Technological Assessment and Predictive Modeling of Probiotic Lactose-Free Fermented Milk with Lacticaseibacillus paracasei GV17
Abstract
1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacteria
2.2. Culture Conditions
2.2.1. Viability of LAB in Glucose and Lactose Medium
2.2.2. Determination of pH
2.2.3. Assessment of the LAB Population
2.3. Kinetic Profiles of LAB Strains in Fermented Milk
2.3.1. Milk Fermentation
2.3.2. Assessment of the LAB Population in Fermented Milk
2.3.3. Determination of Titratable Acidity and pH Throughout Shelf Life
2.3.4. Estimation of LAB Multiplication Parameters During Fermentation
2.3.5. Determination of Syneresis Throughout Shelf Life
2.3.6. Determination of Water-Holding Capacity Throughout Shelf Life
2.3.7. LAB Viability Throughout Shelf Life
2.4. Statistical Analyses
3. Results
3.1. Culture Conditions
3.2. Estimation of LAB Multiplication Parameters During Fermentation
3.3. Determination of Syneresis, Water-Holding Capacity, and Viability of LAB Throughout Shelf Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garnier, L.; Penland, M.; Thierry, A.; Maillard, M.-B.; Jardin, J.; Coton, M.; Salas, M.L.; Coton, E.; Valence, F.; Mounier, J. Antifungal activity of fermented dairy ingredients: Identification of antifungal compounds. Int. J. Food Microbiol. 2020, 322, 108574. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hao, L.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Functional roles and engineering strategies to improve the industrial functionalities of lactic acid bacteria during food fermentation. Biotechnol. Adv. 2024, 74, 108397. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Pereira, J.A.; Pinto, S.S.; Dias, C.O.; Vieira, M.P.; Ribeiro, D.H.; Amboni, R.D.; Fritzen-Freire, C.B. Potentially symbiotic fermented milk: A preliminary approach using lactose-free milk. LWT—Food Sci. Technol. 2020, 118, 108847. [Google Scholar] [CrossRef]
- Moreira, T.C.; da Silva, Á.T.; Fagundes, C.; Ferreira, S.M.R.; Cândido, L.M.B.; Passos, M.; Krüger, C.C.H. Elaboration of yogurt with reduced level of lactose added of carob (Ceratonia siliqua L.). LWT—Food Sci. Technol. 2017, 76, 326–329. [Google Scholar] [CrossRef]
- Ryan, J.; Hutchings, S.C.; Fang, Z.; Bandara, N.; Gamlath, S.; Ajlouni, S.; Ranadheera, C.S. Microbial, physico-chemical and sensory characteristics of mango juice-enriched probiotic dairy drinks. Int. J. Dairy Technol. 2020, 73, 182–190. [Google Scholar] [CrossRef]
- Gonçalves, L.D.d.A.; Piccoli, R.H.; Peres, A.d.P.; Saúde, A.V. Primary and secondary modeling of Brochothrix thermosphacta growth under different temperature and PH values. Food Sci. Technol. 2018, 38, 37–43. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Costa, I.M.; Miranda, T.B.A.; Magalhães, L.M.M.; Fafá, S.M.; Costa, T.J.N.; Magalhães, M.B.; Valente, G.L.C.; Gomes, J.E.G.; de Assis, D.C.S.; Vidal, A.M.C.; et al. Minas artisanal cheese as a reservoir of potentially probiotic Lacticaseibacillus paracasei GV17 and Lactococcus lactis GV103 and their functional properties and kinetic mechanisms. J. Food Meas. Charact. 2024, 19, 424–438. [Google Scholar] [CrossRef]
- Assis, G.B.N.; Pereira, F.L.; Zegarra, A.U.; Tavares, G.C.; Leal, C.A.; Figueiredo, H.C.P. Use of MALDI-TOF Mass Spectrometry for the fast identification of gram-positive fish pathogens. Front. Microbiol. 2017, 8, 1492. [Google Scholar] [CrossRef]
- von Mollendorff, J.W.; Todorov, S.D.; Dicks, L.M.T. Factors affecting the adsorption of bacteriocins to Lactobacillus sakei and Enterococcus sp. Appl. Biochem. Biotechnol. 2007, 142, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Watanabe, R.; Ichimura, T.; Ishida, T.; Kimura, K. Effect of lactose hydrolysis on the milk-fermenting properties of Lactobacillus delbrueckii ssp. bulgaricus 2038 and Streptococcus thermophilus 1131. J. Dairy Sci. 2021, 104, 1454–1464. [Google Scholar] [CrossRef]
- AOAC. Método nº 942.15 e 920. 149. Métodos Oficiais de Análise da AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2006; p. 918. [Google Scholar]
- Alvarenga, V.; Brito, L.M.; Lacerda, I.C.A. Application of mathematical models to validate emerging processing technologies in food. Curr. Opin. Food Sci. 2022, 48, 100928. [Google Scholar] [CrossRef]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of pectin-rich orange fibre on gel characteristics and sensory properties in lactic acid fermented yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Harte, F.; Luedecke, L.; Swanson, B.; Barbosa-Cánovas, G. Low-fat set yogurt made from milk subjected to combinations of high hydrostatic pressure and thermal processing. J. Dairy Sci. 2003, 86, 1074–1082. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzales, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020; Centro de Transferencia InfoStat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020; Available online: http://www.infostat.com.ar (accessed on 20 September 2024).
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Bai, M.; Yang, S.; Zhao, Q.; Wang, D.; Zhang, T.; Kwok, L.Y.; Sun, Z. Fermentation characteristics of Lactobacillus delbrueckii subsp. bulgaricus T50 and Streptococcus thermophilus S10 complex starter: Enhancing fermentation performance, metabolic interaction and storage stability. LWT—Food Sci. Technol. 2024, 208, 116716. [Google Scholar]
- Guan, Y.; Cui, Y.; Qu, X.; Li, B.; Zhang, L. Post-acidification of fermented milk and its molecular regulatory mechanism. Int. J. Food Microbiol. 2024, 426, 110920. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Teixeira, S.; Bertolo, M.R.; Ranadheera, C.; Raices, R.S.; Russo, P.; Spano, G.; Junior, S.B.; Cruz, A.G.; Sant’ana, A.S. Functional benefits of probiotic fermented dairy drink elaborated with sheep milk processed by ohmic heating. Food Biosci. 2024, 59, 103781. [Google Scholar] [CrossRef]
- Mahony, J.; Bottacini, F.; van Sinderen, D. Towards the diversification of lactococcal starter and non-starter species in mesophilic dairy culture systems. Microb. Biotechnol. 2023, 16, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Yu, X.; Zhao, X.; Liu, C.; Li, T.; Um, S.; Zhang, L.; Chen, Z.; Zhang, Z.; Song, Z.; et al. Fermentation characteristics and post acidification of yogurt by Streptococcus thermophilus CICC 6038 and Lactobacillus delbrueckii ssp. bulgaricus CICC 6047 at optimal inoculum ratio. J. Dairy Sci. 2024, 107, 123–140. [Google Scholar]
- Miao, M.; Li, S.; Yang, S.; Yan, Q.; Xiang, Z.; Jiang, Z. Engineering the β-galactosidase from Aspergillus oryzae for making lactose-free and no-sugar-added yogurt. J. Dairy Sci. 2024, 107, 6602–6613. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yao, S.; Zhang, M.; Wu, C. Heat adaptation induced cross protection against ethanol stress in Tetragenococcus halophilus: Physiological characteristics and proteomic analysis. Front. Microbiol. 2021, 12, 686672. [Google Scholar] [CrossRef]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT—Food Sci. Tecnol. 2016, 73, 536–542. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Xu, J.; Yuan, Z.; Liu, X.; Fan, X.; Li, J.; Zhu, D.; Liu, H. Effects of different probiotic fermentations on the quality, soy isoflavone and equol content of soy protein yogurt made from soy whey and soy embryo powder. LWT—Food Sci. Tecnol. 2022, 157, 113096. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, X.; Wen, A.; Qin, L. Development of probiotics beverage using cereal enzymatic hydrolysate fermented with Limosilactobacillus reuteri. Food Sci. Nutr. 2022, 10, 3143–3153. [Google Scholar] [CrossRef]
- Cruz, L.H.d.O.; Nascimento, R.M.; Ramos, G.L.d.P.A.; Gonzalez, A.G.M.; Domingues, J.R. Development of plant-based yogurt from munguba (Pachira aquatica) seeds: Stability and predictive growth of lactic acid cultures. Food Biosci. 2024, 62, 105363. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, Y.; Zhang, S.; Xu, Z. Effect of sugar transporter on galactose utilization in Streptococcus thermophilus. Front. Microbiol. 2023, 14, 1267237. [Google Scholar] [CrossRef]
- Yang, Q.; Yao, H.; Liu, S.; Mao, J. Interaction and application of molds and yeasts in chinese fermented foods. Front. Microbiol. 2022, 12, 664850. [Google Scholar] [CrossRef]
- Iskandar, C.F.; Cailliez-Grimal, C.; Borges, F.; Revol-Junelles, A.-M. Review of lactose and galactose metabolism in lactic acid bacteria dedicated to expert genomic annotation. Trends Food Sci. Technol. 2019, 88, 121–132. [Google Scholar] [CrossRef]
- Machado, S.G.; Freitas, R. Letes fermetados. In Microbiologia de Alimentos Fermentado, 1st ed.; Martin, J.G.P.M., Lindner, J.D., Eds.; Blucher: São Paulo, Brazil, 2022; pp. 223–248. [Google Scholar]
- Dekker, P.J.T.; Koenders, D.; Bruins, M.J. Lactose-free dairy products: Market developments, production, nutrition and health benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Inayat, S.; Gulzar, N.; Khalique, A.; Shahzad, F.; Irshad, I.; Imran, M. Physical, chemical, microbial, and sensory evaluation and fatty acid profiling of value-added drinking yogurt (laban) under various storage conditions. J. Dairy Sci. 2023, 106, 39–46. [Google Scholar] [CrossRef]
- Wilbanks, D.; Lee, M.; Rahimi, Y.; Lucey, J. Comparison of micellar casein isolate and nonfat dry milk for use in the production of high-protein cultured milk products. J. Dairy Sci. 2023, 106, 61–74. [Google Scholar] [CrossRef]
- Zeineb, J.; Olfa, O.; Slah, Z.; Touhami, K.; El Halima, H. Co-fermentation process strongly affects the nutritional, texture, syneresis, fatty acids and aromatic compounds of dromedary UF-yogurt. J. Food Sci. Technol. 2020, 58, 1727–1739. [Google Scholar]
- Molaee, P.; Reza, F.; Mortazavian, A.; Sarem, N.; Ali, M.; Akbar, G.; Khorshidian, N. Comparative effects of probiotic and paraprobiotic addition on microbiological, biochemical and physical properties of yogurt. Food Res. Int. 2020, 140, 110030. [Google Scholar] [CrossRef]
- Tan, C.; Tian, Y.; Tao, L.; Xie, J.; Wang, M.; Zhang, F.; Yu, Z.; Sheng, J.; Zhao, C. Exploring the effect of milk fat on fermented milk flavor based on gas chromatography–ion mobility spectrometry (GC-IMS) and multivariate statistical analysis. Molecules 2024, 29, 1099. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Tiwari, S.; Kumar, A.; Raman, R.K.; Kadyan, S. Review on factors affecting and control of post-acidification in yoghurt and related products. Trends Food Sci. Technol. 2021, 109, 499–512. [Google Scholar] [CrossRef]
- Tao, H.; Li, S.-Q.; Fang, M.-J.; Cai, W.-H.; Zhang, S.; Wang, H.-L. The characterization of a low-calorie and lactose-free brown fermented milk by the hydrolysis of different enzymatic lactose. Foods 2024, 13, 2861. [Google Scholar] [CrossRef]
- Schmidt, C.; Mende, S.; Jaros, D.; Rohm, H. Fermented milk products: Effects of lactose hydrolysis and fermentation conditions on the rheological properties. Dairy Sci. Technol. 2015, 96, 199–211. [Google Scholar] [CrossRef][Green Version]
- Veringa, H.A.; Galesloot, T.E.; Davelaar, H. Symbiosis in yoghurt (II). Isolation and identification of a growth factor for Lactobacillus bulgaricus produced by Streptococcus thermophilus. Milk Dairy J. 1968, 148, 114–120. [Google Scholar]
- Li, S.; Tang, S.; Ren, R.; Gong, J.; Chen, Y. Metabolomic profile of milk fermented with Streptococcus thermophilus cocultured with Bifidobacterium animalis ssp. lactis, Lactiplantibacillus plantarum, or both during storage. J. Dairy Sci. 2021, 104, 8493–8505. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.; Santos, M.C.D.; Martins, O.A.; Pereira, J.G. Microbiological and physicochemical characterization of probiotic fermented milk throughout the shelf life under different storage temperatures. Food Sci. Technol. 2022, 42, e102521. [Google Scholar] [CrossRef]
 ) and Streptococcus thermophilus STI-12 (
) and Streptococcus thermophilus STI-12 ( ) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media (
) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media ( ), lactose (
), lactose ( ), and glucose–lactose (
), and glucose–lactose ( ) (B) Different lowercase letters indicate significant differences (p < 0.05) be-tween the media evaluated for the same strain. * For different strains, they indicate significant differences (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).
) (B) Different lowercase letters indicate significant differences (p < 0.05) be-tween the media evaluated for the same strain. * For different strains, they indicate significant differences (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).
 ) and Streptococcus thermophilus STI-12 (
) and Streptococcus thermophilus STI-12 ( ) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media (
) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media ( ), lactose (
), lactose ( ), and glucose–lactose (
), and glucose–lactose ( ) (B) Different lowercase letters indicate significant differences (p < 0.05) be-tween the media evaluated for the same strain. * For different strains, they indicate significant differences (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).
) (B) Different lowercase letters indicate significant differences (p < 0.05) be-tween the media evaluated for the same strain. * For different strains, they indicate significant differences (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).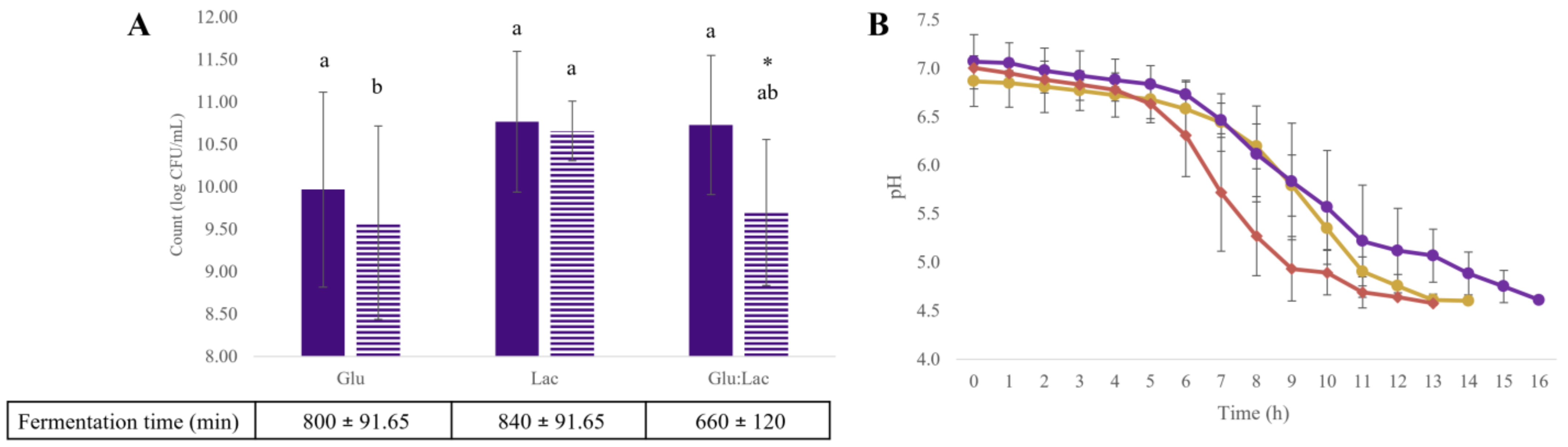
 ) and Streptococcus thermophilus STI-12 (
) and Streptococcus thermophilus STI-12 ( ) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media (
) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media ( ), lactose (
), lactose ( ), and glucose–lactose (
), and glucose–lactose ( ) for monocultures of Lacticaseibacillus paracasei GV17 (B) and Streptococcus thermophilus STI-12 (C). Different lowercase letters indicate significant difference (p < 0.05) between the media evaluated for the same strain. * For different strains, they indicate significant difference (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).
) for monocultures of Lacticaseibacillus paracasei GV17 (B) and Streptococcus thermophilus STI-12 (C). Different lowercase letters indicate significant difference (p < 0.05) between the media evaluated for the same strain. * For different strains, they indicate significant difference (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).
 ) and Streptococcus thermophilus STI-12 (
) and Streptococcus thermophilus STI-12 ( ) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media (
) in glucose media (Glu), lactose (Lac), and glucose–lactose (Glu:Lac) at pH 4.6 (A) and pH during fermentation in glucose media ( ), lactose (
), lactose ( ), and glucose–lactose (
), and glucose–lactose ( ) for monocultures of Lacticaseibacillus paracasei GV17 (B) and Streptococcus thermophilus STI-12 (C). Different lowercase letters indicate significant difference (p < 0.05) between the media evaluated for the same strain. * For different strains, they indicate significant difference (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).
) for monocultures of Lacticaseibacillus paracasei GV17 (B) and Streptococcus thermophilus STI-12 (C). Different lowercase letters indicate significant difference (p < 0.05) between the media evaluated for the same strain. * For different strains, they indicate significant difference (p < 0.05) between strains in the same medium. The results are expressed as mean ± standard deviation (n = 9).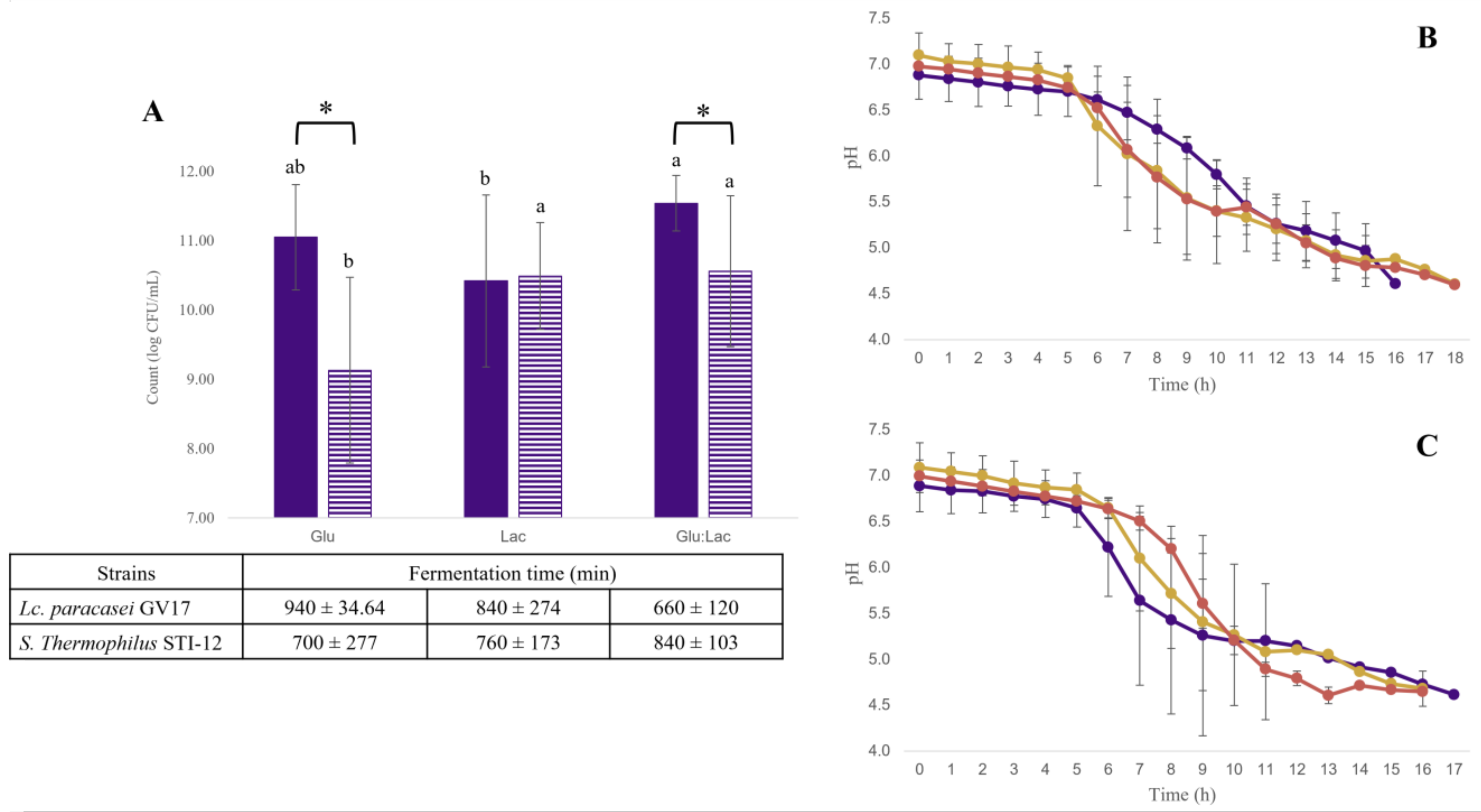

| Strains | µmax (log CFU/h) | LT (h) | R2 | |
|---|---|---|---|---|
| Lc. paracasei | GV17 | 0.912 ± 0.541 | 2.428 ± 1.210 | 0.760 ± 0.085 |
| S. thermophilus | STI-12 | 0.882 ± 0.641 | 1.943 ± 0.961 | 0.852 ± 0.034 |
| Strains | Analysis | 1st | 14th | 28th | |
|---|---|---|---|---|---|
| Lc. paracasei associated with S. thermophilus | GV17 and STI-12 | Syneresis (%) | 56.4 ± 2.79 a | 56.7 ± 2.99 a | 47.8 ± 1.98 b |
| WHC (%) | 41.3 ± 4.82 a | 41.6 ± 2.57 a | 45.0 ± 2.08 a | ||
| Strains | 1st | 14th | 28th | |
|---|---|---|---|---|
| Lc. paracasei | GV17 | 10.40 ± 0.86 b | 11.38 ± 0.56 a | 11.30 ± 0.46 a |
| S. thermophilus | STI-12 | 10.72 ± 0.31 a | 10.72 ± 0.90 a | 11.18 ± 0.65 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, T.J.N.; Costa, I.M.; Magalhães, L.M.M.; de Souza, M.R.; Rossi, G.A.M.; Salotti-Souza, B.M.; Fante, C.A. Technological Assessment and Predictive Modeling of Probiotic Lactose-Free Fermented Milk with Lacticaseibacillus paracasei GV17. Foods 2025, 14, 1176. https://doi.org/10.3390/foods14071176
Costa TJN, Costa IM, Magalhães LMM, de Souza MR, Rossi GAM, Salotti-Souza BM, Fante CA. Technological Assessment and Predictive Modeling of Probiotic Lactose-Free Fermented Milk with Lacticaseibacillus paracasei GV17. Foods. 2025; 14(7):1176. https://doi.org/10.3390/foods14071176
Chicago/Turabian StyleCosta, Taynan Jonatha Neves, Isabella Maciel Costa, Larissa Mirelle Mendes Magalhães, Marcelo Resende de Souza, Gabriel Augusto Marques Rossi, Bruna Maria Salotti-Souza, and Camila Argenta Fante. 2025. "Technological Assessment and Predictive Modeling of Probiotic Lactose-Free Fermented Milk with Lacticaseibacillus paracasei GV17" Foods 14, no. 7: 1176. https://doi.org/10.3390/foods14071176
APA StyleCosta, T. J. N., Costa, I. M., Magalhães, L. M. M., de Souza, M. R., Rossi, G. A. M., Salotti-Souza, B. M., & Fante, C. A. (2025). Technological Assessment and Predictive Modeling of Probiotic Lactose-Free Fermented Milk with Lacticaseibacillus paracasei GV17. Foods, 14(7), 1176. https://doi.org/10.3390/foods14071176






