Potential of Process-Induced Modification of Potato Starch to Modulate Starch Digestibility and Levels of Resistant Starch Type III
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Starch
2.2.1. Starch Modulation via Autoclaving
2.2.2. Starch Modulation via High Hydrostatic Pressure (HHP)
2.3. Physiochemical Characterizations
2.3.1. X-Ray Diffraction (XRD)
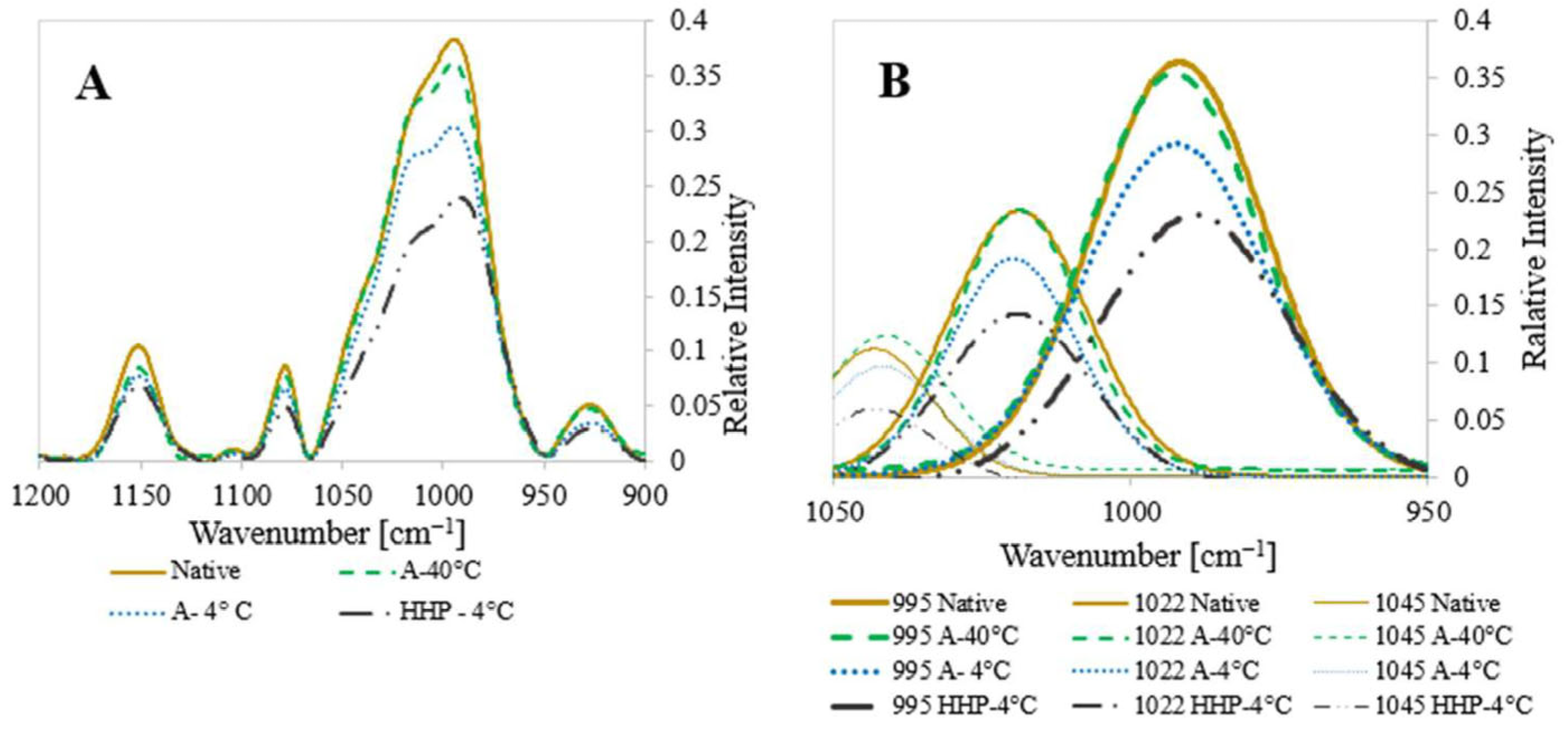
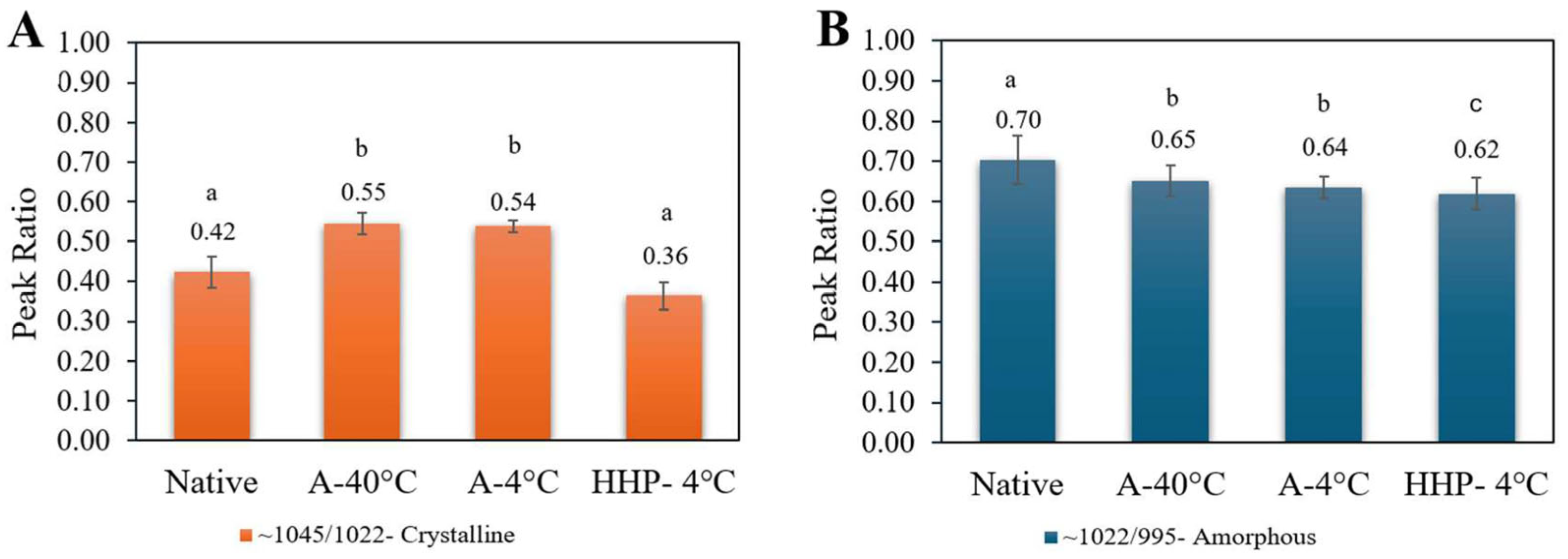
2.3.2. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR)
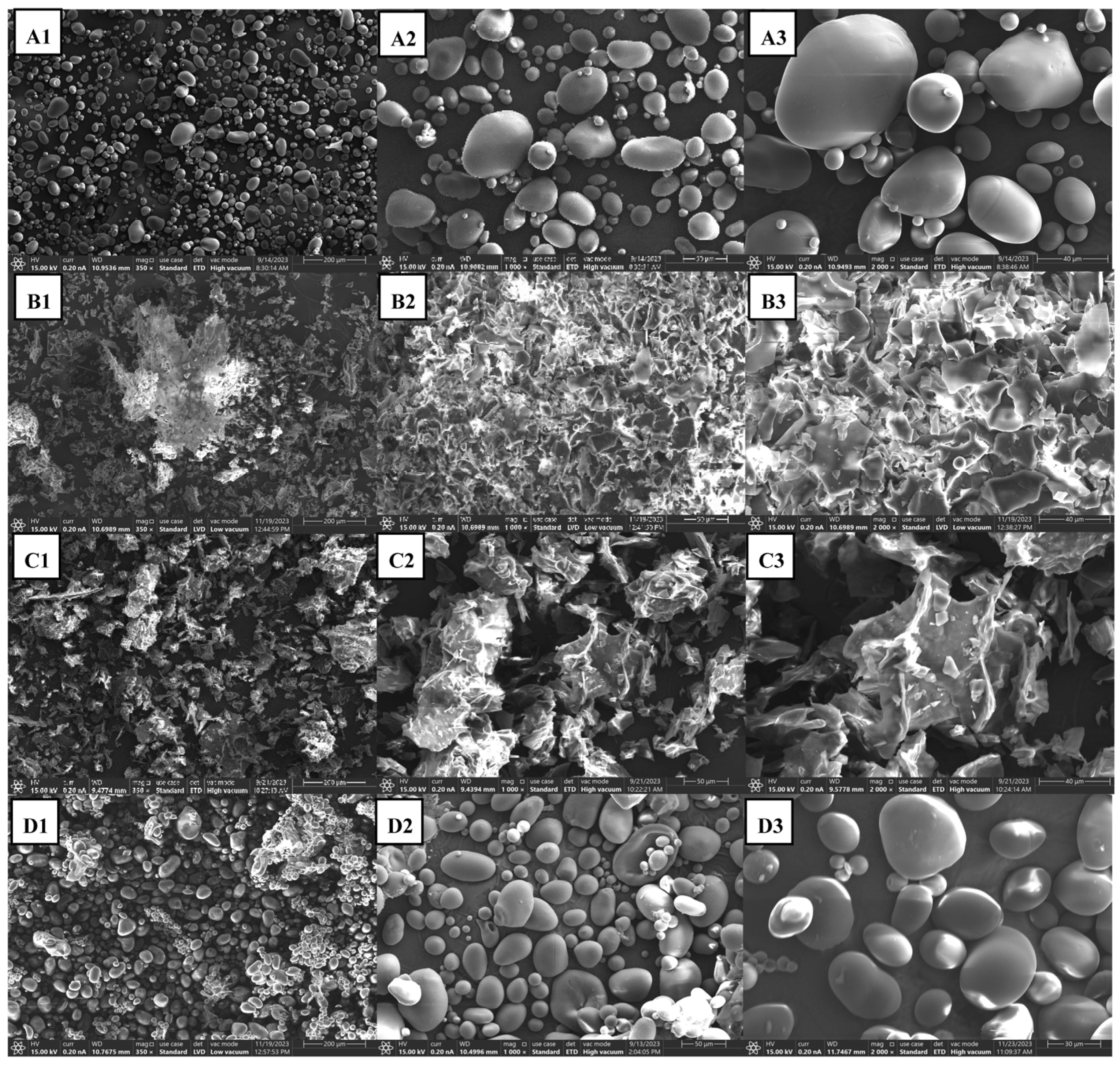
2.3.3. Scanning Electron Microscopy (SEM)
2.4. In Vitro Digestibility of Starch
2.5. Evaluation of Starch Digestion Using Dinitrosalicybe (DNS) Acid Assay
2.6. Assessing the Potential RS Content
3. Results and Discussion
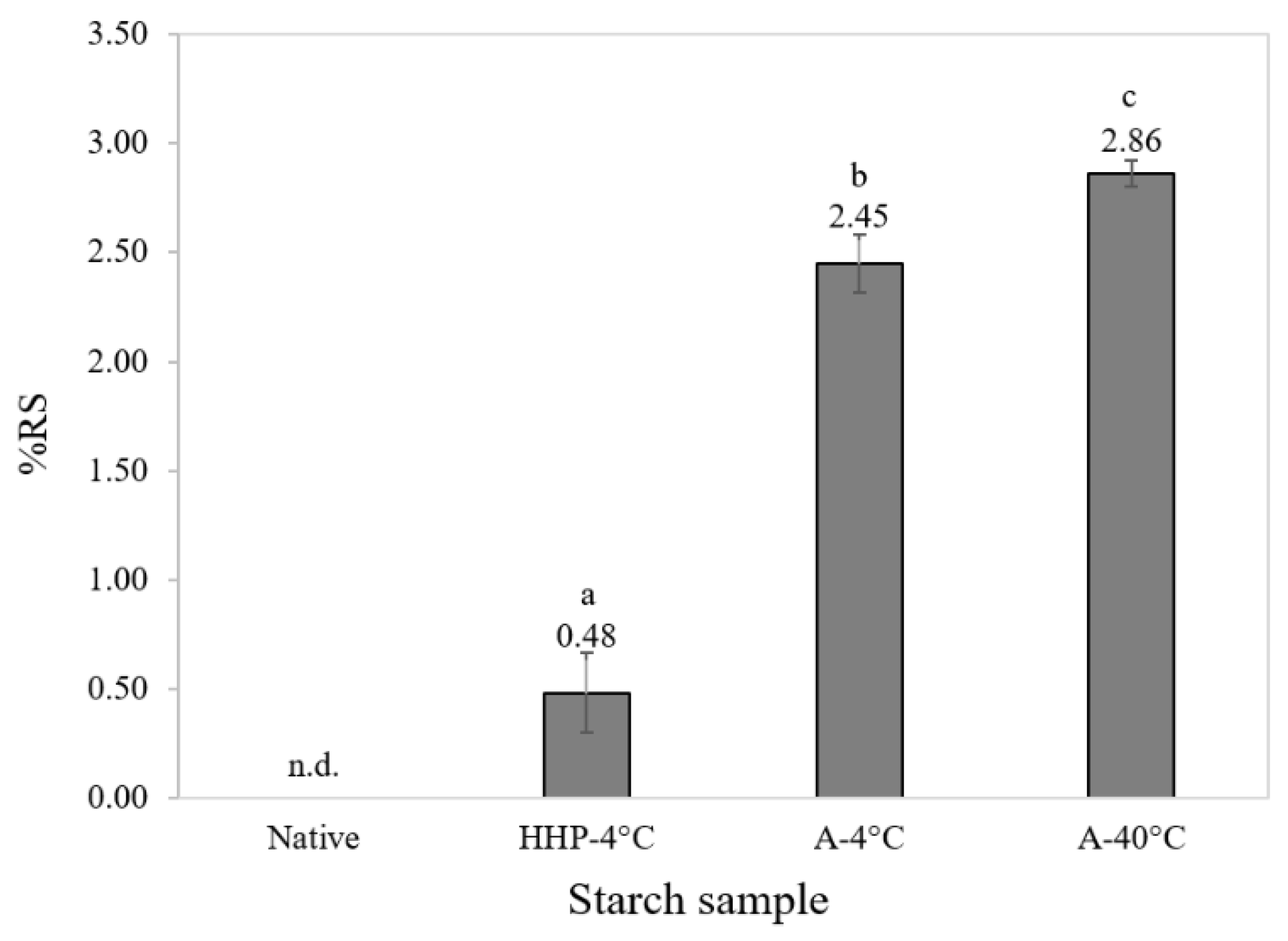
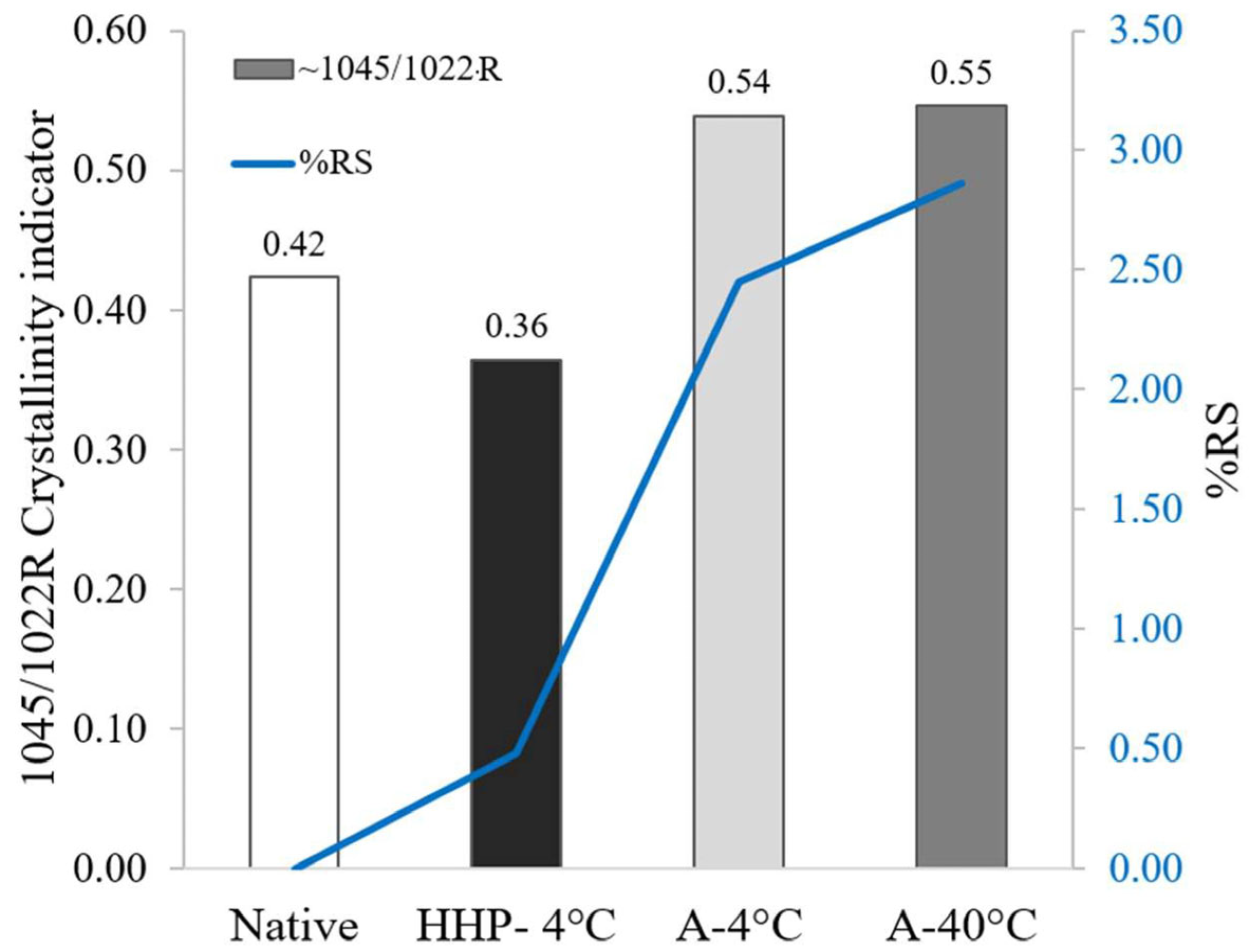
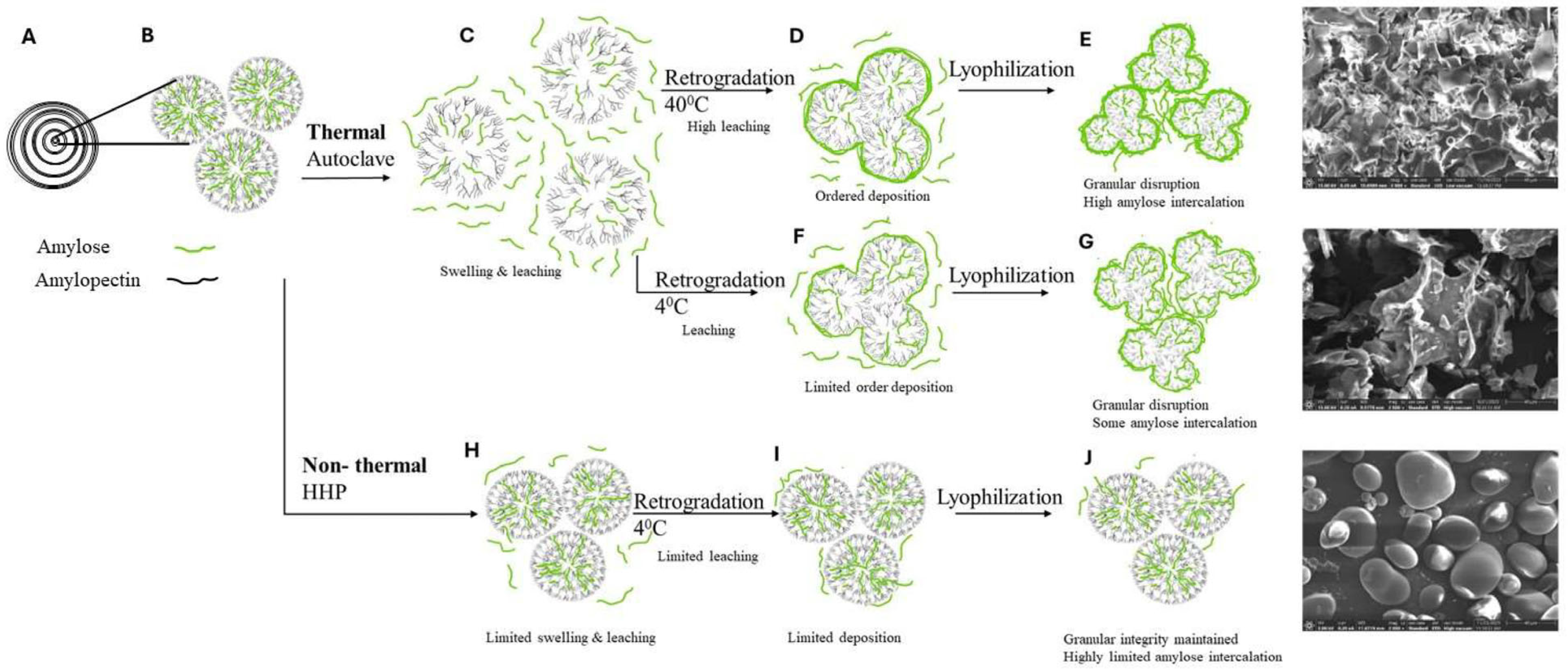
3.1. Characterization of Starch and Processed Starch Samples
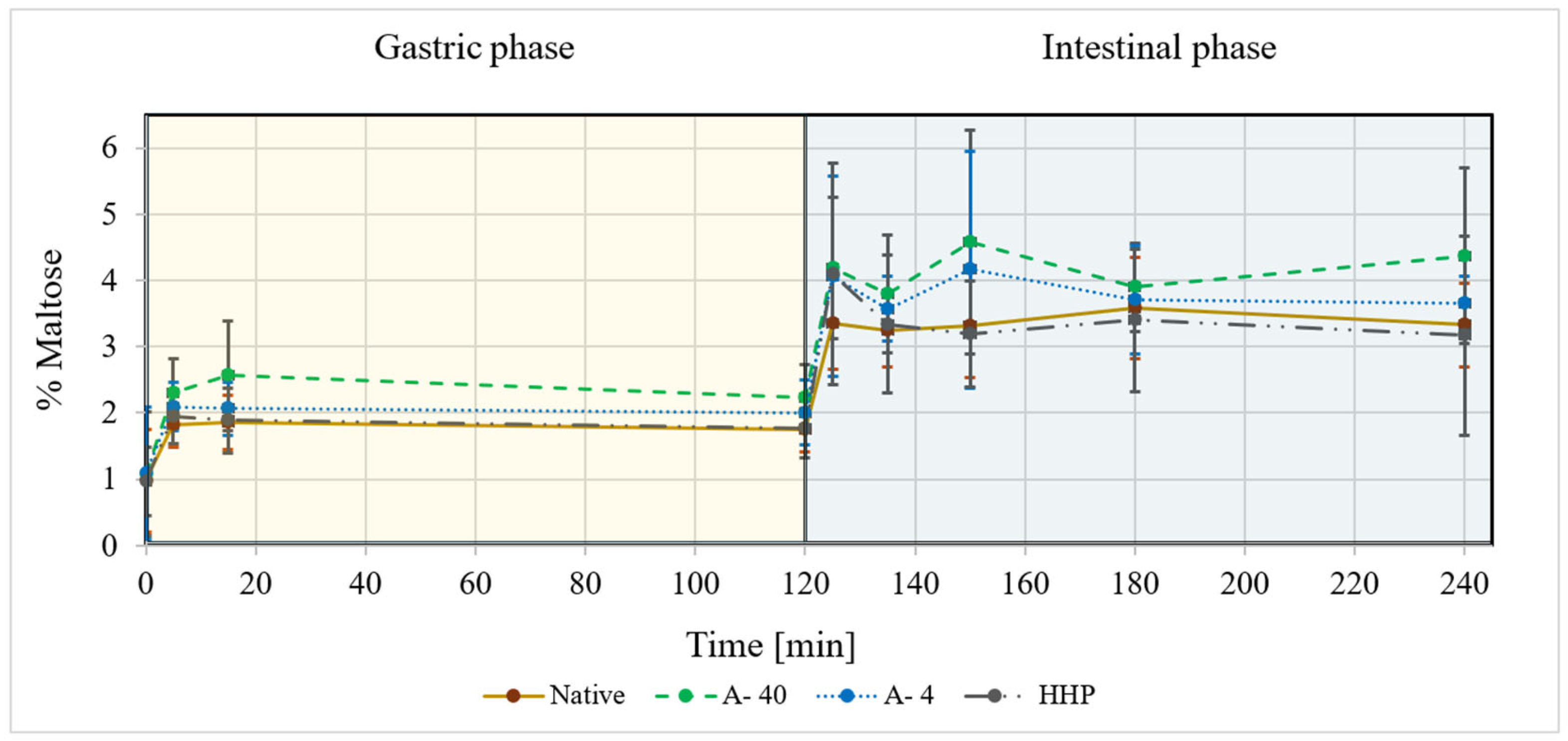
3.2. In Vitro Digestibility of Processed Starch
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RS | Resistant Starch |
| HHP | High Hydrostatic Pressure |
| XRD | X-Ray Diffraction |
| ATR-FTIR | Attenuated Total Reflectance-Fourier Transform Infrared |
| SEM | Scanning Electron Microscopy |
| RDS | Rapidly Digestible Starch |
| GIT | Gastrointestinal Tract |
| SDS | Slowly Digestible Starch |
| DDW | Double-Distilled Water |
| IR | Infrared |
| RC | Relative Crystallinity |
| IVD | In Vitro Digestion |
| SSF | Simulated Salivary Fluid |
| SGF | Simulated Gastric Fluid |
| SDF | Simulated Duodenal Fluid |
| DNS | Di Nitro Salicylic |
| AUC | Area Under Curve |
References
- Pllana, M.; Merovci, N.; Jashari, M.; Tmava, A.; Shaqiri, F. Potato Market and Consumption. Int. J. Sustain. Econ. Manag. 2018, 7, 19–29. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch Structure Influences Its Digestibility: A Review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and Human Health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Cui, S.W. Food Carbohydrates: Chemistry, Physical Properties, and Applications; Taylor & Francis: Boca Raton, FL, USA, 2005; ISBN 0849315743. [Google Scholar]
- Li, C.; Hu, Y.; Li, S.; Yi, X.; Shao, S.; Yu, W.; Li, E. Biological Factors Controlling Starch Digestibility in Human Digestive System. Food Sci. Hum. Wellness 2023, 12, 351–358. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Zhong, Y.; Møller, M.S.; Westh, P.; Svensson, B.; Blennow, A. Interfacial Catalysis during Amylolytic Degradation of Starch Granules: Current Understanding and Kinetic Approaches. Molecules 2023, 28, 3799. [Google Scholar] [CrossRef]
- Englyst, H.N.; Hudson, G.J. The Classification and Measurement of Dietary Carbohydrates. Food Chem. 1996, 57, 15–21. [Google Scholar] [CrossRef]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly Available Glucose in Foods: An in Vitro Measurement That the Glycemic Response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef]
- Dupuis, J.H.; Liu, Q.; Yada, R.Y. Methodologies for Increasing the Resistant Starch Content of Food Starches: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1219–1234. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, B.; Tang, Y.; Chen, L. Starch Concentration Is an Important Factor for Controlling Its Digestibility during Hot-Extrusion 3D Printing. Food Chem. 2022, 379, 132180. [Google Scholar] [CrossRef]
- Zhang, G.; Hamaker, B.R. Slowly Digestible Starch: Concept, Mechanism, and Proposed Extended Glycemic Index. Crit. Rev. Food Sci. Nutr. 2009, 49, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Ovando-Martínez, M.; Whitney, K.; Reuhs, B.L.; Doehlert, D.C.; Simsek, S. Effect of Hydrothermal Treatment on Physicochemical and Digestibility Properties of Oat Starch. Food Res. Int. 2013, 52, 17–25. [Google Scholar] [CrossRef]
- Patterson, M.A.; Maiya, M.; Stewart, M.L. Resistant Starch Content in Foods Commonly Consumed in the United States: A Narrative Review. J. Acad. Nutr. Diet. 2020, 120, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary Bile Acids: An Underrecognized Cause of Colon Cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef]
- Thompson, M.S.; Hui Yan, T.; Saari, N.; Sarbini, S.R. A Review: Resistant Starch, a Promising Prebiotic for Obesity and Weight Management. Food Biosci. 2022, 50, 101965. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Suzuki, J.; Fukuoka, D.; Tokimitsu, I.; Hase, T. RS4-Type Resistant Starch Prevents High-Fat Diet-Induced Obesity via Increased Hepatic Fatty Acid Oxidation and Decreased Postprandial GIP in C57BL/6J Mice. Am. J. Physiol. Endocrinol. Metab. 2010, 298, 652–662. [Google Scholar] [CrossRef]
- Kim, S.K.; Kwak, J.E. Formation of Resistant Starch in Corn Starch and Estimation of Its Content from Physicochemical Properties. Starch/Staerke 2009, 61, 514–519. [Google Scholar] [CrossRef]
- Lesmes, U.; Beards, E.J.; Gibson, G.R.; Tuohy, K.M.; Shimoni, E. Effects of Resistant Starch Type III Polymorphs on Human Colon Microbiota and Short Chain Fatty Acids in Human Gut Models. J. Agric. Food Chem. 2008, 56, 5415–5421. [Google Scholar] [CrossRef]
- Higgins, J.A.; Brown, I.L. Resistant Starch: A Promising Dietary Agent for the Prevention/Treatment of Inflammatory Bowel Disease and Bowel Cancer. Curr. Opin. Gastroenterol. 2013, 29, 190–194. [Google Scholar] [CrossRef]
- Aller, E.E.J.G.; Abete, I.; Astrup, A.; Alfredo, M.J.; van Baak, M.A. Starches, Sugars and Obesity. Nutrients 2011, 3, 341–369. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch—A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Furrer, A.N.; Chegeni, M.; Ferruzzi, M.G. Impact of Potato Processing on Nutrients, Phytochemicals, and Human Health. Crit. Rev. Food Sci. Nutr. 2018, 58, 146–168. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Hylemon, P.B. A Potential Role for Resistant Starch Fermentation in Modulating Colonic Bacterial Metabolism and Colon Cancer Risk. Cancer Biol. Ther. 2006, 5, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, Y.; Tsao, R.; Dong, H.; Zhang, H. Amelioratory Effect of Resistant Starch on Non-Alcoholic Fatty Liver Disease via the Gut-Liver Axis. Front. Nutr. 2022, 9, 861854. [Google Scholar] [CrossRef]
- Kadyan, S.; Sharma, A.; Arjmandi, B.H.; Singh, P.; Nagpal, R. Prebiotic Potential of Dietary Beans and Pulses and Their Resistant Starch for Aging-Associated Gut and Metabolic Health. Nutrients 2022, 14, 1726. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Starch Digestibility: Past, Present, and Future. J. Sci. Food Agric. 2020, 100, 5009–5016. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch Modification through Environmentally Friendly Alternatives: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef]
- Gałkowska, D.; Kapuśniak, K.; Juszczak, L. Chemically Modified Starches as Food Additives. Molecules 2023, 28, 7543. [Google Scholar] [CrossRef]
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and Chemical Modifications in Starch Structure and Reactivity. Chem. Prop. Starch 2020, 9, 13–35. [Google Scholar] [CrossRef]
- Goel, C.; Semwal, A.D.; Khan, A.; Kumar, S.; Sharma, G.K. Physical Modification of Starch: Changes in Glycemic Index, Starch Afractions, Physicochemical and Functional Properties of Heat-Moisture Treated Buckwheat Starch. J. Food Sci. Technol. 2020, 57, 2941–2948. [Google Scholar] [CrossRef]
- Fonseca, L.M.; El Halal, S.L.; Dias, A.R.; da Rosa Zavareze, E. Physical Modification of Starch by Heat-Moisture Treatment and Annealing and Their Applications: A Review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Fei, W.; Wang, Z.; Chen, X.; Wen, H.; Xie, J.; Shen, M. Modulation of Starch Digestibility Using Non-Thermal Processing Techniques: A Review. Grain Oil Sci. Technol. 2024, 7, 209–218. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and Potential Applications: A Review of the Modification of Starches by Heat-Moisture Treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Chai, Z.; Liu, H.; Wei, X.; Ye, X.; Tian, J.; Fang, H. Physicochemical Properties and in Vitro Digestibility of Proso Millet Starch Modified by Heat-Moisture Treatment and Annealing Processing. Int. J. Biol. Macromol. 2023, 235, 123829. [Google Scholar] [CrossRef]
- Liu, C.; Song, M.; Liu, L.; Hong, J.; Guan, E.; Bian, K.; Zheng, X. Effect of Heat-Moisture Treatment on the Structure and Physicochemical Properties of Ball Mill Damaged Starches from Different Botanical Sources. Int. J. Biol. Macromol. 2020, 156, 403–410. [Google Scholar] [CrossRef]
- Soler, A.; Velazquez, G.; Velazquez-Castillo, R.; Morales-Sanchez, E.; Osorio-Diaz, P.; Mendez-Montealvo, G. Retrogradation of Autoclaved Corn Starches: Effect of Water Content on the Resistant Starch Formation and Structure. Carbohydr. Res. 2020, 497, 108137. [Google Scholar] [CrossRef]
- Lee, C.-S.; Chung, H.-J. Enhancing Resistant Starch Content of High Amylose Rice Starch through Heat–Moisture Treatment for Industrial Application. Molecules 2022, 27, 6375. [Google Scholar] [CrossRef]
- Wang, C.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Resistant Starch and Its Nanoparticles: Recent Advances in Their Green Synthesis and Application as Functional Food Ingredients and Bioactive Delivery Systems. Trends Food Sci. Technol. 2022, 119, 90–100. [Google Scholar] [CrossRef]
- Mutlu, S.; Kahraman, K.; Öztürk, S. Optimization of Resistant Starch Formation from High Amylose Corn Starch by Microwave Irradiation Treatments and Characterization of Starch Preparations. Int. J. Biol. Macromol. 2017, 95, 635–642. [Google Scholar] [CrossRef]
- Sievert, D.; Pomeranz, Y. Enzyme-Resistant Starch. I. Characterization and Evaluation by Enzymatic, Thermoanalytical, and Microscopic Methods. Cereal Chem. 1989, 66, 342–347. [Google Scholar]
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of Treatment Methods on the Formation of Resistant Starch in Purple Sweet Potato. Food Chem. 2022, 367, 130580. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S. Improving Starch for Food and Industrial Applications. Curr. Opin. Plant Biol. 2004, 7, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Duijsens, D.; Pälchen, K.; Guevara-Zambrano, J.M.M.; Verkempinck, S.H.E.H.E.; Infantes-Garcia, M.R.R.; Hendrickx, M.E.E.; Van Loey, A.M.M.; Grauwet, T. Strategic Choices for in Vitro Food Digestion Methodologies Enabling Food Digestion Design. Trends Food Sci. Technol. 2022, 126, 61–72. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Soler, A.; Mendez-Montealvo, G.; Velazquez-Castillo, R.; Hernández-Gama, R.; Osorio-Diaz, P.; Velazquez, G. Effect of Crystalline and Double Helical Structures on the Resistant Fraction of Autoclaved Corn Starch with Different Amylose Content. Starch/Staerke 2020, 72, 1900306. [Google Scholar] [CrossRef]
- Su, C.; Saleh, A.S.M.; Zhang, B.; Zhao, K.; Ge, X.; Zhang, Q.; Li, W. Changes in Structural, Physicochemical, and Digestive Properties of Normal and Waxy Wheat Starch during Repeated and Continuous Annealing. Carbohydr. Polym. 2020, 247, 116675. [Google Scholar] [CrossRef]
- Bimo Setiarto, R.H.; Isra, M.; Andrianto, D.; Widhyastuti, N.; Masrukhin. Improvement of Prebiotic Properties and Resistant Starch Content of Corn Flour (Zea mays L.) Momala Gorontalo Using Physical, Chemical and Enzymatic Modification. Trop. Life Sci. Res. 2023, 34, 255–278. [Google Scholar] [CrossRef]
- Dominguez-Ayala, J.E.; Soler, A.; Mendez-Montealvo, G.; Velazquez, G. Supramolecular Structure and Technofunctional Properties of Starch Modified by High Hydrostatic Pressure (HHP): A Review. Carbohydr. Polym. 2022, 291, 119609. [Google Scholar] [CrossRef]
- Zabar, S.; Lesmes, U.; Katz, I.; Shimoni, E.; Bianco-Peled, H. Studying Different Dimensions of Amylose-Long Chain Fatty Acid Complexes: Molecular, Nano and Micro Level Characteristics. Food Hydrocoll. 2009, 23, 1918–1925. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, L.; Yin, J.; Yan, S.; Liu, K.; Zhang, F.; Xu, B.; Li, L. Structure and Physicochemical Properties of Starches in Lotus (Nelumbo nucifera Gaertn.) Rhizome. Food Sci. Nutr. 2013, 1, 273–283. [Google Scholar] [CrossRef]
- Colussi, R.; Singh, J.; Kaur, L.; da Rosa Zavareze, E.; Dias AR, G.; Stewart, R.B.; Singh, H. Microstructural Characteristics and Gastro-Small Intestinal Digestion in Vitro of Potato Starch: Effects of Refrigerated Storage and Reheating in Microwave. Food Chem. 2017, 226, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food-an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Shani-Levi, C.; Levi-Tal, S.; Lesmes, U. Comparative Performance of Milk Proteins and Their Emulsions under Dynamic in Vitro Adult and Infant Gastric Digestion. Food Hydrocoll. 2013, 32, 349–357. [Google Scholar] [CrossRef]
- Levi, C.S.; Lesmes, U. Bi-Compartmental Elderly or Adult Dynamic Digestion Models Applied to Interrogate Protein Digestibility. Food Funct. 2014, 5, 2402–2409. [Google Scholar] [CrossRef]
- Bailey, M.J. A Note on the Use of Dinitrosalicylic Acid for Determining the Products of Enzymatic Reactions. Appl. Microbiol. Biotechnol. 1988, 29, 494–496. [Google Scholar] [CrossRef]
- Shamai, K.; Bianco-Peled, H.; Shimoni, E. Polymorphism of Resistant Starch Type III. Carbohydr. Polym. 2003, 54, 363–369. [Google Scholar] [CrossRef]
- Shamai, K.; Shimoni, E.; Bianco-Peled, H. Small Angle X-Ray Scattering of Resistant Starch Type III. Biomacromolecules 2004, 5, 219–223. [Google Scholar] [CrossRef]
- Lee, C.; Dazen, K.; Kafle, K.; Moore, A.; Johnson, D.K.; Park, S.; Kim, S.H. Correlations of Apparent Cellulose Crystallinity Determined by XRD, NMR, IR, Raman, and SFG Methods. Adv. Polym. Sci. 2015, 271, 115–131. [Google Scholar] [CrossRef]
- Kumari, P.; Luthra, A.; Sethi, P.; Banyal, S. X-Ray Diffraction Analysis for Starch. In Standardized Procedures and Protocols for Starch; Springer: Berlin/Heidelberg, Germany, 2024; pp. 197–220. [Google Scholar]
- Zobel, H.F. Starch Crystal Transformations and Their Industrial Importance. Starch-Stärke 1988, 40, 1–7. [Google Scholar] [CrossRef]
- Conde, L.A.; Kebede, B.; Leong, S.Y.; Oey, I. Effect of High Hydrostatic Pressure Processing on Starch Properties of Cassava Flour. Appl. Sci. 2022, 12, 10043. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared Spectroscopy as a Tool to Characterise Starch Ordered Structure—A Joint FTIR-ATR, NMR, XRD and DSC Study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, Q.Y.; Jiang, W.; Qian, J.Y.; Zhang, L.; Wu, M.; Rao, S.Q.; Wu, C. Sen Effect of Pulsed Electric Field on Structural Properties and Digestibility of Starches with Different Crystalline Type in Solid State. Carbohydr. Polym. 2019, 207, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Sopade, P.A. Modeling Starch Digestograms: Computational Characteristics of Kinetic Models for in Vitro Starch Digestion in Food Research. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1422–1445. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of Starch Digestion by α-Amylase-Structural Basis for Kinetic Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Navaf, M.; Bhasha, S.A.; George, J.; Mounir, S.; Kumar, S.; Sajeevkumar, V.A. Hydrothermal Modifications of Nonconventional Kithul (Caryota urens) Starch: Physico-Chemical, Rheological Properties and in Vitro Digestibility. J. Food Sci. Technol. 2020, 57, 2916–2925. [Google Scholar] [CrossRef]
- Brahma, B.; Sit, N. Physicochemical Properties and Digestibility of Heat Moisture–Treated Potato Starches for Different Treatment Conditions. Potato Res. 2020, 63, 367–383. [Google Scholar] [CrossRef]
- Zheng, M.Z.; Xiao, Y.; Yang, S.; Liu, H.M.; Liu, M.H.; Yaqoob, S.; Xu, X.Y.; Liu, J.S. Effects of Heat–Moisture, Autoclaving, and Microwave Treatments on Physicochemical Properties of Proso Millet Starch. Food Sci. Nutr. 2020, 8, 735–743. [Google Scholar] [CrossRef]
- Shi, X.; Ding, Y.; Wan, J.; Liu, C.; Prakash, S.; Xia, X. Effect of Annealing on Structural, Physicochemical, and In Vitro Digestive Properties of Starch from Castanopsis sclerophylla. Starch/Staerke 2021, 73, 2100005. [Google Scholar] [CrossRef]
- Danjo, K.; Saito, D.; Chinda, D.; Shimoyama, T.; Sakamoto, J.; Fukuda, S.; Nakaji, S.; Munakata, A.; Sugawara, K. The Difference between in Vitro and in Vivo Measurements of Digestion-Resistant Starch Is Far Greater than Those of Pectin. Gastroenterology 2003, 124, A262. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, C.; Huang, H.; Wang, S.; Wang, S.; Wang, S.; Copeland, L. Revisiting Mechanisms Underlying Digestion of Starches. J. Agric. Food Chem. 2019, 67, 8212–8226. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. In-Vitro Digestibility, Rheology, Structure, and Functionality of RS3 from Oat Starch. Food Chem. 2016, 212, 749–758. [Google Scholar] [CrossRef]
- Dorneles, M.S.; de Azevedo, E.S.; Noreña, C.P.Z. Effect of Heat Treatment at Low Moisture on the Increase of Resistant Starch Content in Araucaria Angustifolia Seed Starch. Food Hydrocoll. 2024, 150, 109639. [Google Scholar] [CrossRef]
- Hung, P.; Vien, N.L.; Lan Phi, N.T. Resistant Starch Improvement of Rice Starches under a Combination of Acid and Heat-Moisture Treatments. Food Chem. 2016, 191, 67–73. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaskin Harush, M.; Shani Levi, C.; Lesmes, U. Potential of Process-Induced Modification of Potato Starch to Modulate Starch Digestibility and Levels of Resistant Starch Type III. Foods 2025, 14, 880. https://doi.org/10.3390/foods14050880
Yaskin Harush M, Shani Levi C, Lesmes U. Potential of Process-Induced Modification of Potato Starch to Modulate Starch Digestibility and Levels of Resistant Starch Type III. Foods. 2025; 14(5):880. https://doi.org/10.3390/foods14050880
Chicago/Turabian StyleYaskin Harush, Moshit, Carmit Shani Levi, and Uri Lesmes. 2025. "Potential of Process-Induced Modification of Potato Starch to Modulate Starch Digestibility and Levels of Resistant Starch Type III" Foods 14, no. 5: 880. https://doi.org/10.3390/foods14050880
APA StyleYaskin Harush, M., Shani Levi, C., & Lesmes, U. (2025). Potential of Process-Induced Modification of Potato Starch to Modulate Starch Digestibility and Levels of Resistant Starch Type III. Foods, 14(5), 880. https://doi.org/10.3390/foods14050880






