A Protein-Based Approach for Greek Yogurt Authentication via an HRMS Technique (MALDI-TOF MS) and Milk Powder Detection as a Fraudulent Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals and Reagents
2.3. Sample Preparation
2.4. MALDI-TOF MS Analysis
2.5. Data Treatment and Statistics
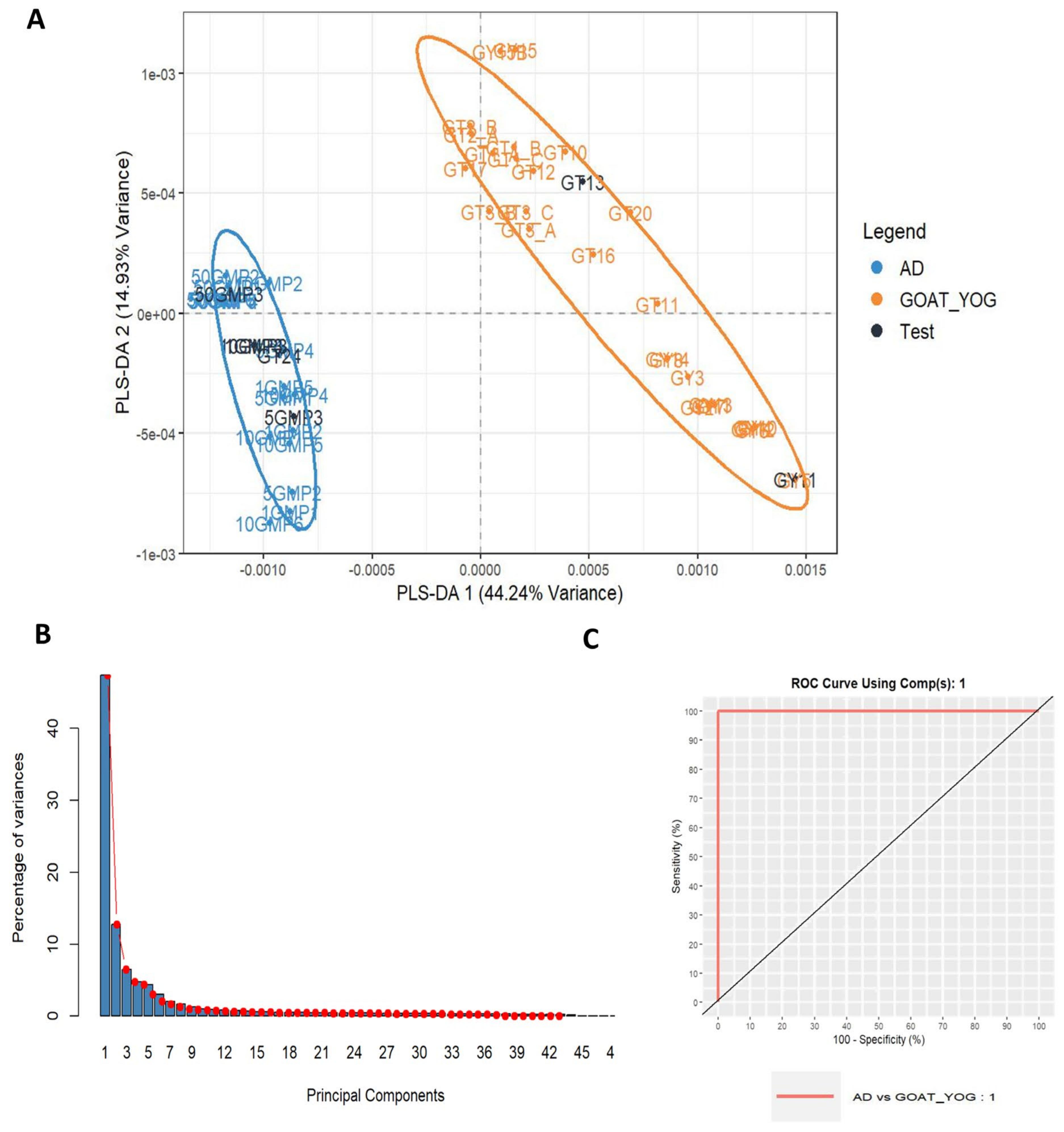
3. Results and Discussion
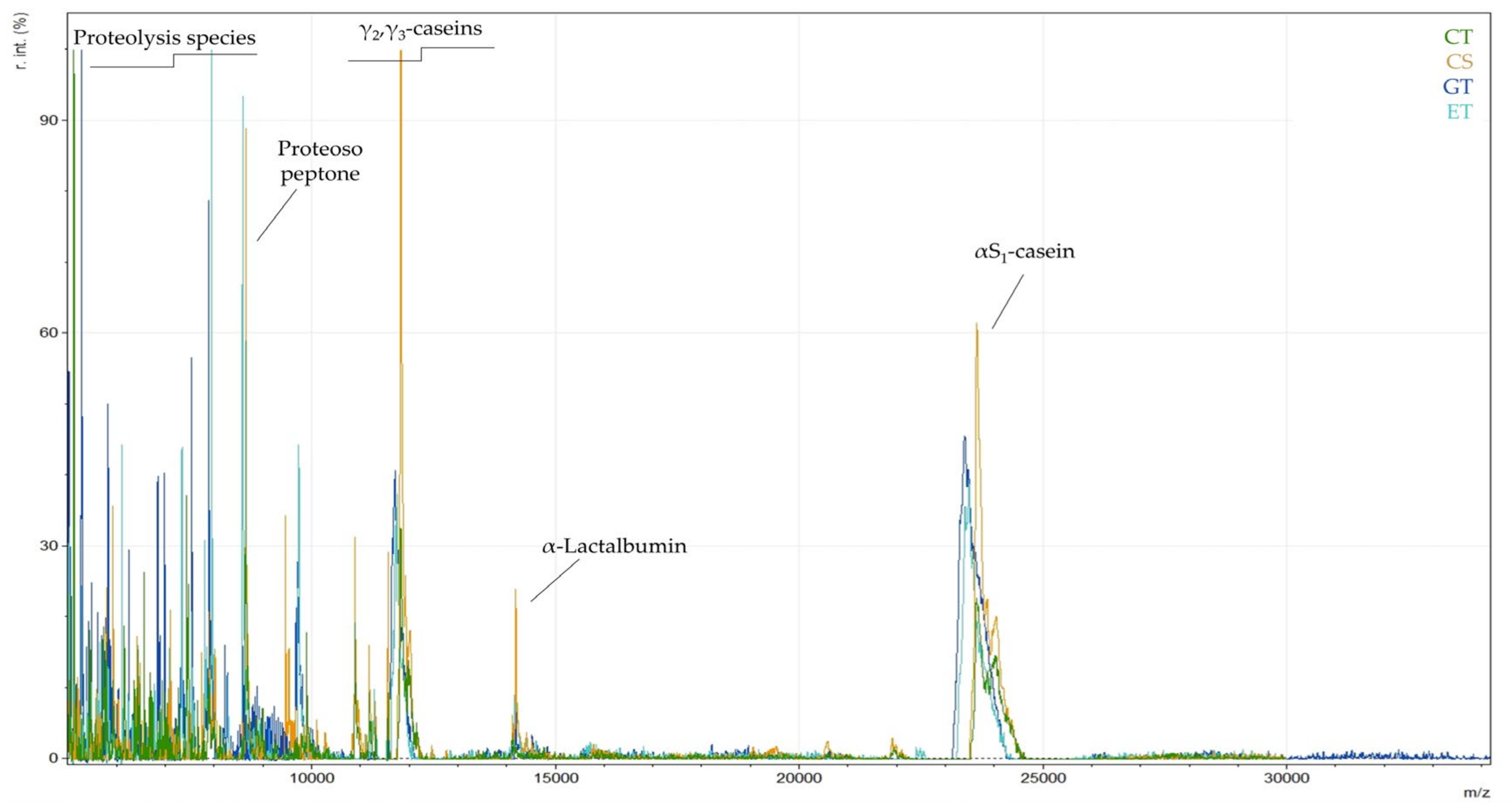
3.1. Discrimination of Milk Animal Origin in Yogurt Samples (Cow/Goat/Ewe)
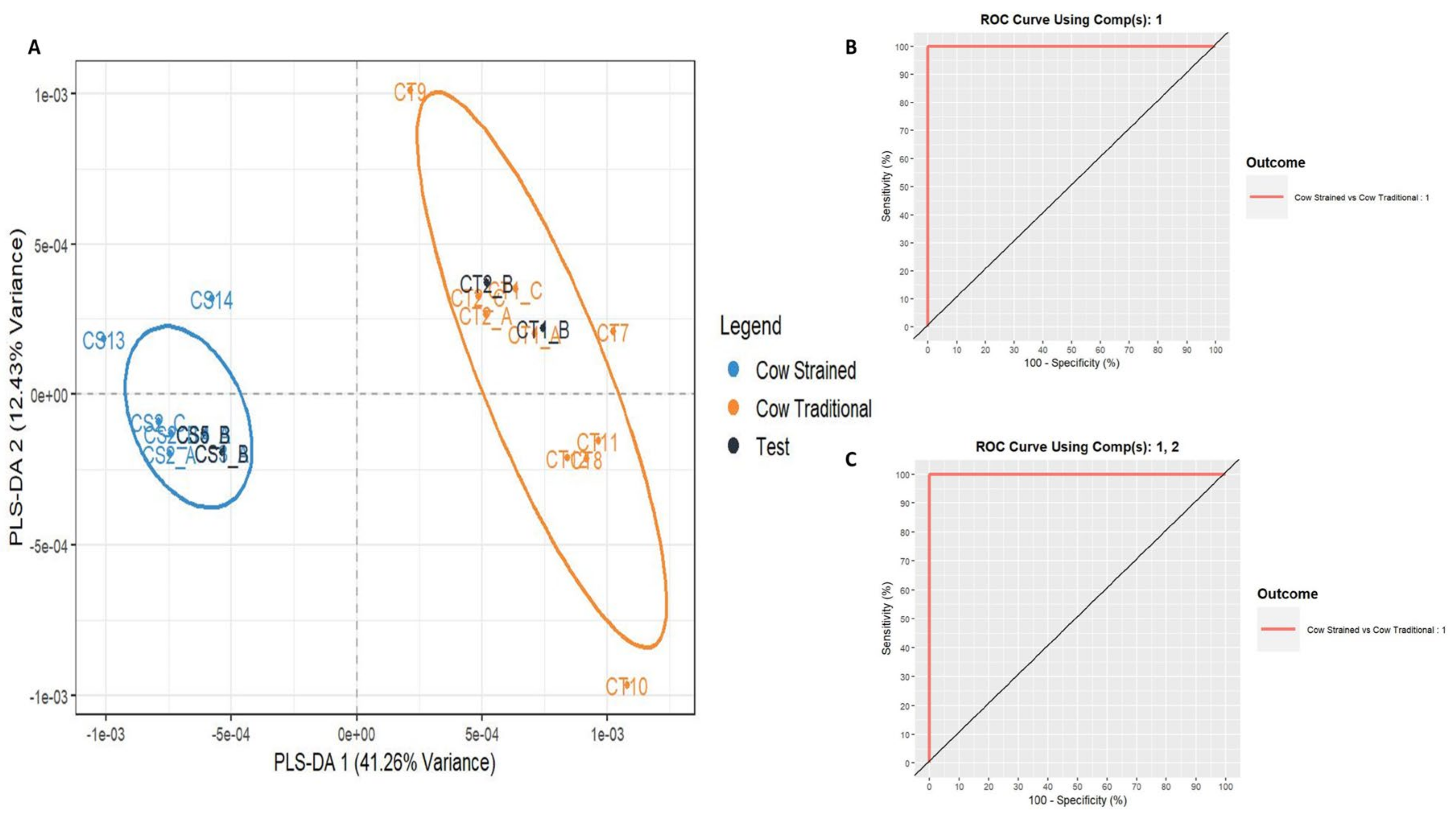
3.2. Discrimination of Greek Cow Milk Yogurt Technological Process (Strained/Traditional)
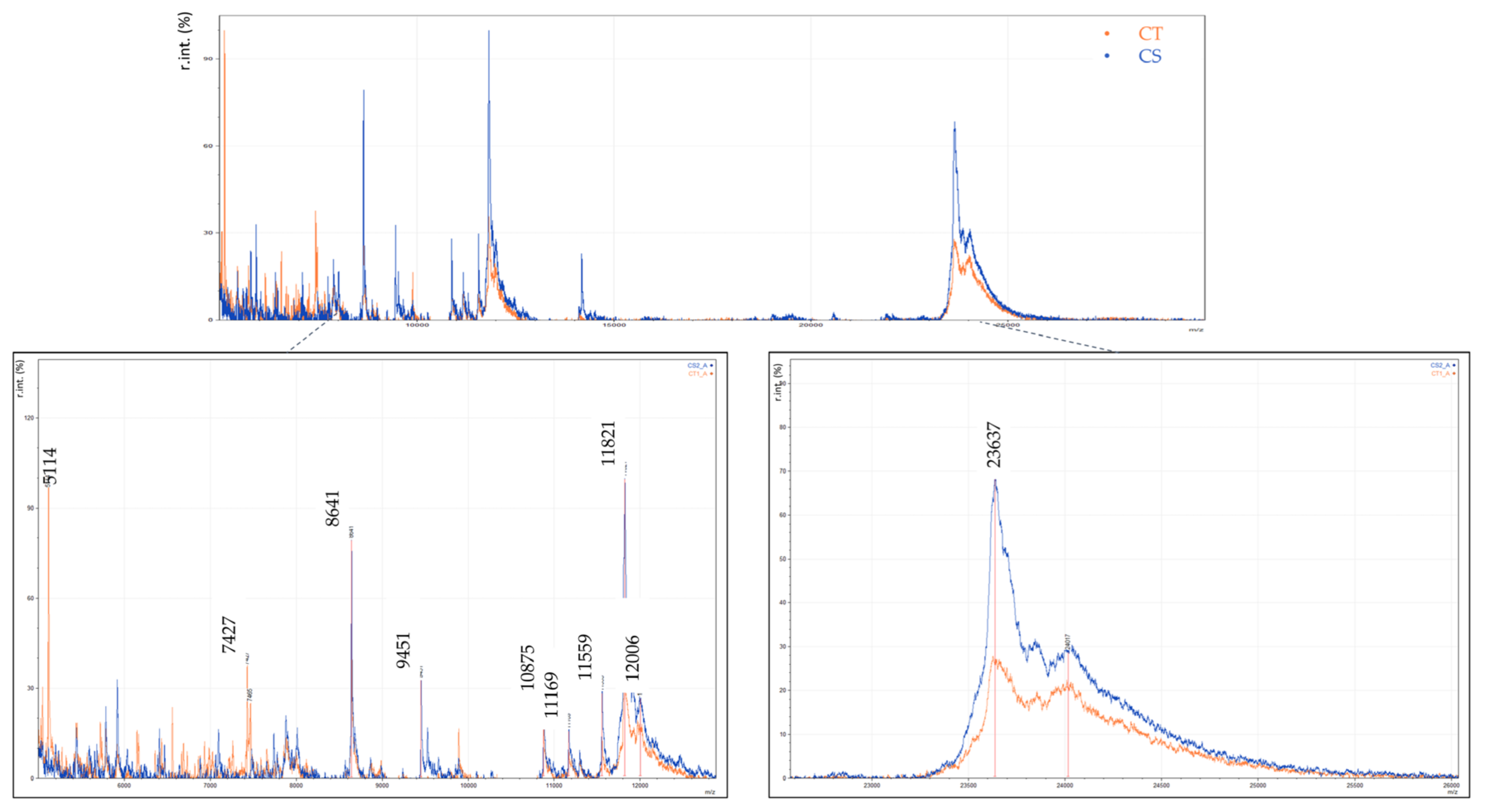
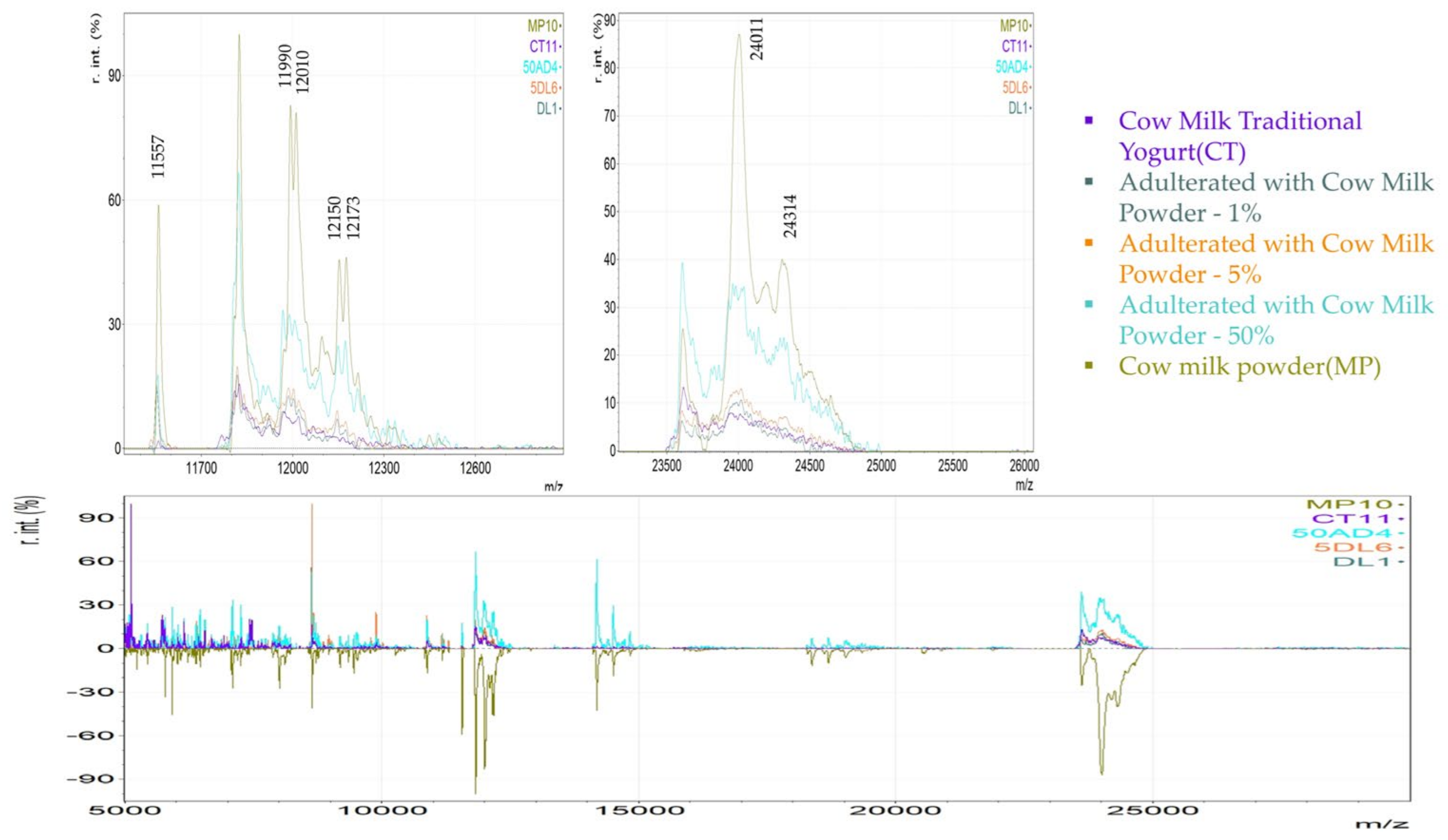
3.3. Discrimination of Cow Milk Powder and Cow Milk Traditional Yogurt
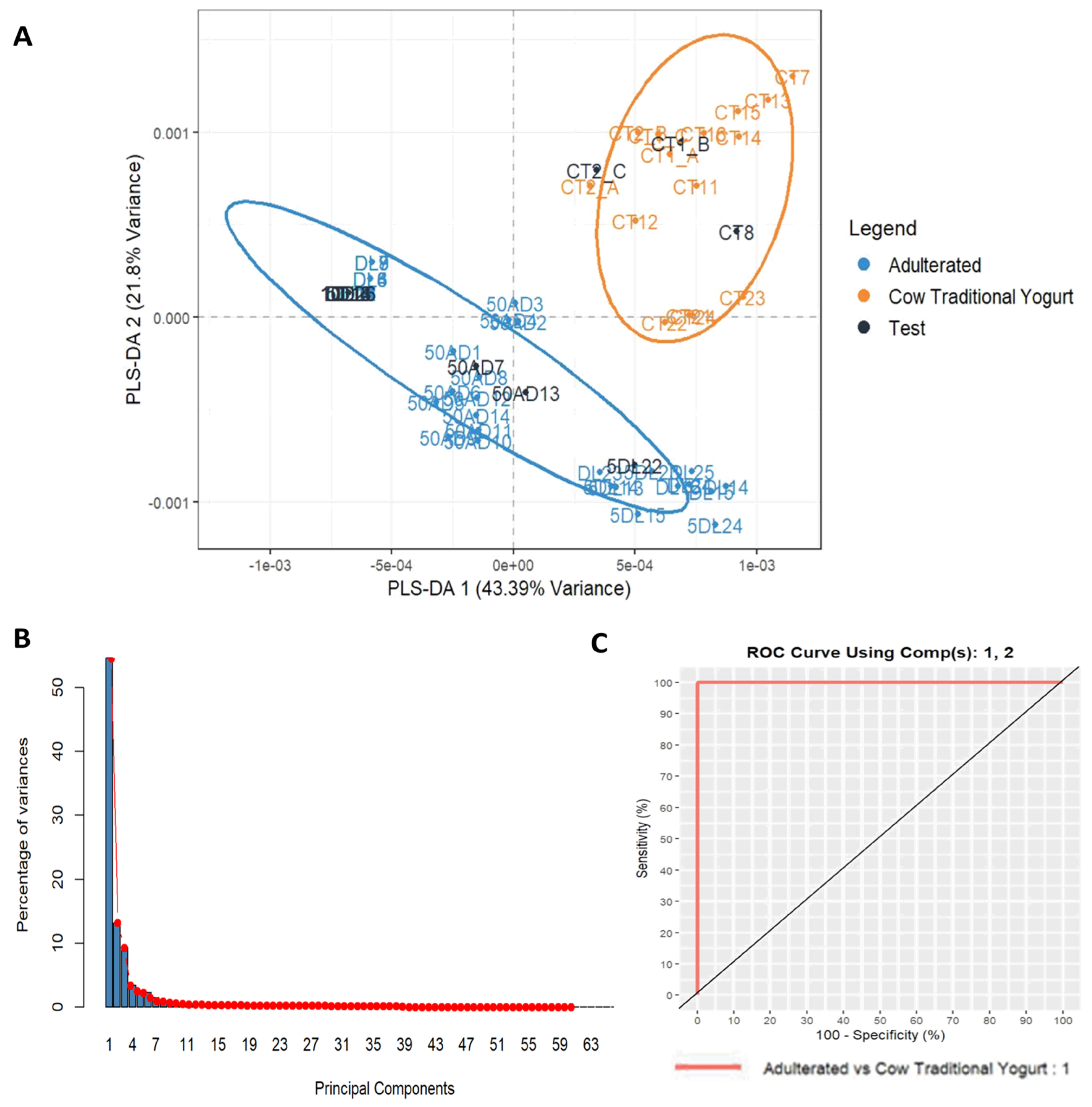
3.4. Adulteration Study: Detection of Cow Milk Powder Addition in Cow and Goat Milk Traditional Yogurt to the Level of 1%
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef] [PubMed]
- CXS 243-2003; Standard For Fermented Milks. CODEX Alimentarius Commission: Rome, Italy, 2018.
- de Oliveira, M.N. Fermented Milks: Fermented Milks and Yogurt, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, ISBN 9780123847331. [Google Scholar]
- Article 82—Yogurt. In Greek Code of Foods and Beverages; Volume IX Milk, Uggs and Their Products, Version 2, Date of Issue August 2016. Available online: https://www.aade.gr/en/himeio/ix-products-animal-origin-other-those-chapter-x (accessed on 6 January 2025).
- Sfakianakis, P.; Tzia, C. Conventional and Innovative Processing of Milk for Yogurt Manufacture; Development of Texture and Flavor: A Review. Foods 2014, 3, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, D.; Hatzithomas, L.; Fotiadis, T.; Gasteratos, A. The Impact of Brand Awareness and Country of Origin in the Advertising Effectiveness of Greek Food Products in the United Kingdom: The Case of Greek Yogurt. Foods 2022, 11, 4019. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.M.; Venkatesh, K.; Reddy, C.V.S. Adulteration of Milk and Its Detection: A Review. Int. J. Chem. Stud. 2017, 5, 613–617. [Google Scholar]
- Sassi, M.; Arena, S.; Scaloni, A. MALDI-TOF-MS Platform for Integrated Proteomic and Peptidomic Profiling of Milk Samples Allows Rapid Detection of Food Adulterations. J. Agric. Food Chem. 2015, 63, 6157–6171. [Google Scholar] [CrossRef]
- Caira, S.; Pinto, G.; Nicolai, M.A.; Chianese, L.; Addeo, F. Simultaneously Tracing the Geographical Origin and Presence of Bovine Milk in Italian Water Buffalo Mozzarella Cheese Using MALDI-TOF Data of Casein Signature Peptides. Anal. Bioanal. Chem. 2016, 408, 5609–5621. [Google Scholar] [CrossRef]
- Camerini, S.; Montepeloso, E.; Casella, M.; Crescenzi, M.; Marianella, R.M.; Fuselli, F. Mass Spectrometry Detection of Fraudulent Use of Cow Whey in Water Buffalo, Sheep, or Goat Italian Ricotta Cheese. Food Chem. 2016, 197, 1240–1248. [Google Scholar] [CrossRef]
- Kritikou, A.S.; Aalizadeh, R.; Damalas, D.E.; Barla, I.V.; Baessmann, C.; Thomaidis, N.S. MALDI-TOF-MS Integrated Workflow for Food Authenticity Investigations: An Untargeted Protein-Based Approach for Rapid Detection of PDO Feta Cheese Adulteration. Food Chem. 2022, 370, 131057. [Google Scholar] [CrossRef]
- Le, T.T.; Deeth, H.C.; Larsen, L.B. Proteomics of Major Bovine Milk Proteins: Novel Insights. Int. Dairy J. 2017, 67, 2–15. [Google Scholar] [CrossRef]
- Manso, M.A.; Léonil, J.; Jan, G.; Gagnaire, V. Application of Proteomics to the Characterisation of Milk and Dairy Products. Int. Dairy J. 2005, 15, 845–855. [Google Scholar] [CrossRef]
- Calvano, C.D.; Monopoli, A.; Loizzo, P.; Faccia, M.; Zambonin, C. Proteomic Approach Based on MALDI-TOF MS to Detect Powdered Milk in Fresh Cow’s Milk. J. Agric. Food Chem. 2013, 61, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Tzouros, N.E. Implementation of Quality Control Methods in Conjunction with Chemometrics toward Authentication of Dairy Products. Crit. Rev. Food Sci. Nutr. 2005, 45, 231–249. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2018/150 of 30 January 2018, Amending Implementing Regulation (EU) 2016/1240 as Regards Methods for the Analysis and Quality Evaluation of Milk and Milk Products Eligible for Public Intervention and Aid for Private Storage. Off. J. Eur. Union 2018, 26, 14–47. [Google Scholar]
- Kaminarides, S.E.; Koukiassa, P. Detection of Bovine Milk in Ovine Yoghurt by Electrophoresis of Para-k-Casein. Food Chem. 2002, 78, 53–55. [Google Scholar] [CrossRef]
- Zachar, P.; Šoltés, M.; Kasarda, R.; Jaroslav, N.; Novikmecová, M.; Marcinčáková, D. Analytical Methods for the Species Identification of Milk and Milk Products. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2007, 61, 199–207. [Google Scholar]
- Kalogianni, D.P. DNA-Based Analytical Methods for Milk Authentication. Eur. Food Res. Technol. 2018, 244, 775–793. [Google Scholar] [CrossRef]
- Jung, Y.K.; Jhon, D.Y.; Kim, K.; Hong, Y.H. Quantitative Detection of Cow Milk in Goat Milk Mixtures by Real-Time PCR. Korean J. Food Sci. Anim. Resour. 2011, 31, 827–833. [Google Scholar] [CrossRef]
- Poonia, A.; Jha, A.; Sharma, R.; Singh, H.B.; Rai, A.K.; Sharma, N. Detection of Adulteration in Milk: A Review. Int. J. Dairy Technol. 2017, 70, 23–42. [Google Scholar] [CrossRef]
- Nagraik, R.; Sharma, A.; Kumar, D.; Chawla, P.; Kumar, A.P. Milk Adulterant Detection: Conventional and Biosensor Based Approaches: A Review. Sens. Biosens. Res. 2021, 33, 100433. [Google Scholar] [CrossRef]
- Calvano, C.D.; De Ceglie, C.; Giorgio, C. Detection of Sheep and Goat Milk Adulterations by Direct MALDI—TOF MS Analysis of Milk Tryptic Digests†. J. Mass Spectrom. 2012, 47, 1141–1149. [Google Scholar] [CrossRef]
- Teixeira, J.L.d.P.; Caramês, E.T.d.S.; Baptista, D.P.; Gigante, M.L.; Pallone, J.A.L. Rapid Adulteration Detection of Yogurt and Cheese Made from Goat Milk by Vibrational Spectroscopy and Chemometric Tools. J. Food Compos. Anal. 2021, 96, 103712. [Google Scholar] [CrossRef]
- Mafra, I.; Honrado, M.; Amaral, J.S. Animal Species Authentication in Dairy Products. Foods 2022, 11, 1124. [Google Scholar] [CrossRef]
- Nicolaou, N.; Xu, Y.; Goodacre, R. Fourier Transform Infrared Spectroscopy and Multivariate Analysis for the Detection and Quantification of Different Milk Species. J. Dairy Sci. 2010, 93, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Hruzikova, J.; Milde, D.; Krajancova, P.; Ranc, V. Discrimination of Cheese Products for Authenticity Control by Infrared Spectroscopy. J. Agric. Food Chem. 2012, 60, 1845–1849. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuckova, S.; Zitkova, K.; Novotny, O.; Smirnova, T. Verification of Cheeses Authenticity by Mass Spectrometry. J. Sep. Sci. 2019, 42, 3487–3496. [Google Scholar] [CrossRef]
- Rau, J.; Korte, N.; Dyk, M.; Wenninger, O.; Schreiter, P.; Hiller, E. Rapid Animal Species Identification of Feta and Mozzarella Cheese Using MALDI-TOF Mass-Spectrometry. Food Control 2020, 117, 107349. [Google Scholar] [CrossRef]
- Kastanos, E.; Papaneophytou, C.; Georgiou, T.; Demoliou, C. A Simple and Fast Triplex-PCR for the Identification of Milk’s Animal Origin in Halloumi Cheese and Yoghurt. J. Dairy Res. 2022, 89, 323–326. [Google Scholar] [CrossRef]
- Guan, R.F.; Liu, D.H.; Ye, X.Q.; Yang, K. Use of Fluorometry for Determination of Skim Milk Powder Adulteration in Fresh Milk. J. Zhejiang Univ. Sci. B 2005, 6, 1101–1106. [Google Scholar] [CrossRef]
- Meltretter, J.; Birlouez-Aragon, I.; Becker, C.M.; Pischetsrieder, M. Assessment of Heat Treatment of Dairy Products by MALDI-TOF-MS. Mol. Nutr. Food Res. 2009, 53, 1487–1495. [Google Scholar] [CrossRef]
- Cozzolino, R.; Passalacqua, S.; Salemi, S.; Malvagna, P.; Spina, E.; Garozzo, D. Identification of Adulteration in Milk by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. J. Mass Spectrom. 2001, 36, 1031–1037. [Google Scholar] [CrossRef]
- Strohalm, M.; Hassman, M.; Košata, B.; Kodíček, M. MMass Data Miner: An Open Source Alternative for Mass Spectrometric Data Analysis. Rapid Commun. Mass Spectrom. 2008, 22, 905–908. [Google Scholar] [CrossRef]
- Strohalm, M.; Kavan, D.; Novák, P.; Volný, M.; Havlicek, V. MMass 3: A Cross-Platform Software Environment for Precise Analysis of Mass Spectrometric Data. Anal. Chem. 2010, 82, 4648–4651. [Google Scholar] [CrossRef]
- Mendez, K.M.; Broadhurst, D.I.; Reinke, S.N. Migrating from Partial Least Squares Discriminant Analysis to Artificial Neural Networks: A Comparison of Functionally Equivalent Visualisation and Feature Contribution Tools Using Jupyter Notebooks. Metabolomics 2020, 16, 17. [Google Scholar] [CrossRef]
- Mahieu, B.; Qannari, E.M.; Jaillais, B. Extension and Significance Testing of Variable Importance in Projection (VIP) Indices in Partial Least Squares Regression and Principal Components Analysis. Chemom. Intell. Lab. Syst. 2023, 242, 104986. [Google Scholar] [CrossRef]
- Sumi, K.; Tagawa, R.; Yamazaki, K.; Nakayama, K.; Ichimura, T.; Sanbongi, C.; Nakazato, K. Nutritional Value of Yogurt as a Protein Source: Digestibility/Absorbability and Effects on Skeletal Muscle. Nutrients 2023, 15, 4366. [Google Scholar] [CrossRef]
- Farkye, N.Y. Cheeses: Chemistry and Microbiology of Maturation. In Encyclopedia of Food Sciences and Nutrition; California Polytechnic State University: San Luis Obispo, CA, USA, 2003; pp. 1062–1066. [Google Scholar]
- Garcia, H.S.; López-Hernandez, A.; Hill, C., Jr. Enzyme Technology—Dairy Industry Applications, 2nd ed.; Moo-Young, M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 1. [Google Scholar]
- Ruprichová, L.; Dračková, M.; Borkovcová, I.; Vorlová, L. Determination of Proteins in Yoghurt. J. Microbiol. 2012, 1, 644–650. [Google Scholar]
- Bu, G.; Luo, Y.; Zhang, Y.; Chen, F. Effects of Fermentation by Lactic Acid Bacteria on the Antigenicity of Bovine Whey Proteins. J. Sci. Food Agric. 2010, 90, 2015–2020. [Google Scholar] [CrossRef]
- Harte, F.; Luedecke, L.; Swanson, B.; Barbosa-Cánovas, G.V. Low-Fat Set Yogurt Made from Milk Subjected to Combinations of High Hydrostatic Pressure and Thermal Processing. J. Dairy Sci. 2003, 86, 1074–1082. [Google Scholar] [CrossRef]
- Tzvetkova, I.; Dalgalarrondo, M.; Danova, S.; Iliev, I.; Ivanova, I.; Chobert, J.-M.; Haertlé, T. Hydrolysis of Major Dairy Proteins by Lactic Acid Bacteria from Bulgarian Yogurts. J. Food Biochem. 2007, 31, 680–702. [Google Scholar] [CrossRef]
- Nicolaou, N.; Xu, Y.; Goodacre, R. MALDI-MS and Multivariate Analysis for the Detection and Quantification of Different Milk Species. Anal. Bioanal. Chem. 2011, 399, 3491–3502. [Google Scholar] [CrossRef]
- Chen, R.K.; Chang, L.W.; Chung, Y.Y.; Lee, M.H.; Ling, Y.C. Quantification of Cow Milk Adulteration in Goat Milk Using High-Performance Liquid Chromatography with Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Mazzeo, M.F.; Arena, S.; Renzone, G.; Scaloni, A. Mass Spectrometry for the Analysis of Protein Lactosylation in Milk Products. Food Res. Int. 2013, 54, 988–1000. [Google Scholar] [CrossRef]
- Carulli, S.; Calvano, C.D.; Palmisano, F.; Pischetsrieder, M. MALDI-TOF MS Characterization of Glycation Products of Whey Proteins in a Glucose/Galactose Model System and Lactose-Free Milk. J. Agric. Food Chem. 2011, 59, 1793–1803. [Google Scholar] [CrossRef] [PubMed]








| Description | Experimental m/z Value | Literature |
|---|---|---|
| Cow milk yogurt | ||
| Unknown | 5115 | |
| Unknown | 5778 | |
| Unknown | 6402 | |
| Unknown | 7427 | |
| Unknown | 7466 | |
| proteose peptone (fragment of β-Casein) | 8640 | |
| Unknown | 10,879 | |
| γ2-casein (fragment of β-Casein) | 11,552 | [8] |
| γ3-casein (fragment of β-Casein) | 11,560 | [8] |
| γ2-casein (fragment of β-Casein) | 11,807 | [8,48] |
| γ2-casein (fragment of β-Casein) | 11,821 | [8] |
| γ2-casein (fragment of β-Casein) | 11,845 | |
| as1-casein | 23,608, 23,628, 23,647 | [8] |
| Ewe milk yogurt | ||
| Unknown | 5101 | |
| Unknown | 5265 | |
| Unknown | 7940 | |
| Unknown | 8275 | |
| Unknown | 8574 | [8] |
| Goat milk yogurt | ||
| Unknown | 5101 | |
| Unknown | 5265 | |
| Unknown | 5280 | |
| Unknown | 5406 | |
| Unknown | 7528 | |
| Unknown | 7795 | |
| Unknown | 7880 | |
| Unknown | 8589 | |
| γ2-casein (fragment of β-Casein) | 11,680 | |
| γ2-casein (fragment of β-Casein) | 11,700 | |
| as1-casein (broad peak) | 23,348, 23,392, 23,408 | |
| Description | Experimental m/z Value | Literature |
|---|---|---|
| Cow milk traditional (set) yogurt | ||
| Unknown | 5115 | |
| Unknown | 5124 | |
| Unknown | 5717 | |
| Unknown | 6143 | |
| Unknown | 6552 | |
| Unknown | 7427 | |
| Unknown | 7466 | |
| γ2-casein (fragment of β-Casein) | 11,807 | [8,45,47] |
| Cow milk strained yogurt | ||
| proteose peptone (fragment of β-Casein) | 8639 | |
| Unknown | 9451 | |
| Unknown | 10,877 | |
| γ2-casein (fragment of β-Casein) | 11,807 | |
| Description | Experimental m/z Value | Literature |
|---|---|---|
| Cow milk powder | ||
| Unknown | 8006 | [8] |
| γ2-Casein (fragment of β-Casein) | 11,557 | [8] |
| Unknown | 11,990 | [8] |
| Unknown | 12,010 | |
| γ2casein + lactose | 12,150 | [8] |
| Unknown | 12,173 | |
| α-Lactalbumin | 14,176 | [8,33] |
| α-Lactalbumin + Lactose | 14,506 | [32,33,48] |
| α-Lactalbumin + 2 × Lactose | 14,825 | [32,33,48] |
| α-Lactalbumin + 3 × Lactose | 15,143 | [32,33,48] |
| β-Lactoglobulin (var 1, 2) | 18,281, 18,358 | [32,33,48] |
| β-Lactoglobulin (var 1, 2) + Lactose | 18,600, 18,689 | [32,33,48] |
| β-Lactoglobulin (var 1, 2) + 2 × Lactose | 18,923, 19,009 | [32,33,48] |
| β-Lactoglobulin (var 1, 2) + 3 × Lactose | 19,246, 19,343 | [32,33,48] |
| as1-casein | 23,620 | [33,48] |
| β-casein | 24,011 | |
| β-casein + Lactose | 24,314 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krystalli, E.; Thomaidis, N.; Kritikou, A.S.; Kokkinos, C. A Protein-Based Approach for Greek Yogurt Authentication via an HRMS Technique (MALDI-TOF MS) and Milk Powder Detection as a Fraudulent Addition. Foods 2025, 14, 693. https://doi.org/10.3390/foods14040693
Krystalli E, Thomaidis N, Kritikou AS, Kokkinos C. A Protein-Based Approach for Greek Yogurt Authentication via an HRMS Technique (MALDI-TOF MS) and Milk Powder Detection as a Fraudulent Addition. Foods. 2025; 14(4):693. https://doi.org/10.3390/foods14040693
Chicago/Turabian StyleKrystalli, Evangelia, Nikolaos Thomaidis, Anastasia S. Kritikou, and Christos Kokkinos. 2025. "A Protein-Based Approach for Greek Yogurt Authentication via an HRMS Technique (MALDI-TOF MS) and Milk Powder Detection as a Fraudulent Addition" Foods 14, no. 4: 693. https://doi.org/10.3390/foods14040693
APA StyleKrystalli, E., Thomaidis, N., Kritikou, A. S., & Kokkinos, C. (2025). A Protein-Based Approach for Greek Yogurt Authentication via an HRMS Technique (MALDI-TOF MS) and Milk Powder Detection as a Fraudulent Addition. Foods, 14(4), 693. https://doi.org/10.3390/foods14040693








