Enhancing Meat Emulsion Quality and Storage Stability During Refrigeration Using Thyme and Oregano Essential Oil Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Materials
2.3. Preparation of Nanoparticles
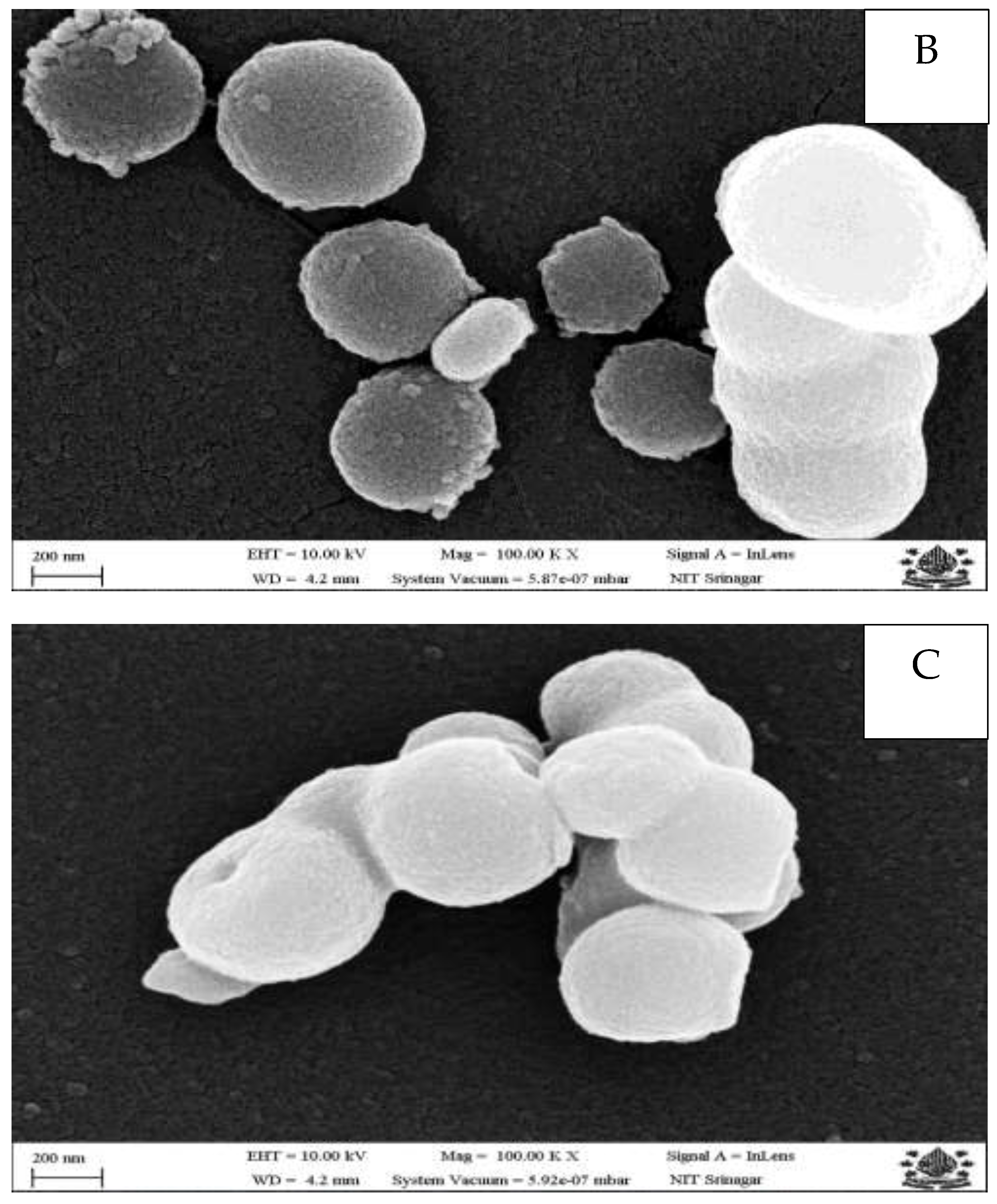
2.4. Morphological Assessment of Nanoparticles
2.5. Emulsion Preparation
2.6. Physico-Chemical Qualities
2.6.1. pH
2.6.2. Peroxide Value
- V= volume (mL) of sodium thiosulphate used;
- N = normality of sodium thiosulphate;
- W = weight of sample (g).
2.6.3. Thiobarbituric Acid Reactive Substances (TBARS)
2.6.4. Protein Carbonyls
2.6.5. 2,2 Diphenyl-1-picryl Hydrazyl Activity
2.6.6. Ferric Reducing Anti-Oxidant Power
2.6.7. Texture Profile Analysis
2.6.8. Colour Analysis
2.7. Microbiological Quality
2.8. Emulsion Stability
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Morphological Properties of Nanoparticles
3.2. Physico-Chemical Qualities
3.2.1. pH
3.2.2. Peroxide Value
3.2.3. TBARS
3.2.4. Protein Carbonyls
3.2.5. DPPH and FRAP
3.2.6. Texture Profile Analysis
3.2.7. Colour Profile Analysis
3.3. Microbiological Quality
3.4. Emulsion Stability
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, S.; Lapčík, L.; Lapčíková, B.; Gál, R. Emulsion-Based Coatings for Preservation of Meat and Related Products. Foods 2023, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.A.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative processes in muscle systems and fresh meat: Sources, markers and remedies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 565–597. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, M.; Wang, T.; Wang, D.; Sun, C.; Bian, H.; Li, P.; Zou, Y.; Xu, W. Lipid oxidation induced by heating in chicken meat and the relationship with oxidants and antioxidant enzymes activities. Poult. Sci. 2020, 99, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Tajik, H.; Aminzare, M.; Raad, T.M.; Hashemi, M.; Azar, H.H.; Raeisi, M.; Naghili, H. Effect of Zataria multiflora Boiss Essential Oil and Grape Seed Extract on the Shelf Life of Raw Buffalo Patty and Fate of Inoculated Listeria monocytogenes. J. Food Process. Preserv. 2015, 39, 3005–3013. [Google Scholar]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities-Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar]
- Soliman, K.; Badeaa, R. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential Oils Encapsulated in Polymer-based Nanocapsules as Potential Candidates for Application in Food Preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Boskovic, M.; Glisic, M.; Djordjevic, J.; Starcevic, M.; Glamoclija, N.; Djordjevic, V.; Baltic, M.Z. Antioxidative activity of thyme (Thymus vulgaris) and oregano (Origanum vulgare) essential oils and their effect on oxidative stability of minced pork packaged under vacuum and modified atmosphere. J. Food Sci. 2019, 84, 2467–2474. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H. Chitosan coatings incorporated with free or nano-encapsulated Paulownia tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. LWT 2019, 116, 108580. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Malekhosseini, P.; Alami, M.; Khomeiri, M.; Esteghlal, S.; Nekoei, A.; Hosseini, S.M.H. Development of casein-based nanoencapsulation systems for delivery of epigallocatechin gallate and folic acid. Food Sci. Nutr. 2019, 7, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Adil, S.; Banday, M.T.; Hussain, S.A.; Wani, M.A.; Al-Olayan, E.; Patra, A.K.; Rasool, S.; Gani, A.; Sheikh, I.U.; Khan, A.A.; et al. Impact of Nanoencapsulated Rosemary Essential Oil as a Novel Feed Additive on Growth Performance, Nutrient Utilization, Carcass Traits, Meat Quality and Gene Expression of Broiler Chicken. Foods 2024, 13, 1515. [Google Scholar] [CrossRef]
- Adil, S.; Banday, M.T.; Wani, M.A.; Hussain, S.A.; Showkat Shah, S.; Sheikh, I.D.; Shafi, M.; Khan, A.A.; Kashoo, Z.A.; Pattoo, R.A.; et al. Nano-protected form of rosemary essential oil has a positive influence on blood biochemistry parameters, haematological indices, immune-antioxidant status, intestinal microbiota and histomorphology of meat-type chickens. Poult. Sci. 2024, 103, 104309. [Google Scholar]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Hussain, S.A.; Jalal, H.; Bhat, M.; Salahuddin, M.; Wani, S.A.; Pal, M. Effect of alpha-tocopherol on the storage stability of Rista. Indian J. Small Rumin. 2015, 21, 311–316. [Google Scholar] [CrossRef]
- Troutt, E.; Hunt, M.; Johnson, D.; Claus, J.; Kastner, C.; Kropf, D. Characteristics of low fat ground beef containing texture modifying ingredients. J. Food Sci. 1992, 57, 19–24. [Google Scholar] [CrossRef]
- Akhter, R.; Masoodi, F.; Wani, T.A.; Rather, S.A.; Hussain, P.R. Synergistic effect of low dose γ-irradiation, natural antimicrobial and antioxidant agents on quality of meat emulsions. Radiat. Phys. Chem. 2021, 189, 109724. [Google Scholar] [CrossRef]
- Serrano, A.; Cofrade, S.; Jime´nez-Colmenero, F. Characteristics of restructured beef steak with different proportionsof walnut during frozen storage. Meat Sci. 2006, 72, 108–115. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hultin, H.O. Chemical, physical and functional properties of cod proteins modified by a nonenzymic free-radical-generating system. J. Agric. Food Chem. 1997, 45, 310–320. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Ghirro, L.C.; Rezende, S.; Ribeiro, A.S.; Rodrigues, N.; Carocho, M.; Pereira, J.A.; Barros, L.; Demczuk, B., Jr.; Barreiro, M.F.; Santamaria-Echart, A. Pickering Emulsions Stabilized with Curcumin-Based Solid Dispersion Particles as Mayonnaise-like Food Sauce Alternatives. Molecules 2022, 27, 1250. [Google Scholar] [CrossRef]
- APHA. Compendium of Methods for the Microbiological Examinations of Foods, 2nd ed.; Speck, M.L., Ed.; American Public Health Association: Washington, DC, USA, 2018. [Google Scholar]
- Horita, C.; Morgano, M.A.; Celeghini, R.; Pollonio, M. Physico-chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci. 2011, 89, 426–433. [Google Scholar] [CrossRef]
- Zhou, X.; Zong, X.; Zhang, M.; Ge, Q.; Qi, J.; Liang, J.; Xu, X.; Xiong, G. Effect of konjac glucomannan/carrageenan-based edible emulsion coatings with camellia oil on quality and shelf-life of chicken meat. Int. J. Biol. Macromol. 2021, 183, 331–339. [Google Scholar] [CrossRef]
- Benjemaa, M.; Marcos, A.; Fallehb, M.; Isodac, H.; Ksourib, R.; Nakajimac, M. Nanoencapsulation of Thymus capitatus essential oil: Formulation process, physical stability characterization and antibacterial efficiency monitoring. Indus. Crops Prod. 2018, 113, 414–421. [Google Scholar]
- Huang, M.; Wang, H.; Xu, X.; Lu, X.; Song, X.; Zhou, G. Effects of nanoemulsion-based edible coatings with composite mixture of rosemary extract and ε-poly-L-lysine on the shelf life of ready-to-eat carbonado chicken. Food Hydrocoll. 2020, 102, 105576. [Google Scholar] [CrossRef]
- Ansarian, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R.; Bimakr, M. Nanoemulsion-based basil seed gum edible film containing resveratrol and clove essential oil: In vitro antioxidant properties and its effect on oxidative stability and sensory characteristic of camel meat during refrigeration storage. Meat Sci. 2022, 185, 108716. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Saricaoglu, F.T.; Turhan, S. Performance of mechanically deboned chicken meat protein coatings containing thyme or clove essential oil for storage quality improvement of beef sucuks. Meat Sci. 2019, 158, 107912. [Google Scholar] [CrossRef]
- Ojeda-Piedra, S.A.; Zambrano-Zaragoza, M.L.; González-Reza, R.M.; García-Betanzos, C.I.; Real-Sandoval, S.A.; Quintanar-Guerrero, D. Nano-Encapsulated Essential Oils as a Preservation Strategy for Meat and Meat Products Storage. Molecules 2022, 27, 8187. [Google Scholar] [CrossRef]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.d.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.; Chawla, S.; Sharma, A. Effects of Chitosan Coating on Shelf-Life of Ready-to-Cook Meat Products during Chilled Storage. LWT 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Sheard, P.; Enser, M.; Wood, J.; Nute, G.; Gill, B.; Richardson, R. Shelf life and quality of pork and pork products with raised n-3 PUFA. Meat Sci. 2000, 55, 213–221. [Google Scholar] [CrossRef]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016, 197 (Pt A), 670–675. [Google Scholar] [CrossRef]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Influence of early post-mortem protein oxidation on beef quality. J. Anim. Sci. 2004, 82, 785–793. [Google Scholar]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Enhancement of Thermal Stability and Antioxidant Activity of Thyme Essential Oil by Encapsulation in Chitosan Nanoparticles. J. Agric. Sci. Technol. 2016, 18, 1781–1792. [Google Scholar]
- Manheem, K.; Adiamo, O.; Roobab, U.; Mohteshamuddin, K.; Hassan, H.M.; Nirmal, N.P.; Maqsood, S. A Comparative Study on Changes in Protein, Lipid and Meat-Quality Attributes of Camel Meat, Beef and Sheep Meat (Mutton) during Refrigerated Storage. Animals 2023, 13, 904. [Google Scholar] [CrossRef]
- Hakimian, F.; Emamifar, A.; Karami, M. Evaluation of microbial and physicochemical properties of mayonnaise containing zinc oxide nanoparticles. LWT 2022, 163, 113517. [Google Scholar] [CrossRef]
- Tabilo, G.; Flores, M.; Fiszman, S.M.; Toldra, F. Post mortem meat quality and sex affect textural properties and protein breakdown of dry-cured ham. Meat Sci. 1999, 51, 255–260. [Google Scholar] [CrossRef]
- Martín-Sánchez, A.M.; Chaves-López, C.; Sendra, E.; Sayas, E.; Fenández-López, J.; Pérez-Álvarez, J.Á. proteolysis and sensory characteristics of a Spanish fermented dry-cured meat product (salchichon) with oregano essential oil used as surface mold inhibitor. Meat Sci. 2011, 89, 35–44. [Google Scholar] [CrossRef]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 2008, 80, 1290–1296. [Google Scholar] [CrossRef]
- Dara, P.K.; Geetha, A.; Mohanty, U.; Raghavankutty, M.; Mathew, S.; Nagarajarao, R.C.; Rangasamy, A. Extraction and Characterization of Myofibrillar Proteins from Different Meat Sources: A Comparative Study. J. Bioresour. Bioprod. 2021, 6, 367–378. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Feng, X.; Tjia, J.Y.Y.; Zhou, Y.; Liu, Q.; Fu, C.; Yang, H. Effects of tocopherol nanoemulsion addition on fish sausage properties and fatty acid oxidation. LWT 2020, 118, 108737. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Hongxia, Z.; Zhiyang, L.; Xin, Y.; Yuan, L. Study on colour classification of pork with computer vision system based on different colour difference formula. Key Eng. Mater. 2011, 474–476, 1638–1642. [Google Scholar]
- Jeon, Y.-J.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an edible invisible film for quality preservation of herring and atlantic cod. J. Agric. Food Chem. 2002, 50, 5167–5178. [Google Scholar] [CrossRef]
- Jay, J.M. (Ed.) Food preservation with chemicals. In Modern Food Microbiology, 4th ed.; CBS Publishers and Distributors: New Delhi, India, 1996; p. 269. [Google Scholar]
- Kamkar, A.; Molaee-Aghaee, E.; Khanjari, A.; Akhondzadeh-Basti, A.; Noudoost, B.; Shariatifar, N.; Sani, M.A.; Soleimani, M. Nanocomposite active packaging based on chitosan biopolymer loaded with nano-liposomal essential oil: Its characterizations and effects on microbial and chemical properties of refrigerated chicken breast fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Numpaque, M.A.; Oviedo, L.A.; Gil, J.H.; García, C.M.; Durango, D.L. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop. Plant Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Shellie, R.; Keast, R.; Palombo, E.; Jadhav, S. Encapsulation of Essential Oils and Their Application in Antimicrobial Active Packaging. Food Control. 2022, 136, 108883. [Google Scholar] [CrossRef]
- Cho, Y.-N.; Chung, S.-H.; Yoo, G.; Ko, S.-W. Water-soluble chitin as a wound healing accelerator. Biomaterials 1999, 20, 2139–2145. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Antimicrobial and Antioxidant Potential of Papain Liver Hydrolysate in Meat Emulsion Model at Chilling Storage Under Aerobic Packaging Condition. Waste Biomass Valorization 2022, 13, 417–429. [Google Scholar] [CrossRef]
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, Y.L.; Chen, J. Protein oxidation enhances hydration but suppresses water-holding capacity in porcine longissimus muscle. J. Agric. Food Chem. 2010, 58, 10697–10704. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Yarnpakdee, S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J. Food Sci. Technol. 2015, 52, 6182–6193. [Google Scholar]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilled pork. LWT-Food Sci. Technol. 2015, 63, 519–526. [Google Scholar] [CrossRef]

| Treatments | Emulsion Type |
|---|---|
| T0 | Control, containing no additives |
| T1 | Incorporated with chitosan nanoparticles (CNPs) @ 500 ppm |
| T2 | Incorporated with thyme essential oil nanoparticles (TNPs) @ 500 ppm |
| T3 | Incorporated with oregano essential oil nanoparticles (ONPs) @ 500 ppm |
| T4 | Positive control, incorporated with free butylated hydroxytoluene (BHT) @ 200 ppm |
| Treatment | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | |
| T0 | 5.98 ± 0.01 A | 6.16 ± 0.04 2B | 6.35 ± 0.03 2C | 6.54 ± 0.06 3D | 6.72 ± 0.07 3E | 6.91 ± 0.03 3F |
| T1 | 5.94 ± 0.03 A | 6.05 ± 0.05 12A | 6.27 ± 0.07 2B | 6.36 ± 0.03 2BC | 6.53 ± 0.04 2C | 6.75 ± 0.08 2D |
| T2 | 5.95 ± 0.04 A | 5.99 ± 0.04 1AB | 6.06 ± 0.05 1AB | 6.15 ± 0.04 1BC | 6.34 ± 0.07 1CD | 6.56 ± 0.07 1D |
| T3 | 5.93 ± 0.05 A | 5.97 ± 0.04 1A | 6.04 ± 0.08 1A | 6.14 ± 0.08 1AB | 6.30 ± 0.06 1BC | 6.51 ± 0.05 1C |
| T4 | 5.95 ± 0.07 A | 6.13 ± 0.06 12AB | 6.31 ± 0.05 2BC | 6.57 ± 0.09 3CD | 6.69 ± 0.05 3DE | 6.87 ± 0.06 3E |
| Treatment | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | |
| Peroxide Value (meq/kg) | ||||||
| T0 | 2.85 ± 0.04 3A | 4.83 ± 0.08 3B | 7.49 ± 0.17 3D | 10.66 ± 0.12 4F | 8.23 ± 0.02 4E | 6.89 ± 0.23 4C |
| T1 | 1.85 ± 0.02 2A | 3.39 ± 0.03 2B | 5.26 ± 0.02 2C | 8.16 ± 0.01 3E | 7.64 ± 0.12 3D | 5.30 ± 0.03 3C |
| T2 | 1.58 ± 0.01 1A | 2.96 ± 0.04 1B | 4.19 ± 0.01 1C | 5.56 ± 0.02 1F | 5.39 ± 0.09 1E | 4.84 ± 0.03 12D |
| T3 | 1.56 ± 0.01 1A | 2.93 ± 0.02 1B | 4.16 ± 0.01 1C | 5.52 ± 0.03 1F | 5.30 ± 0.10 1E | 4.81 ± 0.02 1D |
| T4 | 1.54 ± 0.02 1A | 2.92 ± 0.02 1B | 4.23 ± 0.02 1C | 6.32 ± 0.06 2F | 6.08 ± 0.02 2E | 5.17 ± 0.01 23D |
| TBARS (mg MDA/kg) | ||||||
| T0 | 0.37 ± 0.01 3A | 0.55 ± 0.02 3B | 0.72 ± 0.02 3C | 0.92 ± 0.06 4D | 1.13 ± 0.05 4E | 1.25 ± 0.05 4E |
| T1 | 0.24 ± 0.01 2A | 0.43 ± 0.01 2B | 0.58 ± 0.03 2C | 0.74 ± 0.01 3D | 0.92 ± 0.01 3E | 1.04 ± 0.01 3F |
| T2 | 0.14 ± 0.01 1A | 0.26 ± 0.02 1B | 0.37 ± 0.02 1C | 0.46 ± 0.01 1D | 0.66 ± 0.01 1E | 0.85 ± 0.01 1F |
| T3 | 0.13 ± 0.01 1A | 0.24 ± 0.01 1B | 0.34 ± 0.01 1C | 0.42 ± 0.01 1D | 0.64 ± 0.01 1E | 0.83 ± 0.01 1F |
| T4 | 0.12 ± 0.01 1A | 0.22 ± 0.01 1B | 0.36 ± 0.01 1C | 0.57 ± 0.01 2D | 0.76 ± 0.02 2E | 0.93 ± 0.01 2F |
| Carbonyl Content (nmol/mg protein) | ||||||
| T0 | 0.96 ± 0.01 3A | 1.25 ± 0.01 3B | 1.56 ± 0.01 3C | 2.66 ± 0.02 4D | 3.44 ± 0.11 4E | 4.49 ± 0.02 4F |
| T1 | 0.85 ± 0.02 2A | 1.16 ± 0.02 2B | 1.34 ± 0.02 2C | 1.66 ± 0.01 3D | 1.95 ± 0.01 3E | 2.85 ± 0.02 3F |
| T2 | 0.75 ± 0.01 1A | 0.94 ± 0.01 1B | 1.14 ± 0.01 1C | 1.34 ± 0.01 1D | 1.56 ± 0.01 1E | 1.74 ± 0.01 1F |
| T3 | 0.73 ± 0.01 1A | 0.93 ± 0.01 1B | 1.13 ± 0.01 1C | 1.33 ± 0.01 1D | 1.52 ± 0.02 1E | 1.73 ± 0.01 1F |
| T4 | 0.75 ± 0.01 1A | 0.95 ± 0.01 1B | 1.17 ± 0.01 1C | 1.47 ± 0.01 2D | 1.75 ± 0.01 2E | 1.96 ± 0.01 2F |
| Treatment | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | |
| DPPH (% inhibition) | ||||||
| T0 | 17.14 ± 0.10 1F | 13.15 ± 0.11 1E | 11.14 ± 0.13 1D | 8.41 ± 0.22 1C | 6.20 ± 0.11 1B | 4.33 ± 0.12 1A |
| T1 | 30.34 ± 0.52 2F | 27.26 ± 0.28 2E | 24.06 ± 0.15 2D | 20.08 ± 0.12 2C | 17.40 ± 0.08 2B | 15.07 ± 0.21 1A |
| T2 | 61.08 ± 0.18 4F | 57.19 ± 0.05 4E | 54.19 ± 0.31 4D | 51.04 ± 0.21 4C | 47.08 ± 0.10 4B | 43.20 ± 0.04 4A |
| T3 | 64.46 ± 0.44 5F | 61.09 ± 0.40 5E | 57.16 ± 0.20 5D | 55.14 ± 0.28 5C | 51.09 ± 0.21 5B | 46.20 ± 0.35 5A |
| T4 | 49.70 ± 0.24 3F | 45.41 ± 0.18 3E | 41.75 ± 0.23 3D | 36.72 ± 0.47 3C | 33.12 ± 0.19 3B | 30.08 ± 0.11 3A |
| FRAP (mM Fe2+ Eq) | ||||||
| T0 | 11.56 ± 0.17 1F | 9.39 ± 0.14 1E | 7.38 ± 0.08 1D | 6.50 ± 0.12 1C | 5.53 ± 0.16 1B | 4.51 ± 0.12 1A |
| T1 | 18.65 ± 0.17 2F | 15.52 ± 0.19 2E | 13.62 ± 0.15 2D | 12.22 ± 0.03 2C | 10.55 ± 0.15 2B | 9.62 ± 0.14 2A |
| T2 | 32.50 ± 0.09 4F | 29.62 ± 0.18 4E | 26.44 ± 0.16 4D | 24.64 ± 0.12 4C | 23.48 ± 0.15 4B | 20.67 ± 0.12 4A |
| T3 | 35.55 ± 0.19 5F | 33.47 ± 0.13 5E | 30.43 ± 0.06 5D | 27.67 ± 0.26 5C | 25.53 ± 0.17 5B | 23.29 ± 0.07 5A |
| T4 | 22.41 ± 0.14 3F | 19.52 ± 0.10 3E | 17.40 ± 0.09 3D | 14.63 ± 0.20 3C | 12.31 ± 0.04 3B | 10.25 ± 0.06 3A |
| Treatment | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| Firmness (N) | Consistency (N.s) | |||||
| Day 0 | Day 7 | Day 15 | Day 0 | Day 7 | Day 15 | |
| T0 | 52.37 ± 0.29 C | 39.12 ± 0.80 1B | 30.16 ± 0.39 1A | 2123.36 ± 68.22C | 1025.24 ± 26.88 1B | 568.81 ± 52.87 A |
| T1 | 54.85 ± 0.98 C | 45.10 ± 0.36 3B | 33.71 ± 0.77 23A | 2302.07 ± 310.72B | 1724.13 ± 73.19 2B | 695.35 ± 59.20 A |
| T2 | 54.49 ± 0.66 C | 47.49 ± 0.81 4B | 35.23 ± 0.85 34A | 2164.37 ± 91.07 B | 1871.04 ± 46.93 23B | 729.68 ± 58.72 A |
| T3 | 54.37 ± 0.54 C | 47.54 ± 0.47 4B | 36.42 ± 0.78 4A | 2108.06 ± 256.30B | 1998.78 ± 122.74 3B | 750.59 ± 48.00 A |
| T4 | 53.12 ± 0.98 C | 42.77 ± 0.32 2B | 31.67 ± 0.87 12A | 2108.85 ± 101.78 C | 1042.61 ± 24.611B | 570.68 ± 54.79 A |
| Treatment | Cohesiveness (N) | Work of Cohesion (N.s) | ||||
| Day 0 | Day 7 | Day 15 | Day 0 | Day 7 | Day 15 | |
| T0 | (−)45.73 ± 0.66 C | (−)34.18 ± 0.66 1B | (−)16.63 ± 0.80 1A | (−)37.01 ± 0.16 C | (−)12.21 ± 0.84 1B | (−)5.46 ± 0.15 1A |
| T1 | (−)47.71 ± 1.37 C | (−)42.01 ± 0.28 3B | (−)19.74 ± 1.49 2A | (−)37.81 ± 0.71 C | (−)16.48 ± 0.84 23B | (−)8.37 ± 0.10 2A |
| T2 | (−)46.21 ± 0.88 C | (−)42.25 ± 0.23 3B | (−)20.84 ± 0.36 2A | (−)37.90 ± 0.40 C | (−)16.50 ± 0.64 23B | (−)9.31 ± 0.28 3A |
| T3 | (−)47.63 ± 0.89 C | (−)41.32 ± 0.30 3B | (−)21.11 ± 0.17 2A | (−)37.47 ± 0.53 C | (−)16.88 ± 0.71 3B | (−)9.11 ± 0.02 3A |
| T4 | (−)46.21 ± 0.48 C | (−)35.88 ± 0.85 2B | (−)16.99 ± 0.38 1A | (−)36.37 ± 0.84 C | (−)13.93 ± 0.96 12B | (−)5.59 ± 0.22 1A |
| Treatment | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| Lightness (L*) | Redness (a*) | |||||
| Day 0 | Day 7 | Day 15 | Day 0 | Day 7 | Day 15 | |
| T0 | 52.49 ± 0.09 C | 48.44 ± 0.12 1B | 45.20 ± 0.03 1A | 12.29 ± 0.09 C | 9.33 ± 0.06 1B | 8.12 ± 0.19 1A |
| T1 | 52.57 ± 0.08 C | 50.39 ± 0.06 2B | 47.39 ± 0.07 2A | 12.50 ± 0.07 C | 10.65 ± 0.01 2B | 8.57 ± 0.08 2A |
| T2 | 52.39 ± 0.12 C | 51.52 ± 0.07 3B | 49.37 ± 0.08 4A | 12.43 ± 0.12 C | 11.06 ± 0.01 4B | 9.43 ± 0.01 4A |
| T3 | 52.31 ± 0.09 C | 51.56 ± 0.06 3B | 49.19 ± 0.03 4A | 12.34 ± 0.09 C | 11.84 ± 0.01 5B | 10.70 ± 0.11 5A |
| T4 | 52.45 ± 0.12 C | 51.38 ± 0.05 3B | 48.44 ± 0.08 3A | 12.50 ± 0.04 C | 10.93 ± 0.01 3B | 9.09 ± 0.04 3A |
| Treatment | Yellowness (b*) | Overall Colour Change (ΔE) | ||||
| Day 0 | Day 7 | Day 15 | Day (0–15) | |||
| T0 | 14.48 ± 0.10 A | 15.77 ± 0.31 3B | 17.61 ± 0.29 4C | 8.99 ± 0.15 5 | ||
| T1 | 14.44 ± 0.08 A | 15.10 ± 0.17 2B | 16.90 ± 0.20 3C | 6.99 ± 0.08 4 | ||
| T2 | 14.37 ± 0.10 A | 14.42 ± 0.09 1A | 15.36 ± 0.25 1B | 4.44 ± 0.17 2 | ||
| T3 | 14.29 ± 0.03 A | 14.36 ± 0.08 1A | 15.25 ± 0.04 1B | 3.68 ± 0.11 1 | ||
| T4 | 14.40 ± 0.06 A | 14.65 ± 0.16 12A | 16.14 ± 0.32 2B | 5.58 ± 0.15 3 | ||
| Treatment | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | |
| Total Plate Count (log10CFU/g) | ||||||
| T0 | 4.10 ± 0.14 A | 5.68 ± 0.08 2B | 6.43 ± 0.05 3C | 7.21 ± 0.20 3D | NP | NP |
| T1 | 3.84 ± 0.15 A | 4.14 ± 0.04 1A | 5.29 ± 0.23 2B | 6.62 ± 0.10 2C | 7.10 ± 0.07 2D | NP |
| T2 | 3.75 ± 0.07 A | 3.91 ± 0.10 1A | 4.84 ± 0.09 1B | 5.68 ± 0.15 1C | 6.32 ± 0.07 1D | 7.14 ± 0.01 E |
| T3 | 3.71 ± 0.15 A | 3.87 ± 0.26 1A | 4.78 ± 0.05 1B | 5.72 ± 0.15 1C | 6.38 ± 0.11 1D | 7.12 ± 0.01 E |
| T4 | 4.13 ± 0.08 A | 5.71 ± 0.04 2B | 6.49 ± 0.12 3C | 7.28 ± 0.10 3D | NP | NP |
| Total Psychrotrophic Count (log10CFU/g) | ||||||
| T0 | 3.65 ± 0.04 2A | 4.69 ± 0.06 3B | 5.84 ± 0.04 3C | 6.58 ± 0.08 3D | NP | NP |
| T1 | 3.48 ± 0.03 1A | 3.89 ± 0.10 2B | 4.14 ± 0.02 2C | 5.23 ± 0.08 2D | 5.98 ± 0.06 2E | NP |
| T2 | 3.36 ± 0.08 1A | 3.48 ± 0.05 1A | 3.91 ± 0.04 1B | 4.55 ± 0.05 1C | 4.79 ± 0.06 1D | 5.24 ± 0.07 E |
| T3 | 3.32 ± 0.04 1A | 3.42 ± 0.05 1A | 3.86 ± 0.09 1B | 4.49 ± 0.04 1C | 4.72 ± 0.07 1D | 5.19 ± 0.03 E |
| T4 | 3.73 ± 0.04 2A | 4.74 ± 0.11 3B | 5.89 ± 0.03 3C | 6.62 ± 0.07 3D | NP | NP |
| Coliform Count (log10CFU/g) | ||||||
| T0 | 2.59 ± 0.11 2A | 2.78 ± 0.13 2AB | 2.95 ± 0.06 2BC | 3.12 ± 0.08 2C | NP | NP |
| T1 | 2.38 ± 0.08 12A | 2.47 ± 0.10 12AB | 2.71 ± 0.03 12AB | 2.83 ± 0.02 1AB | 3.08 ± 0.11 2B | NP |
| T2 | 2.26 ± 0.07 1A | 2.35 ± 0.12 1AB | 2.48 ± 0.23 1AB | 2.66 ± 0.11 1AB | 2.86 ± 0.06 1B | 2.74 ± 0.08 AB |
| T3 | 2.22 ± 0.04 1A | 2.30 ± 0.10 1AB | 2.42 ± 0.06 1AB | 2.61 ± 0.07 1AB | 2.81 ± 0.06 1B | 2.68 ± 0.09 AB |
| T4 | 2.54 ± 0.08 2A | 2.73 ± 0.08 2AB | 2.90 ± 0.10 2AB | 3.19 ± 0.04 2B | NP | NP |
| Yeast and Mould Count (log10CFU/g) | ||||||
| T0 | 1.53 ± 0.15 A | 2.32 ± 0.03 2B | 2.43 ± 0.10 2BC | 2.65 ± 0.09 2C | NP | NP |
| T1 | 1.45 ± 0.15 A | 1.93 ± 0.07 1B | 2.20 ± 0.06 1C | 2.38 ± 0.05 1CD | 2.49 ± 0.12 1CD | NP |
| T2 | 1.36 ± 0.03 A | 1.84 ± 0.05 1B | 2.16 ± 0.08 1C | 2.27 ± 0.04 1CD | 2.37 ± 0.03 1D | 2.42 ± 0.04 1D |
| T3 | 1.32 ± 0.02 A | 1.81 ± 0.03 1B | 2.10 ± 0.09 1C | 2.23 ± 0.04 1CD | 2.31 ± 0.02 1DE | 2.39 ± 0.04 1E |
| T4 | 1.57 ± 0.08 A | 2.38 ± 0.05 2B | 2.47 ± 0.04 2B | 2.72 ± 0.05 2C | NP | NP |
| Treatment | Storage Period (Days) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 15 | |
| T0 | 4.79 ± 0.30 2A | 10.53 ± 0.14 3B | 21.37 ± 0.37 3C |
| T1 | 4.21 ± 0.21 1A | 8.43 ± 0.11 2B | 18.53 ± 0.44 2C |
| T2 | 4.26 ± 0.06 1A | 7.53 ± 0.08 1B | 15.75 ± 0.13 1C |
| T3 | 4.24 ± 0.05 1A | 7.49 ± 0.05 1B | 14.66 ± 0.05 1C |
| T4 | 4.85 ± 0.12 2A | 10.80 ± 0.28 3B | 21.81 ± 0.62 3C |
| Treatment | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | |
| Odour | ||||||
| T0 | 4.77 ± 0.13 D | 4.20 ± 0.13 1C | 2.41 ± 0.12 1B | 1.00 ± 0.00 1A | NP | NP |
| T1 | 4.75 ± 0.13 E | 4.42 ± 0.12 2D | 3.67 ± 0.13 3C | 2.54 ± 0.13 2B | 1.00 ± 0.00 1A | NP |
| T2 | 4.73 ± 0.14 F | 4.58 ± 0.14 3E | 3.83 ± 0.12 4D | 3.50 ± 0.13 3C | 2.53 ± 0.13 2B | 1.00 ± 0.00 A |
| T3 | 4.78 ± 0.15 F | 4.53 ± 0.15 3E | 3.89 ± 0.11 4D | 3.58 ± 0.15 3C | 2.59 ± 0.15 2B | 1.00 ± 0.00 A |
| T4 | 4.71 ± 0.14 D | 4.25 ± 0.15 1C | 2.49 ± 0.14 2B | 1.00 ± 0.00 1A | NP | NP |
| Colour | ||||||
| T0 | 5.00 ± 0.00 C | 4.52 ± 0.12 B | 3.21 ± 0.12 1A | NP | NP | NP |
| T1 | 5.00 ± 0.00 D | 4.51 ± 0.12 C | 3.67 ± 0.13 2B | 3.28 ± 0.13 1A | NP | NP |
| T2 | 5.00 ± 0.00 E | 4.54 ± 0.12 D | 3.83 ± 0.13 3C | 3.71 ± 0.15 2B | 3.55 ± 0.13 A | NP |
| T3 | 5.00 ± 0.00 E | 4.56 ± 0.13 D | 3.92 ± 0.15 3C | 3.75 ± 0.13 2B | 3.58 ± 0.15 A | NP |
| T4 | 5.00 ± 0.00 C | 4.50 ± 0.13 B | 3.85 ± 0.13 3A | NP | NP | NP |
| Texture | ||||||
| T0 | 4.75 ± 0.14 C | 4.63 ± 0.13 B | 3.12 ± 0.12 1A | NP | NP | NP |
| T1 | 4.78 ± 0.14 D | 4.65 ± 0.13 C | 3.67 ± 0.15 2B | 3.15 ± 0.13 1A | NP | NP |
| T2 | 4.72 ± 0.13 D | 4.67 ± 0.13 D | 3.75 ± 0.13 3C | 3.58 ± 0.12 2B | 3.24 ± 0.14 A | NP |
| T3 | 4.74 ± 0.13 D | 4.69 ± 0.12 D | 3.71 ± 0.14 23C | 3.55 ± 0.13 2B | 3.27 ± 0.14 A | NP |
| T4 | 4.75 ± 0.14 C | 4.62 ± 0.13 B | 3.17 ± 0.15 1A | NP | NP | NP |
| Overall Acceptability | ||||||
| T0 | 4.82 ± 0.13 D | 4.42 ± 0.12 1C | 2.62 ± 0.11 1B | 1.00 ± 0.00 1A | NP | NP |
| T1 | 4.84 ± 0.12 E | 4.51 ± 0.14 2D | 3.65 ± 0.13 3C | 2.86 ± 0.14 2B | 1.00 ± 0.00 1A | NP |
| T2 | 4.80 ± 0.13 F | 4.59 ± 0.15 23E | 3.80 ± 0.15 4D | 3.56 ± 0.14 3C | 2.91 ± 0.11 2B | 1.00 ± 0.00 A |
| T3 | 4.83 ± 0.15 F | 4.62 ± 0.15 3E | 3.85 ± 0.12 4D | 3.61 ± 0.15 3C | 2.95 ± 0.14 2B | 1.00 ± 0.00 A |
| T4 | 4.81 ± 0.15 D | 4.40 ± 0.15 1C | 2.83 ± 0.14 2B | 1.00 ± 0.00 1A | NP | NP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.A.; Wani, S.A.; Rafeh, S.; Adil, S.; Sofi, A.H.; Ghamry, H.I.; Wani, M. Enhancing Meat Emulsion Quality and Storage Stability During Refrigeration Using Thyme and Oregano Essential Oil Nanoparticles. Foods 2025, 14, 1076. https://doi.org/10.3390/foods14061076
Hussain SA, Wani SA, Rafeh S, Adil S, Sofi AH, Ghamry HI, Wani M. Enhancing Meat Emulsion Quality and Storage Stability During Refrigeration Using Thyme and Oregano Essential Oil Nanoparticles. Foods. 2025; 14(6):1076. https://doi.org/10.3390/foods14061076
Chicago/Turabian StyleHussain, Syed A., Sarfaraz A. Wani, Sheikh Rafeh, Sheikh Adil, Asif H. Sofi, Heba I. Ghamry, and Manzoor Wani. 2025. "Enhancing Meat Emulsion Quality and Storage Stability During Refrigeration Using Thyme and Oregano Essential Oil Nanoparticles" Foods 14, no. 6: 1076. https://doi.org/10.3390/foods14061076
APA StyleHussain, S. A., Wani, S. A., Rafeh, S., Adil, S., Sofi, A. H., Ghamry, H. I., & Wani, M. (2025). Enhancing Meat Emulsion Quality and Storage Stability During Refrigeration Using Thyme and Oregano Essential Oil Nanoparticles. Foods, 14(6), 1076. https://doi.org/10.3390/foods14061076





