Marine-Derived Enterococcus faecalis HY0110 as a Next-Generation Functional Food Probiotic: Comprehensive In Vitro and In Vivo Bioactivity Evaluation and Synergistic Fermentation of Periplaneta americana Extract Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Strains
2.2. Strain Safety Evaluation
2.3. Simulated Gastrointestinal Survival Assay
2.4. Antibacterial Assay
2.5. Antioxidant Activity Assays
2.6. Cancer Cell Cytotoxicity Analysis
2.6.1. Cell Viability Assessment
2.6.2. Colony Formation Analysis with HY0110 Supernatant
2.6.3. Pro-Apoptotic Effects Analysis
2.6.4. Apoptosis Detection by One-Step TUNEL Assay
2.7. Animals and Treatments
2.7.1. Animal Studies and Ethics Authorization
2.7.2. Inflammatory Cytokine Detection in Mouse Serum
2.7.3. Analysis of Changes in Mouse Gut Microbiota
2.8. GC-MS Evaluation of Acid Output Capability
2.9. Metabolomic Analysis of HY0110 Fermented P. americana Powder
2.9.1. Sample Preparation
2.9.2. LC-MS Analysis
2.9.3. Data Processing and Analysis
2.9.4. Statistical and Bioinformatic Analysis
2.10. Statistical Analysis
3. Results and Discussion
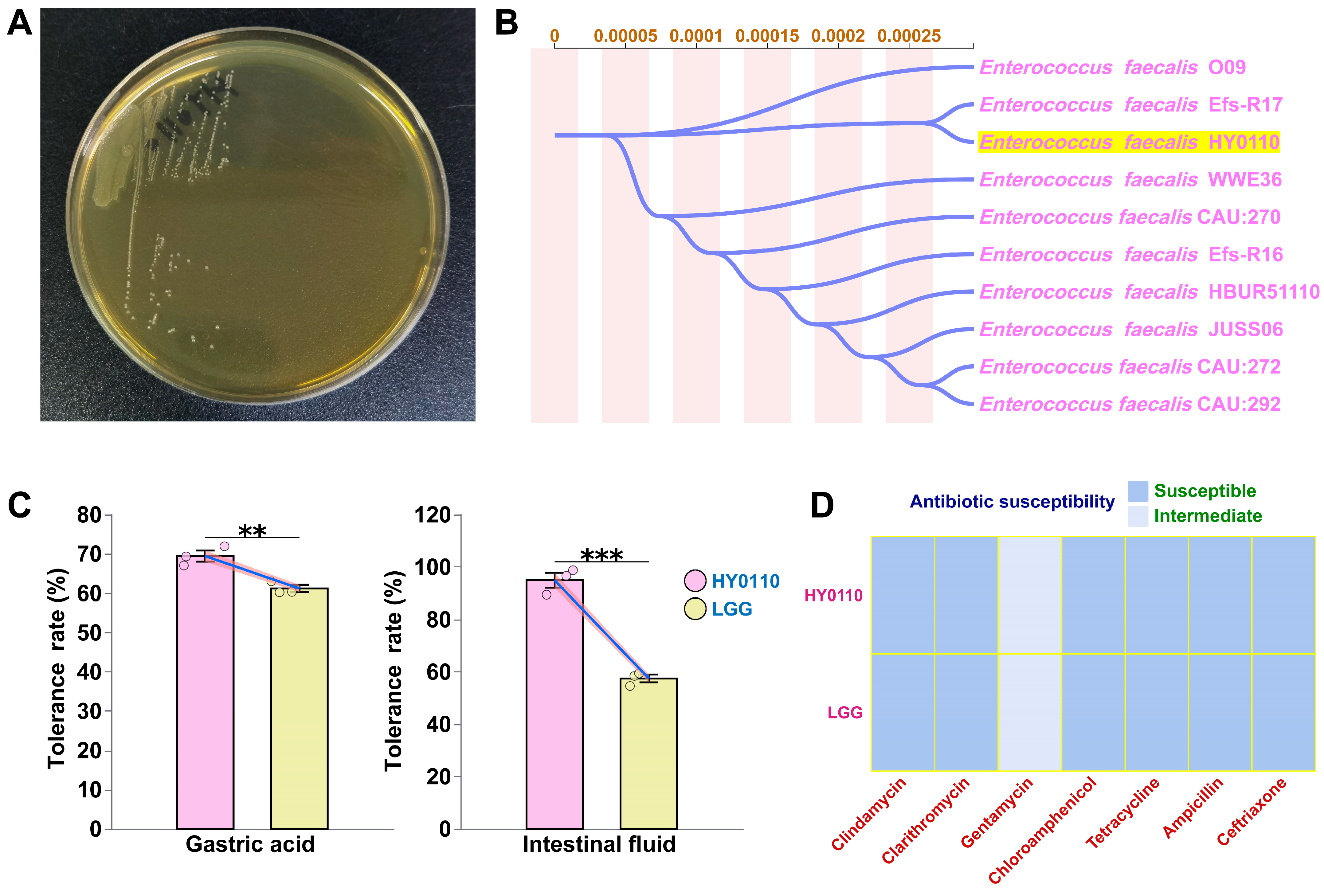
3.1. Taxonomic Characterization of E. faecalis HY0110
3.2. Antibiotic Susceptibility Tests
3.3. Gastrointestinal Transit Tolerance
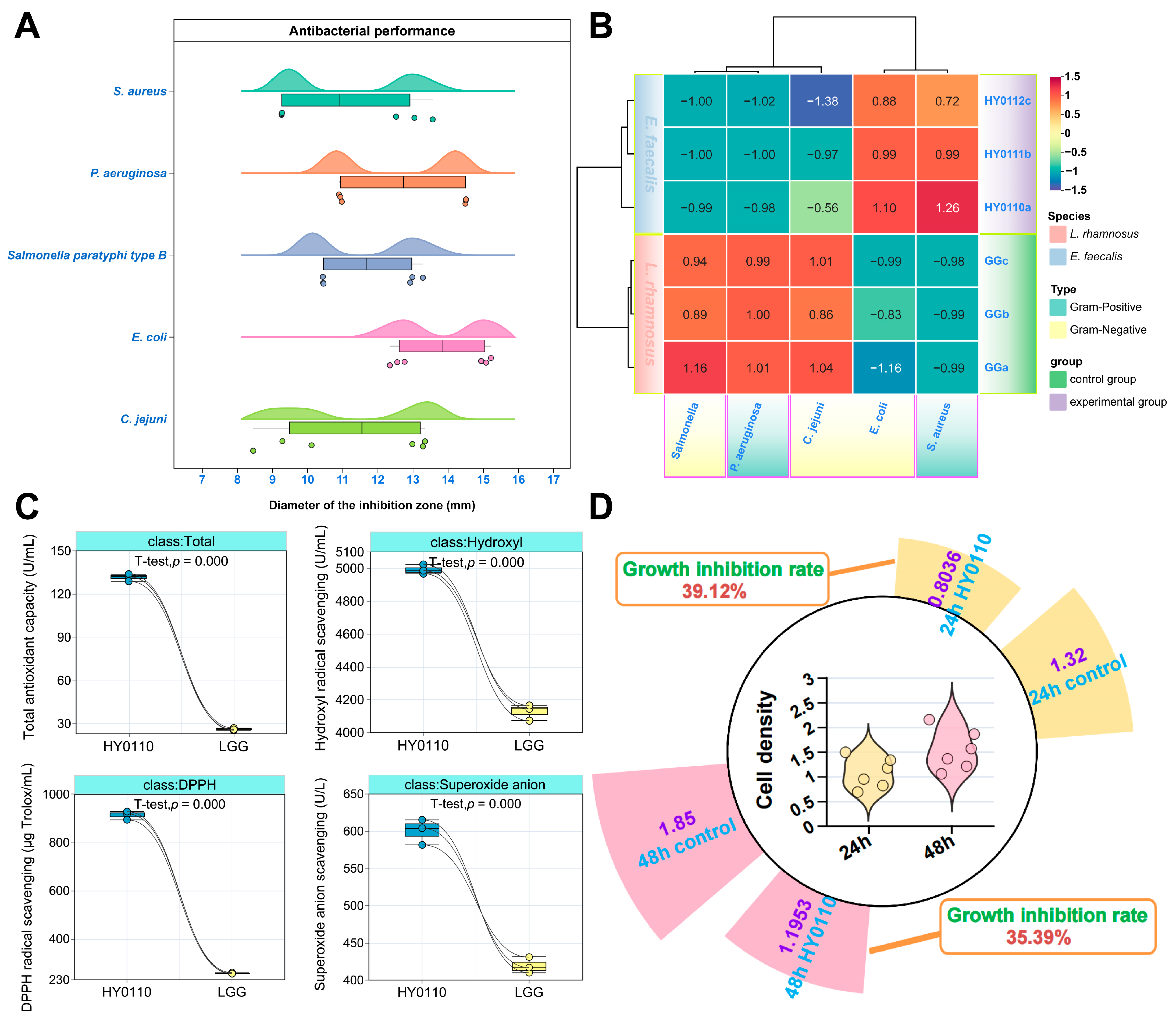
3.4. Antimicrobial, Antioxidant, and Antiproliferative Properties of Probiotic Strain HY0110
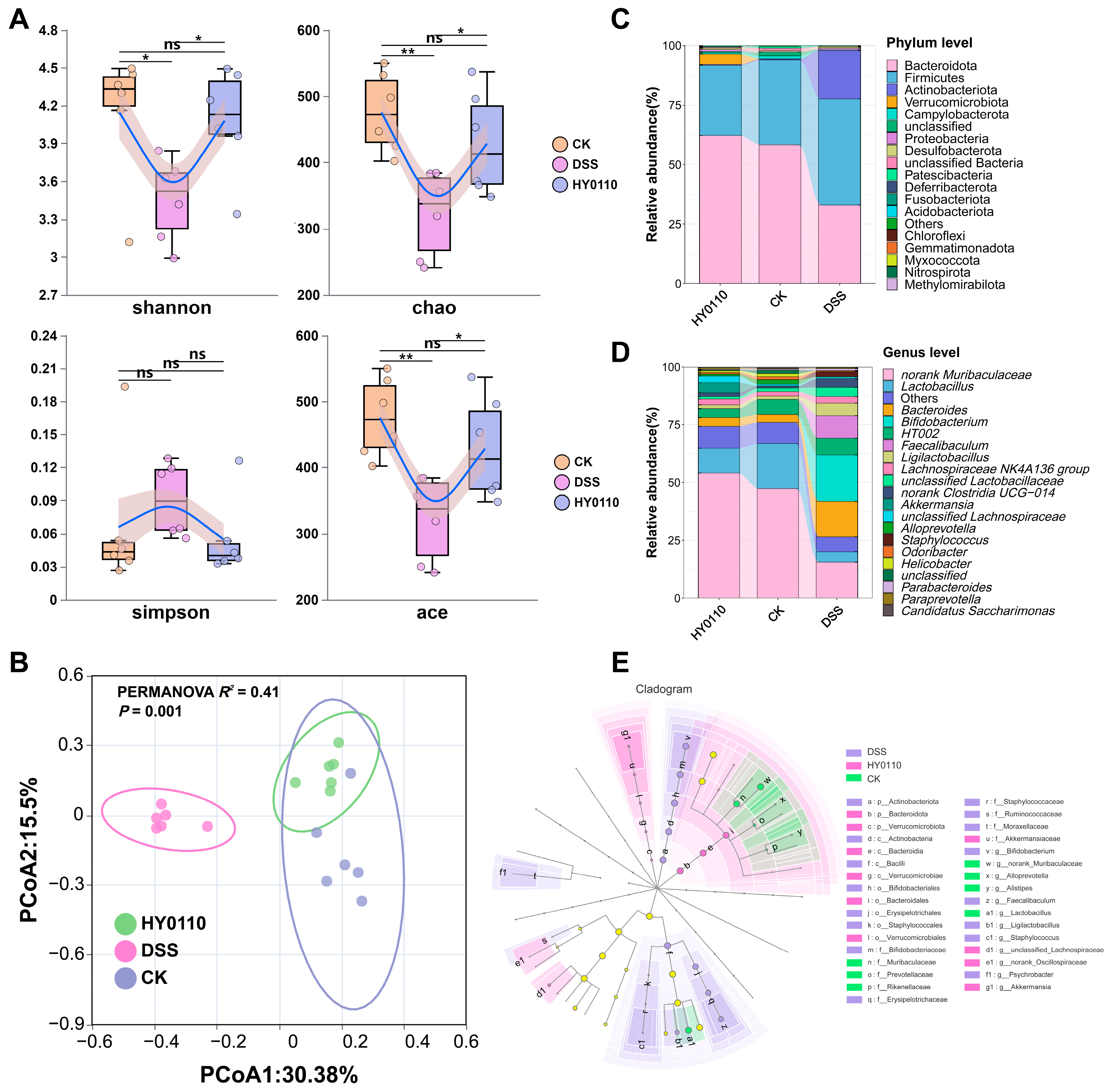
3.5. Effect of HY0110 Strain on Sodium Polysaccharide-Induced Colitis in Mice
3.5.1. Anti-Inflammatory Effects of HY0110 in DSS-Induced Colitis Model
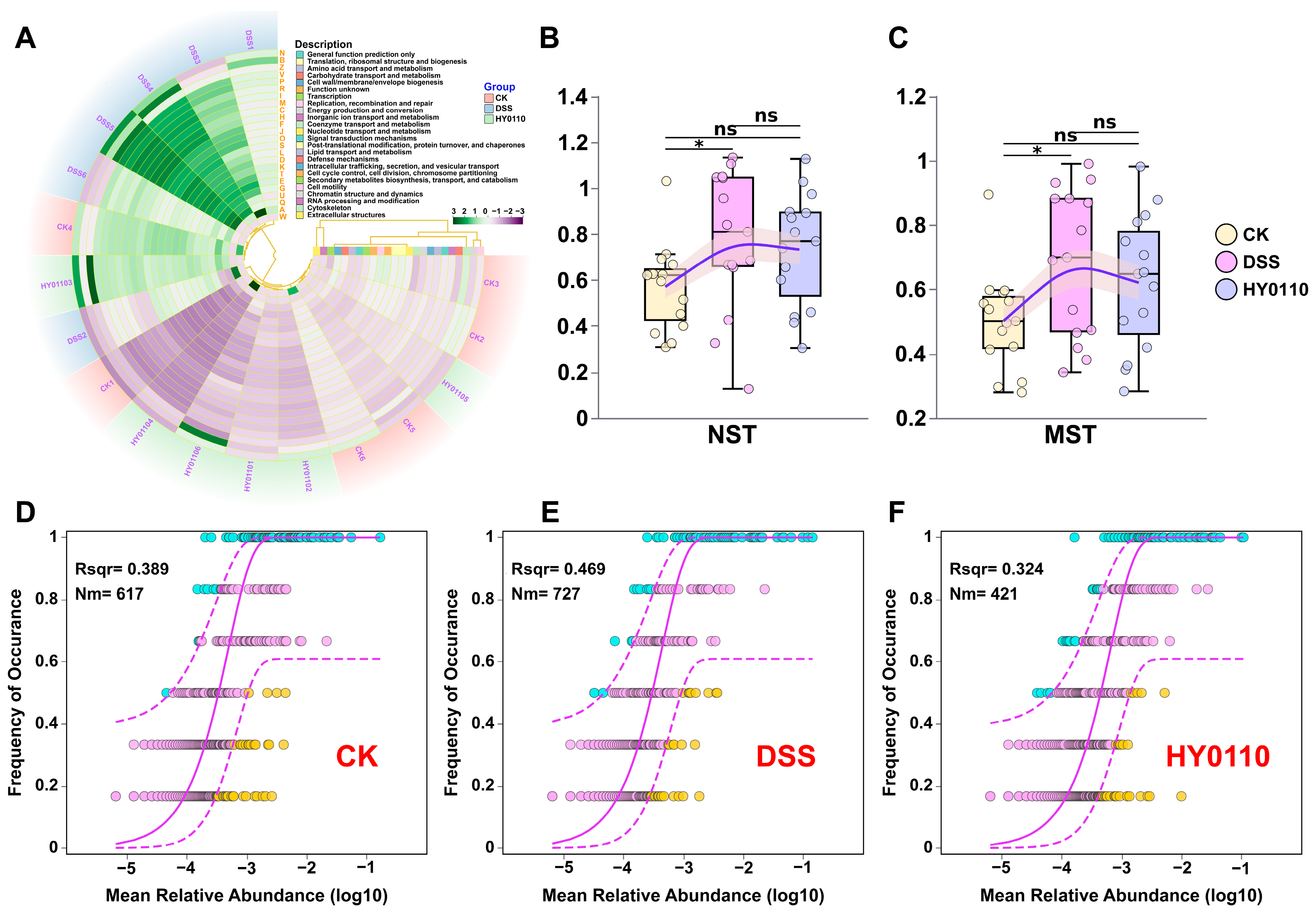
3.5.2. Examination of the Intestinal Microbiome Across Different Mouse Cohorts
3.5.3. Comparison with Existing Probiotic Strategies for Gut Microbiota and Immunomodulation
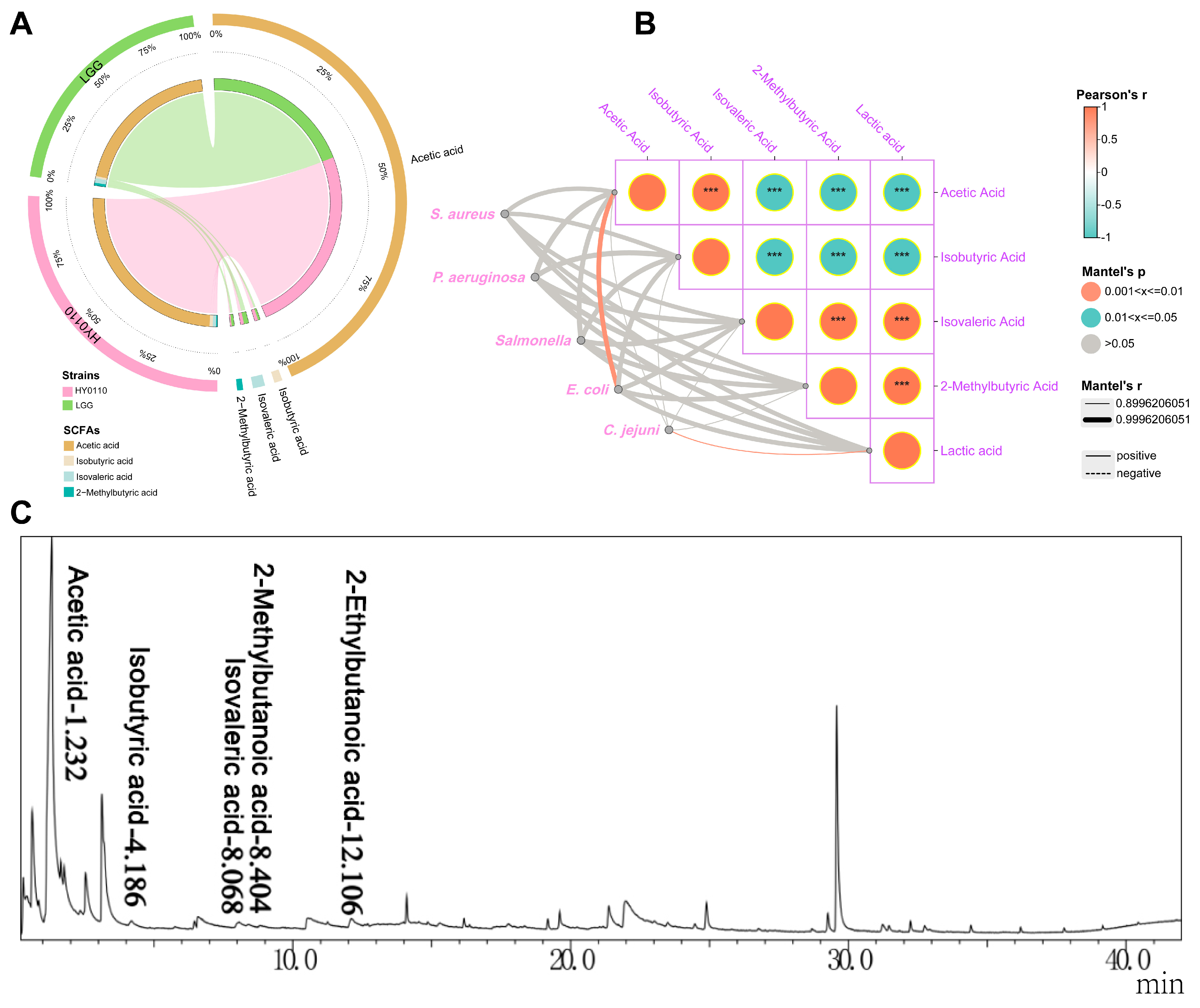
3.6. Major Antibacterial Components–Acid-Producing Capacity and Constituent Analysis
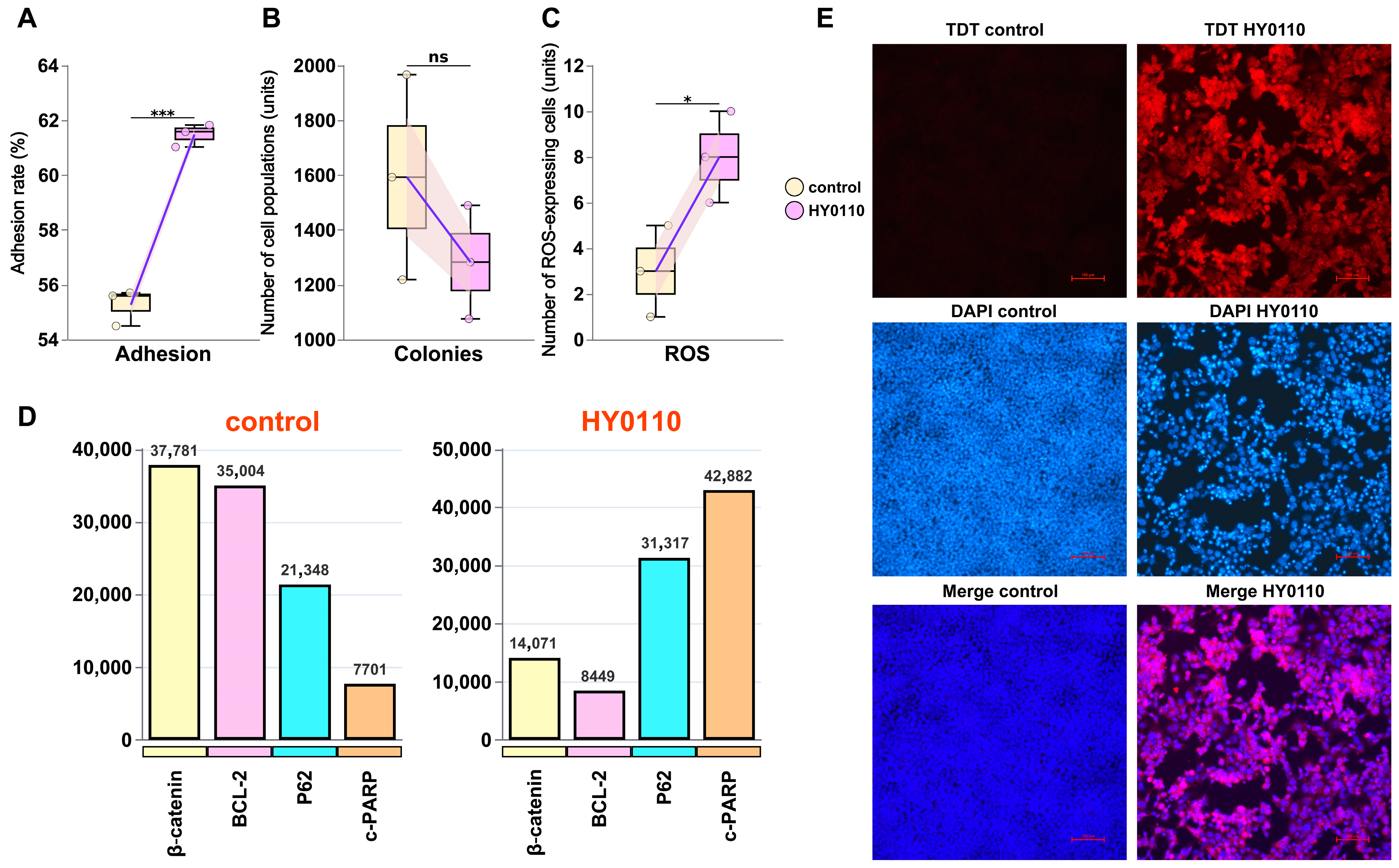
3.7. Mechanism of HT-29 Cell Inhibition
3.8. Metabolic Profiling of HY0110 Fermented and Non-Fermented P. americana Powder
3.8.1. HY0110 Fermentation Improves Bioavailability and Therapeutic Potential of P. americana Powder
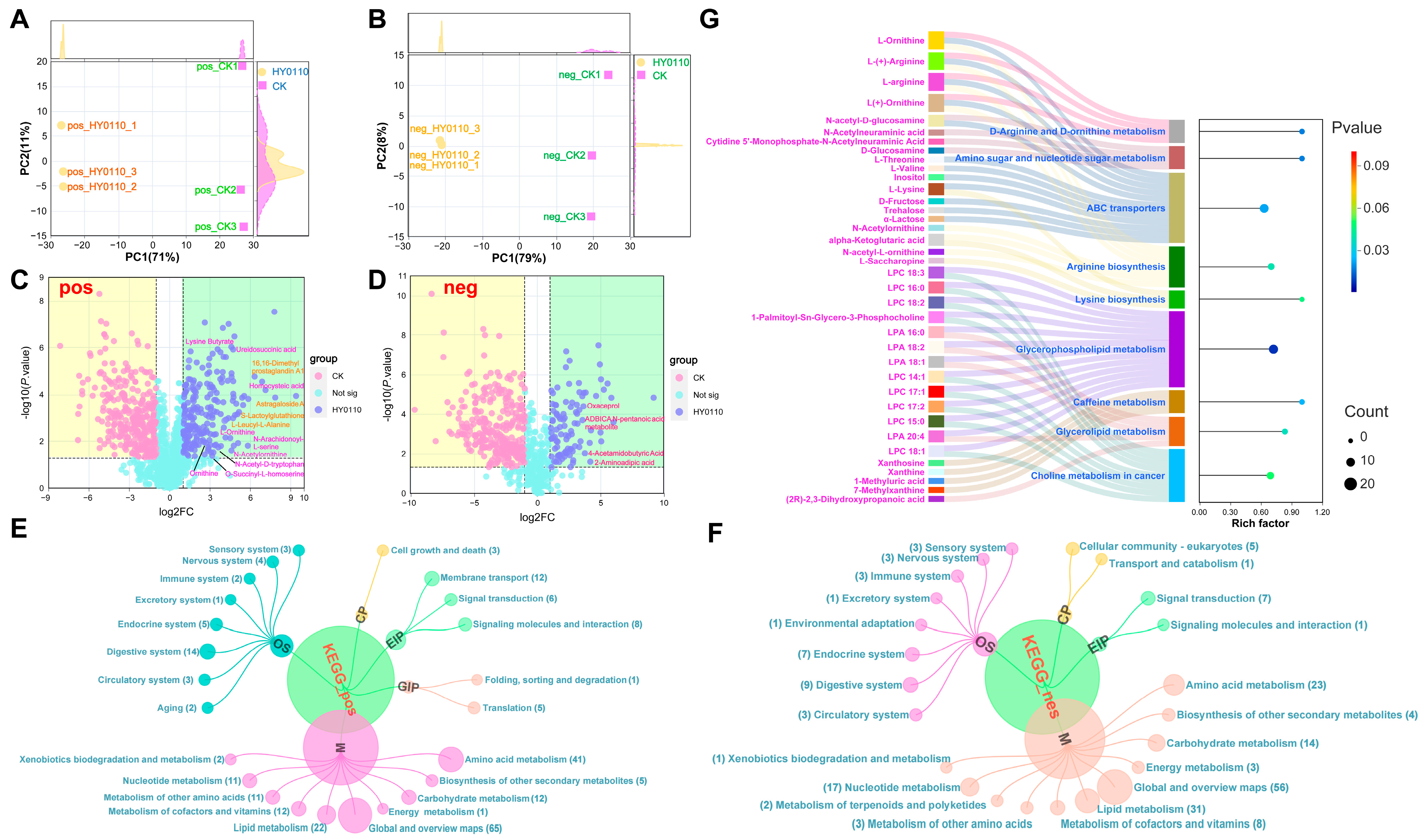
3.8.2. PCA Reveals Metabolic Differentiation
3.8.3. Significant Increases in Amino Acids and Peptides
3.8.4. KEGG Pathway Remodeling
3.8.5. Key Enriched Pathways
3.8.6. Metabolic Reorganization and Commercialization Challenges of HY0110-Fermented P. americana Powder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, C.; Fu, X.; Liu, Y.; Zhao, H.; Wang, G. Burden of infectious diseases and bacterial antimicrobial resistance in China: A systematic analysis for the global burden of disease study 2019. Lancet Reg. Health West Pac. 2024, 43, 100972. [Google Scholar] [PubMed]
- Sharma, V.; Sharma, N.; Sheikh, I.; Kumar, V.; Sehrawat, N.; Yadav, M.; Ram, G.; Sankhyan, A.; Sharma, A.K. Probiotics and Prebiotics Having Broad Spectrum Anticancer Therapeutic Potential: Recent Trends and Future Perspectives. Curr. Pharmacol. Rep. 2021, 7, 67–79. [Google Scholar]
- Pang, L.; Huang, Y.; Li, R.; Guo, L.; Man, C.; Yang, X.; Jiang, Y. Effects of postbiotics produced by Lactobacillus plantarum JM015 isolated from traditional fermented dairy products on Salmonella-induced intestinal inflammation: A preventive strategy. Food Chem. 2025, 469, 142549. [Google Scholar] [CrossRef]
- Salas-Ramírez, C.A.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; González-Laredo, R.F.; González Herrera, S.M.; González-Mercado, M.E.; Herrera-Rocha, K.M.; Cháirez-Ramirez, M.H. Rehydration Beverages Made from Quercus sideroxyla Infusion, Probiotics, and Prebiotics: Antioxidant and Anti-Inflammatory Potential. Foods 2025, 14, 837. [Google Scholar] [CrossRef]
- Li, W.; Xu, M.; Liu, Y.; Zhang, S.; Wang, J.; Zhang, Z.; Xiao, G.; Wang, R.; Zhang, J.; Xue, H. Lactiplantibacillus plantarum GOLDGUT-HNU082 Alleviates CUMS-Induced Depressive-like Behaviors in Mice by Modulating the Gut Microbiota and Neurotransmitter Levels. Foods 2025, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Uraporn, P.; Kanokrat, S.; Onrapak, R.; Pornphan, D. A novel bacteriocin from Enterococcus faecalis 478 exhibits a potent activity against vancomycin-resistant enterococci. PLoS ONE 2017, 12, e0186415. [Google Scholar]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2020, 45, fuaa039. [Google Scholar]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Ohashi, N.; Hirose, M.; Kudo, K.; Tsukamoto, N.; Ito, M.; Kobayashi, N. Antimicrobial resistance, virulence factors, and genotypes of Enterococcus faecalis and Enterococcus faecium clinical isolates in northern Japan: Identification of optrA in ST480 E. faecalis. Antibiotics 2023, 12, 108. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. ‘Multi-omics’ data integration: Applications in probiotics studies. npj Sci. Food 2023, 7, 25. [Google Scholar] [CrossRef]
- Lee, C.-G.; Cha, K.H.; Kim, G.-C.; Im, S.-H.; Kwon, H.-K. Exploring probiotic effector molecules and their mode of action in gut–immune interactions. FEMS Microbiol. Rev. 2023, 47, fuad046. [Google Scholar]
- Haranahalli Nataraj, B.; Behare, P.V.; Yadav, H.; Srivastava, A.K. Emerging pre-clinical safety assessments for potential probiotic strains: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 8155–8183. [Google Scholar] [PubMed]
- Shu, W.-S.; Huang, L.-N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar]
- Li, S.-l.; Deng, J.-c.; Li, C.-s.; Yang, X.-q.; Wu, Y.-y.; Chen, S.-j.; Ma, H.-x. Isolation and identification of biogenic amine-producing bacteria from raw bigeye tuna (Thunnus obesus). Food Ferment. Ind. 2020, 46, 121–126. [Google Scholar]
- Khajuie, F.; Valizadeh, R.; Naserian, A.-A.; Dadvar, P.; Dayani, O. Evaluation of cockroach (Periplaneta americana) powder as a potential feed ingredient for ruminants: Chemical composition, fatty acids profile and ruminal degradability. J. Livest. Sci. Technol. (JLST) 2022, 10, 19–29. [Google Scholar]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 4819–4841. [Google Scholar]
- Koichiro, T.; Glen, S.; Sudhir, K. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar]
- Huang, F.; Ju, Z.; Hou, Y.; Zhao, G.; Yang, Y.; Yue, B.; Zhang, X. Exploring advanced antimicrobial effects of Pediococcus pentosaceus and Lactococcus lactis derived from Bufo gargarizans: In vitro analysis and in vivo evaluation in mice. LWT 2024, 210, 116851. [Google Scholar]
- Steele, C. Lactobacillus rhamnosus GG: A review of clinical use and efficacy. Nutr. Med. J. 2022, 1, 70–116. [Google Scholar]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef]
- Hall, M.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2. Methods Mol. Biol. 2018, 1849, 113–129. [Google Scholar]
- Chang, F.; He, S.; Dang, C. Assisted Selection of Biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in Microbiome Data. J. Vis. Exp. JoVE 2022, 16, e61715. [Google Scholar]
- Yin, Y.; Wang, J. Predictive functional profiling of microbial communities in fermentative hydrogen production system using PICRUSt. Int. J. Hydrog. Energy 2020, 46, 3716–3725. [Google Scholar]
- Xiong, W.; Yang, J.; Zeng, J.; Xiao, D.; Tong, C.; Zeng, Z. Metagenomic analysis of antimicrobial resistance in ducks, workers, and the environment in duck farms, southern China. Ecotoxicol. Environ. Saf. 2023, 262, 115191. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb) 2021, 2, 100141. [Google Scholar] [PubMed]
- Maeda, T.; Furusawa, C. Laboratory evolution of antimicrobial resistance in bacteria to develop rational treatment strategies. Antibiotics 2024, 13, 94. [Google Scholar] [CrossRef]
- Shridhar, P.B.; Amachawadi, R.G.; Tokach, M.; Patel, I.; Gangiredla, J.; Mammel, M.; Nagaraja, T. Whole genome sequence analyses-based assessment of virulence potential and antimicrobial susceptibilities and resistance of Enterococcus faecium strains isolated from commercial swine and cattle probiotic products. J. Anim. Sci. 2022, 100, skac030. [Google Scholar]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Antibiotic resistance: Moving from individual health norms to social norms in one health and global health. Front. Microbiol. 2020, 11, 1914. [Google Scholar] [CrossRef]
- Somayaji, A.; Dhanjal, C.R.; Lingamsetty, R.; Vinayagam, R.; Selvaraj, R.; Varadavenkatesan, T.; Govarthanan, M. An insight into the mechanisms of homeostasis in extremophiles. Microbiol. Res. 2022, 263, 127115. [Google Scholar] [CrossRef]
- Ertürkmen, P.; Fırıncıoğulları, B.; Öner, Z. The Expression levels of genes responsible for the enzymatic activity of bile salt hydrolase (BSH) and the relationship of cholesterol assimilation in L. plantarum and L. paracasei. Curr. Microbiol. 2023, 80, 205. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant activity and probiotic properties of lactic acid bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Aindelis, G.; Chlichlia, K. Modulation of anti-tumour immune responses by probiotic bacteria. Vaccines 2020, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Sirikhwan, T.; Piyatip, K.; Krit, T.; Traidej, C.M. In vitro assessment of Enterococcus faecalis MTC 1032 as the potential probiotic in food supplements. J. Food Sci. Technol. 2018, 55, 2384–2394. [Google Scholar]
- Indranil, C.; Kalyani, K.; Prasenjit, P.; Nath, M.G.; Soumitra, M.; Ranjan, P.B.; Nandan, B. Structural Characterization of an Exopolysaccharide Isolated from Enterococcus faecalis, and Study on its Antioxidant Activity, and Cytotoxicity Against HeLa Cells. Curr. Microbiol. 2020, 77, 3125–3135. [Google Scholar]
- Bahrami, A.; Khalaji, A.; Najafi, M.B.; Sadati, S.; Raisi, A.; Abolhassani, A.; Eshraghi, R.; Mahabady, M.K.; Rahimian, N.; Mirzaei, H. NF-κB pathway and angiogenesis: Insights into colorectal cancer development and therapeutic targets. Eur. J. Med. Res. 2024, 29, 610. [Google Scholar] [CrossRef]
- Hua, Z.; Liu, S.; Yang, G.; Hou, X.; Fang, Y. Next-Generation Probiotics: Innovations in Safety Assessments. Curr. Opin. Food Sci. 2024, 61, 101238. [Google Scholar] [CrossRef]
- Peter, L. Targeting Inflammatory Pathways in Cardiovascular Disease: The Inflammasome, Interleukin-1, Interleukin-6 and Beyond. Cells 2021, 10, 951. [Google Scholar] [CrossRef]
- Finn, D.R. A metagenomic alpha-diversity index for microbial functional biodiversity. FEMS Microbiol. Ecol. 2024, 100, fiae019. [Google Scholar] [CrossRef]
- Jost, S.M.; Cardona, L.; Rohrbach, E.; Mathis, A.; Holliger, C.; Verhulst, N.O. Environment rather than breed or body site shapes the skin bacterial community of healthy sheep as revealed by metabarcoding. Vet. Dermatol. 2023, 35, 273–283. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar]
- Li, L.; Li, M.; Chen, Y.; Yu, Z.; Cheng, P.; Yu, Z.; Cheng, W.; Zhang, W.; Wang, Z.; Gao, X. Function and therapeutic prospects of next-generation probiotic Akkermansia muciniphila in infectious diseases. Front. Microbiol. 2024, 15, 1354447. [Google Scholar]
- Wang, C.; Gu, Y.; Chu, Q.; Wang, X.; Ding, Y.; Qin, X.; Liu, T.; Wang, S.; Liu, X.; Wang, B. Gut microbiota and metabolites as predictors of biologics response in inflammatory bowel disease: A comprehensive systematic review. Microbiol. Res. 2024, 282, 127660. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Geraldi, M.V.; Cedran, M.F.; Bicas, J.L.; Junior, M.R.M.; Pastore, G.M. Prebiotics and probiotics. In Bioactive Food Components Activity in Mechanistic Approach; Elsevier: Amsterdam, The Netherlands, 2022; pp. 55–118. [Google Scholar]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [PubMed]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Vallejos, O.P.; Bueno, S.M.; Kalergis, A.M. Probiotics in inflammatory bowel disease: Microbial modulation and therapeutic prospects. Trends Mol. Med. 2025. [Google Scholar] [CrossRef]
- Qin, S.; Huang, Z.; Wang, Y.; Pei, L.; Shen, Y. Probiotic potential of Lactobacillus isolated from horses and its therapeutic effect on DSS-induced colitis in mice. Microb. Pathog. 2022, 165, 105216. [Google Scholar]
- Mulinari Turin de Oliveira, N.; Schiebel, C.S.; Sauruk da Silva, K.; de Mello Braga, L.L.V.; Bach, C.; Maria-Ferreira, D. Efficacy of Dietary Supplementation in the Relief of Inflammatory Bowel Disease: A Systematic Review of Animal Studies. Nutr. Rev. 2025, nuae224. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.-Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar]
- Wijesekara, T.; Abeyrathne, E.D.N.S.; Ahn, D.U. Effect of bioactive peptides on gut microbiota and their relations to human health. Foods 2024, 13, 1853. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar]
- Łagowska, K.; Drzymała-Czyż, S. A low glycemic index, energy-restricted diet but not Lactobacillus rhamnosus supplementation changes fecal short-chain fatty acid and serum lipid concentrations in women with overweight or obesity and polycystic ovary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 917–926. [Google Scholar] [PubMed]
- Lee, S.-Y.; Kang, D.-H. Survival mechanism of Escherichia coli O157: H7 against combined treatment with acetic acid and sodium chloride. Food Microbiol. 2016, 55, 95–104. [Google Scholar] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [PubMed]
- Lin, Q.; Lin, S.; Fan, Z.; Liu, J.; Ye, D.; Guo, P. A review of the mechanisms of bacterial colonization of the mammal gut. Microorganisms 2024, 12, 1026. [Google Scholar] [CrossRef]
- Vergalito, F.; Bagnoli, D.; Maiuro, L.; Pannella, G.; Palombo, V.; Testa, B.; Coppola, F.; Di Marco, R.M.; Tremonte, P.; Lombardi, S.J. Akkermansia muciniphila: New insights into resistance to gastrointestinal stress, adhesion, and protein interaction with human mucins through optimised in vitro trials and bioinformatics tools. Front. Microbiol. 2024, 15, 1462220. [Google Scholar] [CrossRef]
- Zhou, Z.; Arroum, T.; Luo, X.; Kang, R.; Lee, Y.J.; Tang, D.; Hüttemann, M.; Song, X. Diverse functions of cytochrome c in cell death and disease. Cell Death Differ. 2024, 31, 387–404. [Google Scholar] [CrossRef]
- Gholipour, F.; Amini, M.; Baradaran, B.; Mokhtarzadeh, A.; Eskandani, M. Anticancer properties of curcumin-treated Lactobacillus plantarum against the HT-29 colorectal adenocarcinoma cells. Sci. Rep. 2023, 13, 2860. [Google Scholar]
- Trejo-Solis, C.; Escamilla-Ramirez, A.; Jimenez-Farfan, D.; Castillo-Rodriguez, R.A.; Flores-Najera, A.; Cruz-Salgado, A. Crosstalk of the Wnt/β-catenin signaling pathway in the induction of apoptosis on cancer cells. Pharmaceuticals 2021, 14, 871. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Yang, Y.; Zhao, Z.; Hu, J. Combined effects of natural products and exercise on apoptosis pathways in obesity-related skeletal muscle dysfunction. Apoptosis 2025, 1–16. [Google Scholar] [CrossRef]
- Singh, V.; Shirbhate, E.; Kore, R.; Vishwakarma, S.; Parveen, S.; Veerasamy, R.; Tiwari, A.K.; Rajak, H. Microbial metabolites-induced epigenetic modifications for inhibition of colorectal cancer: Current status and future perspectives. Mini-Rev. Med. Chem. 2025, 25, 76–93. [Google Scholar] [PubMed]
- Thoda, C.; Touraki, M. Probiotic-derived bioactive compounds in colorectal cancer treatment. Microorganisms 2023, 11, 1898. [Google Scholar] [CrossRef]
- Ha, S.; Zhang, X.; Yu, J. Probiotics intervention in colorectal cancer: From traditional approaches to novel strategies. Chin. Med. J. 2024, 137, 8–20. [Google Scholar]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [PubMed]
- Ghabeshi, S.; Mousavizadeh, L.; Ghasemi, S. Enhancing the antiviral potential and anti-inflammatory properties of Astragalus membranaceus: A comprehensive review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Inflamm. Anti-Allergy Agents) 2023, 22, 211–219. [Google Scholar]
- Auestad, N.; Layman, D.K. Dairy bioactive proteins and peptides: A narrative review. Nutr. Rev. 2021, 79 (Suppl. S2), 36–47. [Google Scholar]
- Pan, Y.; Li, H.; Shahidi, F.; Luo, T.; Deng, Z. Interactions among dietary phytochemicals and nutrients: Role of cell membranes. Trends Food Sci. Technol. 2022, 124, 38–50. [Google Scholar]
- Aggarwal, R.; Bains, K. Protein, lysine and vitamin D: Critical role in muscle and bone health. Crit. Rev. Food Sci. Nutr. 2022, 62, 2548–2559. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Yang, N.; Zhang, Q.; Luo, C.; Wang, J.; Yang, Y.; Yue, B.; Chen, P.; Zhang, X. Marine-Derived Enterococcus faecalis HY0110 as a Next-Generation Functional Food Probiotic: Comprehensive In Vitro and In Vivo Bioactivity Evaluation and Synergistic Fermentation of Periplaneta americana Extract Powder. Foods 2025, 14, 1181. https://doi.org/10.3390/foods14071181
Huang F, Yang N, Zhang Q, Luo C, Wang J, Yang Y, Yue B, Chen P, Zhang X. Marine-Derived Enterococcus faecalis HY0110 as a Next-Generation Functional Food Probiotic: Comprehensive In Vitro and In Vivo Bioactivity Evaluation and Synergistic Fermentation of Periplaneta americana Extract Powder. Foods. 2025; 14(7):1181. https://doi.org/10.3390/foods14071181
Chicago/Turabian StyleHuang, Feiyun, Nan Yang, Qingqing Zhang, Cuiling Luo, Jingheng Wang, Yu Yang, Bisong Yue, Peng Chen, and Xiuyue Zhang. 2025. "Marine-Derived Enterococcus faecalis HY0110 as a Next-Generation Functional Food Probiotic: Comprehensive In Vitro and In Vivo Bioactivity Evaluation and Synergistic Fermentation of Periplaneta americana Extract Powder" Foods 14, no. 7: 1181. https://doi.org/10.3390/foods14071181
APA StyleHuang, F., Yang, N., Zhang, Q., Luo, C., Wang, J., Yang, Y., Yue, B., Chen, P., & Zhang, X. (2025). Marine-Derived Enterococcus faecalis HY0110 as a Next-Generation Functional Food Probiotic: Comprehensive In Vitro and In Vivo Bioactivity Evaluation and Synergistic Fermentation of Periplaneta americana Extract Powder. Foods, 14(7), 1181. https://doi.org/10.3390/foods14071181





