [Google Scholar]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- Aguilar-Miranda, E.D.; López, M.G.; Escamilla-Santana, C.; Barba de la Rosa, A.P. Characteristics of Maize Flour Tortilla Supplemented with Ground Tenebrio molitor Larvae. J. Agric. Food Chem. 2002, 50, 192–195. [Google Scholar] [CrossRef]
- Cuj-Laines, R.; Hernández-Santos, B.; Reyes-Jaquez, D.; Delgado-Licon, E.; Juárez-Barrientos, J.M.; Rodríguez-Miranda, J. Physicochemical Properties of Ready-to-eat Extruded Nixtamalized Maize-based Snacks Enriched with Grasshopper. Int. J. Food Sci. Technol. 2018, 53, 1889–1895. [Google Scholar] [CrossRef]
- Xie, X.; Yuan, Z.; Fu, K.; An, J.; Deng, L. Effect of Partial Substitution of Flour with Mealworm (Tenebrio molitor L.) Powder on Dough and Biscuit Properties. Foods 2022, 11, 2156. [Google Scholar] [CrossRef] [PubMed]
- Kurečka, M.; Kulma, M.; Petříčková, D.; Plachý, V.; Kouřimská, L. Larvae and Pupae of Alphitobius diaperinus as Promising Protein Alternatives. Eur. Food Res. Technol. 2021, 247, 2527–2532. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the Nutritional Value of Mysore Thorn Borer (Anoplophora chinensis) and Mealworm Larva (Tenebrio molitor): Amino Acid, Fatty Acid, and Element Profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef] [PubMed]
- Herdeiro, F.M.; Carvalho, M.O.; Nunes, M.C.; Raymundo, A. Development of Healthy Snacks Incorporating Meal from Tenebrio molitor and Alphitobius diaperinus Using 3D Printing Technology. Foods 2024, 13, 179. [Google Scholar] [CrossRef]
- Jonas-Levi, A.; Martinez, J.-J.I. The High Level of Protein Content Reported in Insects for Food and Feed Is Overestimated. J. Food Compos. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- Xu, M.-L.; Gao, Y.; Han, X.X. Structure Information Analysis and Relative Content Determination of Protein and Chitin from Yellow Mealworm Larvae Using Raman Spectroscopy. Int. J. Biol. Macromol. 2024, 272, 132787. [Google Scholar] [CrossRef]
- Jankauskienė, A.; Kiseliovienė, S.; Aleknavičius, D.; Miliūnaitė, I.; Kerzienė, S.; Gaižauskaitė, Ž.; Juknienė, I.; Zaviztanavičiūtė, P.; Kabašinskienė, A. Innovative Applications of Tenebrio molitor Larvae in the Production of Sustainable Meat Sausages: Quality and Safety Aspects. Foods 2024, 13, 1451. [Google Scholar] [CrossRef]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the Physicochemical and Structural Properties and the Sensory Characteristics of Meat Analogues Prepared with Various Non-Animal Based Liquid Additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef]
- Kolobe, S.D.; Manyelo, T.G.; Malematja, E.; Sebola, N.A.; Mabelebele, M. Fats and Major Fatty Acids Present in Edible Insects Utilised as Food and Livestock Feed. Vet. Anim. Sci. 2023, 22, 100312. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat Bread Supplementation with Various Edible Insect Flours. Influence of Chemical Composition on Nutritional and Technological Aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Zhou, S.; Duan, H.; Guo, J.; Yan, W. Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review. Foods 2022, 11, 3961. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential Health Benefits of Edible Insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Yang, W.; Vera Aviles, M. In Vitro Iron Availability from Insects and Sirloin Beef. J. Agric. Food Chem. 2016, 64, 8420–8424. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Liang, L.; Qiao, K.; Luo, J.; Liu, X.; Sun, B.; Zhang, Y. A Comprehensive Review of Plant-Derived Salt Substitutes: Classification, Mechanism, and Application. Food Res. Int. 2024, 194, 114880. [Google Scholar] [CrossRef]
- Dordevic, D.; Capikova, J.; Dordevic, S.; Tremlová, B.; Gajdács, M.; Kushkevych, I. Sulfur Content in Foods and Beverages and Its Role in Human and Animal Metabolism: A Scoping Review of Recent Studies. Heliyon 2023, 9, e15452. [Google Scholar] [CrossRef]
- McLean, R.M.; Wang, N.X. Potassium. Adv. Food Nutr. Res. 2021, 96, 89–121. [Google Scholar]
- Luz, G.M.; Orlando, E.A.; Rebellato, A.P.; Greiner, R.; Pallone, J.A.L. Essential Minerals and Anti-Nutritional Compounds in Plant-Based Burgers Using the Infogest in Vitro Digestion Protocol. J. Food Compos. Anal. 2024, 135, 106574. [Google Scholar] [CrossRef]
- Orkusz, A. Edible Insects versus Meat—Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; European Union: Brussels, Belgium, 2006. [Google Scholar]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Dietary Reference Values for Sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Neuhäuser-Berthold, M.; et al. Dietary Reference Values for Potassium. EFSA J. 2016, 14, e04592. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Phosphorus. EFSA J. 2015, 13, 4185. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of Calcium. EFSA J. 2012, 10, 2814. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iron. EFSA J. 2015, 13, 4254. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected Species of Edible Insects as a Source of Nutrient Composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Woo Kim, S.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Sengottuvelan, M.; Nalini, N. Protective Effect of Glycine Supplementation on the Levels of Lipid Peroxidation and Antioxidant Enzymes in the Erythrocyte of Rats with Alcohol-induced Liver Injury. Cell Biochem. Funct. 2004, 22, 123–128. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free Radicals, Antioxidants, and Nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Kohlmeier, M. Amino Acids and Nitrogen Compounds. In Nutrient Metabolism; Elsevier: Amsterdam, The Netherlands, 2015; pp. 265–477. [Google Scholar]
- Jankauskienė, A.; Aleknavičius, D.; Andrulevičiūtė, V.; Mockus, E.; Bartkienė, E.; Juknienė, I.; Kiseliovienė, S.; Zavistanavičiūtė, P.; Zaborskienė, G.; Kabašinskienė, A. Nutritional Composition and Safety Parameters of Mealworms (Tenebrio molitor) Reared on Substrates Derived from By-Products. Appl. Sci. 2024, 14, 2744. [Google Scholar] [CrossRef]
- Hensel, C.; Becker, M.; Düzel, S.; Demuth, I.; Norman, K.; Steinhagen-Thiessen, E.; Gallinat, J.; Lindenberger, U.; Kühn, S. Influence of Nutritional Tyrosine on Cognition and Functional Connectivity in Healthy Old Humans. Neuroimage 2019, 193, 139–145. [Google Scholar] [CrossRef]
- Fuso, A.; Leni, G.; Caligiani, A. Unravelling the Influence of Extraction Techniques on Protein Yield and Nutritional Value in Lesser Mealworm Larvae. Molecules 2024, 29, 4220. [Google Scholar] [CrossRef]
- Ajomiwe, N.; Boland, M.; Phongthai, S.; Bagiyal, M.; Singh, J.; Kaur, L. Protein Nutrition: Understanding Structure, Digestibility, and Bioavailability for Optimal Health. Foods 2024, 13, 1771. [Google Scholar] [CrossRef]
- Gerrard, J.A.; Lasse, M.; Cottam, J.; Healy, J.P.; Fayle, S.E.; Rasiah, I.; Brown, P.K.; BinYasir, S.M.; Sutton, K.H.; Larsen, N.G. Aspects of Physical and Chemical Alterations to Proteins during Food Processing—Some Implications for Nutrition. Br. J. Nutr. 2012, 108, S288–S297. [Google Scholar] [CrossRef] [PubMed]
- McKerchar, H.J.; Clerens, S.; Dobson, R.C.J.; Dyer, J.M.; Maes, E.; Gerrard, J.A. Protein-Protein Crosslinking in Food: Proteomic Characterisation Methods, Consequences and Applications. Trends Food Sci. Technol. 2019, 86, 217–229. [Google Scholar] [CrossRef]
- Apolzan, J.W.; Stein, J.A.; Rood, J.C.; Beyl, R.A.; Yang, S.; Greenway, F.L.; Lieberman, H.R. Effects of Acute Arginine Supplementation on Neuroendocrine, Metabolic, Cardiovascular, and Mood Outcomes in Younger Men: A Double-Blind, Placebo-Controlled Trial. Nutrition 2022, 101, 111658. [Google Scholar] [CrossRef]
- Cho, S.Y.; Ryu, G.H. Effects of Mealworm Larva Composition and Selected Process Parameters on the Physicochemical Properties of Extruded Meat Analog. Food Sci. Nutr. 2021, 9, 4408–4419. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Kim, T.-K.; Choi, H.-D.; Park, J.-D.; Sung, J.-M.; Jeon, K.-H.; Paik, H.-D.; Kim, Y.-B. Optimization of Replacing Pork Meat with Yellow Worm (Tenebrio molitor L.) for Frankfurters. Korean J. Food Sci. Anim. Resour. 2017, 37, 617–625. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lee, M.H.; Yong, H.I.; Jung, S.; Paik, H.-D.; Jang, H.W.; Choi, Y.-S. Effect of Interaction between Mealworm Protein and Myofibrillar Protein on the Rheological Properties and Thermal Stability of the Prepared Emulsion Systems. Foods 2020, 9, 1443. [Google Scholar] [CrossRef]
- Pasqualin Cavalheiro, C.; Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Avelar de Sousa, C.C.; Sant’Ana Falcão Leite, J.; Costa Alves da Silva, M. Potential of Cricket (Acheta domesticus) Flour as a Lean Meat Replacer in the Development of Beef Patties. Foods 2024, 13, 286. [Google Scholar] [CrossRef]
- Gomes Martins, V.M.; Milano, P.; Rodrigues Pollonio, M.A.; dos Santos, M.; de Oliveira, A.P.; Savay-da-Silva, L.K.; Ferreira Ignácio Câmara, A.K.; de Souza Paglarini, C. Adding Cricket (Gryllus assimilis) Flour in Hybrid Beef Patties: Physicochemical, Technological and Sensory Challenges. Int. J. Food Sci. Technol. 2024, 59, 450–459. [Google Scholar] [CrossRef]
- Lü, J.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Botella-Martínez, C.; Lucas-González, R.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Assessment of Chemical Composition and Antioxidant Properties of Defatted Flours Obtained from Several Edible Insects. Food Sci. Technol. Int. 2021, 27, 383–391. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Nino, M.C.; Reddivari, L.; Osorio, C.; Kaplan, I.; Liceaga, A.M. Insects as a Source of Phenolic Compounds and Potential Health Benefits. J. Insects Food Feed. 2021, 7, 1077–1087. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Gutiérrez-González, J.A. A Comprehensive and Critical Review of the Unstandardized Folin-Ciocalteu Assay to Determine the Total Content of Polyphenols: The Conundrum of the Experimental Factors and Method Validation. Talanta 2024, 272, 125771. [Google Scholar] [CrossRef]
- Bastola, K.P.; Guragain, Y.N.; Bhadriraju, V.; Vadlani, P.V. Evaluation of Standards and Interfering Compounds in the Determination of Phenolics by Folin-Ciocalteu Assay Method for Effective Bioprocessing of Biomass. Am. J. Analyt. Chem. 2017, 08, 416–431. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, Antioxidant Activity, and Inhibitory Effect on Pancreatic Lipase of Extracts from the Edible Insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Son, Y.-J.; Choi, S.Y.; Hwang, I.-K.; Nho, C.W.; Kim, S.H. Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients? Foods 2020, 9, 40. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Yang, H.; Xue, J.; Li, Y.; Wang, S.; Ge, L.; Shen, Q.; Zhang, M. Comparative Study on the Rheological Properties of Myofibrillar Proteins from Different Kinds of Meat. LWT 2022, 153, 112458. [Google Scholar] [CrossRef]
- Aybar, M.; Simões, S.; Sales, J.R.; Santos, J.; Figueira, D.; Raymundo, A. Tenebrio molitor as a Clean Label Ingredient to Produce Nutritionally Enriched Food Emulsions. Insects 2023, 14, 147. [Google Scholar] [CrossRef]
- Krawczyk, A.; Fernández-López, J.; Zimoch-Korzycka, A. Insect Protein as a Component of Meat Analogue Burger. Foods 2024, 13, 1806. [Google Scholar] [CrossRef] [PubMed]
- Ros-Baró, M.; Sánchez-Socarrás, V.; Santos-Pagès, M.; Bach-Faig, A.; Aguilar-Martínez, A. Consumers’ Acceptability and Perception of Edible Insects as an Emerging Protein Source. Int. J. Environ. Res. Public Health 2022, 19, 15756. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Pastrana, I.; Rebollo-Hernanz, M.; Benitez, V.; Álvarez-Rivera, G.; Viejo, J.L.; Martín-Cabrejas, M.A. Investigating Edible Insects as a Sustainable Food Source: Nutritional Value and Techno-Functional and Physiological Properties. Food Funct. 2021, 12, 6309–6322. [Google Scholar] [CrossRef] [PubMed]

 Control,
Control,  10T,
10T,  10A, and
10A, and  10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.
10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.
 Control,
Control,  10T,
10T,  10A, and
10A, and  10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.
10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.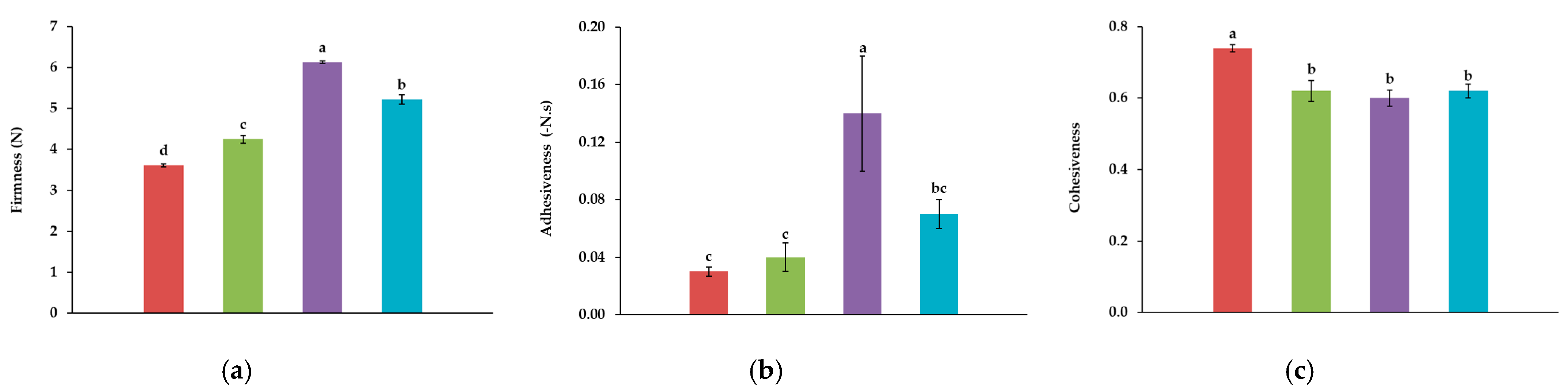
 Control,
Control,  10T,
10T,  10A, and
10A, and  10TA. G′ (full symbol) corresponds to the storage moduli and G″ (open symbol) corresponds to the loss moduli.
10TA. G′ (full symbol) corresponds to the storage moduli and G″ (open symbol) corresponds to the loss moduli.
 Control,
Control,  10T,
10T,  10A, and
10A, and  10TA. G′ (full symbol) corresponds to the storage moduli and G″ (open symbol) corresponds to the loss moduli.
10TA. G′ (full symbol) corresponds to the storage moduli and G″ (open symbol) corresponds to the loss moduli.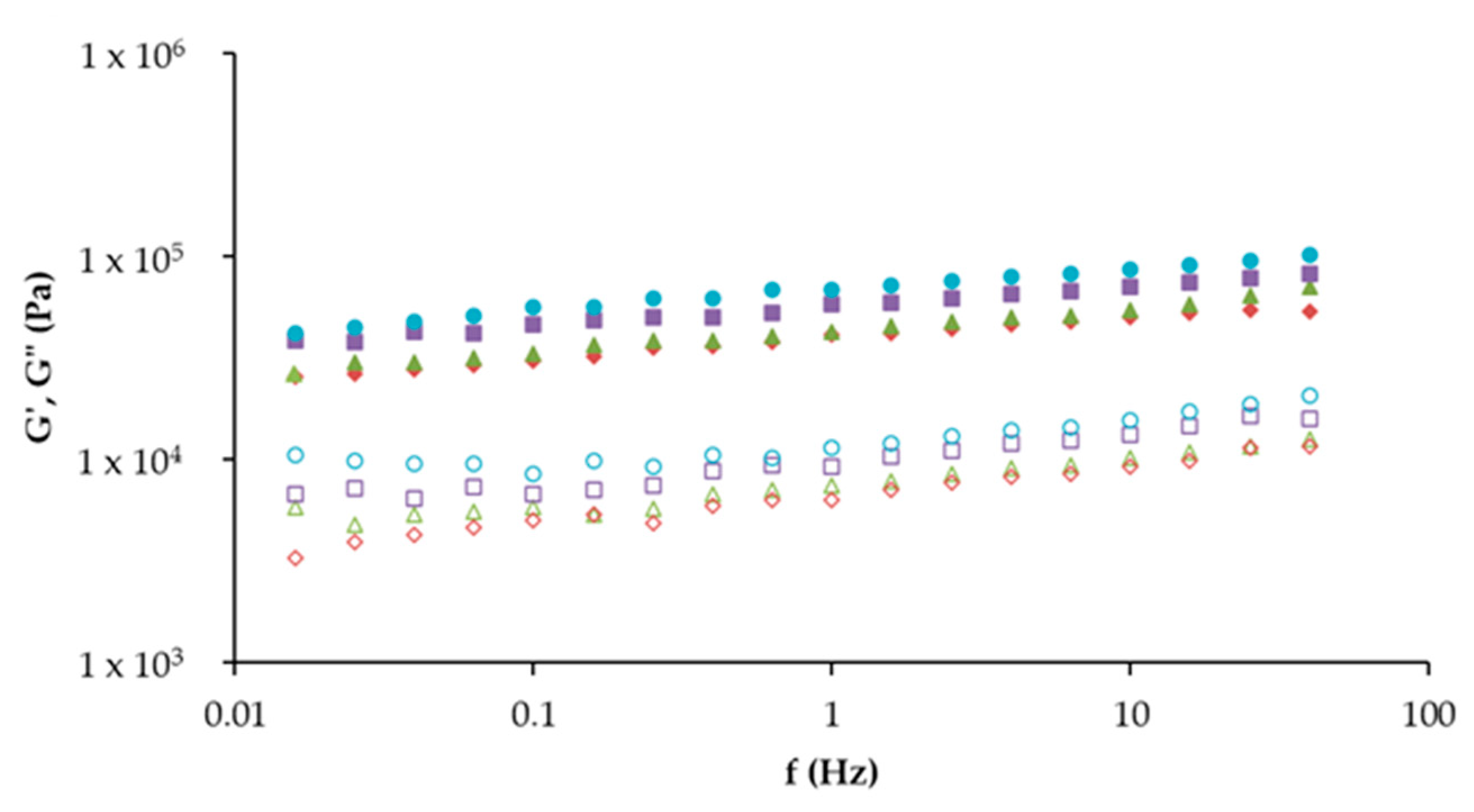
 Control,
Control,  10T,
10T,  10A, and
10A, and  10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.
10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.
 Control,
Control,  10T,
10T,  10A, and
10A, and  10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.
10TA. Different letters indicate significantly different results (p < 0.05) according to the Tukey test.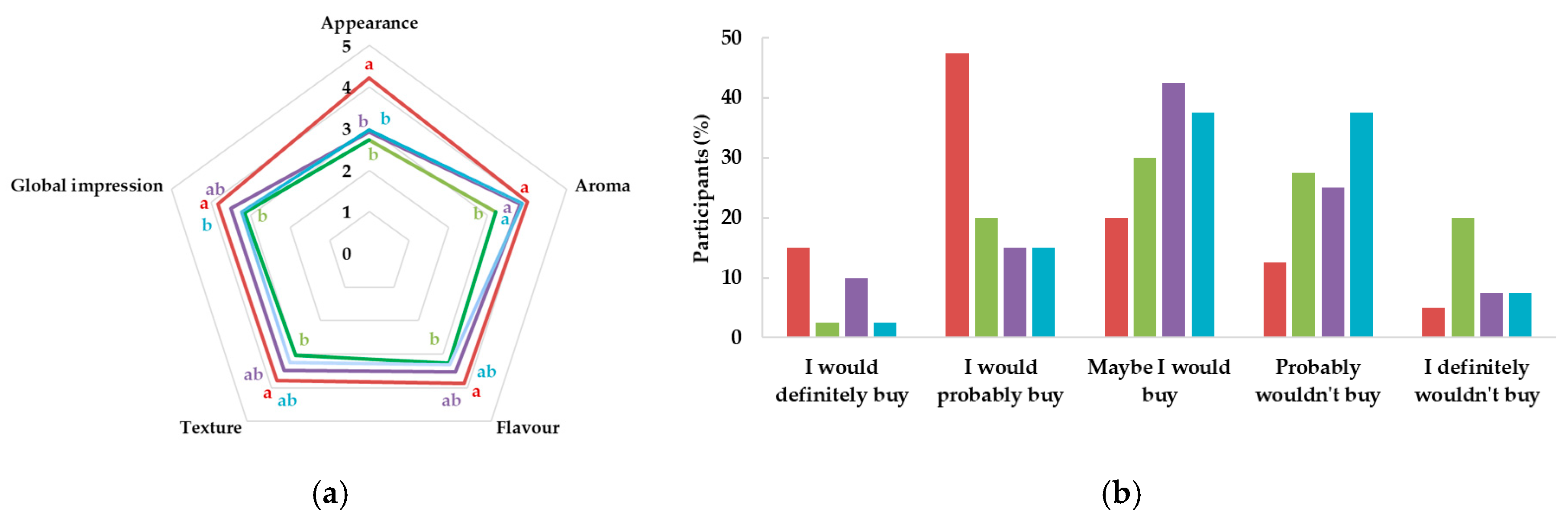
| Parameters | Control | 10T | 10A | 10TA |
|---|---|---|---|---|
| L* | 66.92 ± 0.479 a | 59.77 ± 0.180 c | 60.15 ± 0.363 c | 61.27 ± 0.084 b |
| a* | 10.02 ± 0.235 a | 7.70 ± 0.025 b | 6.49 ± 0.121 c | 6.89 ± 0.060 c |
| b* | 10.89 ± 0.238 b | 12.28 ± 0.568 a | 12.42 ± 0.329 a | 11.90 ± 0.112 a |
| ∆E | - | 7.65 | 7.79 | 6.55 |
| Parameters (%) | T. molitor Powder | A. diaperinus Powder | Control | 10T | 10A | 10TA |
|---|---|---|---|---|---|---|
| Protein | 37.9 ± 1.74 B | 61.1 ± 0.68 A | 34.9 ± 0.83 d | 48.9 ± 1.42 a | 46.3 ± 0.67 b | 40.4 ± 1.93 c |
| Lipids | 29.1 ± 0.13 A | 16.6 ± 0.28 B | 24.2 ± 0.75 c | 40.1 ± 0.08 a | 36.6 ± 0.09 b | 36.8 ± 1.74 b |
| Carbohydrates | 30.3 | 19.2 | 30.1 | 1.6 | 7.5 | 13.2 |
| Ash | 2.7 ± 0.06 B | 3.2 ± 0.07 A | 7.8 ± 0.39 b | 9.5 ± 0.03 a | 9.6 ± 0.09 a | 9.6 ± 0.20 a |
| Moisture | 5.1 ± 0.12 A | 4.1 ± 0.05 B | 66.1 ± 0.02 a | 58.4 ± 0.11 b | 57.3 ± 0.31 c | 57.5 ± 0.11 c |
| Minerals (mg/100 g) | T. molitor Powder | A. diaperinus Powder | Control | 10T | 10A | 10TA |
|---|---|---|---|---|---|---|
| Na | 51.4 ± 1.45 B | 95.2 ± 0.32 A | 189.8 ± 0.44 c | 212.6 ± 4.53 b | 221.1 ± 3.78 a | 225.0 ± 1.88 a |
| K | 778.7 ± 5.70 B | 1003.5 ± 8.70 A | 198.3 ± 0.86 d | 229.1 ± 1.24 c | 253.1 ± 0.50 a | 237.4 ± 0.17 b |
| Ca | 48.4 ± 1.09 B | 55.6 ± 0.72 A | 9.0 ± 0.01 c | 10.7 ± 0.57 b | 12.7 ± 0.74 a | 11.8 ± 0.63 ab |
| Mg | 313.9 ± 0.33 A | 186.0 ± 2.71 B | 18.9 ± 0.29 d | 35.0 ± 0.59 a | 25.7 ± 0.30 c | 30.1 ± 0.80 b |
| P | 754.7 ± 2.74 B | 857.7 ± 10.9 A | 106.5 ± 0.69 c | 148.6 ± 5.52 b | 164.5 ± 2.72 a | 151.0 ± 1.04 b |
| S | 318.7 ± 0.58 B | 488.33 ± 7.48 A | 157.6 ± 1.14 d | 162.1 ± 0.05 c | 184.5 ± 2.71 a | 169.7 ± 0.26 b |
| Fe | 6.2 ± 0.27 A | 5.1 ± 0.01 B | 1.2 ± 0.01 b | 2.1 ± 0.03 a | 2.1 ± 0.08 a | 2.1 ± 0.04 a |
| Cu | 1.7 ± 0.04 B | 2.1 ± 0.07 A | 0.0 ± 0.00 b | 0.2 ±0.01 a | 0.2 ±0.00 a | 0.2 ±0.00 a |
| Zn | 13.7 ± 0.48 B | 22.9 ± 0.00 A | 0.9 ± 0.03 d | 1.7 ± 0.07 c | 2.6 ± 0.05 a | 2.2 ± 0.07 b |
| Mn | 1.1 ± 0.02 A | 0.7 ± 0.05 B | 0.0 ± 0.00 b | 0.1 ± 0.01 a | 0.1 ± 0.00 a | 0.1 ± 0.01 a |
| Amino Acid (g/100 g) | Control | 10T | 10A | 10TA |
|---|---|---|---|---|
| Asp + Asn | 5.04 ± 0.59 a | 5.44 ± 0.18 a | 5.64 ± 0.18 a | 5.65 ± 0.37 a |
| Ser | 1.24 ± 0.03 b | 1.38 ± 0.09 ab | 1.49 ± 0.01 a | 1.47 ± 0.03 a |
| Glu + Gln | 7.42 ± 0.43 a | 7.27 ± 0.18 a | 7.56 ± 0.17 a | 7.60 ± 0.24 a |
| His | 2.47 ± 0.06 b | 2.55 ± 0.15 ab | 3.13 ± 0.09 a | 2.94 ± 0.26 ab |
| Gly | 0.84 ± 0.02 ab | 0.76 ± 0.07 b | 1.10 ± 0.06 a | 1.06 ± 0.11 a |
| Arg | 4.83 ± 0.23 b | 5.11 ± 0.01 ab | 5.56 ± 0.03 a | 5.57 ± 0.10 a |
| Thr | 0.60 ± 0.02 c | 0.62 ± 0.01 c | 1.71 ± 0.28 a | 0.91 ± 0.07 bc |
| Ala | 1.60 ± 0.03 a | 1.67 ± 0.07 a | 1.74 ± 0.04 a | 1.73 ± 0.05 a |
| Pro | 0.81 ± 0.03 b | 1.07 ± 0.03 a | 1.14 ± 0.03 a | 1.16 ± 0.05 a |
| Val | 1.40 ± 0.03 b | 1.59 ± 0.02 ab | 1.73 ± 0.04 a | 1.76 ± 0.10 a |
| Tyr | 26.49 ± 0.88 c | 39.31 ± 2.06 ab | 43.85 ± 4.08 a | 33.96 ± 0.00 bc |
| Trp | 1.51 ± 0.02 ab | 1.48 ± 0.13 ab | 1.67 ± 0.10 a | 1.22 ± 0.09 b |
| Hybrid Hams | TPC (mgGAE/g) | FRAP (mgTE/g) | DPPH (mgTE/g) |
|---|---|---|---|
| Control | 0.37 ± 0.02 b | 0.20 ± 0.00 b | 0.14 ± 0.00 a |
| 10T | 0.39 ± 0.02 b | 0.16 ± 0.00 c | 0.14 ± 0.01 a |
| 10A | 0.70 ± 0.03 a | 0.22 ± 0.00 a | 0.15 ± 0.00 a |
| 10TA | 0.69 ± 0.03 a | 0.20 ± 0.02 b | 0.15 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, L.; Ferreira, A.; Barros, A.N.; Carvalho, M.O.; Matos, T.J.S.; Raymundo, A.; Sousa, I. Sustainable Meat Alternatives: Incorporation of Tenebrio molitor and Alphitobius diaperinus Powders into Pork-Based Hybrid Hams. Foods 2025, 14, 1192. https://doi.org/10.3390/foods14071192
Carvalho L, Ferreira A, Barros AN, Carvalho MO, Matos TJS, Raymundo A, Sousa I. Sustainable Meat Alternatives: Incorporation of Tenebrio molitor and Alphitobius diaperinus Powders into Pork-Based Hybrid Hams. Foods. 2025; 14(7):1192. https://doi.org/10.3390/foods14071192
Chicago/Turabian StyleCarvalho, Lisiane, Adriana Ferreira, Ana Novo Barros, Maria Otília Carvalho, Teresa J. S. Matos, Anabela Raymundo, and Isabel Sousa. 2025. "Sustainable Meat Alternatives: Incorporation of Tenebrio molitor and Alphitobius diaperinus Powders into Pork-Based Hybrid Hams" Foods 14, no. 7: 1192. https://doi.org/10.3390/foods14071192
APA StyleCarvalho, L., Ferreira, A., Barros, A. N., Carvalho, M. O., Matos, T. J. S., Raymundo, A., & Sousa, I. (2025). Sustainable Meat Alternatives: Incorporation of Tenebrio molitor and Alphitobius diaperinus Powders into Pork-Based Hybrid Hams. Foods, 14(7), 1192. https://doi.org/10.3390/foods14071192











