Combined Effect of Low-Temperature Stress and Slightly Acidic Electrolyzed Water (SAEW) on the Microbial Control of Oat Sprout Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Oat Seeds
2.2. Seed Preparation
2.3. Preparation of Treatment Solutions
2.4. Preparation of Bacterial Suspension
2.5. Sample Inoculation and Treatments
2.6. Microbiological Analysis
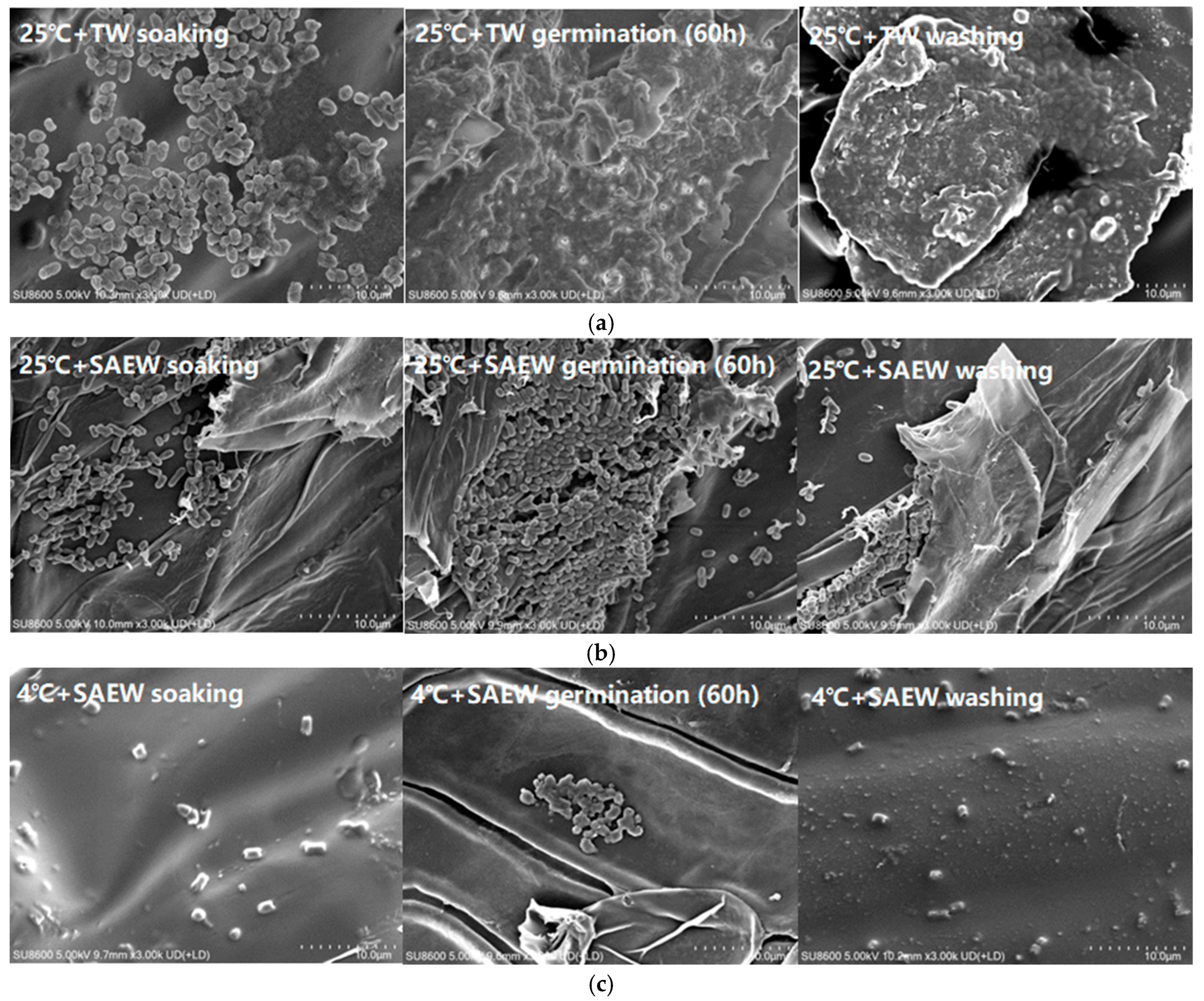
2.7. Observation of Scanning Electron Microscopy (SEM)
2.8. Data Processing and Analysis
3. Results and Discussion
3.1. Combined Effect of Low Temperature Stress and SAEW on E. coli Artificially Inoculated During Oat Sprout Production
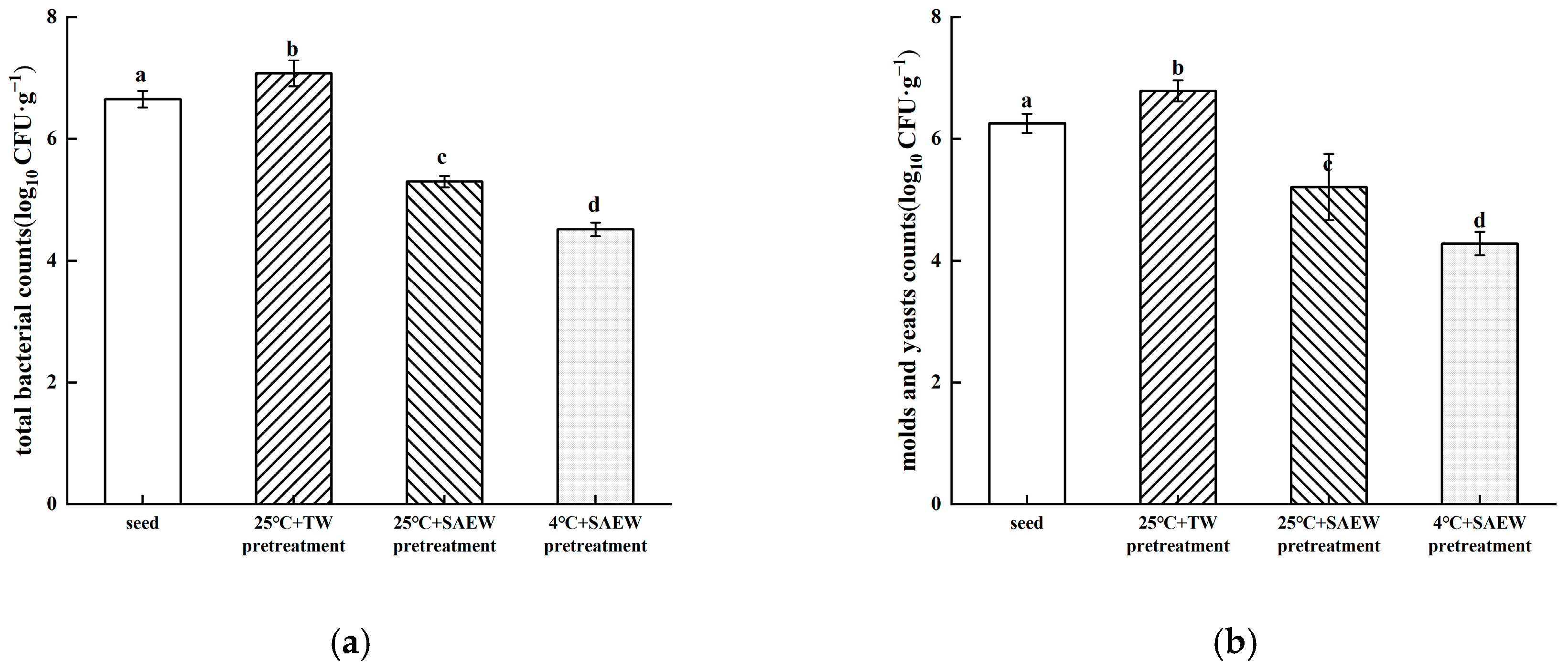
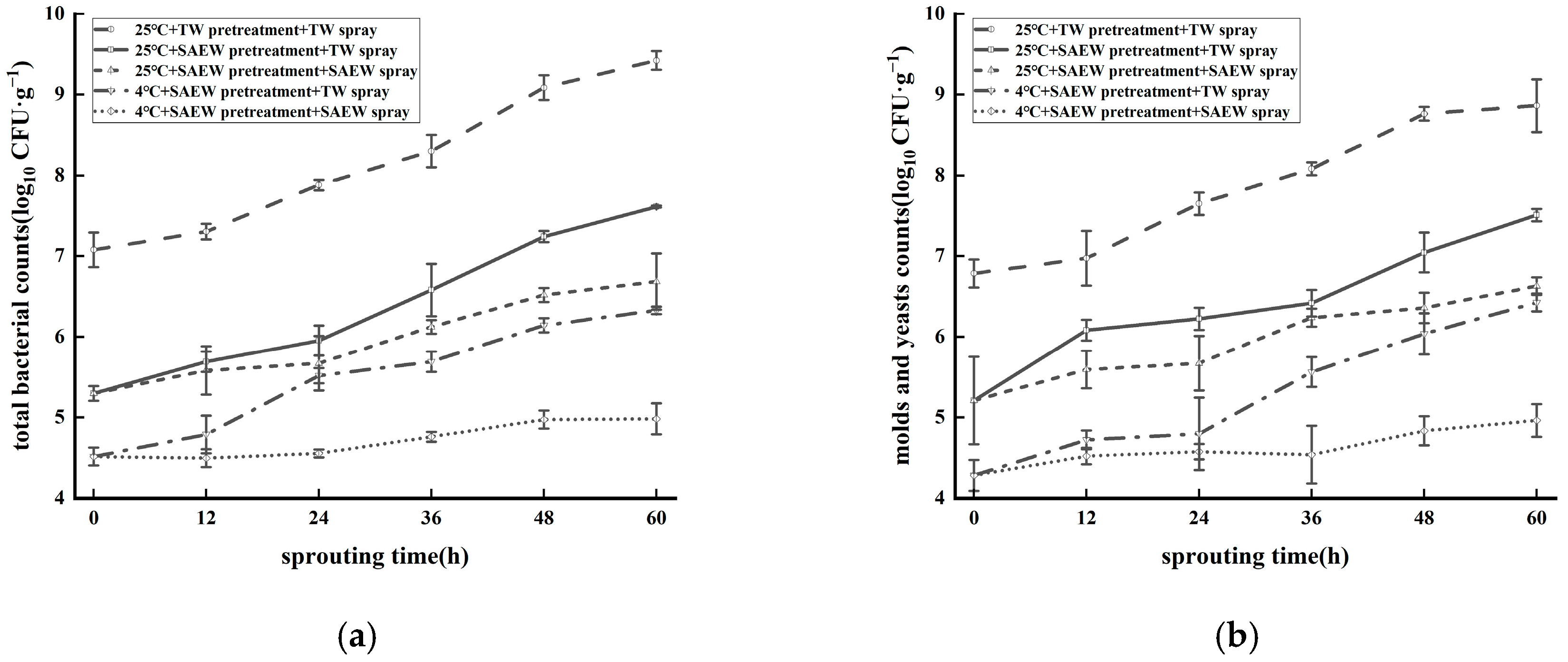
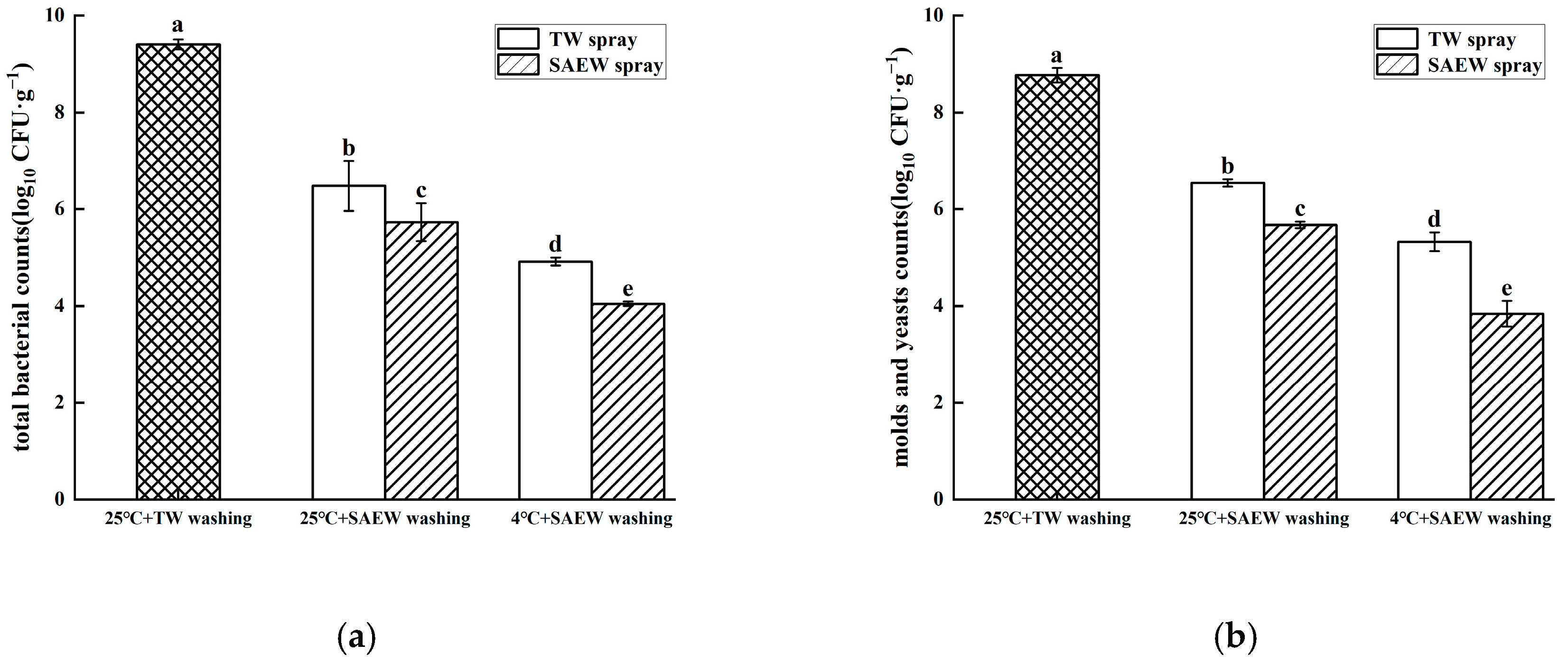
3.2. Combined Effect of Low-Temperature Stress and SAEW for Reducing Natural Microflora During Oat Sprout Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Chen, Z.; Lu, W.; Wen, J.; Lei, Y.; Wu, Z.; Huang, Z. Survey and analysis on knowledge-attitude-practice of medical workers on foodborne diseases surveillance. J. Med. Pest Control 2022, 38, 734–739. [Google Scholar] [CrossRef]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Sprouted oat as a potential gluten-free ingredient with enhanced nutritional and bioactive properties. Food Chem. 2021, 338, 127972. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Miyahira, R.F.; Antunes, A.E.C. Bacteriological safety of sprouts: A brief review. Int. J. Food Microbiol. 2021, 352, 109266. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; García-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Sarno, E.; Pezzutto, D.; Rossi, M.; Liebana, E.; Rizzi, V. A Review of Significant European Foodborne Outbreaks in the Last Decade. J. Food Prot. 2021, 84, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, A.; Ravel, A.; Murray, R.; McCormick, R.; Savelli, C.; Finley, R.; Parmley, J.; Agunos, A.; Majowicz, S.E.; Gilmour, M. Integrated surveillance and potential sources of Salmonella Enteritidis in human cases in Canada from 2003 to 2009. Epidemiol. Infect. 2011, 140, 1757–1772. [Google Scholar] [CrossRef]
- Beutin, L.; Martin, A. Outbreak of Shiga Toxin–Producing Escherichia coli (STEC) O104:H4 Infection in Germany Causes a Paradigm Shift with Regard to Human Pathogenicity of STEC Strains. J. Food Prot. 2012, 75, 408–418. [Google Scholar] [CrossRef]
- Du, Y.; Tian, Z.; Zhou, B. Research advance on germinated oat Storage and Process. Storage Process 2019, 19, 169–173. [Google Scholar]
- Gao, T.; Yin, K.; Shao, D.; Zhao, D.; Dai, W. Research progress on cold plasma technology for killing foodborne pathogens. Farm Prod. Process. 2024, 100–103. [Google Scholar] [CrossRef]
- Neo, S.Y.; Lim, P.Y.; Phua, L.K.; Khoo, G.H.; Kim, S.J.; Lee, S.C.; Yuk, H.G. Efficacy of chlorine and peroxyacetic acid on reduction of natural microflora, Escherichia coli O157:H7, Listeria monocyotgenes and Salmonella spp. on mung bean sprouts. Food Microbiol. 2013, 36, 475–480. [Google Scholar] [CrossRef]
- Gu, G.; Bolten, S.; Mowery, J.; Luo, Y.; Gulbronson, C.; Nou, X. Susceptibility of foodborne pathogens to sanitizers in produce rinse water and potential induction of viable but non-culturable state. Food Control 2020, 112, 107138. [Google Scholar] [CrossRef]
- Gan, X.; Tan, X.; Zou, S.; Zhang, W.; Cheng, M. Study on the effect of irradiation sterilization on the fresh-keeping effect of beef. Chin. Foreign Food Ind. 2024, 86–88. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Li, X.; Guo, S.; Li, S.; Chen, B.; Cheng, Y.; Xu, H.; Yan, W. Optimization of electrolysis process, storage conditions and sterilization effect of slightly acidic electrolytic water prepared by titanium suboxide electrode. J. Environ. Chem. Eng. 2023, 11, 109679. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Chen, X.; Zheng, K.; Li, J.; Li, X. Effect of slightly acidic electrolyzed water (SAEW) combined with ultrasound sterilization on quality of Bigeye tuna (Thunnus obesus) during cryogenic storage. J. Food Compos. Anal. 2023, 115, 104999. [Google Scholar] [CrossRef]
- Zang, Y.T.; Bing, S.; Li, Y.J.; Shu, D.Q. Application of slightly acidic electrolyzed water and ultraviolet light for Salmonella enteritidis decontamination of cell suspensions and surfaces of artificially inoculated plastic poultry transport coops and other facility surfaces. Poult. Sci. 2019, 98, 6445–6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Z.; Yang, G.; Shi, Y.; Zhang, Y.; Shi, C.; Xia, X. Effect of slightly acidic electrolyzed water on natural Enterobacteriaceae reduction and seed germination in the production of alfalfa sprouts. Food Microbiol. 2021, 97, 103414. [Google Scholar] [CrossRef]
- Ding, N.; Xia, F.; Zhao, P.; Zhang, Z.; Zhang, X. Disinfection effect and safety evaluation of slightly acidic electrolyzed water on cucumber. J. Food Saf. Qual. 2018, 9, 930–934. [Google Scholar]
- Lei, J.; Ren, H.; Li, Y.; Wu, T.; Hao, J. The combination of non-electrolytic hypochlorite water and ultrasonic treatment on the cleaning and preservation of baby cabbage. LWT 2024, 205, 116528. [Google Scholar] [CrossRef]
- Ai, K. Effects of Slightly Acidic Electrolyzed Water on the Growth and Drought Resistance of Tomato Seedlings. Master’s Thesis, Northwest A&F University, Xianyang, China, 2020. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Bi, B.; He, J. Effects of Slightly Acidic Electrolyzed Water on the Quality of Fresh-Cut Apple. Foods 2022, 12, 39. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.Y.; Lee, H.-W.; Ha, J.-H. Inactivation of bacteria causing soft rot disease in fresh cut cabbage using slightly acidic electrolyzed water. Food Control 2021, 128, 108217. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Chen, X.; Li, M.; Lin, M.; Chen, Y.; Lin, H. Slightly acidic electrolyzed water treatment improves the quality and storage properties of carambola fruit. Food Chem. X 2023, 17, 100555. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wang, Y.-C.; Xue, Y.-M.; Tong, Z.; Jiang, G.-Y.; Hu, X.-R.; Crittenden, J.C.; Wang, C. Decoding the influence of low temperature on biofilm development: The hidden roles of c-di-GMP. Sci. Total Environ. 2024, 927, 172376. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, P.; Zhang, Y.; Shi, D.; Li, A.; Shi, Y.; Zhang, C. Effect of slightly acidic electrolyzed water on soybean germination. Food Ferment. Ind. 2020, 46, 135–140. [Google Scholar] [CrossRef]
- Issa-Zacharia, A.; Kamitani, Y.; Miwa, N.; Muhimbula, H.; Iwasaki, K. Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for decontamination of fresh ready-to-eat vegetables and sprouts. Food Control 2011, 22, 601–607. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Q.; Zhao, D.; Han, X.; Hao, J. Systematic application of slightly acidic electrolyzed water (SAEW) for natural microbial reduction of buckwheat sprouts. LWT 2019, 108, 14–20. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Jiang, H.; Cao, J.; Jiang, W. A comprehensive review of effects of electrolyzed water and plasma-activated water on growth, chemical compositions, microbiological safety and postharvest quality of sprouts. Trends Food Sci. Technol. 2022, 129, 449–462. [Google Scholar] [CrossRef]
- Hao, J.; Wu, T.; Li, H.; Wang, W.; Liu, H. Dual effects of slightly acidic electrolyzed water (SAEW) treatment on the accumulation of γ-aminobutyric acid (GABA) and rutin in germinated buckwheat. Food Chem. 2016, 201, 87–93. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Application of electrolyzed water in postharvest fruits and vegetables storage: A review. Trends Food Sci. Technol. 2021, 114, 599–607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Ren, H.; Chen, L.; Wu, T.; Hao, J. Combined Effect of Low-Temperature Stress and Slightly Acidic Electrolyzed Water (SAEW) on the Microbial Control of Oat Sprout Production. Foods 2025, 14, 1083. https://doi.org/10.3390/foods14071083
Liu S, Ren H, Chen L, Wu T, Hao J. Combined Effect of Low-Temperature Stress and Slightly Acidic Electrolyzed Water (SAEW) on the Microbial Control of Oat Sprout Production. Foods. 2025; 14(7):1083. https://doi.org/10.3390/foods14071083
Chicago/Turabian StyleLiu, Shaokang, Hongrui Ren, Lin Chen, Tongjiao Wu, and Jianxiong Hao. 2025. "Combined Effect of Low-Temperature Stress and Slightly Acidic Electrolyzed Water (SAEW) on the Microbial Control of Oat Sprout Production" Foods 14, no. 7: 1083. https://doi.org/10.3390/foods14071083
APA StyleLiu, S., Ren, H., Chen, L., Wu, T., & Hao, J. (2025). Combined Effect of Low-Temperature Stress and Slightly Acidic Electrolyzed Water (SAEW) on the Microbial Control of Oat Sprout Production. Foods, 14(7), 1083. https://doi.org/10.3390/foods14071083







