From Dormancy to Eradication: Strategies for Controlling Bacterial Persisters in Food Settings
Abstract
1. Introduction
2. Literature Search Strategy
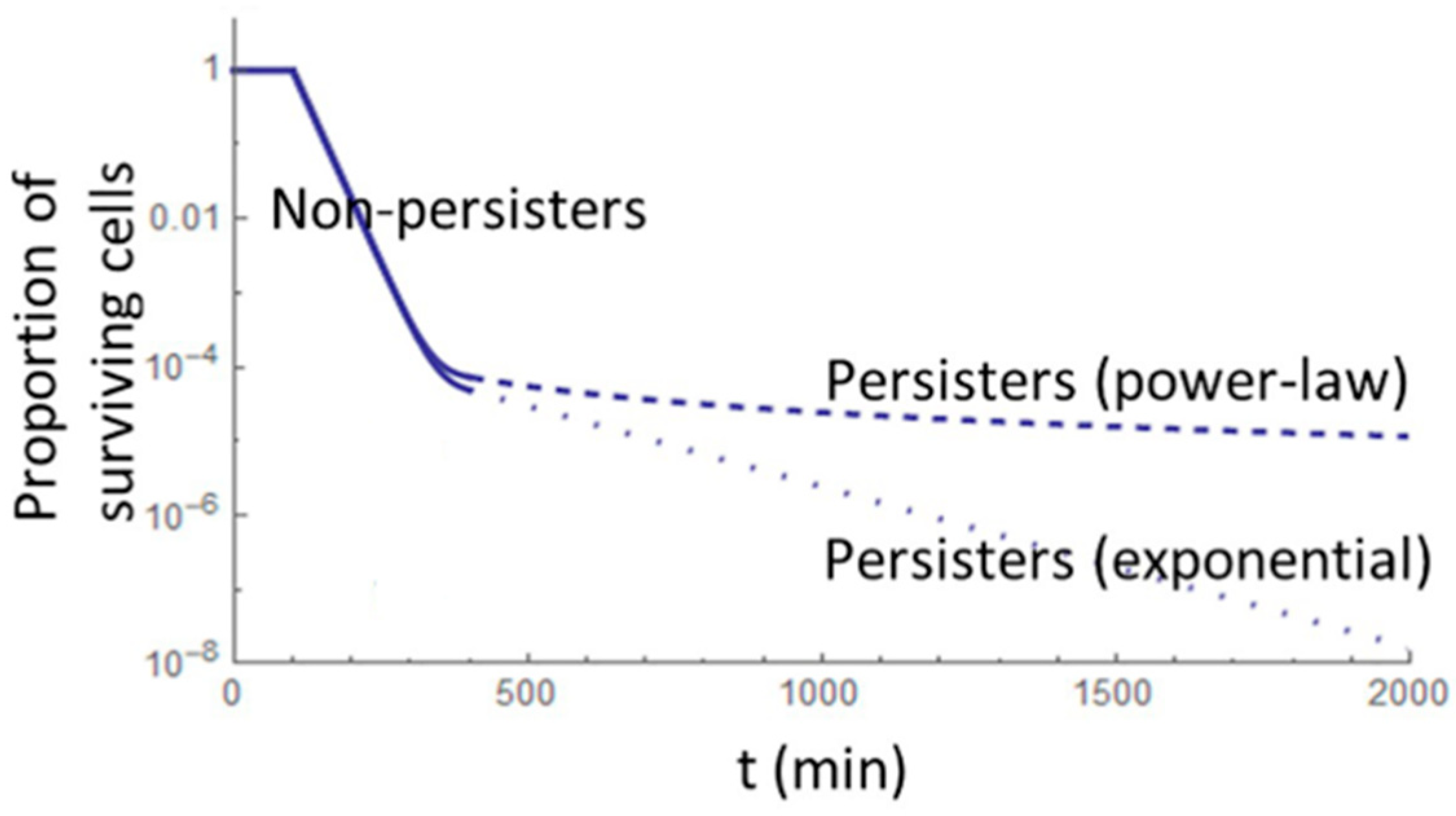
3. Formation, Survival, and Regrowth
4. Types of Persisters
4.1. Type I or Triggered Persisters
4.2. Type II or Stochastic Persisters
4.3. Type III or Specialized Persisters
5. Mechanisms of Persister Cell Formation
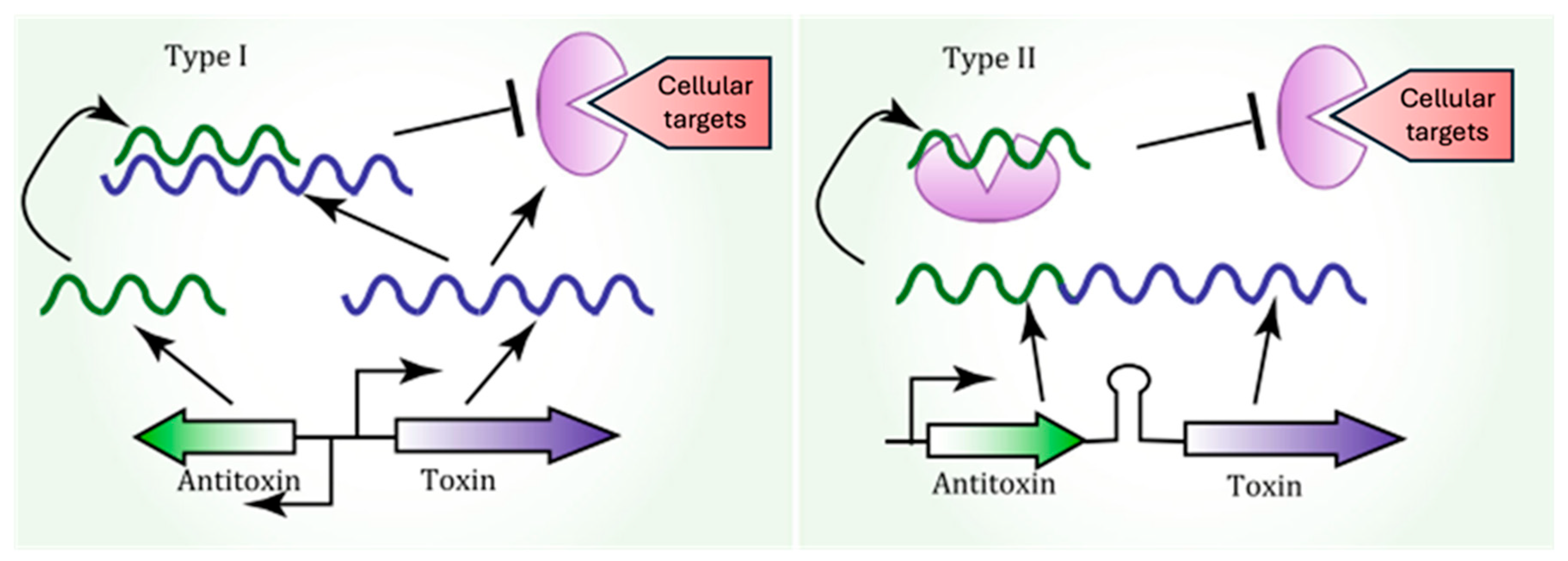
5.1. Toxin–Antitoxin System-Induced Persisters
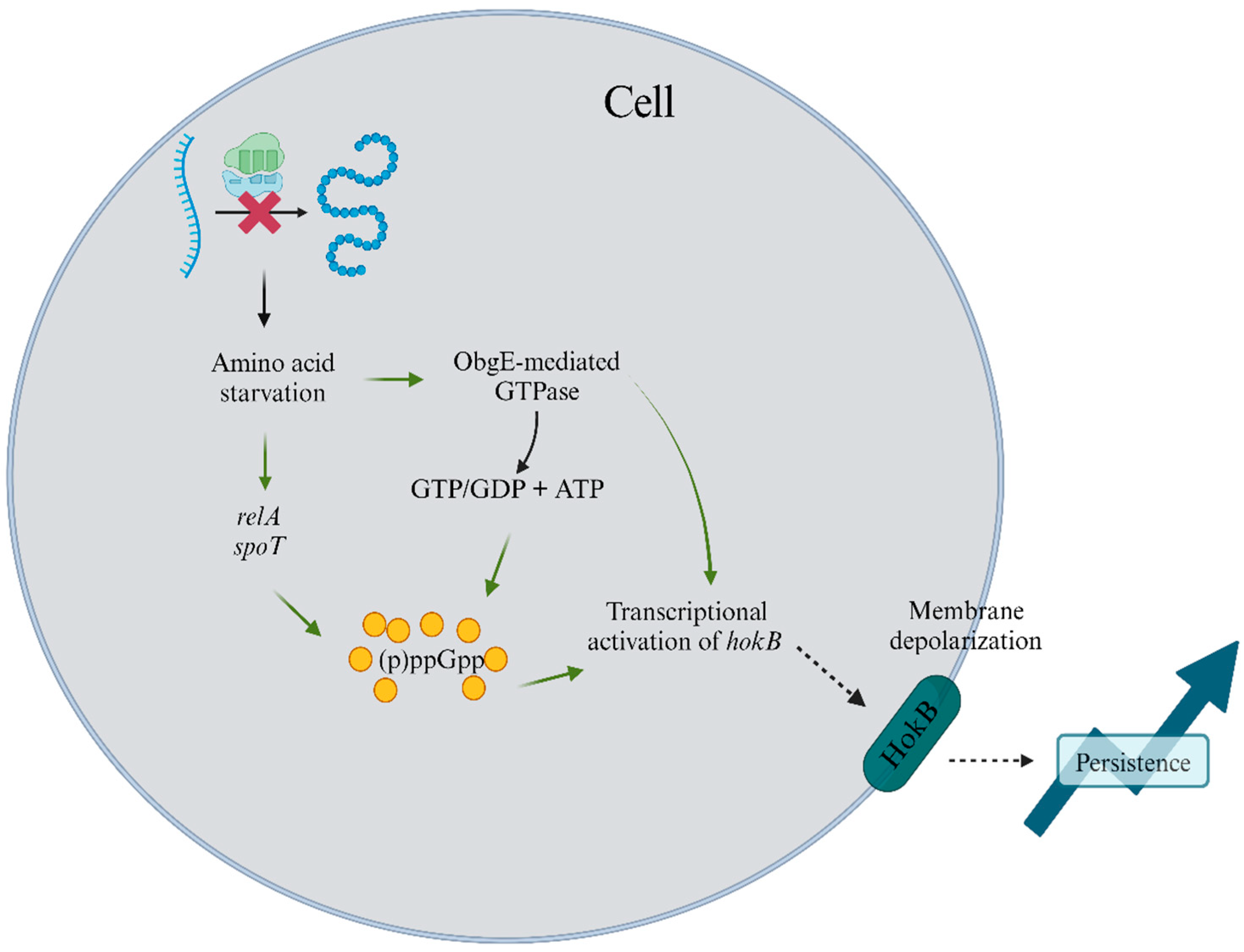
5.2. Stringent Response
5.3. Hunker Theory of Persistence
5.4. SOS Response Connected with Both TA Systems and Efflux Pumps
5.5. Persistence as “Stuff That Happens”
5.6. Other Systems and Forms Contributing to Persistence
| Type of Persister | Production Stage | Formation Mechanism | References |
|---|---|---|---|
| Type I | Stationary phase | TA systems, SOS response (connected with TA system and efflux pumps), and spores | [15,19,20] |
| Type II | Continuous growth at slow rate | TA systems, stringent response, and SCVs | [15] |
| Type III | Induced by specific antibiotics | TA systems, hunker theory, PaSH, and L-form bacteria | [17,18,20] |
6. Implications of Persister Cells in the Food Industry
| Microorganism | Production Inducers | Persister Cells Development Mechanism | Food Type | References |
|---|---|---|---|---|
| L. monocytogenes | Environmental triggers and/or stressful conditions associated with temperature, NaCl, pH, or the presence of antimicrobials | TA systems Stringent response Biofilms | Food-processing environment, meat, dairy (milk, soft cheese, and butter), leafy greens (vegetables and fruits), seafood, bakery products, and sandwiches | [46,49,50,51,52,53] |
| B. cereus | Heat and desiccation | Spore formation and biofilms | Cooked foods, rice, canned products, salted and smoked fish, milk and dairy, and meat | [45,46] |
| S. aureus | ATP depletion | Stochastically Biofilms | Food-processing environment, fish, seafood, bakery and canned products, eggs, milk, plant-based foods, and meat | [46,54,55] |
| P. fluorescens P. aeruginosa | Environmental triggers and/or stressful conditions associated with temperature, NaCl, pH, or the presence of antimicrobials | TA systems Stringent response Biofilms | Dairy, vegetables, meat, and ready-to-eat foods | [44] |
| E. coli | Environmental triggers and/or stressful conditions associated with temperature, NaCl, pH, or the presence of antimicrobials | TA systems Stringent response Biofilms | Cooked meat, vegetables, berries, fruits, milk, and eggs | [46] |
6.1. General Preventive Measures
6.2. Targeted Eradication Approaches
- (i)
- The direct killing of dormant persister cells: This involves targeting cellular structures such as the cell wall, the membrane, and DNA. By disrupting membrane potential or altering permeability, persisters become susceptible to antimicrobials. Physical methods, such as heat, UV radiation, and sonication, directly damage cellular structures, complementing this approach. Chemical agents, including surfactants and reactive oxygen species (ROS), can further enhance membrane disruption, while biological methods, such as bacteriophage-derived enzymes, target cell walls with precision.
- (ii)
- Awakening dormant cells: Some approaches aim to “wake” persister cells, making them metabolically active and, therefore, more vulnerable to antibiotics. Metabolic triggers like pyruvate, often used as chemical agents, can effectively induce cellular activity. Physical methods, such as alternating temperatures or pressures, can also provoke metabolic changes. Additionally, biological tools, including certain enzymes or signaling molecules, may assist in reactivating dormant cells.
- (iii)
- Combination therapies: Combining anti-persister agents with conventional antibiotics enhances treatment effectiveness. This diversified approach attacks persisters through multiple mechanisms. Physical methods can act synergistically with chemical antimicrobials, e.g., heat-enhanced antibiotic activity. Similarly, biological methods, such as combining quorum-sensing inhibitors with antibiotics, amplify the impact of chemical treatments.
- (iv)
- Quorum-sensing interference: Targeting quorum-sensing circuits can prevent persister cells from communicating and forming biofilms. Biological strategies, such as enzymes that degrade quorum-sensing molecules or peptides that block receptors, are highly effective and have been described in previous publications [52,53]. Chemical agents can inhibit quorum-sensing molecule synthesis, while physical methods, like ultrasound, may disrupt biofilm structures, indirectly interfering with quorum-sensing pathways [52,53]. This diversified approach attacks persisters through multiple mechanisms improving the chances of success.
6.3. Physical Treatments
6.4. Chemical Treatments
6.5. Biological Treatments
| Treatments | Subtype | Target | Mechanism of Action | Practical Application | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Physical | High-pressure processing (HPP) | Vegetative cells, biofilms | Disrupts cell walls, membranes, and biofilm matrix | Used in meat, juices, and dairy products seafood, ready-to-eat meals, and functional foods | Preserves the organoleptic and nutritional properties of the food matrix | High initial cost of equipment and limited impact on bacterial spores | [68,71,73] |
| Steam sterilization | Spores | Denatures proteins and destroys spore core structures | Sterilization of canned foods, equipment, and packaging | Highly effective in eliminating bacteria, viruses, and spores, utilizing water as the primary sterilizing agent | Can alter texture and flavor, not suitable for dry, powdery, or heat-sensitive products, risk of overcooking | [128,129] | |
| UV radiation | Vegetative cells, biofilms | Induces DNA damage and ROS generation | Sanitization of surfaces, water treatment, and packaging sterilization, fruits, vegetables, meat, fish, dairy, and cereal products | Ideal for heat-sensitive foods, preserve texture, flavor, and nutritional value, extending shelf life and reducing spoilage | Only effective for surface sterilization since it does not penetrate deep into solid or opaque foods; prolonged exposure and high doses can degrade certain nutrients | [61,129,130] | |
| Pulsed electric fields (PEFs) | Vegetative cells | Disrupts membranes and electroporates cells | Applied in liquid foods like juices and soups without altering sensory properties | Zero adverse effects on the nutritional value and sensory properties of food materials | Less effective on solid or complex structures and does not inactivate bacterial spores | [78] | |
| Ultrasonic waves | Biofilms | Cavitation effect disrupts biofilm matrix and detaches cells from surfaces | Cleaning of processing lines and utensils in food production facilities; suitable for fruits, vegetables, meat, fish, dairy, cereal, and emulsified products | Helps retain the sensory and nutritional qualities of food, effectively inactivating bacteria, yeasts, and mold sand improving the efficiency of food emulsification and homogenization | Solid and complex foods are less responsive compared to liquids; ultrasonic equipment can be costly | [78,129] | |
| UV-C light emitting diodes (LEDs) | Vegetative and biofilms | Induces DNA damage and ROS generation | Fresh fruit and vegetables, washing water, salad leaves, and stainless-steel surfaces | Sustainability, longer lifetimes, lower costs, reduced energy consumption, and minimal maintenance, wavelength diversity | Limited penetration, effectiveness restricted to surfaces, potential food quality degradation, and reduced efficiency on irregular surfaces | [131] | |
| Chemical | Acidic solutions (e.g., lactic acid and acetic acid) | Vegetative cells, biofilms | Lowers pH, disrupting metabolic activity and biofilm stability | Surface decontamination and addition to marinades, fermented, and pickled foods | Extending shelf life, enhancing flavor, and being cost-effective, safe, and easy to use | May alter taste, be corrosive to equipment, cause nutrient loss, have limited effectiveness on certain microbes, or require regulatory compliance | [85] |
| Chlorine dioxide | Vegetative cells, biofilms | Oxidative stress damages biofilm matrix and cellular components | Sanitization of processing equipment and water; washing fruits and vegetables | Leaves no harmful residues, does not produce the strong odor, neither produces toxic by-products nor does alters the nutritive and organoleptic qualities of food products, and is effective over a wide pH range (pH 3–8) | Toxic and explosive at high concentrations, can cause health risks; produce surface properties can affect ClO2 accessibility to microbes, residual moisture after the water rinsing can promote microbial growth, and not suitable for dried foods | [86,87,132] | |
| Hydrogen peroxide | Spores, biofilms | Disrupts spore coat and biofilm structure through oxidative damage | Used in food-contact surfaces and packaging sterilization | Highly versatile with no toxic residues | Unstable and decomposes upon standing, agitation, and exposure to light or heating | [86] | |
| Enzymatic detergents (e.g., proteases and DNases) | Biofilms | Degrades biofilm matrix by breaking down proteins and extracellular DNA | Applied in cleaning protocols for stubborn biofilm removal in drains and equipment | Rich variety, ability to function under various industrial and even extreme conditions (such as high temperatures), offer targeted, effective, and environmentally friendly cleaning solutions | Require careful handling and proper conditions for effectiveness and can be more costly than conventional chemical detergents | [133] | |
| Peracetic acid | Vegetative cells, biofilms | Oxidative stress damages cellular components | Sanitizing surfaces and utensils | Highly effective, fast-acting sanitizer with strong antimicrobial properties | Corrosive nature, potential irritants, and short shelf life | [134] | |
| Biological | Probiotics (e.g., Lactobacillus spp.) | Vegetative cells | Compete for nutrients and produce antimicrobial compounds | Used in fermented foods and meat and dairy products to prevent pathogen establishment | Enhancing gut health, improving food quality, and extending shelf life | Stability, regulatory approval, and individual variability | [135] |
| Antimicrobial peptides (AMPs) | Vegetative cells | Disrupts cell membranes and inhibits growth | Inclusion in food coatings or processing liquids for enhanced safety | A natural and effective way to enhance food safety, extend shelf life, and prevent microbial contamination | Stability, cost, regulatory approval, and potential resistance | [136,137] | |
| Bacteriophages | Vegetative cells and biofilms | Specifically lyses targeted bacteria and biofilms | Biofilm removal on surfaces and equipment; targeted pathogen elimination in ready-to-eat products | Highly specific and natural approach, reduce the need for chemical preservatives and antibiotics while maintaining the taste, texture, and nutritional value of food | Effective only against certain bacteria, bacterial resistance may develop over time, need for regulatory approval | [111,115,138,139] | |
| Bacteriocins (e.g., nisin) | Spores, vegetative cells | Inhibits spore germination and vegetative cell growth | Applied in cheese, canned foods, and vacuum-packed products | Natural and non-toxic | Limited activity to specific bacteria, lose effectiveness in complex food matrices or under high temperatures, and high production costs | [137,140] | |
| Spore lytic enzymes | Spores | Breaks down spore coats and weakens spore resistance mechanisms | Used in high-risk food products to control spore-forming pathogens | Powerful, biological method for improving food safety and extending shelf life by targeting spore-forming pathogens | Careful consideration of their specificity, cost, stability, and regulatory hurdles | [43] |
6.6. Implementation of Sanitation Techniques Recommendations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayrapetyan, M.; Williams, T.C.; Baxter, R.; Oliver, J.D. Viable but Nonculturable and Persister Cells Coexist Stochastically and Are Induced by Human Serum. Infect. Immun. 2015, 83, 4194–4203. [Google Scholar] [CrossRef]
- Balaban, N. Persistence: Mechanisms for Triggering and Enhancing Phenotypic Variability. Curr. Opin. Genet. Dev. 2011, 21, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister Cells, Dormancy and Infectious Disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial Persister Cell Formation and Dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef] [PubMed]
- Bigger, J. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 1944, 244, 497–500. [Google Scholar] [CrossRef]
- Moyed, H.S.; Bertrand, K.P. HipA, a Newly Recognized Gene of Escherichia coli K-12 That Affects Frequency of Persistence after Inhibition of Murein Synthesis. J. Bacteriol. 1983, 155, 768–775. [Google Scholar] [CrossRef]
- Moyed, H.S.; Broderick, S.H. Molecular Cloning and Expression of HipA, a Gene of Escherichia coli K-12 That Affects Frequency of Persistence after Inhibition of Murein Synthesis. J. Bacteriol. 1986, 166, 399–403. [Google Scholar] [CrossRef]
- Johnson, P.J.T.; Levin, B.R. Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in Staphylococcus aureus. PLoS Genet. 2013, 9, e1003123. [Google Scholar] [CrossRef]
- Levin, B.R.; Concepción-Acevedo, J.; Udekwu, K.I. Persistence: A Copacetic and Parsimonious Hypothesis for the Existence of Non-Inherited Resistance to Antibiotics. Curr. Opin. Microbiol. 2014, 21, 18–21. [Google Scholar] [CrossRef]
- Rebelo, J.S.; Domingues, C.P.F.; Monteiro, F.; Nogueira, T.; Dionisio, F. Bacterial Persistence Is Essential for Susceptible Cell Survival in Indirect Resistance, Mainly for Lower Cell Densities. PLoS ONE 2021, 16, e0246500. [Google Scholar] [CrossRef]
- Şimşek, E.; Kim, M. Power-Law Tail in Lag Time Distribution Underlies Bacterial Persistence. Proc. Natl. Acad. Sci. USA 2019, 116, 17635–17640. [Google Scholar] [CrossRef] [PubMed]
- Blattman, S.B.; Jiang, W.; McGarrigle, E.R.; Liu, M.; Oikonomou, P.; Tavazoie, S. Identification and Genetic Dissection of Convergent Persister Cell States; Springer: Berlin/Heidelberg, Germany, 2024; Volume 636, ISBN 4158602408. [Google Scholar]
- van den Bergh, B.; Fauvart, M.; Michiels, J. Formation, Physiology, Ecology, Evolution and Clinical Importance of Bacterial Persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and Guidelines for Research on Antibiotic Persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial Persistence as a Phenotypic Switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef]
- Urbaniec, J.; Xu, Y.; Hu, Y.; Hingley-Wilson, S.; McFadden, J. Phenotypic Heterogeneity in Persisters: A Novel “hunker” Theory of Persistence. FEMS Microbiol. Rev. 2022, 46, fuab042. [Google Scholar] [CrossRef]
- Wakamoto, Y.; Dhar, N.; Chait, R.; Schneider, K.; Signorino-Gelo, F.; Leibler, S.; McKinney, J.D. Dynamic Persistence of Antibiotic-Stressed Mycobacteria. Science 2013, 339, 91–95. [Google Scholar] [CrossRef]
- Goormaghtigh, F.; Van Melderen, L. Single-Cell Imaging and Characterization of Escherichia coli Persister Cells to Ofloxacin in Exponential Cultures. Sci. Adv. 2019, 5, eaav9462. [Google Scholar] [CrossRef]
- Gefen, O.; Balaban, N.Q. The Importance of Being Persistent: Heterogeneity of Bacterial Populations under Antibiotic Stress: Review Article. FEMS Microbiol. Rev. 2009, 33, 704–717. [Google Scholar] [CrossRef]
- Dörr, T.; Vulić, M.; Lewis, K. Ciprofloxacin Causes Persister Formation by Inducing the TisB Toxin in Escherichia coli. PLoS Biol. 2010, 8, 29–35. [Google Scholar] [CrossRef]
- Trastoy, R.; Manso, T.; Fernández-García, L.; Blasco, L.; Ambroa, A.; Pérez del Molino, M.L.; Bou, G.; García-Contreras, R.; Wood, T.K.; Tomás, M. Mechanisms of Bacterial Tolerance and Persistence in the Gastrointestinal and Respiratory Environments. Clin. Microbiol. Rev. 2018, 31, e00023-18. [Google Scholar] [CrossRef]
- Kim, Y.; Wood, T.K. Toxins Hha and CspD and Small RNA Regulator Hfq Are Involved in Persister Cell Formation through MqsR in Escherichia coli. Biochem. Biophys. Res. Commun. 2010, 391, 209–213. [Google Scholar] [CrossRef]
- Guglielmini, J.; Van Melderen, L. Bacterial Toxin-Antitoxin Systems: Translation Inhibitors Everywhere. Mob. Genet. Elem. 2011, 1, 283–306. [Google Scholar] [CrossRef]
- Song, S.; Wood, T.K. A Primary Physiological Role of Toxin/Antitoxin Systems Is Phage Inhibition. Front. Microbiol. 2020, 11, 1895. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato-Cezar, L.R.; Spira, B.; Machini, M.T. Bacterial Toxin-Antitoxin Systems: Novel Insights on Toxin Activation across Populations and Experimental Shortcomings. Curr. Res. Microb. Sci. 2023, 5, 100204. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, Targets, and Triggers: An Overview of Toxin-Antitoxin Biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef]
- Zhang, S.-P.; Wang, Q.; Quan, S.-W.; Yu, X.-Q.; Wang, Y.; Guo, D.-D.; Peng, L.; Feng, H.-Y.; He, Y.-X. Type II Toxin–Antitoxin System in Bacteria: Activation, Function, and Mode of Action. Biophys. Rep. 2020, 6, 68–79. [Google Scholar] [CrossRef]
- Paul, P.; Sahu, B.R.; Suar, M. Plausible Role of Bacterial Toxin–Antitoxin System in Persister Cell Formation and Elimination. Mol. Oral Microbiol. 2019, 34, 97–107. [Google Scholar]
- Giramma, C.N.; DeFoer, M.B.; Wang, J.D. The Alarmone (p)PpGpp Regulates Primer Extension by Bacterial Primase. J. Mol. Biol. 2021, 433, 167189. [Google Scholar] [CrossRef]
- Song, S.; Wood, T.K. Combatting Persister Cells With Substituted Indoles. Front. Microbiol. 2020, 11, 1565. [Google Scholar] [CrossRef]
- Chowdhury, N.; Kwan, B.W.; Wood, T.K. Persistence Increases in the Absence of the Alarmone Guanosine Tetraphosphate by Reducing Cell Growth. Sci. Rep. 2016, 6, 20519. [Google Scholar] [CrossRef]
- Verstraeten, N.; Knapen, W.J.; Kint, C.I.; Liebens, V.; Van den Bergh, B.; Dewachter, L.; Michiels, J.E.; Fu, Q.; David, C.C.; Fierro, A.C.; et al. Obg and Membrane Depolarization Are Part of a Microbial Bet-Hedging Strategy That Leads to Antibiotic Tolerance. Mol. Cell 2015, 59, 9–21. [Google Scholar] [CrossRef]
- Verstraeten, N.; Gkekas, S.; Kint, C.I.; Deckers, B.; Van den Bergh, B.; Herpels, P.; Louwagie, E.; Knapen, W.; Wilmaerts, D.; Dewachter, L.; et al. Biochemical Determinants of ObgE-Mediated Persistence. Mol. Microbiol. 2019, 112, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Ambroa, A.; López, M.; Bou, G.; Cantón, R.; Garcia-Contreras, R.; Wood, T.K.; et al. (P)PpGpp and Its Role in Bacterial Persistence: New Challenges. Antimicrob. Agents Chemother. 2020, 64, e01283-20. [Google Scholar] [CrossRef] [PubMed]
- Hussain Chan, M.W.; Mirani, Z.A.; Khan, M.N.; Ali, A.; Khan, A.B.; Asadullah; Rauf, N. Isolation and Characterization of Small Colony Variants of Staphylococcus aureus in Various Food Samples. Biocatal. Agric. Biotechnol. 2021, 35, 102097. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Role of Persisters and Small-Colony Variants in Antibiotic Resistance of Planktonic and Biofilm-Associated Staphylococcus aureus: An in Vitro Study. J. Med. Microbiol. 2009, 58, 1067–1073. [Google Scholar] [CrossRef]
- Curtis, T.D.; Gram, L.; Knudsen, G.M. The Small Colony Variant of Listeria monocytogenes Is More Tolerant to Antibiotics and Has Altered Survival in RAW 264.7 Murine Macrophages. Front. Microbiol. 2016, 7, 1056. [Google Scholar] [CrossRef]
- Frenzel, E.; Kranzler, M.; Stark, T.D.; Hofmann, T.; Ehling-Schulz, M. The Endospore-Forming Pathogen Bacillus cereus Exploits a Small Colony Variant-Based Diversification Strategy in Response to Aminoglycoside Exposure. mBio 2015, 6, e01172-15. [Google Scholar] [CrossRef]
- Besse, A.; Groleau, M.-C.; Déziel, E. Emergence of Small Colony Variants Is an Adaptive Strategy Used by Pseudomonas aeruginosa to Mitigate the Effects of Redox Imbalance. mSphere 2023, 8, e00057-23. [Google Scholar] [CrossRef]
- Glover, W.A.; Yang, Y.; Zhang, Y. Insights into the Molecular Basis of L-Form Formation and Survival in Escherichia coli. PLoS ONE 2009, 4, e7316. [Google Scholar] [CrossRef]
- Liu, S.; Brul, S.; Zaat, S.A.J. Bacterial Persister-Cells and Spores in the Food Chain: Their Potential Inactivation by Antimicrobial Peptides (AMPs). Int. J. Mol. Sci. 2020, 21, 8967. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liang, L.; Deng, S.; Wu, Y.; Yuan, Y.; Gao, M. Novel Spore Lytic Enzyme from a Bacillus Phage Leading to Spore Killing. Enzym. Microb. Technol. 2020, 142, 109698. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Gomes, I.B.; Sousa, S.F.; Simões, M. Antimicrobial Susceptibility of Persister Biofilm Cells of Bacillus cereus and Pseudomonas fluorescens. Microorganisms 2022, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, Y.; Effarizah, M.E. Post-Cooking Growth and Survival of Bacillus cereus Spores in Rice and Their Enzymatic Activities Leading to Food Spoilage Potential. Foods 2023, 12, 626. [Google Scholar] [CrossRef]
- Lyashchuk, Y.O.; Novak, A.I.; Kostrova, Y.B.; Shibarshina, O.Y.; Evdokimova, O.V.; Kanina, I.V. The Study of Persistence of Microorganisms and Parasites in Food Products. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 062002. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Hillig, N.; Hamedy, A.; Koethe, M. Listeria monocytogenes Detection on Food Contact Surfaces: Suitability of Different Swab Materials. J. Consum. Prot. Food Saf. 2023, 18, 443–450. [Google Scholar] [CrossRef]
- Harter, E.; Wagner, E.M.; Zaiser, A.; Halecker, S.; Wagner, M.; Rychli, K. Stress Survival Islet 2, Predominantly Present in Listeria monocytogenes Strains of Sequence Type 121, Is Involved in the Alkaline and Oxidative Stress Responses. Appl. Environ. Microbiol. 2017, 83, e00827-17. [Google Scholar] [CrossRef]
- Narimisa, N.; Kalani, B.S.; Mohammadzadeh, R.; Jazi, F.M. Combination of Antibiotics-Nisin Reduces the Formation of Persister Cell in Listeria monocytogenes. Microb. Drug Resist. 2021, 27, 137–144. [Google Scholar] [CrossRef]
- Li, X.; Hospital, X.F.; Hierro, E.; Fernández, M.; Sheng, L.; Wang, L. Formation of Listeria monocytogenes Persister Cells in the Produce-Processing Environment. Int. J. Food Microbiol. 2023, 390, 110106. [Google Scholar] [CrossRef]
- Tuytschaever, T.; Raes, K.; Sampers, I. Listeria monocytogenes in Food Businesses: From Persistence Strategies to Intervention/Prevention Strategies—A Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3910–3950. [Google Scholar] [CrossRef] [PubMed]
- Belias, A.; Sullivan, G.; Wiedmann, M.; Ivanek, R. Factors That Contribute to Persistent Listeria in Food Processing Facilities and Relevant Interventions: A Rapid Review. Food Control 2022, 133, 108579. [Google Scholar] [CrossRef]
- Miao, J.; Lin, S.; Soteyome, T.; Peters, B.M.; Li, Y.; Chen, H.; Su, J.; Li, L.; Li, B.; Xu, Z.; et al. Biofilm Formation of Staphylococcus aureus under Food Heat Processing Conditions: First Report on CML Production within Biofilm. Sci. Rep. 2019, 9, 1312. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister Formation in Staphylococcus aureus Is Associated with ATP Depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef]
- Morcrette, H.; Kovacs-Simon, A.; Tennant, R.K.; Love, J.; Wagley, S.; Yang, Z.R.; Studholme, D.J.; Soyer, O.S.; Champion, O.L.; Butler, C.S.; et al. Campylobacter Jejuni 11168H Exposed to Penicillin Forms Persister Cells and Cells with Altered Redox Protein Activity. Front. Cell Infect. Microbiol. 2020, 10, 565975. [Google Scholar] [CrossRef]
- Ovsepian, A.; Larsen, M.H.; Vegge, C.S.; Ingmer, H. Ciprofloxacin-Induced Persister-Cells in Campylobacter Jejuni. Microbiology 2020, 166, 849–853. [Google Scholar] [CrossRef] [PubMed]
- ISO 22000:2018; Food Safety Management Systems—A Practical Guide. ISO: Geneva, Switzerland, 2018.
- National Advisory Committee on Microbiological Criteria for Foods. HACCP Principles & Application Guidelines. Available online: https://www.fda.gov/food/hazard-analysis-critical-control-point-haccp/haccp-principles-application-guidelines (accessed on 7 January 2025).
- Khan, F.; Pham, D.T.N.; Tabassum, N.; Oloketuyi, S.F.; Kim, Y.M. Treatment Strategies Targeting Persister Cell Formation in Bacterial Pathogens. Crit. Rev. Microbiol. 2020, 46, 665–688. [Google Scholar] [CrossRef]
- Tran, V.N.; Dasagrandhi, C.; Truong, V.G.; Kim, Y.M.; Kang, H.W. Antibacterial Activity of Staphylococcus aureus Biofilm under Combined Exposure of Glutaraldehyde, near-Infrared Light, and 405-Nm Laser. PLoS ONE 2018, 13, e0202821. [Google Scholar] [CrossRef]
- Manivasagan, P.; Khan, F.; Hoang, G.; Mondal, S.; Kim, H.; Hoang Minh Doan, V.; Kim, Y.M.; Oh, J. Thiol Chitosan-Wrapped Gold Nanoshells for near-Infrared Laser-Induced Photothermal Destruction of Antibiotic-Resistant Bacteria. Carbohydr. Polym. 2019, 225, 115228. [Google Scholar] [CrossRef]
- Dash, K.K.; Fayaz, U.; Dar, A.H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Deka, P.; Khan, S.A. A Comprehensive Review on Heat Treatments and Related Impact on the Quality and Microbial Safety of Milk and Milk-Based Products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Rifna, E.J.; Singh, S.K.; Chakraborty, S.; Dwivedi, M. Effect of Thermal and Non-Thermal Techniques for Microbial Safety in Food Powder: Recent Advances. Food Res. Int. 2019, 126, 108654. [Google Scholar] [CrossRef]
- Khalid, W.; Maggiolino, A.; Kour, J.; Arshad, M.S.; Aslam, N.; Afzal, M.F.; Meghwar, P.; Zafar, K.-W.; De Palo, P.; Korma, S.A. Dynamic Alterations in Protein, Sensory, Chemical, and Oxidative Properties Occurring in Meat during Thermal and Non-Thermal Processing Techniques: A Comprehensive Review. Front. Nutr. 2023, 9, 1057457. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Sit, N. A Review on Fruit and Vegetable Processing Using Traditional and Novel Methods. Futur. Postharvest Food 2024. [Google Scholar] [CrossRef]
- Catherine, M.G.C.; Renard, J.F.M. Thermal Food Processing New Technologies and Qualities Issues: Thermal Processing of Fruits and Fruit Juices, 2nd ed.; Sun, D.-W., Ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 1439876789. [Google Scholar]
- Evelyn; Silva, F.V.M. High Pressure Processing of Milk: Modeling the Inactivation of Psychrotrophic Bacillus cereus Spores at 38–70 °C. J. Food Eng. 2015, 165, 141–148. [Google Scholar] [CrossRef]
- Luu-Thi, H.; Corthouts, J.; Passaris, I.; Grauwet, T.; Aertsen, A.; Hendrickx, M.; Michiels, C.W. Carvacrol Suppresses High Pressure High Temperature Inactivation of Bacillus cereus Spores. Int. J. Food Microbiol. 2015, 197, 45–52. [Google Scholar] [CrossRef]
- Santos, L.M.; Oliveira, F.A.; Ferreira, E.H.; Rosenthal, A. Application and Possible Benefits of High Hydrostatic Pressure or High-Pressure Homogenization on Beer Processing: A Review. Food Sci. Technol. Int. 2017, 23, 561–581. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of High Hydrostatic Pressure Food Processing: Perspectives on Technology and Food Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current Status and Future Trends of High-Pressure Processing in Food Industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Naveena, B.; Nagaraju, M. Review on Principles, Effects, Advantages and Disadvantages of High Pressure Processing of Food. Int. J. Chem. Stud. 2020, 8, 2964–2967. [Google Scholar] [CrossRef]
- Niepa, T.H.R.; Gilbert, J.L.; Ren, D. Controlling Pseudomonas aeruginosa Persister Cells by Weak Electrochemical Currents and Synergistic Effects with Tobramycin. Biomaterials 2012, 33, 7356–7365. [Google Scholar] [CrossRef]
- Niepa, T.H.R.; Snepenger, L.M.; Wang, H.; Sivan, S.; Gilbert, J.L.; Jones, M.B.; Ren, D. Sensitizing Pseudomonas aeruginosa to Antibiotics by Electrochemical Disruption of Membrane Functions. Biomaterials 2016, 74, 267–279. [Google Scholar] [CrossRef] [PubMed]
- USFDA CFR—Code of Federal Regulations Title 21 the Information on This Page Is Current as of 1 April 2016. 2019. Available online: https://www.ecfr.gov (accessed on 5 July 2024).
- Liu, D.; Huang, Q.; Gu, W.; Zeng, X.-A. A Review of Bacterial Biofilm Control by Physical Strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 3453–3470. [Google Scholar] [CrossRef] [PubMed]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Strategies and Novel Technologies to Control Campylobacter in the Poultry Chain: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1353–1377. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.H. Basic Elements of Equipment Cleaning and Sanitizing in Food Processing and Handling Operations 1; Institute of Food and Agricultural Sciences (IFAS), University of Florida: Gainesville, FL, USA, 2018. [Google Scholar]
- Bayoumi, M.A.; Kamal, R.M.; Abd El Aal, S.F.; Awad, E.I. Assessment of a Regulatory Sanitization Process in Egyptian Dairy Plants in Regard to the Adherence of Some Food-Borne Pathogens and Their Biofilms. Int. J. Food Microbiol. 2012, 158, 225–231. [Google Scholar] [CrossRef]
- Atasoy, M.; Álvarez Ordóñez, A.; Cenian, A.; Djukić-Vuković, A.; Lund, P.A.; Ozogul, F.; Trček, J.; Ziv, C.; De Biase, D. Exploitation of Microbial Activities at Low PH to Enhance Planetary Health. FEMS Microbiol. Rev. 2024, 48, fuad062. [Google Scholar] [CrossRef]
- Wood, J.M. Bacterial Responses to Osmotic Challenges. J. Gen. Physiol. 2015, 145, 381–388. [Google Scholar] [CrossRef]
- Mari, A.; Parisouli, D.N.; Krokida, M. Exploring Osmotic Dehydration for Food Preservation: Methods, Modelling, and Modern Applications. Foods 2024, 13, 2783. [Google Scholar] [CrossRef]
- Xiong, X.; Kong, J.; Qi, D.; Xiong, X.; Liu, Y.; Cui, X. Presence, Formation, and Elimination of Foodborne Pathogen Persisters. JSFA Rep. 2022, 2, 4–16. [Google Scholar] [CrossRef]
- Karki, P.; Mohiuddin, S.G.; Kavousi, P.; Orman, M.A. Investigating the Effects of Osmolytes and Environmental PH on Bacterial Persisters. Antimicrob. Agents Chemother. 2020, 64, e02393-19. [Google Scholar] [CrossRef]
- Aryal, M.; Muriana, P.M. Efficacy of Commercial Sanitizers Used in Food Processing Facilities for Inactivation of Listeria monocytogenes, E. coli O157:H7, and Salmonella Biofilms. Foods 2019, 8, 639. [Google Scholar] [CrossRef]
- Duze, S.T.; Marimani, M.; Patel, M. Tolerance of Listeria monocytogenes to Biocides Used in Food Processing Environments. Food Microbiol. 2021, 97, 103758. [Google Scholar]
- Lioy, V.S.; Rey, O.; Balsa, D.; Pellicer, T.; Alonso, J.C. A Toxin–Antitoxin Module as a Target for Antimicrobial Development. Plasmid 2010, 63, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Son, W.S.; Lee, B.-J. Structural Overview of Toxin–Antitoxin Systems in Infectious Bacteria: A Target for Developing Antimicrobial Agents. Biochim. Biophys. Acta-Proteins Proteom. 2013, 1834, 1155–1167. [Google Scholar] [CrossRef]
- Barraud, N.; Buson, A.; Jarolimek, W.; Rice, S.A. Mannitol Enhances Antibiotic Sensitivity of Persister Bacteria in Pseudomonas aeruginosa Biofilms. PLoS ONE 2013, 8, e84220. [Google Scholar] [CrossRef]
- Leszczynska, D.; Matuszewska, E.; Kuczynska-Wisnik, D.; Furmanek-Blaszk, B.; Laskowska, E. The Formation of Persister Cells in Stationary-Phase Cultures of Escherichia coli Is Associated with the Aggregation of Endogenous Proteins. PLoS ONE 2013, 8, e54737. [Google Scholar] [CrossRef]
- Mizzi, L.; Maniscalco, D.; Gaspari, S.; Chatzitzika, C.; Gatt, R.; Valdramidis, V.P. Assessing the Individual Microbial Inhibitory Capacity of Different Sugars against Pathogens Commonly Found in Food Systems. Lett. Appl. Microbiol. 2020, 71, 251–258. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M.S. Suitability of Sugar Alcohols as Antidiabetic Supplements: A Review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef]
- Medina-Rodríguez, A.C.; Ávila-Sierra, A.; Ariza, J.J.; Guillamón, E.; Baños-Arjona, A.; Vicaria, J.M.; Jurado, E. Clean-in-Place Disinfection of Dual-Species Biofilm (Listeria and Pseudomonas) by a Green Antibacterial Product Made from Citrus Extract. Food Control 2020, 118, 107422. [Google Scholar] [CrossRef]
- Cacciatore, F.A.; Brandelli, A.; Malheiros, P.d.S. Combining Natural Antimicrobials and Nanotechnology for Disinfecting Food Surfaces and Control Microbial Biofilm Formation. Crit. Rev. Food Sci. Nutr. 2021, 61, 3771–3782. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Joković, N.; Jokanović, M.; Ivić, M.; Šojić, B.; Škaljac, S.; Stojanović, P.; Mihajilov-Krstev, T. Inhibition of Salmonella Enteritidis Growth and Storage Stability in Chicken Meat Treated with Basil and Rosemary Essential Oils Alone or in Combination. Food Control 2018, 90, 332–343. [Google Scholar] [CrossRef]
- Jokanović, M.; Ivić, M.; Škaljac, S.; Tomović, V.; Pavlić, B.; Šojić, B.; Zeković, Z.; Peulić, T.; Ikonić, P. Essential Oil and Supercritical Extracts of Winter Savory (Satureja montana L.) as Antioxidants in Precooked Pork Chops during Chilled Storage. LWT 2020, 134, 110260. [Google Scholar] [CrossRef]
- Tomović, V.; Šojić, B.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Đorđević, V.; Parunović, N.; Martinović, A.; Vujadinović, D. Caraway (Carum carvi L.) Essential Oil Improves Quality of Dry-fermented Sausages Produced with Different Levels of Sodium Nitrite. J. Food Process Preserv. 2022, 46, e15786. [Google Scholar] [CrossRef]
- Muruzović, M.; Mladenović, K.G.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Extracts of Agrimonia eupatoria L. as Sources of Biologically Active Compounds and Evaluation of Their Antioxidant, Antimicrobial, and Antibiofilm Activities. J. Food Drug Anal. 2016, 24, 539–547. [Google Scholar] [CrossRef]
- Lu, M.; Wang, S.; Wang, T.; Hu, S.; Bhayana, B.; Ishii, M.; Kong, Y.; Cai, Y.; Dai, T.; Cui, W.; et al. Bacteria-Specific Phototoxic Reactions Triggered by Blue Light and Phytochemical Carvacrol. Sci. Transl. Med. 2021, 13, eaba3571. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, M.; Elias, M.; Fraqueza, M.J. The Use of Starter Cultures in Traditional Meat Products. J. Food Qual. 2017, 2017, 9546026. [Google Scholar] [CrossRef]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and Disadvantages of Non-starter Lactic Acid Bacteria from Traditional Fermented Foods: Potential Use as Starters or Probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Henderson, L.O.; Erazo Flores, B.J.; Skeens, J.; Kent, D.; Murphy, S.I.; Wiedmann, M.; Guariglia-Oropeza, V. Nevertheless, She Resisted—Role of the Environment on Listeria monocytogenes Sensitivity to Nisin Treatment in a Laboratory Cheese Model. Front. Microbiol. 2020, 11, 635. [Google Scholar] [CrossRef]
- Gut, I.M.; Blanke, S.R.; van der Donk, W.A. Mechanism of Inhibition of Bacillus anthracis Spore Outgrowth by the Lantibiotic Nisin. ACS Chem. Biol. 2011, 6, 744–752. [Google Scholar] [CrossRef]
- Balandin, S.V.; Sheremeteva, E.V.; Ovchinnikova, T.V. Pediocin-Like Antimicrobial Peptides of Bacteria. Biochemistry 2019, 84, 464–478. [Google Scholar] [CrossRef]
- Rishi, P.; Bhagat, N.R.; Thakur, R.; Pathania, P. Tackling Salmonella Persister Cells by Antibiotic–Nisin Combination via Mannitol. Indian J. Microbiol. 2018, 58, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage Therapy: An Alternative to Antibiotics in the Age of Multi-Drug Resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Caflisch, K.M.; Patel, R. Implications of Bacteriophage- and Bacteriophage Component-Based Therapies for the Clinical Microbiology Laboratory. J. Clin. Microbiol. 2019, 57, e00229-19. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Donovan, D.M. Antimicrobial Bacteriophage-Derived Proteins and Therapeutic Applications. Bacteriophage 2015, 5, e1062590. [Google Scholar] [CrossRef]
- Schuch, R.; Khan, B.K.; Raz, A.; Rotolo, J.A.; Wittekind, M. Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent. Antimicrob. Agents Chemother. 2017, 61, e02666-16. [Google Scholar] [CrossRef]
- Singh, A.; Padmesh, S.; Dwivedi, M.; Kostova, I. How Good Are Bacteriophages as an Alternative Therapy to Mitigate Biofilms of Nosocomial Infections. Infect. Drug Resist. 2022, 15, 503–532. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of Environmental Factors on Phage-Bacteria Interaction and on the Efficacy and Infectivity of Phage P100. Front. Microbiol. 2016, 7, 1152. [Google Scholar] [CrossRef]
- Iacumin, L.; Manzano, M.; Comi, G. Phage Inactivation of Listeria monocytogenes on San Daniele Dry-Cured Ham and Elimination of Biofilms from Equipment and Working Environments. Microorganisms 2016, 4, 4. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Ruas-Madiedo, P.; Martínez, B.; Rodríguez, A.; García, P. Effective Removal of Staphylococcal Biofilms by the Endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 Is a Highly Efficient Antibacterial against Multidrug-Resistant Strains and Persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef]
- Defraine, V.; Schuermans, J.; Grymonprez, B.; Govers, S.K.; Aertsen, A.; Fauvart, M.; Michiels, J.; Lavigne, R.; Briers, Y. Efficacy of Artilysin Art-175 against Resistant and Persistent Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Ko, K.S. Eradication of Persister Cells of Acinetobacter baumannii through Combination of Colistin and Amikacin Antibiotics. J. Antimicrob. Chemother. 2019, 74, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Mizan, M.F.R.; Ha, A.J.; Ha, S. Do Advances and Future Prospects of Enzyme-Based Biofilm Prevention Approaches in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1484–1502. [Google Scholar] [CrossRef] [PubMed]
- Meireles, A.; Borges, A.; Giaouris, E.; Simões, M. The Current Knowledge on the Application of Anti-Biofilm Enzymes in the Food Industry. Food Res. Int. 2016, 86, 140–146. [Google Scholar] [CrossRef]
- Sikdar, R.; Elias, M. Quorum Quenching Enzymes and Their Effects on Virulence, Biofilm, and Microbiomes: A Review of Recent Advances. Expert Rev. Anti. Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef]
- Salman, M.K.; Abuqwider, J.; Mauriello, G. Anti-Quorum Sensing Activity of Probiotics: The Mechanism and Role in Food and Gut Health. Microorganisms 2023, 11, 793. [Google Scholar] [CrossRef]
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.M.; Sulaiman, A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Appl. Sci. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, Y.; Lin, X.; Zhang, S. Recent Applications and Prospects of Enzymes in Quality and Safety Control of Fermented Foods. Foods 2024, 13, 3804. [Google Scholar] [CrossRef]
- Abril, B.; Bou, R.; García-Pérez, J.V.; Benedito, J. Role of Enzymatic Reactions in Meat Processing and Use of Emerging Technologies for Process Intensification. Foods 2023, 12, 1940. [Google Scholar] [CrossRef]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Pectin and Polygalacturonic Acid-Coated Liposomes as Novel Delivery System for Nisin: Preparation, Characterization and Release Behavior. Food Hydrocoll. 2017, 70, 1–7. [Google Scholar] [CrossRef]
- Srividya, N.; Ghoora, M.D.; Padmanabh, P.R. Antimicrobial Nanotechnology: Research Implications and Prospects in Food Safety. In Food Preservation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 125–165. [Google Scholar]
- Jildeh, Z.B.; Wagner, P.H.; Schöning, M.J. Sterilization of Objects, Products, and Packaging Surfaces and Their Characterization in Different Fields of Industry: The Status in 2020. Phys. Status Solidi Appl. Mater. Sci. 2021, 218, 2000732. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Abana, C.M.; Brannon, J.R.; Ebbott, R.A.; Dunigan, T.L.; Guckes, K.R.; Fuseini, H.; Powers, J.; Rogers, B.R.; Hadjifrangiskou, M. Characterization of Blue Light Irradiation Effects on Pathogenic and Nonpathogenic Escherichia coli. Microbiologyopen 2017, 6, e00466. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Santos, C.; Sério, J.; Crespo, M.T.B.; Pereira, V.J. Enhancing Food Safety: Employing Ultraviolet-C Light Emitting Diodes for Water, Leaf, and Surface Disinfection. Innov. Food Sci. Emerg. Technol. 2024, 98, 103848. [Google Scholar] [CrossRef]
- Malka, S.K.; Park, M.-H. Fresh Produce Safety and Quality: Chlorine Dioxide’s Role. Front. Plant Sci. 2022, 12, 775629. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial Proteases and Their Applications. Front. Microbiol. 2023, 14, 1236368. [Google Scholar] [CrossRef]
- Zoellner, C.; Aguayo-Acosta, A.; Siddiqui, M.W.; Dávila-Aviña, J.E. Peracetic Acid in Disinfection of Fruits and Vegetables. In Postharvest Disinfection of Fruits and Vegetables; Siddiqui, M.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–66. [Google Scholar]
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial Peptides and Their Application in Food Packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Yuan, L.; Sadiq, F.A.; Wang, N.; Yang, Z.; He, G. Recent Advances in Understanding the Control of Disinfectant-Resistant Biofilms by Hurdle Technology in the Food Industry. Crit. Rev. Food Sci. Nutr. 2021, 61, 3876–3891. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.B.; Trampuz, A.; Di Luca, M. Bacteriophage Sb-1 Enhances Antibiotic Activity against Biofilm, Degrades Exopolysaccharide Matrix and Targets Persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 842–853. [Google Scholar] [CrossRef]
- Lu, T.K.; Collins, J.J. Engineered Bacteriophage Targeting Gene Networks as Adjuvants for Antibiotic Therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 4629–4634. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Delshadi, R.; Jafari, S.M.; Williams, L. Nanoencapsulated Nisin: An Engineered Natural Antimicrobial System for the Food Industry. Trends Food Sci. Technol. 2019, 94, 20–31. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, S.; Grujović, M.Ž.; Marković, K.G.; Barreto-Crespo, M.T.; Semedo-Lemsaddek, T. From Dormancy to Eradication: Strategies for Controlling Bacterial Persisters in Food Settings. Foods 2025, 14, 1075. https://doi.org/10.3390/foods14061075
Serrano S, Grujović MŽ, Marković KG, Barreto-Crespo MT, Semedo-Lemsaddek T. From Dormancy to Eradication: Strategies for Controlling Bacterial Persisters in Food Settings. Foods. 2025; 14(6):1075. https://doi.org/10.3390/foods14061075
Chicago/Turabian StyleSerrano, Susana, Mirjana Ž. Grujović, Katarina G. Marković, Maria Teresa Barreto-Crespo, and Teresa Semedo-Lemsaddek. 2025. "From Dormancy to Eradication: Strategies for Controlling Bacterial Persisters in Food Settings" Foods 14, no. 6: 1075. https://doi.org/10.3390/foods14061075
APA StyleSerrano, S., Grujović, M. Ž., Marković, K. G., Barreto-Crespo, M. T., & Semedo-Lemsaddek, T. (2025). From Dormancy to Eradication: Strategies for Controlling Bacterial Persisters in Food Settings. Foods, 14(6), 1075. https://doi.org/10.3390/foods14061075







