Effects of Different Pretreatments on Wheat Bran and Its Arabinoxylan Obtained by Sequential Extraction with Dilute Alkali and Alkali–Urea Mixture
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Pretreatment of Wheat Bran
2.3. Extraction of Arabinoxylan from Pretreated Wheat Bran
2.4. Major Chemical Composition of Pretreated Wheat Brans
2.5. Characterization of Pretreated Wheat Brans
2.6. Characterization of Arabinoxylan Extracts
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Different Pretreated Wheat Brans
3.2. Extraction Yield and Sugar Composition of Arabinoxylan Extracts
3.3. Phenolic Acid Composition and Antioxidant Capacity of Arabinoxylan Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Mense, A.L.; Brewer, L.R.; Shi, Y.-C. Wheat Bran Layers: Composition, Structure, Fractionation, and Potential Uses in Foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 6636–6659. [Google Scholar] [CrossRef]
- Li, M.; Ma, S. Effects of Interaction between Wheat Bran Dietary Fiber and Gluten Protein on Gluten Protein Aggregation Behavior. Int. J. Biol. Macromol. 2024, 283, 137692. [Google Scholar] [CrossRef] [PubMed]
- Pietiäinen, S.; Moldin, A.; Ström, A.; Malmberg, C.; Langton, M. Effect of Physicochemical Properties, Pre-Processing, and Extraction on the Functionality of Wheat Bran Arabinoxylans in Breadmaking—A Review. Food Chem. 2022, 383, 132584. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Simsek, S. A Novel Combination of Methods for the Extraction and Purification of Arabinoxylan from Byproducts of the Cereal Industry. Food Meas. 2019, 13, 1049–1057. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Chen, Z.; Li, Y.; Li, J. Facile and Green Preparation of Diverse Arabinoxylan Hydrogels from Wheat Bran by Combining Subcritical Water and Enzymatic Crosslinking. Carbohydr. Polym. 2020, 241, 116317. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, S. A Review of Healthy Role of Dietary Fiber in Modulating Chronic Diseases. Food Res. Int. 2024, 191, 114682. [Google Scholar] [CrossRef] [PubMed]
- He, H.-J.; Qiao, J.; Liu, Y.; Guo, Q.; Ou, X.; Wang, X. Isolation, Structural, Functional, and Bioactive Properties of Cereal Arabinoxylan─A Critical Review. J. Agric. Food Chem. 2021, 69, 15437–15457. [Google Scholar] [CrossRef]
- Mouzakitis, C.-K.; Kotsiou, K.; Pontikakos, G.; Matzapetakis, M.; Zervou, M.; Biliaderis, C.G.; Lazaridou, A. Unravelling the Structural Heterogeneity and Diversity in Rheological Behavior of Alkali-Extractable Wheat Bran Arabinoxylans Using Ammonia Pretreatment. Food Hydrocoll. 2024, 151, 109888. [Google Scholar] [CrossRef]
- Chen, Z.; Mense, A.L.; Brewer, L.R.; Shi, Y.-C. Wheat Bran Arabinoxylans: Chemical Structure, Extraction, Properties, Health Benefits, and Uses in Foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13366. [Google Scholar] [CrossRef]
- Rudjito, R.C.; Ruthes, A.C.; Jiménez-Quero, A.; Vilaplana, F. Feruloylated Arabinoxylans from Wheat Bran: Optimization of Extraction Process and Validation at Pilot Scale. ACS Sustain. Chem. Eng. 2019, 7, 13167–13177. [Google Scholar] [CrossRef]
- Mishler-Elmore, J.; Zhou, Y.; Sukul, A.; Oblak, M.; Tan, L.; Faik, A.; Held, M. Extensins: Self-Assembly, Crosslinking, and the Role of Peroxidases. Front. Plant Sci. 2021, 12, 664738. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Wang, C. Enzymatic Preparation of Wheat Bran Xylooligosaccharides and Their Stability during Pasteurization and Autoclave Sterilization at Low pH. Carbohydr. Polym. 2009, 77, 816–821. [Google Scholar] [CrossRef]
- Wang, J.; Fan, M.; Li, Y.; Qian, H.; Wang, L. Structural and Emulsion-Stabilizing Properties of the Alkali-Extracted Arabinoxylans from Corn and Wheat Brans. Int. J. Biol. Macromol. 2023, 251, 126190. [Google Scholar] [CrossRef] [PubMed]
- Ruthes, A.C.; Rudjito, R.C.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Jiménez-Quero, A.; Vilaplana, F. Comparative Recalcitrance and Extractability of Cell Wall Polysaccharides from Cereal (Wheat, Rye, and Barley) Brans Using Subcritical Water. ACS Sustain. Chem. Eng. 2020, 8, 7192–7204. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Drăgoi, E.N.; Danu, M.; Ailiesei, G.-L.; Balan, C.D.; Chicet, D.-L.; Nechita, M.T. Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles. Polymers 2023, 15, 1038. [Google Scholar] [CrossRef]
- Jiang, Y.; Bai, X.; Lang, S.; Zhao, Y.; Liu, C.; Yu, L. Optimization of Ultrasonic-Microwave Assisted Alkali Extraction of Arabinoxylan from the Corn Bran Using Response Surface Methodology. Int. J. Biol. Macromol. 2019, 128, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Yang, Q.; Zhang, L.; Liebert, T.; Heinze, T. The Dissolution of Cellulose in NaOH-Based Aqueous System by Two-Step Process. Cellulose 2011, 18, 237–245. [Google Scholar] [CrossRef]
- Mense, A.L.; Shi, Y.-C. Dissolution of Wheat Bran by NaOH/Urea Solutions and Structure of Soluble Materials. ACS Sustain. Chem. Eng. 2018, 6, 4264–4271. [Google Scholar] [CrossRef]
- Paesani, C.; Lammers, T.C.G.L.; Sciarini, L.S.; Moiraghi, M.; Pérez, G.T.; Fabi, J.P. Effect of Chemical, Thermal, and Enzymatic Processing of Wheat Bran on the Solubilization, Technological and Biological Properties of Non-Starch Polysaccharides. Carbohydr. Polym. 2024, 328, 121747. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Karlsson, E.N.; Adlercreutz, P. Extraction of Soluble Arabinoxylan from Enzymatically Pretreated Wheat Bran and Production of Short Xylo-Oligosaccharides and Arabinoxylo-Oligosaccharides from Arabinoxylan by Glycoside Hydrolase Family 10 and 11 Endoxylanases. J. Biotechnol. 2017, 260, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hromádková, Z.; Paulsen, B.S.; Polovka, M.; Košťálová, Z.; Ebringerová, A. Structural Features of Two Heteroxylan Polysaccharide Fractions from Wheat Bran with Anti-Complementary and Antioxidant Activities. Carbohydr. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical Functional Groups of Extractives, Cellulose and Lignin Extracted from Native Leucaena Leucocephala Bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Y.; Guo, L.; Li, D.; Liu, A.; Bilal, M.; Xie, C.; Yang, R.; Gu, Z.; Jiang, D.; et al. Antifreeze Polysaccharides from Wheat Bran: The Structural Characterization and Antifreeze Mechanism. Biomacromolecules 2024, 25, 3877–3892. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) AOAC Official Method 954.02 Fat (Crude) or Ether Extract in Pet Food: Gravimetric Method. In Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; Oxford University Press: Oxford, UK, 2023; pp. 4–40. [Google Scholar]
- Latimer, G.W., Jr. (Ed.) AOAC Official Method 954.01 Protein (Crude) in Animal Feed and Pet food: Kjeldahl Method. In Official Methods of Analysis of AOAC INTERNATIONAL; Oxford University Press: Oxford, UK, 2023; ISBN 978-0-19-761013-8. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure; NREL: Denver, CO, USA, 2012. [Google Scholar]
- Du, Y.; Zhang, S.; Sun-Waterhouse, D.; Zhou, T.; Xu, F.; Waterhouse, G.I.N.; Wu, P. Physicochemical, Structural and Emulsifying Properties of RG-I Enriched Pectin Extracted from Unfermented or Fermented Cherry Pomace. Food Chem. 2023, 405, 134985. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, M.; Coldea, T.E.; Yang, H.; Zhao, H. Emulsifying Properties of Arabinoxylans Derived from Brewers’ Spent Grain by Ultrasound-Assisted Extraction: Structural and Functional Properties Correlation. Cellulose 2023, 30, 359–372. [Google Scholar] [CrossRef]
- Yilmaz-Turan, S.; Jiménez-Quero, A.; Moriana, R.; Arte, E.; Katina, K.; Vilaplana, F. Cascade Extraction of Proteins and Feruloylated Arabinoxylans from Wheat Bran. Food Chem. 2020, 333, 127491. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tian, F.; Wang, H.; Wu, S.; Jin, W.; Shen, W.; Hu, Z.; Cai, Q.; Liu, G. Comparative Assessment of Extraction, Composition, and in Vitro Antioxidative Properties of Wheat Bran Polyphenols. LWT 2023, 180, 114706. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Liu, Y.-S.; Zeng, Y.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How Does Plant Cell Wall Nanoscale Architecture Correlate with Enzymatic Digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef]
- Guo, X.; Chen, M.; Li, Y.; Dai, T.; Shuai, X.; Chen, J.; Liu, C. Modification of Food Macromolecules Using Dynamic High Pressure Microfluidization: A Review. Trends Food Sci. Technol. 2020, 100, 223–234. [Google Scholar] [CrossRef]
- Lai, S.; Chen, Z.; Zhang, Y.; Li, G.; Wang, Y.; Cui, Q. Micronization Effects on Structural, Functional, and Antioxidant Properties of Wheat Bran. Foods 2023, 12, 98. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Cai, Z.; Zhang, J.; Guan, D.; Ji, D.; Gao, W. Effect of Ammonia Fiber Expansion Combined with NaOH Pretreatment on the Resource Efficiency of Herbaceous and Woody Lignocellulosic Biomass. ACS Omega 2022, 7, 18761–18769. [Google Scholar] [CrossRef]
- Mense, A.L.; Zhang, C.-Q.; Liu, Q.; Shi, Y.-C. Hydrothermal Treatment and Enzymatic Hydrolysis of Wheat Bran and Their Effects on Physical Structure. ACS Food Sci. Technol. 2024, 4, 642–655. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Boese, M.; Mader, W.; Barthlott, W.; Koch, K. Crystallinity of Plant Epicuticular Waxes: Electron and X-Ray Diffraction Studies. Chem. Phys. Lipids 2006, 144, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, X.; Zhuang, M.; Ke, S.; Ning, M.; Yu, P.; Zhou, Z. Size-Dependent Physicochemical Property and Functionality of Insoluble Dietary Fiber Derived from Wheat Bran. LWT 2024, 193, 115747. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, D.; Zhang, X.; Qu, L.; Zhang, Y.; Huang, Q. Effects of the Removal of Lipids and Surface Proteins on the Physicochemical and Structural Properties of Green Wheat Starches. Starch Stärke 2021, 73, 2000046. [Google Scholar] [CrossRef]
- Zhou, J.; Du, X.; Zhou, S.; Wu, S. Selectively Isolated Hemicellulose with High Whiteness and Molecular Weight from Poplar by Sodium Perborate-Assisted Alkali Extraction. Cellulose 2023, 30, 4855–4871. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated Cellulose Obtained from Soybean Hull Using Simple and Eco-Friendly Processes Based on Reactive Extrusion. Cellulose 2020, 27, 1975–1988. [Google Scholar] [CrossRef]
- Guo, R.; Xu, Z.; Wu, S.; Li, X.; Li, J.; Hu, H.; Wu, Y.; Ai, L. Molecular Properties and Structural Characterization of an Alkaline Extractable Arabinoxylan from Hull-Less Barley Bran. Carbohydr. Polym. 2019, 218, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Chen, J. Mechanisms of Sequential Dissolution and Hydrolysis for Lignocellulosic Waste Using a Multilevel Hydrothermal Process. Chem. Eng. J. 2015, 273, 37–45. [Google Scholar] [CrossRef]
- Requena, R.; Jiménez-Quero, A.; Vargas, M.; Moriana, R.; Chiralt, A.; Vilaplana, F. Integral Fractionation of Rice Husks into Bioactive Arabinoxylans, Cellulose Nanocrystals, and Silica Particles. ACS Sustain. Chem. Eng. 2019, 7, 6275–6286. [Google Scholar] [CrossRef]
- Biely, P.; Singh, S.; Puchart, V. Towards Enzymatic Breakdown of Complex Plant Xylan Structures: State of the Art. Biotechnol. Adv. 2016, 34, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Deralia, P.K.; Jensen, A.; Felby, C.; Thygesen, L.G. Chemistry of Lignin and Hemicellulose Structures Interacts with Hydrothermal Pretreatment Severity and Affects Cellulose Conversion. Biotechnol. Prog. 2021, 37, e3189. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Onyango, S.O. Ultrasound Irradiation Alters the Spatial Structure and Improves the Antioxidant Activity of the Yellow Tea Polysaccharide. Ultrason. Sonochem. 2021, 70, 105355. [Google Scholar] [CrossRef]
- Chang, X.; Shen, C.-Y.; Jiang, J.-G. Structural Characterization of Novel Arabinoxylan and Galactoarabinan from Citron with Potential Antitumor and Immunostimulatory Activities. Carbohydr. Polym. 2021, 269, 118331. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, Q.; Yang, S.; Zhang, S.; Chen, G. Comparative Study on the Impact of Different Extraction Technologies on Structural Characteristics, Physicochemical Properties, and Biological Activities of Polysaccharides from Seedless Chestnut Rose (Rosa Sterilis) Fruit. Foods 2024, 13, 772. [Google Scholar] [CrossRef] [PubMed]

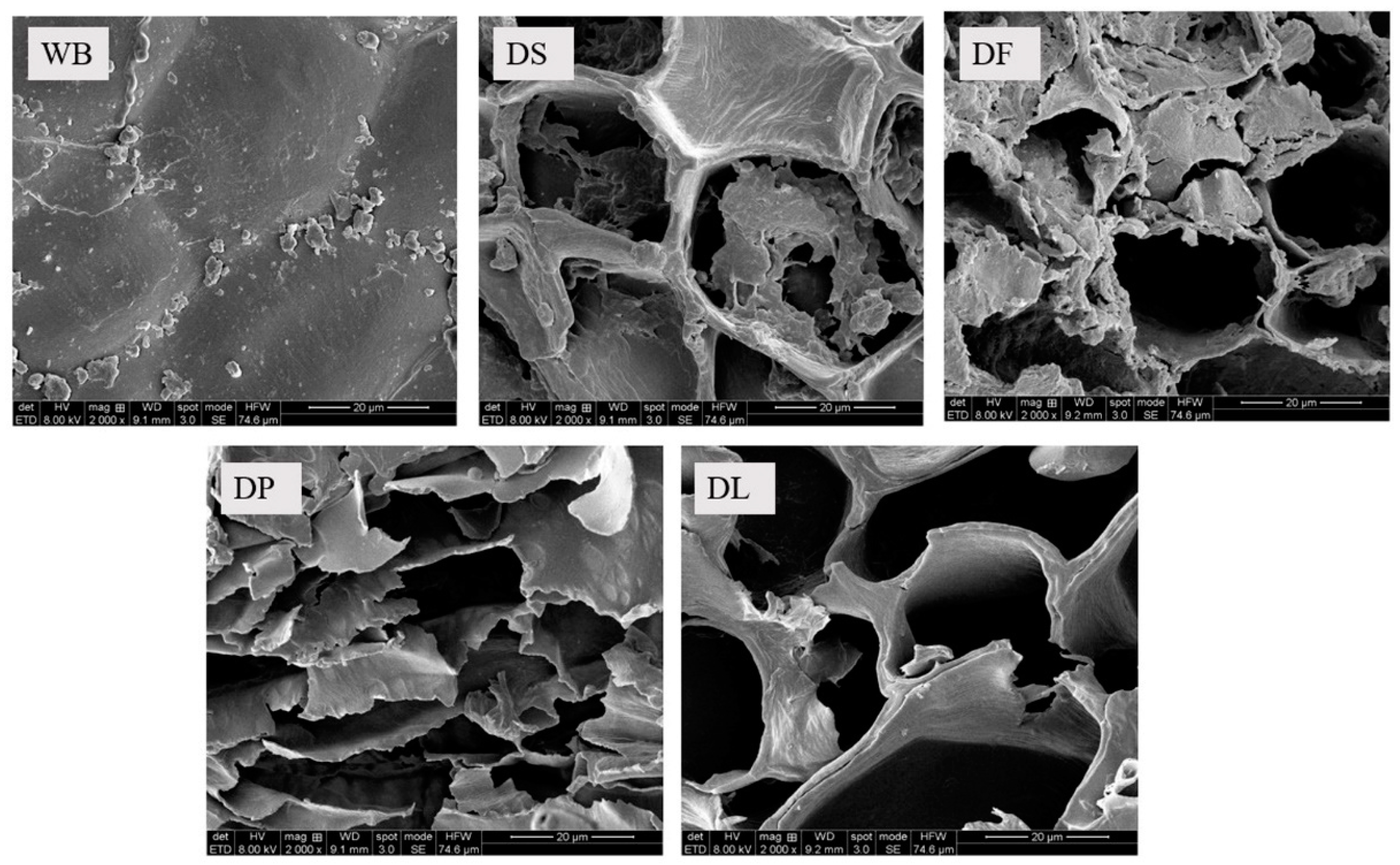
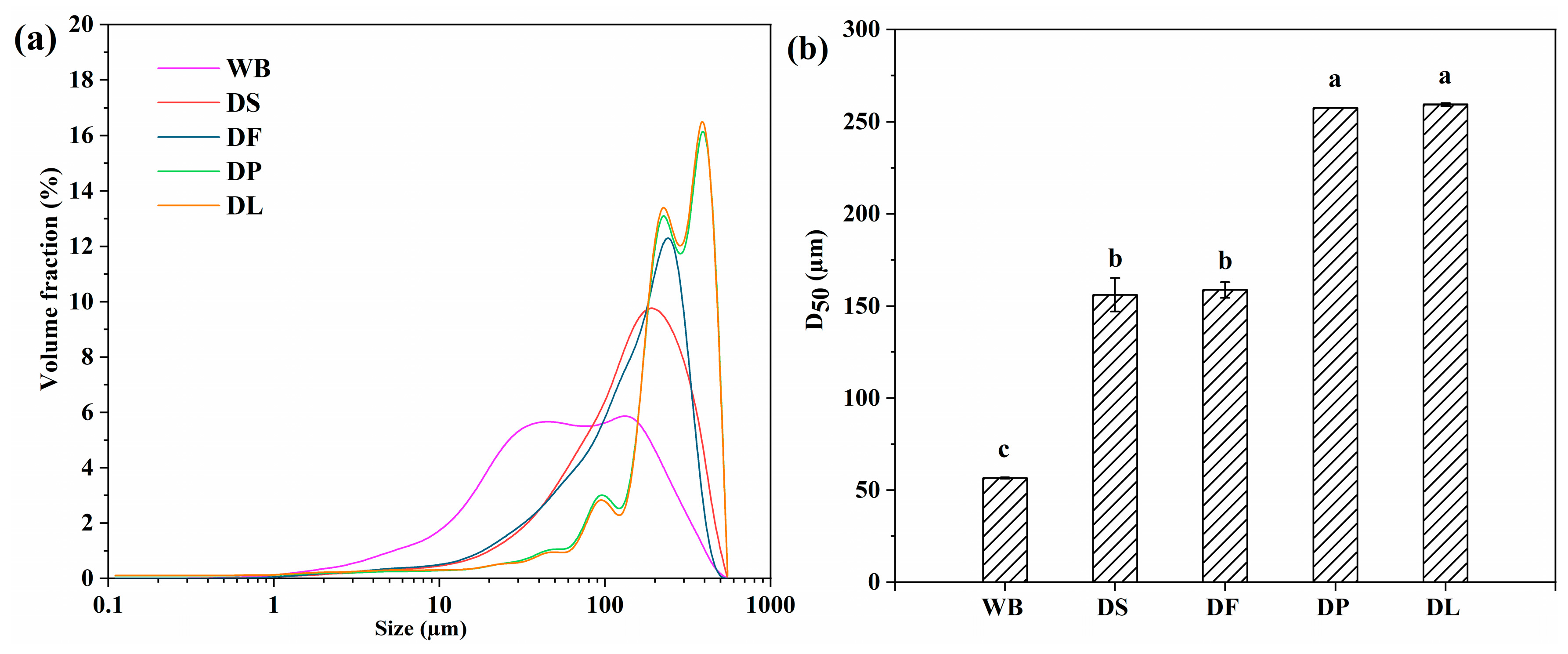

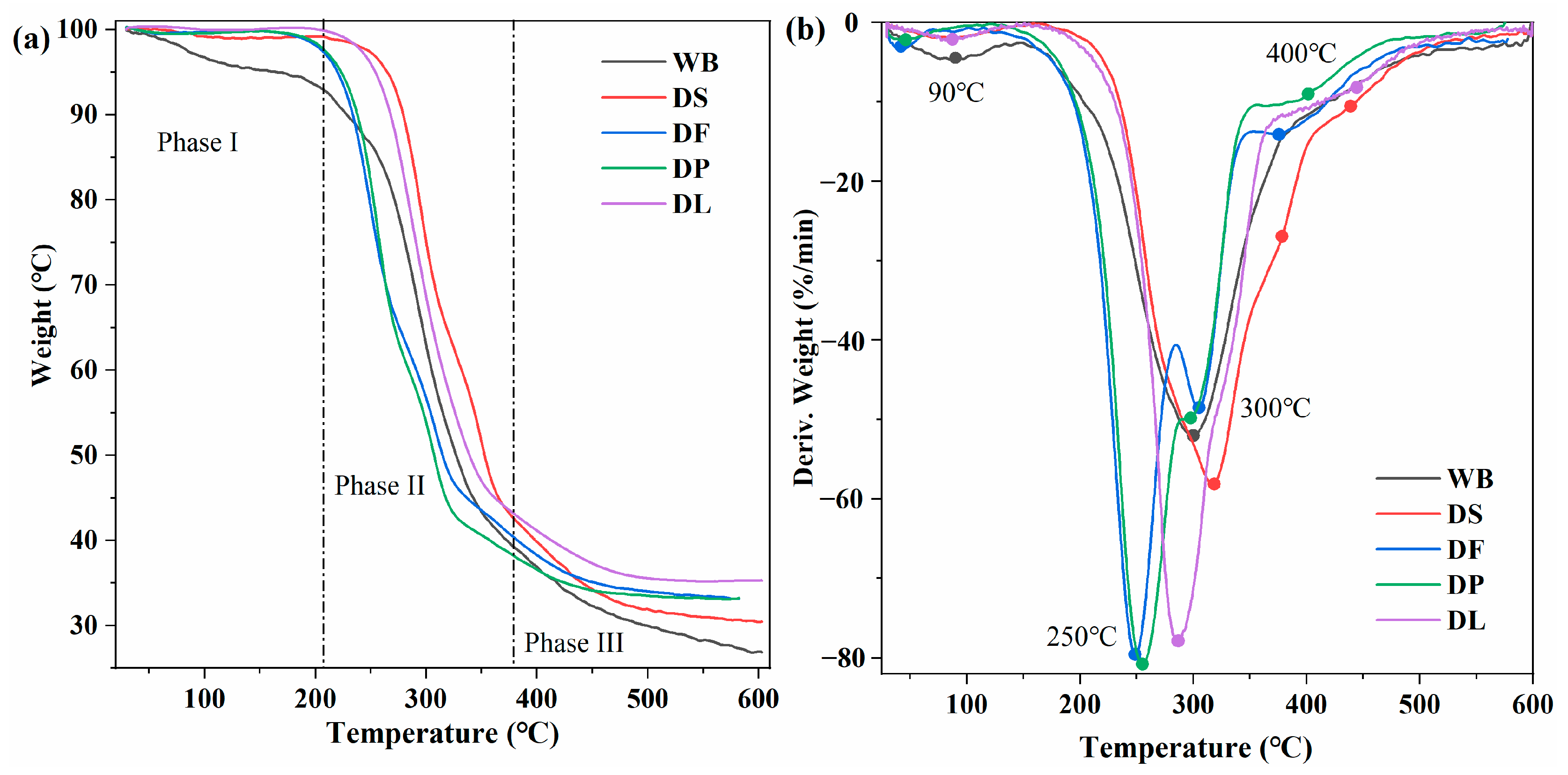
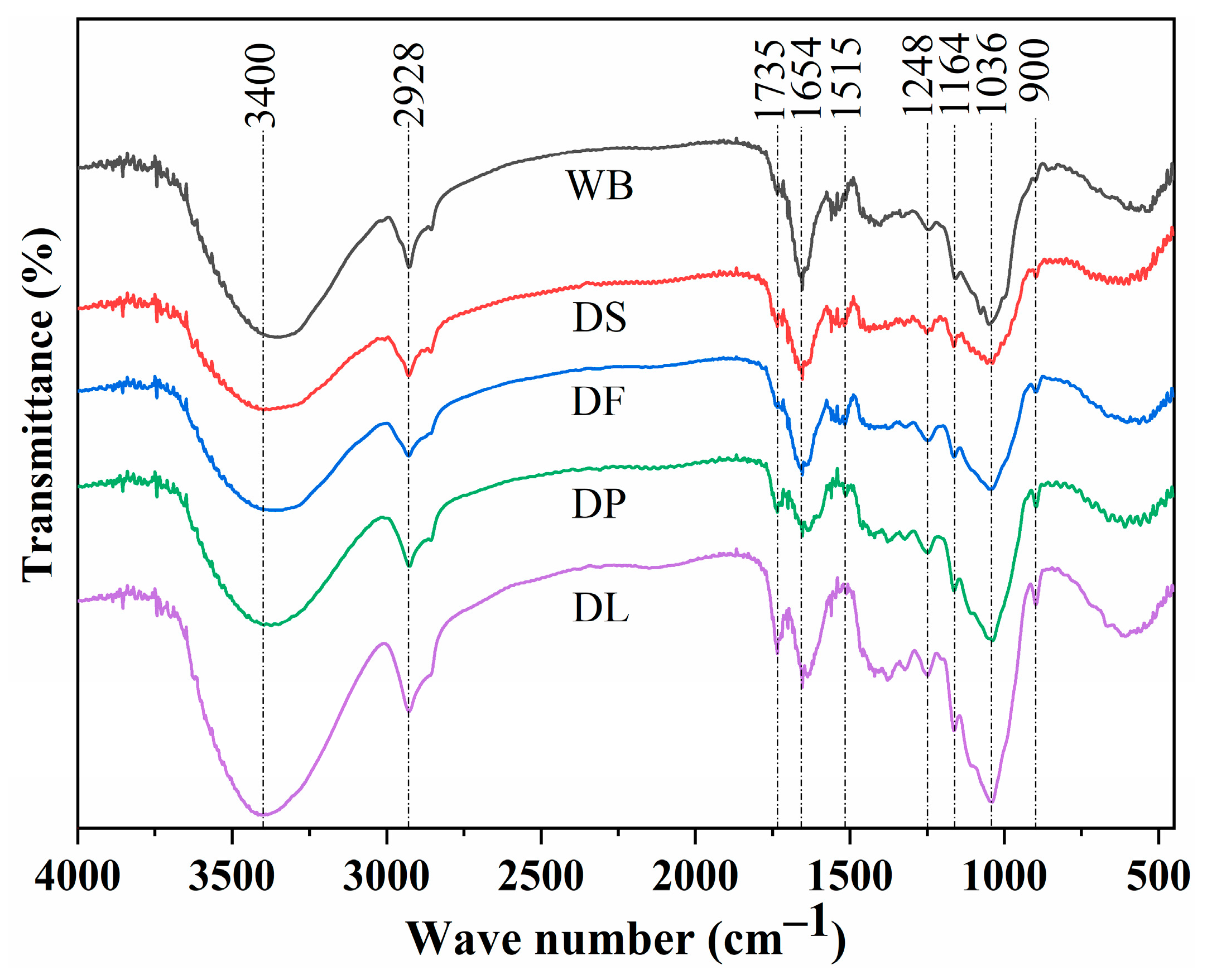
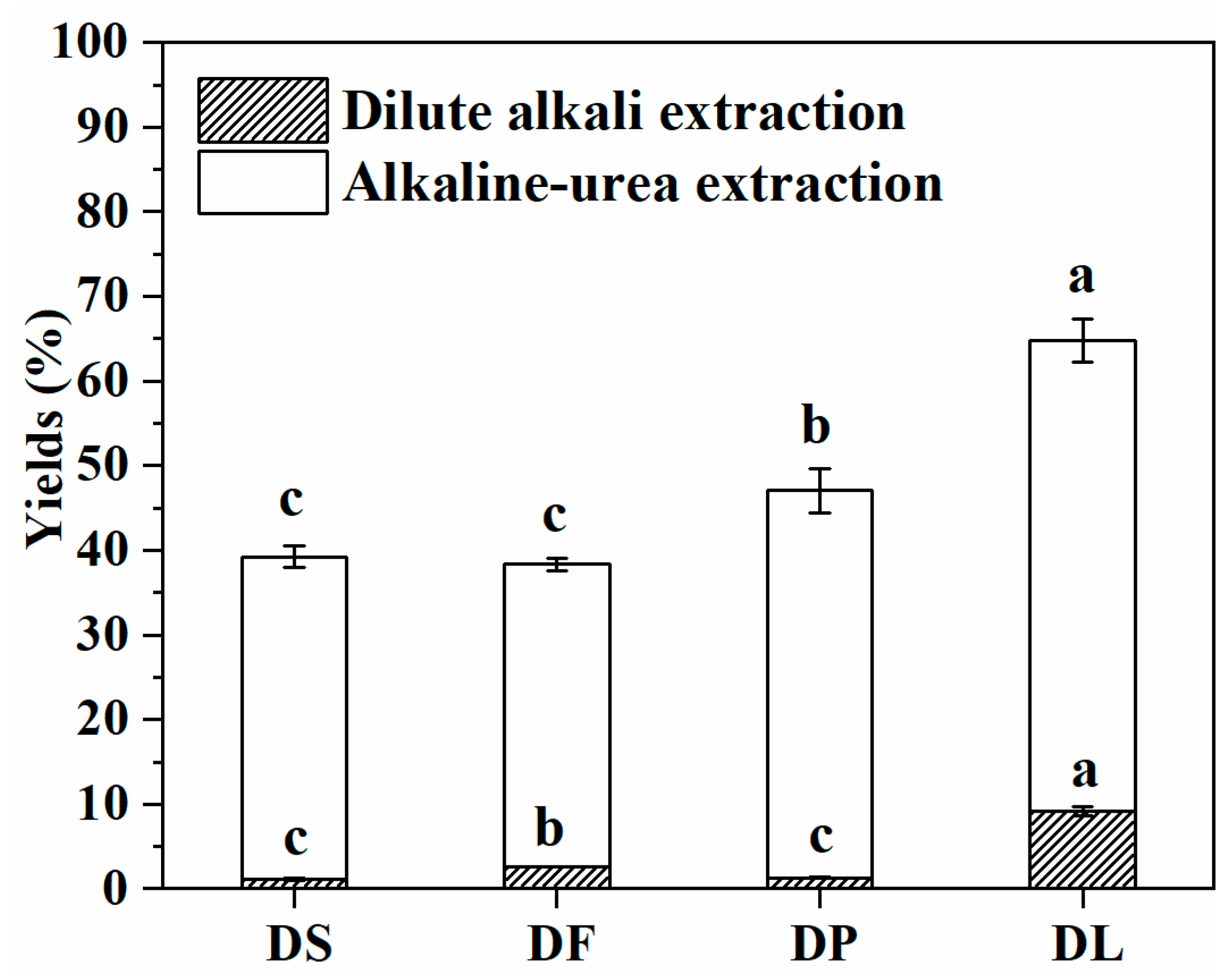
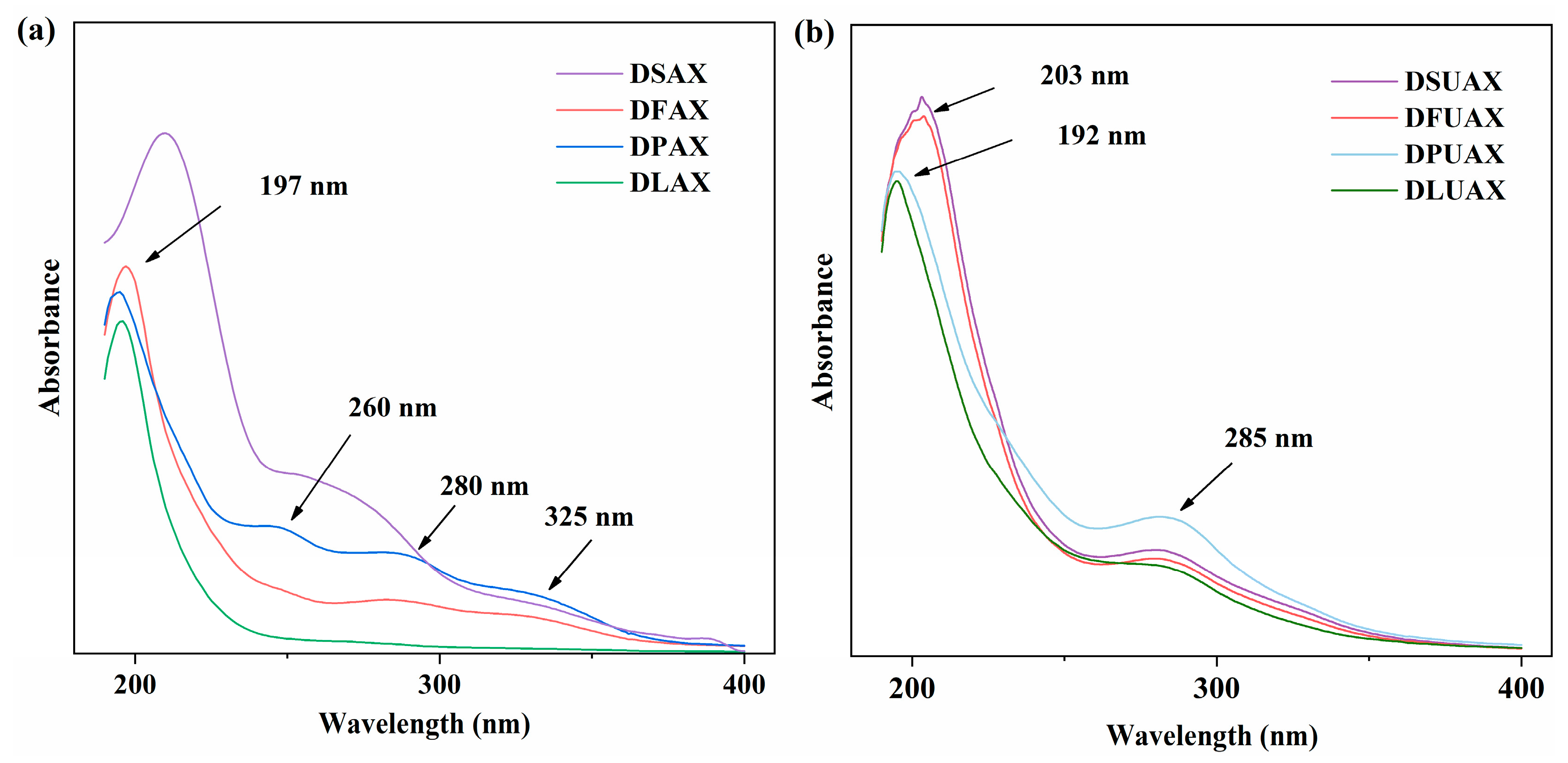
| Constituents | WB | DS | DF | DP | DL |
|---|---|---|---|---|---|
| Starch | 50.22 ± 0.08 a | 5.66 ± 0.16 b | 3.16 ± 0.40 d | 2.72 ± 0.12 d | 4.09 ± 0.18 c |
| Fat | 4.32 ± 0.05 a | 4.26 ± 0.48 a | 0.85 ± 0.16 c | 1.90 ± 0.06 b | 1.19 ± 0.03 c |
| Protein | 17.30 ± 0.09 c | 18.91 ± 0.10 b | 20.48 ± 0.11 a | 4.89 ± 0.03 d | 4.29 ± 0.03 e |
| Lignin | 8.33 ± 0.13 d | 15.08 ± 0.23 a | 12.25 ± 0.13 b | 15.54 ± 0.17 a | 11.04 ± 0.26 c |
| Cellulose | 11.94 ± 0.21 c | 20.68 ± 0.44 b | 21.35 ± 0.35 b | 24.15 ± 0.62 a | 25.02 ± 0.69 a |
| Hemicellulosic sugar composition | |||||
| Arabinose | 8.87 ± 0.11 d | 16.06 ± 0.20 c | 15.75 ± 0.04 c | 23.03 ± 0.43 a | 22.10 ± 0.13 b |
| Xylose | 9.62 ± 0.08 d | 17.42 ± 0.14 c | 16.50 ± 0.17 c | 20.44 ± 0.53 a | 18.74 ± 0.67 b |
| Galactose | 4.07 ± 0.04 d | 7.37 ± 0.07 c | 10.39 ± 0.50 a | 10.93 ± 0.04 a | 9.13 ± 0.95 b |
| A/X ratio * | 0.92 | 0.92 | 0.95 | 1.13 | 1.18 |
| Extracts | Sugar Composition | Total Sugar (%) | A/X Ratio | Protein (%) | |||
|---|---|---|---|---|---|---|---|
| Arabinose (%) | Galactose (%) | Glucose (%) | Xylose (%) | ||||
| DSAX | 10.34 ± 1.23 e | 0.79 ± 0.08 e | 4.94 ± 0.88 de | 17.12 ± 2.65 e | 33.20 ± 2.42 d | 0.59 | 9.24 ± 0.21 a |
| DFAX | 6.03 ± 0.21 f | 0.62 ± 0.01 f | 3.62 ± 0.12 e | 11.31 ± 0.15 f | 21.58 ± 0.24 e | 0.52 | 6.97 ± 0.15 c |
| DPAX | 16.10 ± 0.53 d | 1.38 ± 0.11 cd | 6.28 ± 0.30 d | 27.69 ± 1.25 d | 51.46 ± 1.10 c | 0.57 | 4.62 ± 0.05 d |
| DLAX | 3.01 ± 0.21 g | 0.18 ± 0.01 g | 1.16 ± 0.12 f | 4.70 ± 0.45 g | 9.04 ± 0.39 f | 0.63 | 3.78 ± 0.12 e |
| DSUAX | 20.77 ± 0.09 b | 1.64 ± 0.06 b | 14.69 ± 0.82 b | 37.95 ± 0.43 a | 75.04 ± 0.70 a | 0.53 | 7.89 ± 0.08 b |
| DFUAX | 18.54 ± 0.29 c | 1.30 ± 0.05 d | 12.56 ± 0.99 c | 32.60 ± 0.49 b | 65.02 ± 0.62 b | 0.56 | 6.90 ± 0.09 c |
| DPUAX | 25.14 ± 0.42 a | 2.05 ± 0.10 a | 17.83 ± 0.67 a | 29.93 ± 0.29 c | 74.95 ± 0.74 a | 0.82 | 3.98 ± 0.30 e |
| DLUAX | 20.75 ± 1.61 b | 1.56 ± 0.22 bc | 12.12 ± 1.67 c | 33.24 ± 1.16 b | 67.66 ± 2.33 b | 0.61 | 1.35 ± 0.10 f |
| Samples | Ferulic Acid (μg/g) | Sinapic Acid (μg/g) | P-Coumaric Acid (μg/g) | Total Phenolic Acid (μg/g) |
|---|---|---|---|---|
| DSAX | 34.74 ± 2.83 b | 0.22 ± 0.05 b | 0.78 ± 0.11 b | 35.78 ± 0.09 b |
| DFAX | 32.22 ± 1.92 c | 0.18 ± 0.07 b | 0.90 ± 0.16 b | 33.37 ± 0.13 c |
| DPAX | 87.23 ± 1.63 a | 0.61 ± 0.12 a | 1.84 ± 0.05 a | 88.99 ± 0.71 a |
| DLAX | 0.50 ± 0.14 d | 0.05 ± 0.01 c | 0.47 ± 0.08 c | 0.86 ± 0.21 e |
| DSUAX | 1.86 ± 0.06 d | 0.20 ± 0.01 b | 0.53 ± 0.05 c | 2.40 ± 0.24 d |
| DFUAX | 1.51 ± 0.14 d | 0.17 ± 0.01 b | 0.42 ± 0.02 c | 1.96 ± 0.01 d |
| DPUAX | 1.34 ± 0.40 d | 0 | 0.47 ± 0.03 c | 1.86 ± 0.02 d |
| DLUAX | 0.78 ± 0.12 d | 0 | 0.42 ± 0.03 c | 1.11 ± 0.06 e |
| Samples | DPPH Radical Scavenging Rate (%) | ABTS Radical Scavenging Rate (%) | Fe2+ Chelating Rate (%) |
|---|---|---|---|
| DSAX | 43.43 ± 1.81 b | 24.64 ± 21.52 abc | 47.96 ± 0.68 a |
| DFAX | 26.50 ± 1.89 d | 21.52 ± 3.04 bcd | 9.81 ± 1.11 e |
| DPAX | 59.62 ± 0.26 a | 25.18 ± 1.24 ab | 13.41 ± 1.53 e |
| DLAX | 23.91 ± 1.43 d | 13.57 ± 1.75 e | 11.68 ± 1.18 e |
| DSUAX | 46.04 ± 0.62 b | 19.55 ± 2.63 d | 32.84 ± 1.77 b |
| DFUAX | 44.78 ± 4.18 b | 21.43 ± 1.08 bcd | 28.40 ± 0.86 c |
| DPUAX | 55.60 ± 2.69 a | 26.34 ± 0.58 a | 35.34 ± 3.12 b |
| DLUAX | 34.21 ± 0.36 c | 20.71 ± 1.43 cd | 18.13 ± 0.83 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Zhang, S.; Wang, W.; Hou, H.; Dai, Y.; Li, C.; Zhang, H. Effects of Different Pretreatments on Wheat Bran and Its Arabinoxylan Obtained by Sequential Extraction with Dilute Alkali and Alkali–Urea Mixture. Foods 2025, 14, 696. https://doi.org/10.3390/foods14040696
Liu A, Zhang S, Wang W, Hou H, Dai Y, Li C, Zhang H. Effects of Different Pretreatments on Wheat Bran and Its Arabinoxylan Obtained by Sequential Extraction with Dilute Alkali and Alkali–Urea Mixture. Foods. 2025; 14(4):696. https://doi.org/10.3390/foods14040696
Chicago/Turabian StyleLiu, Axiang, Shengjie Zhang, Wentao Wang, Hanxue Hou, Yangyong Dai, Cheng Li, and Hui Zhang. 2025. "Effects of Different Pretreatments on Wheat Bran and Its Arabinoxylan Obtained by Sequential Extraction with Dilute Alkali and Alkali–Urea Mixture" Foods 14, no. 4: 696. https://doi.org/10.3390/foods14040696
APA StyleLiu, A., Zhang, S., Wang, W., Hou, H., Dai, Y., Li, C., & Zhang, H. (2025). Effects of Different Pretreatments on Wheat Bran and Its Arabinoxylan Obtained by Sequential Extraction with Dilute Alkali and Alkali–Urea Mixture. Foods, 14(4), 696. https://doi.org/10.3390/foods14040696






