Dietary Components Associated with the Risk of Gastric Cancer in the Latin American Population: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
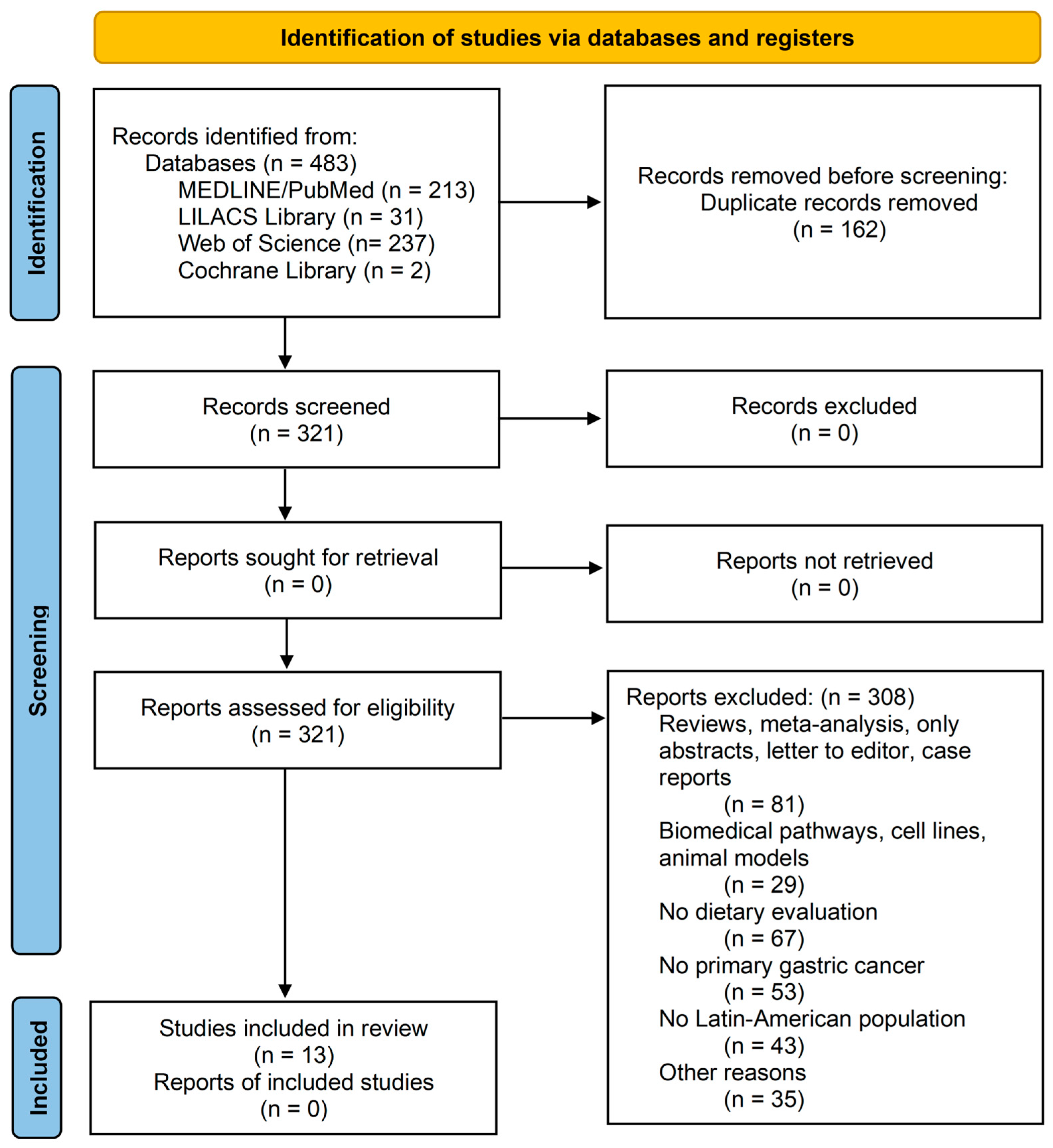
2. Materials and Methods
2.1. Study Design and Search Strategy/Data Source
2.2. Selection of Studies
2.3. Data Extraction
2.4. Study Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Studies
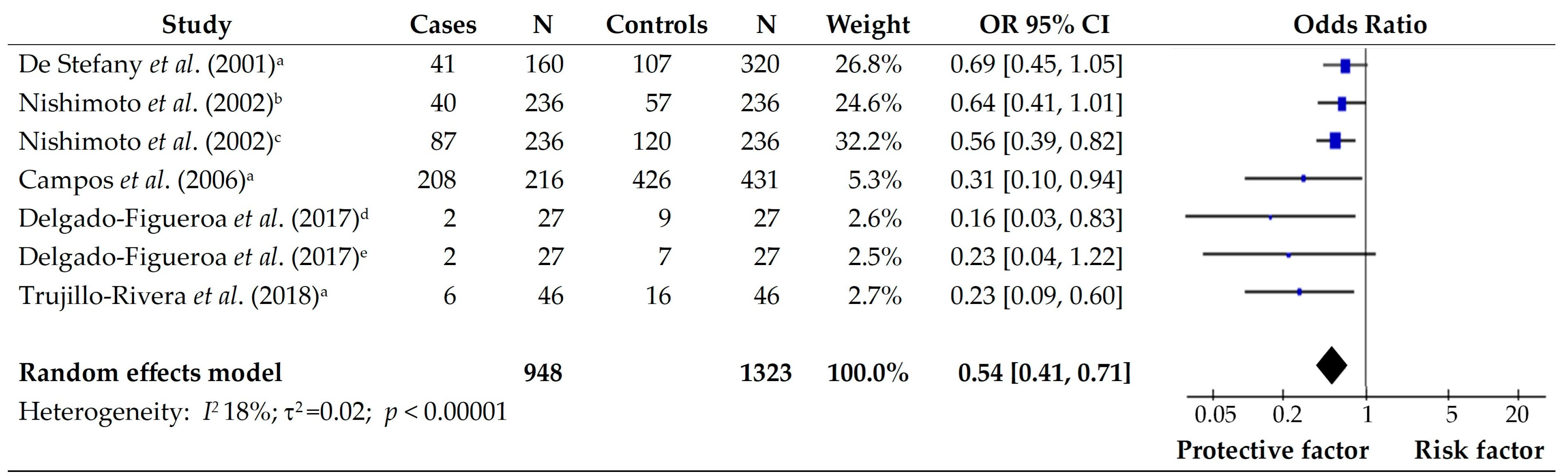
3.2. Vegetables
3.3. Fruits
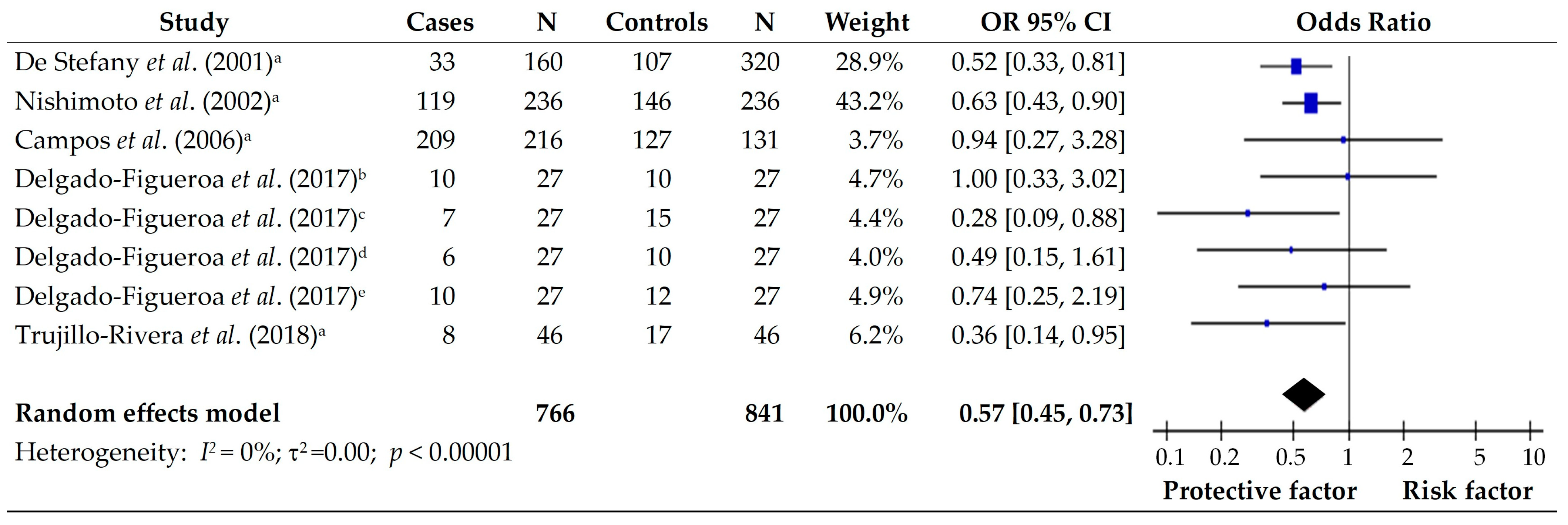
3.4. Fresh Meat and Eggs
3.5. Processed Meat
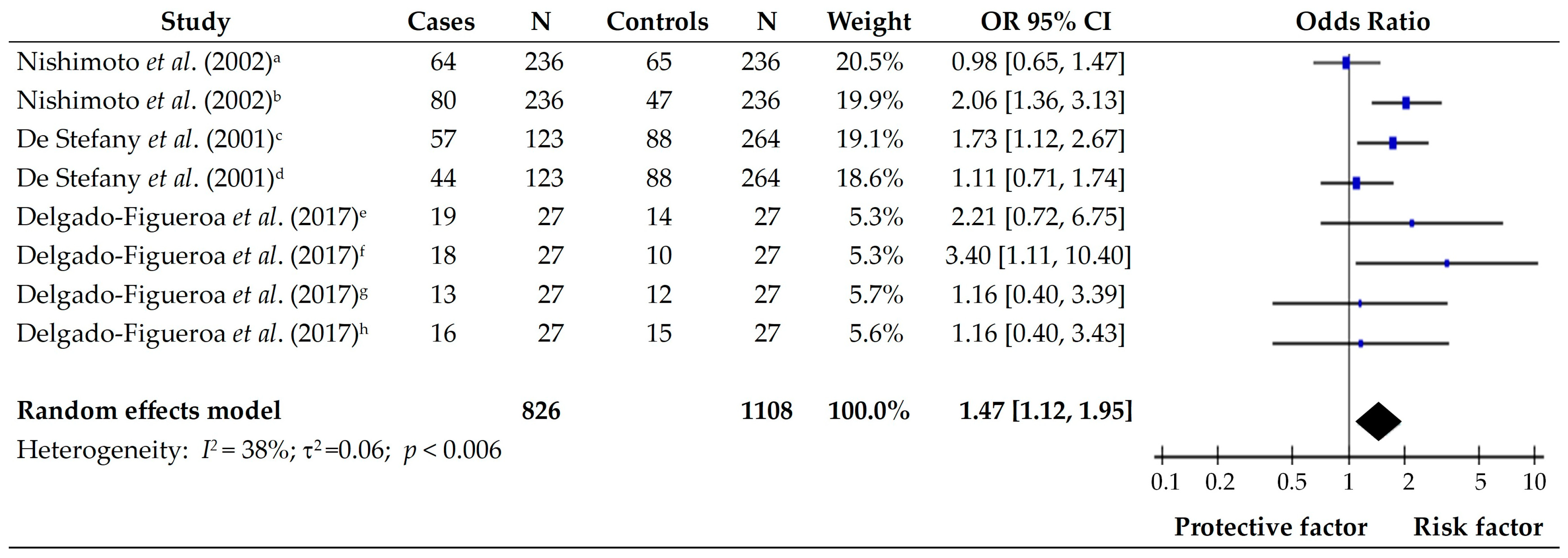
3.6. Cereals and Tubers
3.7. Fish and Seafood
3.8. Salted, Canned, and Pickled
3.9. Micronutrients
3.10. Macronutrients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freile, B.; van Schooten, T.; Derks, S.; Carneiro, F.; Figueiredo, C.; Barros, R.; Gauna, C.; Pareira, R.; Romero, M.; Riquelme, A. Gastric cancer hospital-based registry: Real-world gastric cancer data from Latin America and Europe. ESMO Gastrointest. Oncol. 2024, 6, 100088. [Google Scholar] [CrossRef]
- WHO. International Agency for Research on Cancer. Age-Standardized Rate (World) per 100 000, Incidence and Mortality, Both sexes, in 2022. Latin American and the Caribbean. 2022. Available online: https://gco.iarc.fr/today/en/dataviz/bars?types=0_1&mode=cancer&group_populations=1&sort_by=value1&populations=904 (accessed on 10 January 2023).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.U.A.; Boccolini, C.S. Social inequalities and complementary feeding in Latin America and the Caribbean. Cien. Saude Coletiva 2022, 27, 619–630. [Google Scholar] [CrossRef]
- Singh, P.N. The Impact of Dietary Changes on Non-communicable Diseases in Latin America. Front. Media SA 2022, 9, 890873. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose(-)Response Meta-Analysis. Nutrients 2019, 11, 826. [Google Scholar] [CrossRef]
- Fagundes, M.A.; Silva, A.R.C.; Fernandes, G.A.; Curado, M.P. Dietary Polyphenol Intake and Gastric Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5878. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- JBI. Critical Appraisal Tools. Checklist for Case Control Studies. 2010. Available online: https://jbi.global/critical-appraisal-tools (accessed on 12 May 2023).
- De Stefani, E.; Ronco, A.; Brennan, P.; Boffetta, P. Meat consumption and risk of stomach cancer in Uruguay: A case-control study. Nutr. Cancer 2001, 40, 103–107. [Google Scholar] [CrossRef]
- De Stefani, E.; Correa, P.; Boffetta, P.; Ronco, A.; Brennan, P.; Deneo-Pellegrini, H.; Mendilaharsu, M. Plant foods and risk of gastric cancer: A case-control study in Uruguay. Eur. J. Cancer Prev. 2001, 10, 357–364. [Google Scholar] [CrossRef]
- Munoz, N.; Plummer, M.; Vivas, J.; Moreno, V.; De Sanjose, S.; Lopez, G.; Oliver, W. A case-control study of gastric cancer in Venezuela. Int. J. Cancer 2001, 93, 417–423. [Google Scholar] [CrossRef]
- Nishimoto, I.N.; Hamada, G.S.; Kowalski, L.P.; Rodrigues, J.G.; Iriya, K.; Sasazuki, S.; Hanaoka, T.; Tsugane, S. Risk factors for stomach cancer in Brazil (I): A case-control study among non-Japanese Brazilians in Sao Paulo. Jpn. J. Clin. Oncol. 2002, 32, 277–283. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Correa, P.; Boffetta, P.; Deneo-Pellegrini, H.; Ronco, A.L.; Mendilaharsu, M. Dietary patterns and risk of gastric cancer: A case-control study in Uruguay. Gastric Cancer 2004, 7, 211–220. [Google Scholar] [CrossRef]
- Campos, F.; Carrasquilla, G.; Koriyama, C.; Serra, M.; Carrascal, E.; Itoh, T.; Nomoto, M.; Akiba, S. Risk factors of gastric cancer specific for tumor location and histology in Cali, Colombia. World J. Gastroenterol. 2006, 12, 5772–5779. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Figueroa, N.; Casas-Junco, P.; Torres-Jasso, J.H.; Bustos-Carpinteyro, A.R.; Santiago-Luna, E.; Marin-Contreras, M.E.; Sanchez-Lopez, J.Y. [Risk factors associated to diffuse gastric cancer and intestinal histological patterns in an adult population from Western Mexico]. Gac. Med. Mex. 2017, 153, 173–178. [Google Scholar] [PubMed]
- Trujillo Rivera, A.; Sampieri, C.L.; Morales Romero, J.; Montero, H.; Acosta Mesa, H.G.; Cruz Ramirez, N.; Novoa Del Toro, E.M.; Leon Cordoba, K. Risk factors associated with gastric cancer in Mexico: Education, breakfast and chili. Rev. Esp. Enferm. Dig. 2018, 110, 372–379. [Google Scholar] [CrossRef]
- Peres, S.V.; Silva, D.R.M.; Coimbra, F.J.F.; Fagundes, M.A.; Auzier, J.J.N.; Pelosof, A.G.; Araujo, M.S.; Assumpcao, P.P.; Curado, M.P. Consumption of processed and ultra-processed foods by patients with stomach adenocarcinoma: A multicentric case-control study in the Amazon and southeast regions of Brazil. Cancer Causes Control 2022, 33, 889–898. [Google Scholar] [CrossRef]
- De Stefani, E.; Boffetta, P.; Brennan, P.; Deneo-Pellegrini, H.; Carzoglio, J.C.; Ronco, A.; Mendilaharsu, M. Dietary carotenoids and risk of gastric cancer: A case-control study in Uruguay. Eur. J. Cancer Prev. 2000, 9, 329–334. [Google Scholar] [CrossRef]
- De Stefani, E.; Boffetta, P.; Ronco, A.L.; Brennan, P.; Deneo-Pellegrini, H.; Carzoglio, J.C.; Mendilaharsu, M. Plant sterols and risk of stomach cancer: A case-control study in Uruguay. Nutr. Cancer 2000, 37, 140–144. [Google Scholar] [CrossRef]
- Lopez-Carrillo, L.; Lopez-Cervantes, M.; Robles-Diaz, G.; Ramirez-Espitia, A.; Mohar-Betancourt, A.; Meneses-Garcia, A.; Lopez-Vidal, Y.; Blair, A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int. J. Cancer 2003, 106, 277–282. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, R.U.; Galvan-Portillo, M.V.; Ward, M.H.; Agudo, A.; Gonzalez, C.A.; Onate-Ocana, L.F.; Herrera-Goepfert, R.; Palma-Coca, O.; Lopez-Carrillo, L. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int. J. Cancer 2009, 125, 1424–1430. [Google Scholar] [CrossRef]
- Li, G.Z.; Doherty, G.M.; Wang, J. Surgical Management of Gastric Cancer: A Review. JAMA Surg. 2022, 157, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Solis, A.L.; Pacheco-Can, O.D.; Vazquez-Segura, H.S.L.; Pech-Aguilar, A.G.; Franco-Gonzalez, C.D.; Avila-Nava, A.; Lugo, R. Impact of surgical resection on the survival in Mexican patients with gastric cancer: A meta-analysis and systematic review. Medicine 2023, 102, e33915. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- WHO. International Agency for Research on Cancer. Age-Standardized Rate (World) per 100 000, Incidence and Mortality, Both sexes, in 2022. Latin American Hub by Country. 2022. Available online: https://gco.iarc.fr/today/en/dataviz/bars?types=0_1&mode=population&group_populations=0&sort_by=value1&populations=152_170_188_192_218_222_32_320_340_484_558_591_600_604_630_68_76_858_862&cancers=7 (accessed on 10 January 2023).
- Rodríguez Temesio, G. Obesidad en Uruguay. Es tiempo de actuar. Rev. Cir. Urug. 2024, 8, 1–3. [Google Scholar] [CrossRef]
- Wu, X.; Chen, L.; Cheng, J.; Qian, J.; Fang, Z.; Wu, J. Effect of Dietary Salt Intake on Risk of Gastric Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies. Nutrients 2022, 14, 4260. [Google Scholar] [CrossRef]
- Ge, S.; Feng, X.; Shen, L.; Wei, Z.; Zhu, Q.; Sun, J. Association between Habitual Dietary Salt Intake and Risk of Gastric Cancer: A Systematic Review of Observational Studies. Gastroenterol. Res. Pract. 2012, 2012, 808120. [Google Scholar] [CrossRef]
- Xie, Y.; Geng, Y.; Yao, J.; Ji, J.; Chen, F.; Xiao, J.; Hu, X.; Ma, L. N-nitrosamines in processed meats: Exposure, formation and mitigation strategies. J. Agric. Food Res. 2023, 13, 100645. [Google Scholar] [CrossRef]
- Bryan, N.S.; van Grinsven, H. The role of nitrate in human health. Adv. Agron. 2013, 119, 153–182. [Google Scholar]
- Said Abasse, K.; Essien, E.E.; Abbas, M.; Yu, X.; Xie, W.; Sun, J.; Akter, L.; Cote, A. Association between Dietary Nitrate, Nitrite Intake, and Site-Specific Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 666. [Google Scholar] [CrossRef]
- Norat, T.; Scoccianti, C.; Boutron-Ruault, M.C.; Anderson, A.; Berrino, F.; Cecchini, M.; Espina, C.; Key, T.; Leitzmann, M.; Powers, H.; et al. European Code against Cancer 4th Edition: Diet and cancer. Cancer Epidemiol. 2015, 39 (Suppl. S1), S56–S66. [Google Scholar] [CrossRef]
- Nunez de Gonzalez, M.T.; Osburn, W.N.; Hardin, M.D.; Longnecker, M.; Garg, H.K.; Bryan, N.S.; Keeton, J.T. A survey of nitrate and nitrite concentrations in conventional and organic-labeled raw vegetables at retail. J. Food Sci. 2015, 80, C942–C949. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Ferreira, N.R.; Rocha, B.S.; Barbosa, R.M.; Laranjinha, J. The redox interplay between nitrite and nitric oxide: From the gut to the brain. Redox Biol. 2013, 1, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Ivy, J.L. Inorganic nitrite and nitrate: Evidence to support consideration as dietary nutrients. Nutr. Res. 2015, 35, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Niklas, A.A.; Borge, G.I.A.; Rodbotten, R.; Berget, I.; Muller, M.H.B.; Herrmann, S.S.; Granby, K.; Kirkhus, B. Levels of nitrate, nitrite and nitrosamines in model sausages during heat treatment and in vitro digestion—The impact of adding nitrite and spinach (Spinacia oleracea L.). Food Res. Int. 2023, 166, 112595. [Google Scholar] [CrossRef]
- Azizi, N.; Zangiabadian, M.; Seifi, G.; Davari, A.; Yekekhani, E.; Safavi-Naini, S.A.A.; Berger, N.A.; Nasiri, M.J.; Sohrabi, M.R. Gastric Cancer Risk in Association with Underweight, Overweight, and Obesity: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2778. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Moreno-Banda, G.L.; Carnalla, M.; Rivera, J.A.; Romero-Martinez, M.; Barquera, S.; Barrientos-Gutiérrez, T. Prevalencia de prediabetes y diabetes en México: Ensanut 2022. Salud Publ. Mex. 2023, 65, s163–s168. [Google Scholar] [CrossRef]
- Campos-Nonato, I.; Galván-Valencia, Ó.; Hernández-Barrera, L.; Oviedo-Solís, C.; Barquera, S. Prevalencia de obesidad y factores de riesgo asociados en adultos mexicanos: Resultados de la Ensanut 2022. Salud Publ. Mex. 2023, 65, s238–s247. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Wilson, R.B. Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. Int. J. Mol. Sci. 2020, 21, 6451. [Google Scholar] [CrossRef]
- Seyyedsalehi, M.S.; Mohebbi, E.; Tourang, F.; Sasanfar, B.; Boffetta, P.; Zendehdel, K. Association of Dietary Nitrate, Nitrite, and N-Nitroso Compounds Intake and Gastrointestinal Cancers: A Systematic Review and Meta-Analysis. Toxics 2023, 11, 190. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrillo, L.; Camargo, M.C.; Schneider, B.G.; Sicinschi, L.A.; Hernandez-Ramirez, R.U.; Correa, P.; Cebrian, M.E. Capsaicin consumption, Helicobacter pylori CagA status and IL1B-31C>T genotypes: A host and environment interaction in gastric cancer. Food Chem. Toxicol. 2012, 50, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Yu, S.; Ruan, X.; Liu, H.; Zong, G.; Cheng, P.; Tao, R.; Chen, W.; Wang, A.; Zhao, Y.; et al. Capsaicin orchestrates metastasis in gastric cancer via modulating expression of TRPV1 channels and driving gut microbiota disorder. Cell Commun. Signal. 2023, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.D.; Avila-Nava, A.; Lugo, R.; Chim Ake, R.; Gutierrez Solis, A.L. Association Between Low-Grade Inflammation and Hyperuricemia in Adults with Metabolic Syndrome in Yucatan, Mexico. Can. J. Diabetes 2022, 46, 369–374. [Google Scholar] [CrossRef]

| Num | Author/Year/Study | Country | Study Design | Population, n (%) | Cases vs. Control OR [95% CI] | |
|---|---|---|---|---|---|---|
| Protective Factor | Risk Factor | |||||
| 1 | De Stefani et al., (2001) [10]. Meat Consumption and Risk of Stomach Cancer in Uruguay: A Case–Control Study | Uruguay | Case–Control | Cases Males, 91 (74) Females, 32 (26) Control Males, 173 (65.5) Females, 91(34.5) | NR | Consumption of Red meat (>152.5 g/d) 2.4 [1.3–4.4] Processed meat (>29.6 g/d) 2.3 [1.3–4.2] Total meat (164.4–219.8 g/d) 2.6 [1.3–5.2] |
| 2 | De Stefani et al., (2001) [10]. Plant foods and risk of gastric cancer: a case–control study in Uruguay | Uruguay | Case- Control | Cases Males, 114 (71.3) Females, 46 (28.8) Control Males, 225 (70.3) Females, 95 (29.7) | Consumption of Raw vegetables (>29.5 g/d) 0.52 [0.31–0.86] Allium vegetables (>21.0 g/d) 0.46 [0.29–0.76] All fruits (>195.9 g/d) 0.33 [0.20–0.56] Citrus fruit (>47.5 g/d) 0.51 [0.31–0.85] Other fruits (>150.2 g/d) 0.34 [0.20–0.57] All vegetables and fruits (>321.1 g/d) 0.33 [0.19–0.55] Plant foods (>427.9 g/d) 0.31 [0.18–0.54] | NR |
| 3 | Muñoz et al., (2001) [12]. A case–control study of gastric cancer in Venezuela | Venezuela | Case- Control | Cases 292 (37.5) Control 485 (62.5) | Consumption of Chili (<once/w) 0.50 [0.30–0.90] Garlic (every day) 0.50 [0.30–0.70] Onion (every day) 0.40 [0.20–0.70] | NR |
| 4 | Nishimoto et al., (2002) [13]. Risk factors for Stomach Cancer in Brazil (I): a Case–control study among Non-Japanese Brazilian in São Paulo | Brazil | Case- Control | Cases Males, 170 (72.0) Females, 66 (28.0) Control Males, 170 (72.0) Females, 66 (28.0) | Consumption of Fruit (daily) 0.4 [0.2–0.8] Yellow vegetables (daily) 0.4 [0.2–0.8] Other vegetables (daily) 0.4 [0.2–0.8] | Consumption of Egg (daily) 2.7 [1.5–4.9] |
| 5 | De Stefani et al., (2004) [14]. Dietary patterns and risk of gastric cancer: a case–control study in Uruguay | Uruguay | Case- Control | Cases Males, 168 (70) Females, 72 (30) Control Males, 672 (70) Females, 288 (30) | Consumption of *+ Egg 0.67 [0.47–0.97] Other fruits 0.46 [0.31–0.71] Total fruits 0.51 [0.35–0.74] Total vegetables and fruits 0.54 [0.41–0.85] | Consumption of *+ Total grains 1.63 [1.07–2.46] Starchy food 1.55 [1.01–2.38] All tubers 1.69 [1.13–2.52] |
| 6 | Campos et al., (2006) [15]. Risk factors of gastric cancer specific for tumor location and histology in Cali, Colombia | Colombia | Case- Control | Cases 216 (33.3) Control 431 (66.7) | Consumption of + Fruits intake 0.3 [0.1–1.0] Vegetable intake 0.3 [0.1–1.0] | Consumption of + Salting meals 3.5 [1.6–7.3] Frying foods 1.9 [1.0–3.6] |
| 7 | Delgado-Figueroa et al., (2017) [16]. Risk factors associated with diffuse gastric cancer and intestinal histological patterns in adult population from Western Mexico | Mexico | Case- Control | Cases Diffuse gastric cancer 27 (50.9) Intestinal gastric cancer 26 (40.1) Control Diffuse gastric cancer 27 (50.9) Intestinal gastric cancer 26 (40.1) | Diffuse gastric cancer: Consumption of Fruit (>1/d) 0.28 [0.08–0.88] Green vegetables (>1/d) 0.16 [0.03–0.83] | Diffuse gastric cancer: Consumption of Pork meat (>1/w) 3.4 [1.11–10.40] Intestinal gastric cancer: Consumption of Canned sardine (>1/mo) 4.07 [1.25–13.24] |
| 8 | Trujillo-Rivera et al., (2018) [17]. Risk factors associated with gastric cancer in Mexico: education, breakfast, and chili | Mexico | Case- Control | Cases Males, 27 (58.7) Females, 19 (41.3) Control Males, 27 (58.7) Females, 19 (41.3) | Consumption of Fresh fruits (≥1 piece/d) 0.31 [0.09–0.89] Fresh vegetables (≥1 piece/d) 0.25 [0.06–0.78] | Consumption of Pickled food (>32.42 g/d) 4.7 [1.30–25.33] |
| 9 | Peres et al., (2022) [18]. Consumption of processed and ultra-processed foods by patients with stomach adenocarcinoma: a multicentric case–control study in the Amazon and southeast region of Brazil | Brazil São Paulo (Southeast region) | Case- Control | Cases Males, 214 (63.1) Females, 79 (36.9) Control Males, 315 (57.1) Females, 236 (42.9) | Consumption of Whole grain bread (>6.7 g/d) 0.62 [0.43–0.90] Pasta (>32 g/d) 0.53 [0.35–0.80] | Consumption of Salted bread (> 44 g/d) 2.45 [1.63–3.67] Leguminous (beans and lentils) (>102 g/d) 1.55 [1.02–2.34] French fries (cassava fries) (>5 g/d) 1.96 [1.31–2.94] Fried and roasted meats (>185 g/d) 1.97 [1.27–3.07] Processed meat, sausage, cold cuts and ‘tropeiro’ beans (>44 g/d) 2.98 [1.93–4.59] Pizza, fried and baked snacks, popcorn, and snack (>37 g/d) 1.91 [1.27–2.87] Processed and Ultra-processed food (>1448 g/d) 2.11 [1.39–3.22] |
| Brazil Belém (Amazon region) | Case- Control | Cases Males, 86 (61.4) Females, 54 (38.6) Control Males, 86 (61.4) Females, 54 (38.6) | Consumption of Whole grain bread (>1.7 g/d) 0.08 [0.02–0.36] Pasta (>54 g/d) 0.48 [0.26–0.87] French fries (cassava fries) (>5 g/d) 0.29 [0.17–0.50] Pizza, fried and baked snacks, popcorn, and snack (>27.6 g/d) 0.29 [0.16–0.53] | Consumption of Salted bread (>101 g/d) 4.44 [2.33–8.47] White rice (>189 g/d) 11.85 [6.05–23.21] Leguminous (beans and lentils) (>66.6 g/d) 12.14 [5.76–25.58] Fried and roasted meats (>166 g/d) 2.45 [1.36–4.42] Processed and Ultra-processed food (>913 g/d) 13.21 [6.56–26.62] | ||
| Num | Author/Year/Study | Country | Study Design | Population, n (%) | Cases vs. Control OR [95% CI] | |
|---|---|---|---|---|---|---|
| Protective Factor | Risk Factor | |||||
| 1 | De Stefani et al., (2000) [20]. Dietary carotenoids and risk of gastric cancer: a case–control study in Uruguay | Uruguay | Case- Control | Cases Males, 85 (70.8) Females, 35 (29.2) Control Males, 255 (70.8) Females, 105 (29.2) | Consumption of Vitamin C (>16,945 mg/d) 0.50 [0.30–0.99] α-carotene (>130 mg/d) 0.26 [0.17–0.65] Lycopene (>3448 mg/d) 0.24 [0.19–0.73] | NR |
| 2 | De Stefani et al., (2000) [20]. Plant Sterols and Risk of Stomach Cancer: A Case–Control Study in Uruguay | Uruguay | Case- Control | Cases Males, 85 (70.8) Females, 35 (29.2) Control Males, 255 (70.8) Females, 105 (29.2) | Consumption of β-Sitosterol (≥56.1 mg/d) 0.34 [0.15–0.76] Total phytosterols (≥82.6 mg/d) 0.33 [0.15–0.75] | NR |
| 3 | Muñoz et al., (2001) [12]. A case–control study of gastric cancer in Venezuela | Venezuela | Case- Control | Cases 292 (37.5) Control 485 (62.5) | Consumption of *+ Protein 0.56 [0.37–0.86] Polyunsaturated fat 0.63 [0.41–0.97] Niacin 0.62 [0.41–0.94] | NR |
| 4 | López-Carrillo et al., (2003) [21]. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico | Mexico | Case- Control | Cases Males, 133 (56.84) Females, 101 (43.16) Control Males, 266 (56.84) Females, 202 (43.16) | NR | Consumption of Capsaicin (30–89.9 mg/d) 1.60 [1.06–2.41] |
| 5 | Hernández-Ramírez et al., (2009) [22]. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City | Mexico | Case- Control | Cases 248 (34.1) Control 478 (65.9) | Consumption of Cinnamic acids (>127.0 μg/d) 0.52 [0.34–0.81] Lignans (75.5 μg/d) 0.42 [0.27–0.65] Coumestrol (1.9 mg/d) 0.45 [0.29–0.70] Total nitrate (>141.7 mg/d) 0.61 [0.39–0.96] Nitrate in fruits and vegetables (>134.9 mg/d) 0.62 [0.40–0.97] | Consumption of Nitrate in animal (>3.9 mg/d) 1.92 [1.23–3.02] Nitrite in animal (>0.4 mg/d) 1.56 [1.02–2.40] |
| 6 | Trujillo-Rivera et al., (2018) [17]. Risk factors associated with gastric cancer in Mexico: education, breakfast, and chili | Mexico | Case- Control | Cases Males, 27 (58.7) Females, 19 (41.3) Control Males, 27 (58.7) Females, 19 (41.3) | NR | Consumption of Capsaicin (>29.9 mg/d) 3.00 [1.14–9.23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Nava, A.; Gutiérrez-Solis, A.L.; Pacheco-Can, O.D.; Sagols-Tanoira, I.Y.; González-Marenco, R.; Cabrera-Lizarraga, A.G.; Castillo-Avila, J.A.; Aguilar-Franco, M.A.; Chim-Aké, R.; Rubio-Zapata, H.; et al. Dietary Components Associated with the Risk of Gastric Cancer in the Latin American Population: A Systematic Review and Meta-Analysis. Foods 2025, 14, 1052. https://doi.org/10.3390/foods14061052
Avila-Nava A, Gutiérrez-Solis AL, Pacheco-Can OD, Sagols-Tanoira IY, González-Marenco R, Cabrera-Lizarraga AG, Castillo-Avila JA, Aguilar-Franco MA, Chim-Aké R, Rubio-Zapata H, et al. Dietary Components Associated with the Risk of Gastric Cancer in the Latin American Population: A Systematic Review and Meta-Analysis. Foods. 2025; 14(6):1052. https://doi.org/10.3390/foods14061052
Chicago/Turabian StyleAvila-Nava, Azalia, Ana Ligia Gutiérrez-Solis, Oscar Daniel Pacheco-Can, Ian Yeshua Sagols-Tanoira, Roberto González-Marenco, Ana Gabriela Cabrera-Lizarraga, Jesús Abraham Castillo-Avila, Miguel Alberto Aguilar-Franco, Rodolfo Chim-Aké, Héctor Rubio-Zapata, and et al. 2025. "Dietary Components Associated with the Risk of Gastric Cancer in the Latin American Population: A Systematic Review and Meta-Analysis" Foods 14, no. 6: 1052. https://doi.org/10.3390/foods14061052
APA StyleAvila-Nava, A., Gutiérrez-Solis, A. L., Pacheco-Can, O. D., Sagols-Tanoira, I. Y., González-Marenco, R., Cabrera-Lizarraga, A. G., Castillo-Avila, J. A., Aguilar-Franco, M. A., Chim-Aké, R., Rubio-Zapata, H., Reyes-Sosa, M., Medina-Vera, I., Guevara-Cruz, M., Sánchez-Pozos, K., & Lugo, R. (2025). Dietary Components Associated with the Risk of Gastric Cancer in the Latin American Population: A Systematic Review and Meta-Analysis. Foods, 14(6), 1052. https://doi.org/10.3390/foods14061052









