How Does Extended Maceration Affect Tannin and Color of Red Wines from Cold-Hardy Grape Cultivars?
Abstract
1. Introduction
2. Materials and Methods
- Chemicals
- Winemaking
- Chemical parameter measurement
- Total Iron-Reactive Phenolics, SPP, and Anthocyanin Concentrations
- Tannin Concentration
- Statistical Analysis
3. Results and Discussion
3.1. Basic Chemical Parameters
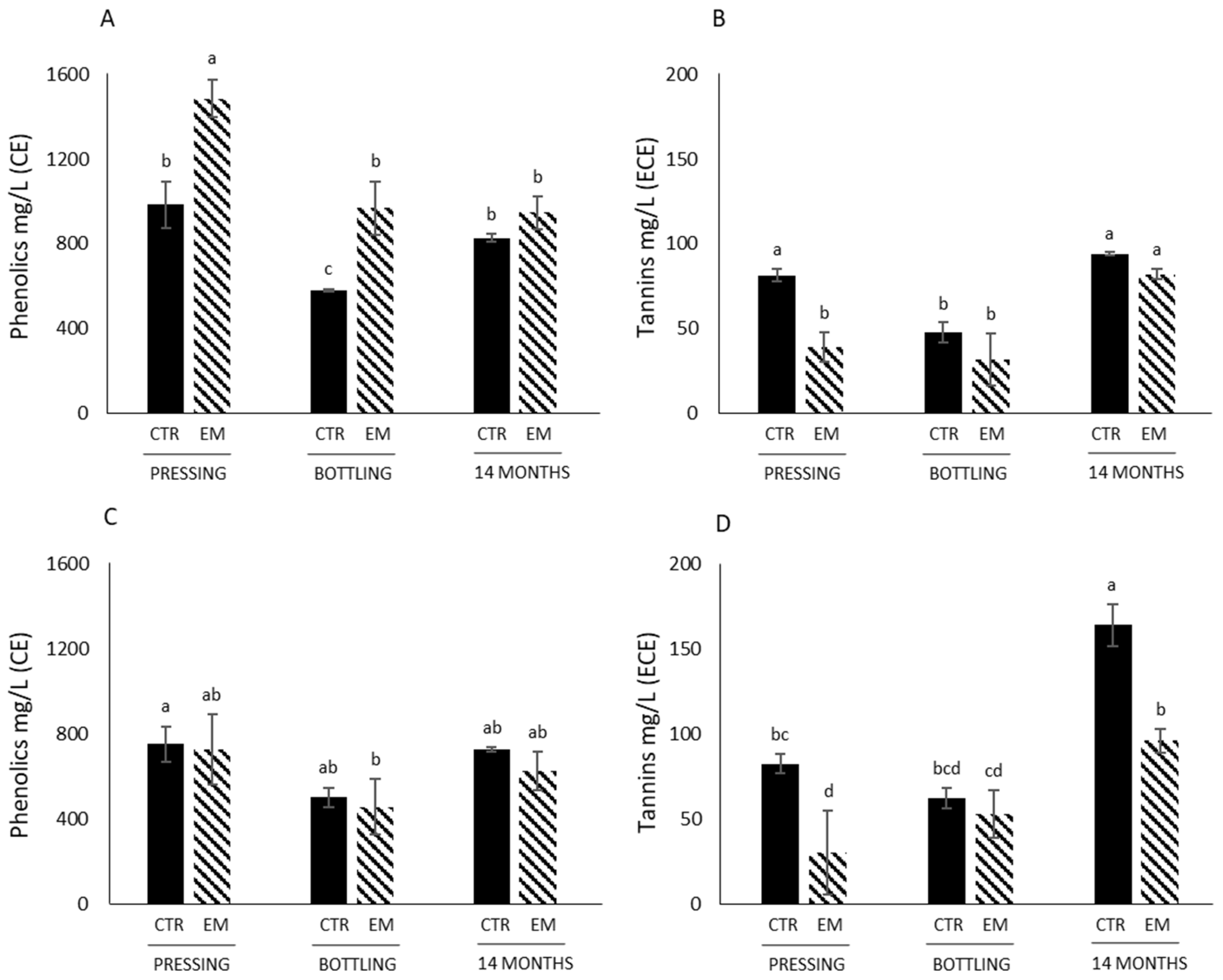
3.2. Phenolics and Tannins Concentrations
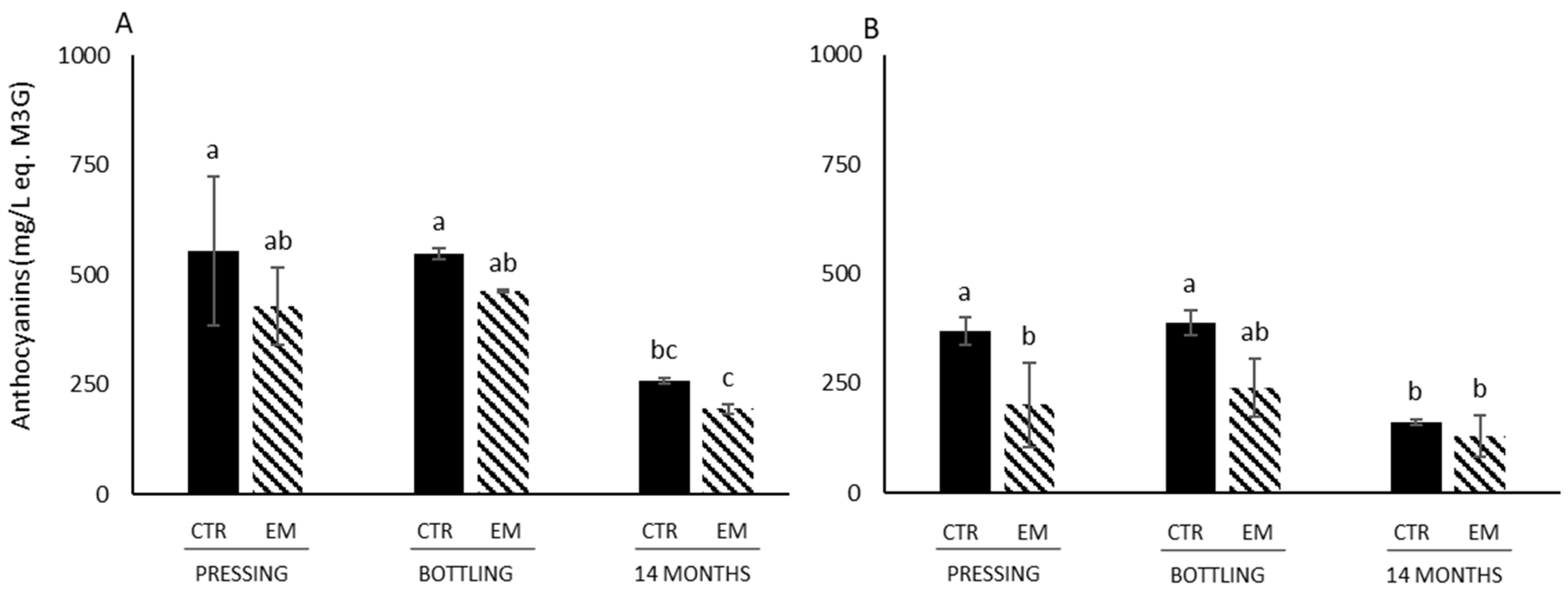
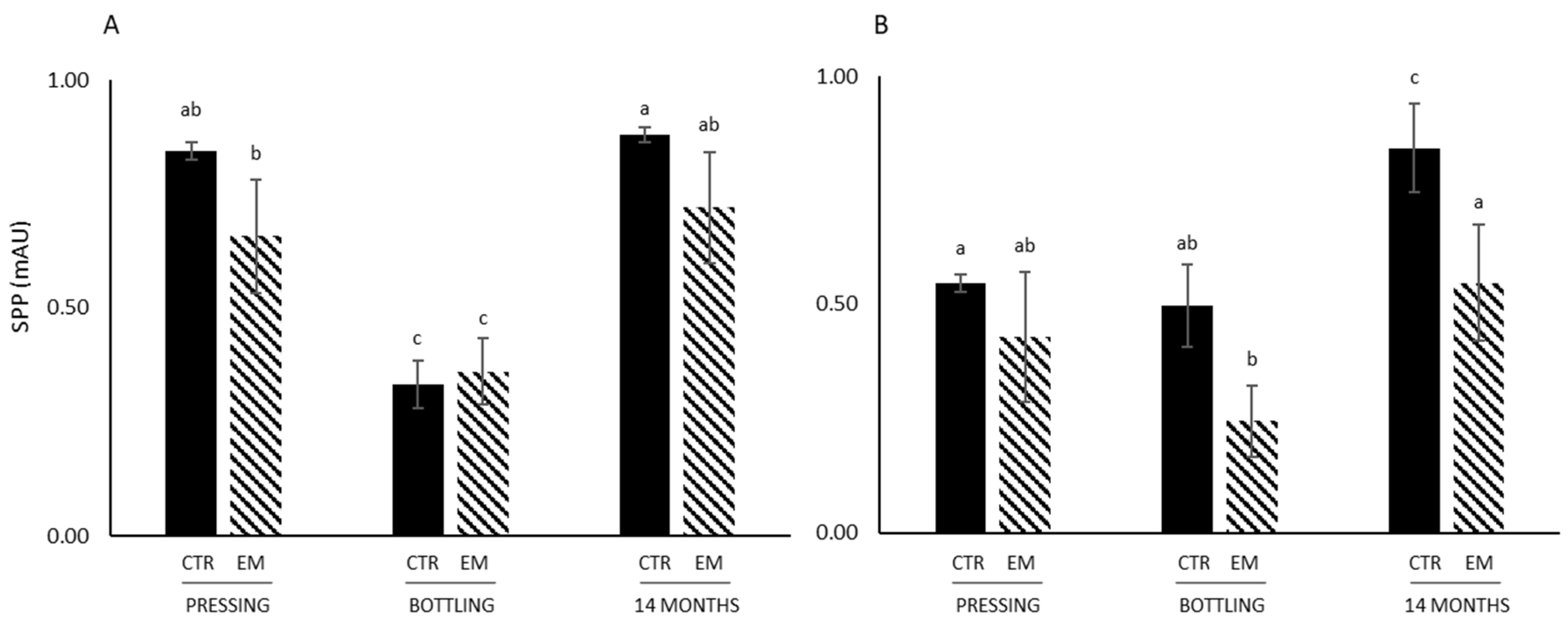
3.3. Color Parameters, Anthocyanin Concentration, SPP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPP | Small Polymeric Pigment |
| mDP | mean Degree of Polymerization |
| EM | extended maceration |
| IRP | iron-reactive phenolic |
| TA | titratable acidity |
References
- Schrader, J.A.; Cochran, D.R.; Domoto, P.A.; Nonnecke, G.R. Phenology and Winter Hardiness of Cold-Climate Grape Cultivars and Advanced Selections in Iowa Climate. HortTechnology 2019, 29, 906–922. [Google Scholar] [CrossRef]
- Cheng, Y.; Gapinski, A.D.; Buren, L.; Nonnecke, G.R.; Watrelot, A.A. Impact of Post-Fruit Set Leaf Removal on Marquette Phenolic Compounds during Berry Development and Ripening. Am. J. Enol. Vitic. 2023, 74, 0740027. [Google Scholar] [CrossRef]
- Gapinski, A.D.; Delchier, N.; Watrelot, A.A. Tannin and Iron-Reactive Phenolics Content in Red Cold-Hardy Hybrid Grape Tissues throughout Development and Ripening. Foods 2024, 13, 986. [Google Scholar] [CrossRef]
- Pedneault, K.; Dorais, M.; Angers, P. Flavor of Cold-Hardy Grapes: Impact of Berry Maturity and Environmental Conditions. J. Agric. Food Chem. 2013, 61, 10418–10438. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Norton, E.L. Chemistry and Reactivity of Tannins in Vitis Spp.: A Review. Molecules 2020, 25, 2110. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roca, J.M.; Gil-Muñoz, R.; Bautista-Ortín, A.B. The Extraction of Anthocyanins and Proanthocyanidins from Grapes to Wine during Fermentative Maceration Is Affected by the Enological Technique. J. Agric. Food Chem. 2011, 59, 5450–5455. [Google Scholar] [CrossRef]
- Cheng, Y.; Savits, J.R.; Watrelot, A.A. Effect of the Application Time of Accentuated Cut Edges (ACE) on Marquette Wine Phenolic Compounds. Molecules 2022, 27, 542. [Google Scholar] [CrossRef]
- Manns, D.C.; Lenerz, C.T.M.C.; Mansfield, A.K. Impact of Processing Parameters on the Phenolic Profile of Wines Produced from Hybrid Red Grapes Maréchal Foch, Corot noir, and Marquette. J. Food Sci. 2013, 78, C696–C702. [Google Scholar] [CrossRef]
- Cheng, Y.; Watrelot, A.A. Synergetic Effect of Accentuated Cut Edges (ACE) and Macerating Enzymes on the Phenolic Composition of Marquette Red Wines. Food Res. Int. 2024, 195, 114968. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold Maceration Application in Red Wine Production and Its Effects on Phenolic Compounds: A Review. LWT 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Reynolds, A.; Cliff, M.; Girard; Kopp, T.G. Influence of Fermentation Temperature on Composition and Sensory Properties of Semillon and Shiraz Wine. Am. J. Enol. Vitic. 2001, 52, 235–240. [Google Scholar]
- Casassa, L.F.; Kuster, S.T.; Gannet, P.; Watrelot, A.A. Temperature and Cap Management Effects on the Chemical, Phenolic, and Chromatic Composition of Pinot Noir Wines from the Central Coast of California. Am. J. Enol. Vitic. 2023, 74, 0740031. [Google Scholar] [CrossRef]
- Frost, S.C.; Blackman, J.W.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H. Extended Maceration and Cap Management Impacts on the Phenolic, Volatile, and Sensory Profile of Merlot Wine. Am. J. Enol. Vitic. 2018, 69, 360–370. [Google Scholar] [CrossRef]
- Scepanovic, R.P.; Vuletic, D.; Christofi, S.; Kallithraka, S. Maceration Duration and Grape Variety: Key Factors in Phenolic Compound Enrichment of Montenegrin Red Wine. OENO One 2024, 58, 3. [Google Scholar] [CrossRef]
- Auw, J.M.; Blanco, V.; O’Keefe, S.F.; Sims, C.A. Effect of Processing on the Phenolics and Color of Cabernet Sauvignon, Chambourcin, and Noble Wines and Juices. Am. J. Enol. Vitic. 1996, 47, 279–286. [Google Scholar] [CrossRef]
- Gil, M.; Quirós, M.; Fort, F.; Morales, P.; Gonzalez, R.; Canals, J.-M.; Zamora, F. Influence of Grape Maturity and Maceration Length on Polysaccharide Composition of Cabernet Sauvignon Red Wines. Am. J. Enol. Vitic. 2015, 66, 393–397. [Google Scholar] [CrossRef]
- Belen Bautista-Ortin, A.; Martinez-Hernandez, A.; Ruiz-Garcia, Y.; Gil-Munoz, R.; Gomez-Plaza, E. Anthocyanins Influence Tannin-Cell Wall Interactions. Food Chem. 2016, 206, 239–248. [Google Scholar] [CrossRef]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.S.; Harbertson, J.F. Effect of Extended Maceration and Ethanol Concentration on the Extraction and Evolution of Phenolics, Colour Components and Sensory Attributes of Merlot Wines. Aust. J. Grape Wine Res. 2013, 19, 25–39. [Google Scholar] [CrossRef]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.; Larsen, R.C.; Hopfer, H.; Heymann, H.; Harbertson, J.F. Influence of Fruit Maturity, Maceration Length, and Ethanol Amount on Chemical and Sensory Properties of Merlot Wines. Am. J. Enol. Vitic. 2013, 64, 437–449. [Google Scholar] [CrossRef]
- Gapinski, A.D.; Horton, A.C.; Watrelot, A.A. Effect of Whole Cluster Fermentation on Phenolics in Cold-Hardy Hybrid Wines. Food Bioprocess Technol. 2023, 6, 1595–1608. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Hodgins, R.E.; Thurston, L.N.; Schaffer, L.J.; Reid, M.S.; Landon, J.L.; Ross, C.F.; Adams, D.O. Variability of Tannin Concentration in Red Wines. Am. J. Enol. Vitic. 2008, 59, 210–214. [Google Scholar] [CrossRef]
- Heredia, T.M.; Adams, D.O.; Fields, K.C.; Held, P.G.; Harbertson, J.F. Evaluation of a Comprehensive Red Wine Phenolics Assay Using a Microplate Reader. Am. J. Enol. Vitic. 2006, 57, 6. [Google Scholar] [CrossRef]
- Revelette, M.R.; Barak, J.A.; Kennedy, J.A. High-Performance Liquid Chromatography Determination of Red Wine Tannin Stickiness. J. Agric. Food Chem. 2014, 62, 6626–6631. [Google Scholar] [CrossRef]
- Riesterer-Loper, J.; Workmaster, B.A.; Atucha, A. Impact of Fruit Zone Sunlight Exposure on Ripening Profiles of Cold Climate Interspecific Hybrid Winegrapes. Am. J. Enol. Vitic. 2019, 70, 286–296. [Google Scholar] [CrossRef]
- Scharfetter, J.; Workmaster, B.A.; Atucha, A. Preveraison Leaf Removal Changes Fruit Zone Microclimate and Phenolics in Cold Climate Interspecific Hybrid Grapes Grown under Cool Climate Conditions. Am. J. Enol. Vitic. 2019, 70, 297–307. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Cheng, Y.; Watrelot, A.A. Effects of Saignée and Bentonite Treatment on Phenolic Compounds of Marquette Red Wines. Molecules 2022, 27, 3482. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kassara, S.; Smith, P.A. Towards a Model of Grape Tannin Extraction under Wine-like Conditions: The Role of Suspended Mesocarp Material and Anthocyanin Concentration. Aust. J. Grape Wine Res. 2017, 23, 22–32. [Google Scholar] [CrossRef]
- Watrelot, A.A. Tannin Content in Vitis Species Red Wines Quantified Using Three Analytical Methods. Molecules 2021, 26, 4923. [Google Scholar] [CrossRef]
- Vernhet, A.; Dupre, K.; Boulange-Petermann, L.; Cheynier, V.; Pellerin, P.; Moutounet, M. Composition of Tartrate Precipitates Deposited on Stainless Steel Tanks During the Cold Stabilization of Wines. Part II. Red Wines. Am. J. Enol. Vitic. 1999, 50, 398–403. [Google Scholar] [CrossRef]
- Ghanem, C.; Taillandier, P.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Evolution of Polyphenols during Syrah Grapes Maceration: Time versus Temperature Effect. Molecules 2019, 24, 2845. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Mireles, M.S.; Harwood, E.D.; Weller, K.M.; Ross, C.F. Chemical and Sensory Effects of Saignée, Water Addition, and Extended Maceration on High Brix Must. Am. J. Enol. Vitic. 2009, 60, 450–460. [Google Scholar] [CrossRef]
- Casassa, L.F.; Huff, R.; Steele, N.B. Chemical Consequences of Extended Maceration and Post-Fermentation Additions of Grape Pomace in Pinot Noir and Zinfandel Wines from the Central Coast of California (USA). Food Chem. 2019, 300, 125147. [Google Scholar] [CrossRef]
- Smith, P.A.; McRae, J.M.; Bindon, K.A. Impact of Winemaking Practices on the Concentration and Composition of Tannins in Red Wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Bouska, L. Optimization of the Ultrasound-Assisted Extraction of Polyphenols from Aronia and Grapes. Food Chem. 2022, 386, 132703. [Google Scholar] [CrossRef]
- Cheng, Y.; Wimalasiri, P.M.; Tian, B.; Watrelot, A.A. Influence of Grape Flesh on the Retention and Composition of Polyphenols from Skins and Seeds. J. Agric. Food Chem. 2024, 72, 9351–9364. [Google Scholar] [CrossRef]
- Kassara, S.; Norton, E.L.; Mierczynska-Vasilev, A.; Lavi Sacks, G.; Bindon, K.A. Quantification of Protein by Acid Hydrolysis Reveals Higher than Expected Concentrations in Red Wines: Implications for Wine Tannin Concentration and Colloidal Stability. Food Chem. 2022, 385, 132658. [Google Scholar] [CrossRef]
- Springer, L.F.; Sacks, G.L. Protein-Precipitable Tannin in Wines from Vitis Vinifera and Interspecific Hybrid Grapes (Vitis Ssp.): Differences in Concentration, Extractability, and Cell Wall Binding. J. Agric. Food Chem. 2014, 62, 7515–7523. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Kuhlman, B.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; du Toit, W.J. Untangling the Impact of Red Wine Maceration Times on Wine Ageing. A Multidisciplinary Approach Focusing on Extended Maceration in Shiraz Wines. Food Res. Int. 2021, 150, 110697. [Google Scholar] [CrossRef]
- Burtch, C.; Mansfield, A.K. Comparing Red Wine Color in V. vinifera and Hybrid Cultivars. Appell. Cornell Res. Focus 2016, 6, 1–6. [Google Scholar]
- Casassa, L.F.; Larsen, R.C.; Beaver, C.W.; Mireles, M.S.; Keller, M.; Riley, W.R.; Smithyman, R.; Harbertson, J.F. Impact of Extended Maceration and Regulated Deficit Irrigation (RDI) in Cabernet Sauvignon Wines: Characterization of Proanthocyanidin Distribution, Anthocyanin Extraction, and Chromatic Properties. J. Agric. Food Chem. 2013, 61, 6446–6457. [Google Scholar] [CrossRef] [PubMed]
- Medina-Plaza, C.; Beaver, J.W.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions. Molecules 2019, 24, 3350. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of Polyphenols to Plant Cell Wall Analogues–Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Merrell, C.P.; Larsen, R.C.; Harbertson, J.F. Effects of Berry Maturity and Wine Alcohol on Phenolic Content during Winemaking and Aging. Am. J. Enol. Vitic. 2018, 69, 1–11. [Google Scholar] [CrossRef]
| Marquette | Petite Pearl | p-Value | |
|---|---|---|---|
| Brix | 22.43 ± 1.29 ‡ | 19.00 ± 0.10 | 0.021 |
| pH | 2.99 ± 0.08 | 3.15 ± 0.02 | 0.961 |
| Titratable acidity (g/L) | 13.55 ± 1.15 ‡ | 8.21 ± 0.30 | 0.005 |
| Tartaric acid (g/L) | 8.89 ± 0.52 ‡ | 7.10 ± 0.10 | 0.012 |
| Malic acid (g/L) | 8.06 ± 1.40 ‡ | 2.53 ± 0.12 | 0.010 |
| Citric acid (mg/L) | 0.50 ± 0.06 ‡ | 0.34 ± 0.01 | 0.019 |
| Hue | 0.93 ± 0.52 | 0.49 ± 0.07 | 0.139 |
| Color intensity (AU) | 4.42 ± 1.02 | 7.25 ± 3.03 | 0.878 |
| L* | 45.47 ± 6.39 | 30.77 ± 9.78 | 0.053 |
| a* | 52.06 ± 11.36 | 53.60 ± 6.50 | 0.572 |
| b* | 52.95 ± 2.57 ‡ | 40.15 ± 6.43 | 0.029 |
| Total phenolics (mg/L) | 308.3 ± 111.9 | 342.30 ± 16.30 | 0.673 |
| Tannin (mg/L) | 16.33 ± 4.85 | 21.51 ± 5.91 | 0.846 |
| Anthocyanins (mg/L) | 542.2 ± 11.4‡ | 109.90 ± 47.00 | 0.001 |
| SPP | 1.10 ± 0.50 | 0.33 ± 0.14 | 0.052 |
| Marquette | Petite Pearl | ||||
|---|---|---|---|---|---|
| Control | EM | Control | EM | ||
| pH | Pressing | 3.20 ± 0.03 a | 3.30 ± 0.11 a | 3.22 ± 0.04 a | 3.33 ± 0.07 a |
| Bottling | 3.26 ± 0.04 a | 3.39 ± 0.06 a | 3.18 ± 0.04 a | 3.25 ± 0.03 a | |
| 14 months | 3.20 ± 0.01 a | 3.28 ± 0.14 a | 3.18 ± 0.07 a | 3.23 ± 0.09 a | |
| Titratable acidity (g/L) | Pressing | 12.58 ± 0.74 a | 10.66 ± 1.41 ab | 9.57 ± 0.98 a | 9.21 ± 0.68 ab |
| Bottling | 8.74 ± 0.24 bc | 8.55 ± 0.15 bc | 8.07 ± 0.28 ab | 7.80 ± 0.36 ab | |
| 14 months | 7.91 ± 0.27 c | 9.20 ± 1.66 bc | 7.75 ± 1.13 ab | 7.35 ± 0.65 b | |
| Tartaric acid (g/L) | Pressing | 5.87 ± 0.15 a | 5.05 ± 0.42 b | 6.36 ± 0.56 a | 5.05 ± 0.85 ab |
| Bottling | 3.47 ± 0.19 d | 3.11 ± 0.30 d | 3.90 ± 0.44 b | 3.61 ± 0.37 b | |
| 14 months | 4.22 ± 0.01 c | 4.48 ± 0.25 bc | 4.93 ± 0.30 ab | 4.32 ± 0.91 b | |
| Malic acid (g/L) | Pressing | 5.02 ± 0.07 a | 0.02 ± 0.01 b | 0.85 ± 0.02 a | 0.02 ± 0.01 b |
| Bottling | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 0.02 ± 0.01 b | |
| 14 months | nd | nd | nd | nd | |
| Lactic acid (g/L) | Pressing | 0.08 ± 0.01 b | 1.37 ± 0.23 a | 0.28 ± 0.07 b | 0.64 ± 0.04 a |
| Bottling | 1.38 ± 0.01 a | 1.37 ± 0.21 a | 0.60 ± 0.07 a | 0.65 ± 0.04 a | |
| 14 months | 1.63 ± 0.01 a | 1.57 ± 0.34 a | 0.73 ± 0.15 a | 0.80 ± 0.05 a | |
| Acetic acid (g/L) | Pressing | 0.32 ± 0.02 e | 0.45 ± 0.00 b | 0.29 ± 0.01 b | 0.42 ± 0.01 a |
| Bottling | 0.37 ± 0.01 d | 0.47 ± 0.01 b | 0.38 ± 0.01 ab | 0.42 ± 0.02 ab | |
| 14 months | 0.41 ± 0.00 c | 0.54 ± 0.02 a | 0.29 ± 0.12 b | 0.50 ± 0.06 a | |
| Ethanol(vol %) | Pressing | 11.38 ± 0.07 a | 11.53 ± 0.61 a | 9.42 ± 0.48 a | 8.62 ± 0.96 a |
| Bottling | 11.99 ± 0.20 a | 12.04 ± 0.89 a | 9.55 ± 0.37 a | 8.73 ± 0.96 a | |
| 14 months | 11.97 ± 0.29 a | 11.95 ± 0.97 a | 9.60 ± 0.26 a | 9.39 ± 0.85 a | |
| Marquette | Petite Pearl | ||||
|---|---|---|---|---|---|
| Control | EM | Control | EM | ||
| Hue | Pressing | 0.39 ± 0.01 c | 0.44 ± 0.04 bc | 0.39 ± 0.01 d | 0.50 ± 0.04 cd |
| Bottling | 0.45 ± 0.01 bc | 0.53 ± 0.04 b | 0.45 ± 0.02 d | 0.57 ± 0.01 bc | |
| 14 months | 0.75 ± 0.01 a | 0.77 ± 0.10 a | 0.62 ± 0.03 ab | 0.73 ± 0.09 a | |
| Color Intensity | Pressing | 16.40 ± 0.96 a | 13.53 ± 3.34 ab | 15.42 ± 1.51 a | 6.66 ± 2.04 b |
| Bottling | 10.53 ± 0.89 bc | 8.13 ± 1.93 c | 10.48 ± 0.95 b | 5.43 ± 2.04 b | |
| 14 months | 10.29 ± 0.07 bc | 8.10 ± 1.37 c | 10.17 ± 0.95 b | 6.20 ± 1.03 b | |
| L* | Pressing | 16.30 ± 1.45 b | 19.37 ± 4.11 ab | 13.70 ± 1.67 b | 28.93 ± 7.53 ab |
| Bottling | 23.30 ± 1.97 ab | 26.80 ± 5.31 a | 18.97 ± 0.29 ab | 31.00 ± 11.17 a | |
| 14 months | 15.00 ± 0.10 b | 21.30 ± 3.47 ab | 15.70 ± 2.82 ab | 26.67 ± 4.48 ab | |
| a* | Pressing | 45.35 ± 1.69 bc | 48.08 ± 4.46 abc | 42.48 ± 1.88 b | 54.76 ± 4.79 a |
| Bottling | 52.28 ± 2.19 ab | 54.34 ± 4.07 a | 47.63 ± 0.33 ab | 52.71 ± 7.24 ab | |
| 14 months | 41.82 ± 0.02 c | 48.54 ± 2.97 abc | 44.01 ± 3.54 ab | 52.14 ± 2.30 ab | |
| b* | Pressing | 28.06 ± 2.53 b | 32.50 ± 6.68 ab | 23.53 ± 2.75 c | 32.71 ± 4.55 ab |
| Bottling | 38.99 ± 2.78 a | 39.51 ± 2.03 a | 31.53 ± 0.29 abc | 25.02 ± 2.32 bc | |
| 14 months | 25.60 ± 0.21 b | 34.26 ± 4.09 ab | 26.41 ± 4.29 bc | 35.26 ± 0.56 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watrelot, A.A.; Delchier, N. How Does Extended Maceration Affect Tannin and Color of Red Wines from Cold-Hardy Grape Cultivars? Foods 2025, 14, 1187. https://doi.org/10.3390/foods14071187
Watrelot AA, Delchier N. How Does Extended Maceration Affect Tannin and Color of Red Wines from Cold-Hardy Grape Cultivars? Foods. 2025; 14(7):1187. https://doi.org/10.3390/foods14071187
Chicago/Turabian StyleWatrelot, Aude A., and Nicolas Delchier. 2025. "How Does Extended Maceration Affect Tannin and Color of Red Wines from Cold-Hardy Grape Cultivars?" Foods 14, no. 7: 1187. https://doi.org/10.3390/foods14071187
APA StyleWatrelot, A. A., & Delchier, N. (2025). How Does Extended Maceration Affect Tannin and Color of Red Wines from Cold-Hardy Grape Cultivars? Foods, 14(7), 1187. https://doi.org/10.3390/foods14071187






