Antimicrobial Activity of Probiotic Bacteria Isolated from Plants: A Review
Abstract
1. Introduction
2. Probiotic Bacteria and Antimicrobial Activity
2.1. Bacteriocin and Bacteriocin-like Inhibitory Substances
2.2. Non-Bacteriocin Types
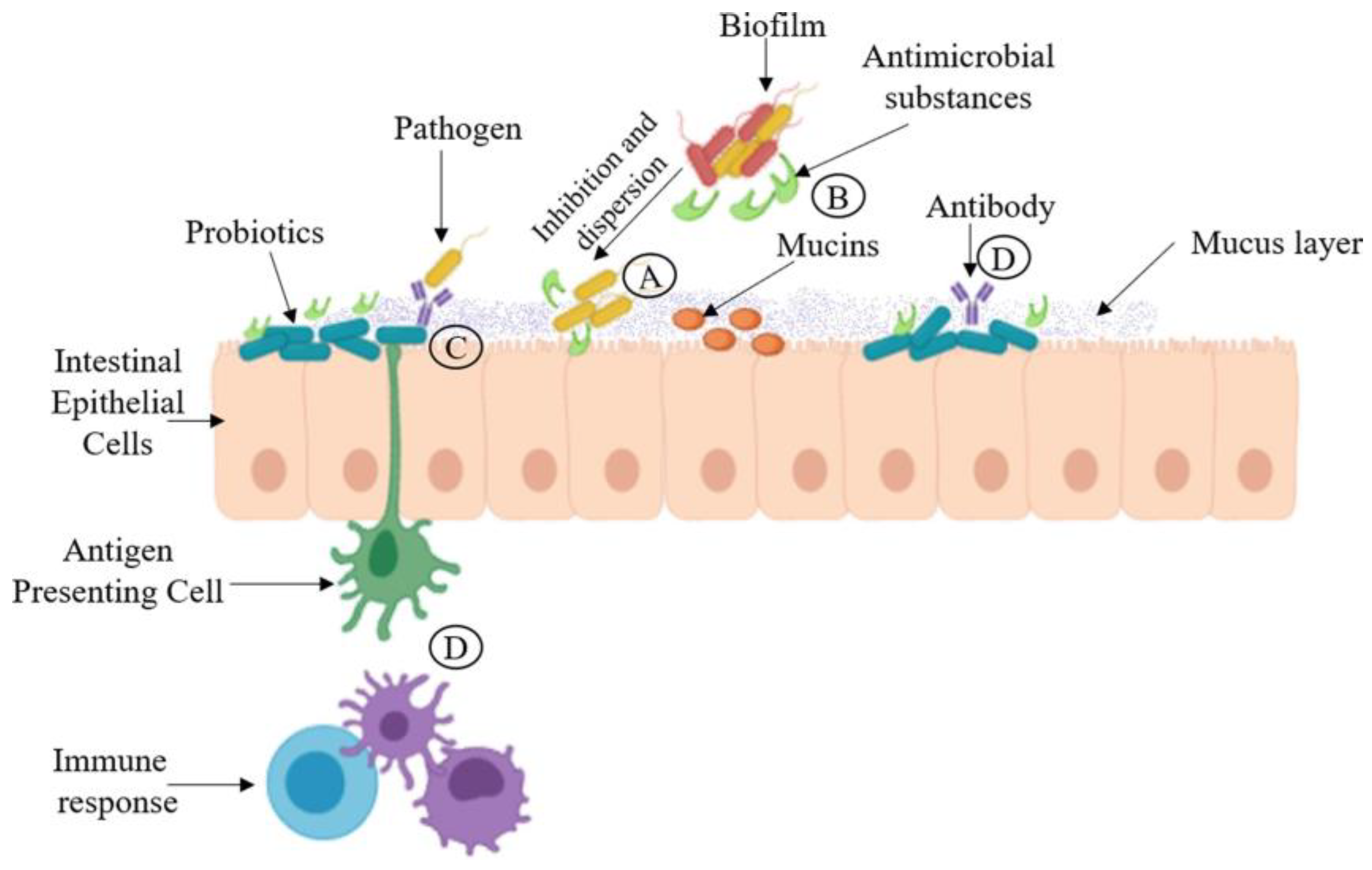
2.3. Indirect Antimicrobial Activity
3. Literature Search Strategy
4. Antimicrobial Activity of Probiotic Bacteria
4.1. Lactobacillus
4.2. Leuconostoc
4.3. Weissella
4.4. Enterococcus
| Isolation Source | LAB Strains and Products | Main Findings | Ref. |
|---|---|---|---|
| Pulp processing of Annona muricata L., Malpighia glabra L., Mangifera indica L., Ananas comosus L., and Fragaria vesca L. | Lactiplantibacillus plantarum 49, Levilactobacillus brevis 59, Lacticaseibacillus paracasei 108, Lactiplantibacillus pentosus 129, and Limosilactobacillus fermentum 111 | All strains exhibited antibacterial Activity against Salmonella Enteritidis > Salmonella Typhimurium > L. monocytogenes > E. coli > S. aureus, based on average ZOI from spot and well-diffusion assays | [75] |
| Pulp processing of Annona muricata L. | Lactiplantibacillus plantarum 53 and Lacticaseibacillus paracasei 106 | All strains showed antagonistic activity against Salmonella Enteritidis, Salmonella Typhimurium, L. monocytogenes, E. coli, and S. aureus. Strongest activity was shown by strains 53 and 60 | [76] |
| Pulp processing of Malpighia glabra L. | Limosilactobacillus fermentum 56, 60 | ||
| Pulp processing of Mangifera indica L. | L. fermentum 139, 141 | ||
| Pulp processing of Ananas comosus L. | L. fermentum 250, 263 | ||
| Pulp processing of Fragaria vesca L. | L. fermentum 296 | ||
| Cabbage and cucumber | EPS (80% methanol, 50% methanol, and aqueous extracts) produced by Lactobacillus acidophilus and Lactobacillus bulgaricus | All extracts exhibit antibacterial activity against E. coli (MTCC 1687), S. aureus (MTCC 7443), Salmonella enterica (MTCC3219), and Shigella flexeneri (MTCC 1457) Inhibition by all the extracts S. aureus > S. enterica > E. coli > S. flexneri | [77] |
| Pear | EPS (negatively charged and acidic) produced by Lactiplantibacillus plantarum SN35N | EPS suppressed the infectivity of Feline calicivirus (Vesivirus Feline calicivirus) and Influenza virus (Alphainfluenzavirus Influenza A virus) | [78] |
| Italian ryegrass (Lolium multiflorum) forage | Lactiplantibacillus plantarum KCC-24 | The strain showed antifungal activity against strains of Aspergillus fumigatus, Penicillium chrysogenum, P. roqueforti, Botrytis elliptica, and Fusarium oxysporum Highest inhibition = A. fumigatus | [80] |
| Rice plants | CFS of Leu. citreum KCC-57, KCC-58 | KCC-57 (ZOI) E. coli = 34.3, E. faecalis = 18.5, P. aeruginosa = 17.5, and S. aureus = 22.0 KCC-58 (ZOI) E. coli = 32.6, E. faecalis = 22.5, P. aeruginosa = 18.5, and S. aureus = 27.3 | [89] |
| Persimmon fruit | Bacteriocins (Leucocin C-607 and bacteriocin 607A) of Leu. pseudomesenteroides | Bacteriocins inhibited the growth of Listeria monocytogenes ATCC 19111 | [90] |
| Orange, sapota, banana, cherry, and plum smashed fruits | CFS and extracellular protein concentrate (FX5) of Weissella paramesenteroides | Showed antimicrobial activity against E. coli and S. aureus | [94] |
| Soybeans | Peptide from Enterococcus mundtii ST4V | Peptide inhibited growth of E. faecalis, Streptococcus spp., P. aeruginosa, Klebsiella pneumoniae, S. pneumoniae, and S. aureus | [99] |
| Peptide concentration (40 and 400 μg/mL) | At 400 μg/mL (% inhibition): HSV-1 (strain F): 99.99% HSV-2 (strain G): 99.98% Measles virus (strain MV/BRAZIL/001/91, an attenuated strain of MV): 95.5% Polio virus (PV3, strain Sabin): 50% | ||
| Leaves, flowers, and roots of Herniaria glabra L. | Enterococcus mundtii | ZOI (mm, 24, 48, and 72 h) B. subtilis: 11–14 mm Klebsiella pneumoniae: 9–13 mm Yersinia pseudotuberculosis: bacteriostatic activity | [100] |
| LAB from jalapeno peppers | Different strains of Enterococcus lactis | Antimicrobial activity of heat-resistant bacteriocin-like component from strain 67 against L. monocytogenes ATCC 7644, S. aureus ATCC 6538, E. coli O157:H7 K3999, and S. Typhimurium | [102] |
| Assam tea plants (Camellia sinensis var. assamica) | B. clausii, B. subtilis ML066-3, B. licheniformis ML071-1, ML073-1, ML075-1, ML076-2, and B. siamensis ML122-2, ML123-1, ML124-1 | Showed activity against S. aureus ATCC 25923, MRSA DMST 20625 B. cereus TISTR 687, and E. coli O157:H7 DMST 12743 | [105] |
| Wild Bromelia sp. flowers | B. subtilis Fa17.2 | Inhibited foodborne pathogens: S. aureus, E. coli, Shigella dysenteriae, and Kosaconia cowanii | [106] |
| Nectar of Butea monosperma flower | Fructobacillus fructosus MCC 3996 | Antagonistic activity against E. coli (NCIM 2109), S. aureus (NCIM 2079), S. Typhimurium (NCIM 2501), Proteus vulgaris (NCIM 2172), B. pumilus (NCIM 2327), and P. aeruginosa (NCIM 2036) | [107] |
| Orange juice | F. tropaeoli KKP 3032 | CFS of KKP 3032 inhibited E. coli (KKP 987), L. monocytogenes (KKP 1058), P. aeruginosa (KKP 994), S. aureus (KKP 995), Salmonella enterica (KKP 1044), and B. cereus (KKP 358) | [108] |
4.5. Bacillus
4.6. Fructobacillus
4.7. Multi-Strain LAB Isolations
| Isolation Source | LAB Strains | Main Findings | Ref. |
|---|---|---|---|
| Fresh papaya leaves | Lactiplantibacillus paraplantarum P01 | All strains showed antagonistic activity against E. coli, B. cereus, L. monocytogenes, and S. aureus | [122] |
| Yam | Weissella (W.) paramesenteroides Y05 | ||
| Taro | Lactiplantibacillus plantarum T03 | ||
| Sugarcane | E. faecalis S02 | ||
| Cassava | W. paramesenteroides C04 | ||
| Grass silage | Lactiplantibacillus plantarum SBR64.1, SBR64.2, SBR64.5, SBR64.7, SBR64.12 | Antagonistic activity against pathogenic and spoilage microorganisms: E. coli, Klebsiella sp., Pseudomonas sp., Staphylococcus sp., Shigella sp., Salmonella sp., and Proteus mirabilis | [123] |
| Alfalfa silage | Lactiplantibacillus pentosus SA64.2 P. acidilactici SA64.6 | ||
| Elephant grass silage | Lacticaseibacillus paracasei SCE50.5 | ||
| Peanut silage | Lacticaseibacillus zeae SAM50.5 | ||
| Sorghum silage | Lentilactobacillus buchneri SS50.1, SS50.4 Limosilactobacillus fermentum SS50.9, SS50.10 | ||
| Two grape varieties (cardinal and red globe) | P. pentosaceus RG7B, C11C Lactiplantibacillus plantarum RG8A | RG7B, C11C, and RG8A showed antifungal activities against Aspergillus niger and A. carbonarius | [125] |
| Fresh vegetables (French beans, cauliflower, gherkins, fenugreek, bitter gourd, cluster beans, tomato, ridged gourd, and bottle gourd) | Twenty-five LAB isolates: Lactobacillus sp. LAB8V, LAB6V, J122V, J129V, J131V, ID12V, ID7V, ID13V and others Enterococcus sp. ID8V, ID11V, ID18V, ID19V, and AGIV Weissella sp. ID10V | Cell-free supernatant (CFS) produced bacteriocin- and bacteriocin-like substances. Extracts showed activity against human pathogens: E. coli, K. pneumoniae, Staphylococcus epidermidis, B. cereus, Citrobacter freundii, and Enterobacter cloaceae | [128] |
| Fresh fruits (banana, Chinese peach, and kiwi fruit) and flowers (narcissus, pink rose, yellow rose, and sunflower) | Fructobacillus pseudoficulneus JNGBKS, JNGBKS3, F. fructosus JNGBKS2, JNGBKS4, F. durionis JNGBKS5, and Lactobacillus kunkeei JNGBKS6, JNGBKS7, JNGBKS8 | The fructophilic LAB strains inhibited E. coli, S. Typhimurium, and S. aureus pathogens | [130] |
| Corn stover silage | Lactiplantibacillus plantarum subsp. plantarum ZZU 204, 273, 274, 278, 203 283, and 299, P. pentosaceus ZZU 64, 223, E. mundtii ZZU 205, W. cibaria ZZU 50, and Leu. pseudomesenteroides ZZU 170 | Isolated LAB species inhibited Salmonella enterica, Micrococcus luteus, and E. coli | [131] |
| Cilantro and cantaloupe melons | P. pentosaceus CM175 and Latilactobacillus graminis C15 | CFS displayed antagonistic activity against S. Typhimurium, Salmonella Saintpaul, S. aureus, L. monocytogenes, and E. coli O157:H7 | [132] |
| Donax canniformis, Dysoxylum parasiticum, Tabernaemontana aurantiaca, Ficus arfakensis, Galearia celebica, Pinanga sp., Lasianthus sp., Dracaena angustifolia, and Myristica subalulata | Lactococcus lactis HM1.1 HM1.2, H12.1, H12.2, HM7, H10.1, H10.2, H3.1, and H3.2 | All isolated strains displayed antagonistic activity against E. coli InaCC B5, Mycobacterium smegmatis NBRC 3082, and S. aureus InaCC B4 | [133] |
| Capparis sp. | W. confusa H14.2 | ||
| Syzygium sp. | Lactococcus garvieae H9.1 | ||
| Tetrastigma papillosum | Enterococcus faecalis H4.1 | ||
| Cordyline sp. and Helicia moluccana | W. oryzae H13.2, H11.2 | ||
| Bell pepper | Leu. mesenteroides PIM5 | CFS displayed antimicrobial activity against Gram-positive bacteria (B. cereus, L. monocytogenes), LAB (L. lactis, L. casei), molds (A. niger, F. oxysporum, and P. expansum), and yeasts (Candida albicans, C. tropicalis, and S. cerevisiae). No activity against Gram-negative bacteria | [134] |
| Zucchini | Leu. mesenteroides CAL14 | ||
| Tangerine | Leu. mesenteroides MAD3 | ||
| Guava | Leu. mesenteroides GUA13 | ||
| Cucumber | Leu. mesenteroides PEP12 | ||
| Cucumber | Enterococcus faecium PEP11 | ||
| Bell pepper | E. faecium PIM4 | ||
| Corn | Enterococcus mundtii ELO8 | ||
| G. tomato | E. mundtii TOV9 | ||
| Orange | E. mundtii NAR1 | ||
| Red apple | E. mundtii MR15 | ||
| Jalapeño | E. mundtii JAV15 | ||
| Açai fruits | Lactiplantibacillus plantarum B144, B143, B142, B141, B140, B135, B150, Z183, and Z170 and P. pentosaceus C21, B134, B125, C52, B139, B137, B109, B113, and B138 | Twenty-seven strains showed antagonistic activity against E. coli, S. Typhimurium, E. faecalis, and S. aureus. Strain C52 had no activity against E. coli | [135] |
| Bacupari-do-cerrado, gabiroba (M1, M2), guapeva, pequi peel (M1, M2, M3), pequi mesocarp (M1, M2, M3), mangaba, and puç’a (M1, M2, M3) | Lactiplantibacillus plantarum, L. pentosus, Lacticaseibacillus casei, Lacticaseibacillus paracasei, P. acidilactici, W. cibaria, and W. confusa | Eleven isolates showed activity against E. coli, S. aureus, and Salmonella sp. | [136] |
| Tomato, peach, cucumber, strawberry, cabbage, lettuce, and parsley | Enterococcus faecium F1, F13, F15, F18, F25, F31, and F37, E. durans F23, F26, F40, F41, and F43, E. faecalis F46, E. lactis F8, P. acidilactici F21, F28, Both heat-killed and probiotic LAB | Both LAB and heat-killed bacteria inhibited growth of E. coli, S. aureus, S. Typhi, and L. monocytogenes | [137] |
5. Challenges and Regulatory Considerations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Lee, S.; Park, Y.-S. Molecular typing tools for identifying and characterizing lactic acid bacteria: A review. Food Sci. Biotechnol. 2020, 29, 1301–1318. [Google Scholar] [CrossRef]
- da Silva, T.F.; Glória, R.d.A.; Americo, M.F.; Freitas, A.d.S.; de Jesus, L.C.L.; Barroso, F.A.L.; Laguna, J.G.; Coelho-Rocha, N.D.; Tavares, L.M.; le Loir, Y.; et al. Unlocking the Potential of Probiotics: A Comprehensive Review on Research, Production, and Regulation of Probiotics. Probiotics Antimicrob. Proteins 2024, 16, 1687–1723. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo[a]pyrene: A review. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Venmathi Maran, B.A.; Rajendran, R.L.; Jogalekar, M.P.; Gurunagarajan, S.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.-C. An update on the effectiveness of probiotics in the prevention and treatment of cancer. Life 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.B.; Almada, C.N.; Pereira, E.P.R.; Ferreira, B.M.; Balthazar, C.F.; Khorshidian, N.; Rocha, R.S.; Xavier-Santos, D.; Cruz, A.G.; Ranadheera, C.S.; et al. Review—Sporeforming probiotic bacteria: Characteristics, health benefits, and technological aspects for their applications in foods and beverages. Trends Food Sci. Technol. 2023, 138, 453–469. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-generation probiotics as novel therapeutics for improving human health: Current trends and future perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Lin, T.-L.; Shu, C.-C.; Lai, W.-F.; Tzeng, C.-M.; Lai, H.-C.; Lu, C.-C. Investiture of next generation probiotics on amelioration of diseases–Strains do matter. Med. Microecol. 2019, 1, 100002. [Google Scholar] [CrossRef]
- Grand View Research. Probiotics Market Size, Share & Trends Analysis Report by Product (Food & Beverages, Dietary Supplements), by Ingredient (Bacteria, Yeast), by Distribution Channel, by End Use, by Region, and Segment Forecasts, 2023–2030; Grand View Research: San Francisco, CA, USA, 2023. [Google Scholar]
- Liang, D.; Wu, F.; Zhou, D.; Tan, B.; Chen, T. Commercial probiotic products in public health: Current status and potential limitations. Crit. Rev. Food Sci. Nutr. 2024, 64, 6455–6476. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, H.; Zhong, H.; Zhao, X.; Li, C.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; Yang, J. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: A double blinded placebo controlled randomized study. Gut Microbes 2022, 14, 2003176. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Park, S.-J.; Sharma, A.; Lee, H.-J. Postbiotics against obesity: Perception and overview based on pre-clinical and clinical studies. Int. J. Mol. Sci. 2023, 24, 6414. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Park, Y.-S.; Kang, D.-K.; Paik, H.-D. Paraprobiotics: Definition, manufacturing methods, and functionality. Food Sci. Biotechnol. 2023, 32, 1981–1991. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Z.; Gao, Y.; Yang, G.; Liu, X.; Huang, R.; Liang, W.; Li, S. Assessment of autochthonous lactic acid bacteria as starter culture for improving traditional Chinese Dongbei Suancai fermentation. LWT 2023, 178, 114615. [Google Scholar] [CrossRef]
- Valan Arasu, M.; Jung, M.W.; Ilavenil, S.; Jane, M.; Kim, D.H.; Lee, K.D.; Park, H.S.; Hur, T.Y.; Choi, G.J.; Lim, Y.C. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013, 115, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, A.; Lee, H.-J. Antioxidant potential of exopolysaccharides from lactic acid bacteria: A comprehensive review. Int. J. Biol. Macromol. 2024, 281, 135536. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Sardi, J.d.C.O.; Pitangui, N.d.S.; Roque, S.M.; Silva, A.C.B.d.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Daba, G.M.; Elkhateeb, W.A. Ribosomally synthesized bacteriocins of lactic acid bacteria: Simplicity yet having wide potentials—A review. Int. J. Biol. Macromol. 2024, 256, 128325. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Probiotics and Their Antimicrobial Effect. Microorganisms 2023, 11, 528. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-K.; Son, S.-H.; Jeon, E.B.; Jung, G.H.; Lee, J.-Y.; Paik, H.-D. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods 2015, 14, 513–518. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W. Probiotics: From isolation to application. J. Am. Coll. Nutr. 2017, 36, 666–676. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal activities by Lactobacillus species: An update on mechanisms of action. Front. Oral Health 2021, 2, 689382. [Google Scholar] [CrossRef]
- Gratia, A. Sur un remarquable exemple d’antagonisme entre deux souches de coilbacille. CR Seances Soc. Biol. Fil. 1925, 93, 1040–1041. [Google Scholar]
- Jacob, F.; Lwoff, A.; Siminovitch, A.; Wollman, E. Definition of some terms relative to lysogeny. Ann. Inst. Pasteur. 1953, 84, 222–224. [Google Scholar]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Labib, B.A.; Chigbu, D.I. Clinical management of herpes simplex virus keratitis. Diagnostics 2022, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Ghrairi, T.; Hani, K. Enhanced bactericidal effect of enterocin A in combination with thyme essential oils against L. monocytogenes and E. coli O157:H7. J. Food Sci. Technol. 2015, 52, 2148–2156. [Google Scholar] [CrossRef]
- Cheruvari, A.; Kammara, R. Bacteriocins future perspectives: Substitutes to antibiotics. Food Control 2025, 168, 110834. [Google Scholar] [CrossRef]
- Fang, H.-R.; Zhang, G.-Q.; Cheng, J.-Y.; Li, Z.-Y. Efficacy of Lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: A meta-analysis of randomized controlled trials. Eur. J. Pediatr. 2019, 178, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, S.-H.; Ko, Y.; Sim, J.-H.; Kim, K.S.; Lee, S.-H.; Park, S.; Kim, Y.J. Effect of bacteriocin produced by Lactococcus sp. HY 449 on skin-inflammatory bacteria. Food Chem. Toxicol. 2006, 44, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.A.C.; Domínguez, J.M.; Converti, A.; de Souza Oliveira, R.P. Production of bacteriocin-like inhibitory substance by Bifidobacterium lactis in skim milk supplemented with additives. J. Dairy Res. 2015, 82, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- León Peláez, A.M.; Serna Cataño, C.A.; Quintero Yepes, E.A.; Gamba Villarroel, R.R.; De Antoni, G.L.; Giannuzzi, L. Inhibitory activity of lactic and acetic acid on Aspergillus flavus growth for food preservation. Food Control 2012, 24, 177–183. [Google Scholar] [CrossRef]
- Denkova, R.; Goranov, B.; Teneva, D.; Denkova, Z.; Kostov, G. Antimicrobial activity of probiotic microorganisms: Mechanisms of interaction and methods of examination. Antimicrob. Res. Nov. Bioknowledge Educ. Programs 2017, 1, 201–212. [Google Scholar]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLOS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Hu, Q.; Luo, Z.; Ban, Z.; Li, L. Hydrogen peroxide from traditional sanitizer to promising disinfection agent in food industry. Food Rev. Int. 2024, 40, 658–690. [Google Scholar] [CrossRef]
- Otero, M.C.; Nader-Macías, M.E. Inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus gasseri isolated from the vaginal tract of cattle. Anim. Reprod. Sci. 2006, 96, 35–46. [Google Scholar] [CrossRef]
- Hertzberger, R.; Arents, J.; Dekker, H.L.; Pridmore, R.D.; Gysler, C.; Kleerebezem, M.; de Mattos, M.J.T. H2O2 production in species of the Lactobacillus acidophilus group: A central role for a novel NADH-dependent flavin reductase. Appl. Environ. Microbiol. 2014, 80, 2229–2239. [Google Scholar] [CrossRef]
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Landete, J.M.; Medina, M.; Arqués, J.L. Short communication: Combined antimicrobial activity of reuterin and diacetyl against foodborne pathogens. J. Dairy Sci. 2014, 97, 6116–6121. [Google Scholar] [CrossRef]
- Hugenholtz, J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 165–178. [Google Scholar] [CrossRef]
- Naidu, A.; Bidlack, W.; Clemens, R. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 1999, 39, 13–126. [Google Scholar] [CrossRef]
- Ray, B. Diacetyl of lactic acid bacteria as a food biopreservative. In Food Biopreservatives of Microbial Origin; CRC Press: Boca Raton, FL, USA, 2019; pp. 137–153. [Google Scholar]
- Asare, P.T.; Zurfluh, K.; Greppi, A.; Lynch, D.; Schwab, C.; Stephan, R.; Lacroix, C. Reuterin demonstrates potent antimicrobial activity against a broad panel of human and poultry meat Campylobacter spp. isolates. Microorganisms 2020, 8, 78. [Google Scholar] [CrossRef]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L.; Chung, T.; Dobrogosz, W.; Lindgren, S. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 1989, 2, 131–136. [Google Scholar] [CrossRef]
- Talarico, T.L.; Dobrogosz, W.J. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1989, 33, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Debonne, E.; Vermeulen, A.; Bouboutiefski, N.; Ruyssen, T.; Van Bockstaele, F.; Eeckhout, M.; Devlieghere, F. Modelling and validation of the antifungal activity of DL-3-phenyllactic acid and acetic acid on bread spoilage moulds. Food Microbiol. 2020, 88, 103407. [Google Scholar] [CrossRef]
- Ponzio, A.; Rebecchi, A.; Zivoli, R.; Morelli, L. Reuterin, Phenyllactic Acid, and Exopolysaccharides as Main Antifungal Molecules Produced by Lactic Acid Bacteria: A Scoping Review. Foods 2024, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Šušković, J.; Kos, B.; Beganović, J.; Leboš Pavunc, A.; Habjanič, K.; Matošić, S. Antimicrobial activity–the most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Tiwari, S.; Shah, I.A.; Devi, P.B.; Delattre, C.; Reddy, G.B.; Shetty, P.H. Antipathogenic potentials of exopolysaccharides produced by lactic acid bacteria and their food and health applications. Food Control 2023, 152, 109850. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Meriluoto, J.; Salminen, S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, A.; Bagchi, T. The expression of adhesin EF-Tu in response to mucin and its role in Lactobacillus adhesion and competitive inhibition of enteropathogens to mucin. J. Appl. Microbiol. 2013, 115, 546–554. [Google Scholar] [CrossRef]
- Van Zyl, W.; Deane, S.; Dicks, L. Bacteriocin production and adhesion properties as mechanisms for the anti-listerial activity of Lactobacillus plantarum 423 and Enterococcus mundtii ST4SA. Benef. Microbes 2019, 10, 329–349. [Google Scholar] [CrossRef]
- Heinemann, C.; van Hylckama Vlieg, J.E.T.; Janssen, D.B.; Busscher, H.J.; van der Mei, H.C.; Reid, G. Purification and characterization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiol. Lett. 2000, 190, 177–180. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yang, Y.-r.; Cheng, G.; Yang, Z.-q. Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yuan, L.; Deng, J.; Yang, Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front. Cell. Infect. Microbiol. 2015, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Faas, M.M.; Zhang, H.; de Vos, P. Protective effects of lactic acid bacteria on gut epithelial barrier dysfunction are Toll like receptor 2 and protein kinase C dependent. Food Funct. 2020, 11, 1230–1234. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Bonavida, B. Activation of natural killer cells by probiotics. Onco Ther. 2016, 7, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ramírez, L.; Pérez-Solano, R.; Castañón-Alonso, S.; Moreno Guerrero, S.; Ramírez Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic significance of Lactobacillus strains: A comprehensive review on health impacts, research gaps, and future prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and probiosis: Antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef]
- Soltani, N.; Abbasi, S.; Baghaeifar, S.; Taheri, E.; Farhoudi Sefidan Jadid, M.; Emami, P.; Abolhasani, K.; Aslanshirzadeh, F. Antibacterial and antibiofilm activity of Lactobacillus strains secretome and extraction against Escherichia coli isolated from urinary tract infection. Biotechnol. Rep. 2022, 36, e00760. [Google Scholar] [CrossRef]
- Mörschbächer, A.P.; Granada, C.E. Mapping the worldwide knowledge of antimicrobial substances produced by Lactobacillus spp.: A bibliometric analysis. Biochem. Eng. J. 2022, 180, 108343. [Google Scholar] [CrossRef]
- Garcia, E.F.; Luciano, W.A.; Xavier, D.E.; da Costa, W.C.; de Sousa Oliveira, K.; Franco, O.L.; de Morais Junior, M.A.; Lucena, B.T.; Picao, R.C.; Magnani, M. Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected Lactobacillus strains. Front. Microbiol. 2016, 7, 1371. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, T.M.R.; Garcia, E.F.; de Oliveira Araújo, A.; Magnani, M.; Saarela, M.; de Souza, E.L. In vitro characterization of Lactobacillus strains isolated from fruit processing by-products as potential probiotics. Probiotics Antimicrob. Proteins 2018, 10, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Saini, P.; Puranik, V.; Gupta, S.; Dubey, S. Optimization and characterization of exopolysaccharides produced by lactobacillus strains isolated from cabbage and cucumber. J. Microbiol. Biotechnol. Res 2016, 6, 27–35. [Google Scholar]
- Noda, M.; Danshiitsoodol, N.; Sakaguchi, T.; Kanno, K.; Sugiyama, M. Exopolysaccharide produced by plant-derived Lactobacillus plantarum SN35N exhibits antiviral activity. Biol. Pharm. Bull. 2021, 44, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, L.G.; Baker, B.A.A.; Kadhem, B.M.; Mahdi, L.H.; Mater, H.N. Exopolysaccharide as antiviral, antimicrobial and as immunostimulants: A review. Plant Arch 2020, 20, 5859–5875. [Google Scholar]
- Vijayakumar, M.; Ilavenil, S.; Kim, D.H.; Arasu, M.V.; Priya, K.; Choi, K.C. In-vitro assessment of the probiotic potential of Lactobacillus plantarum KCC-24 isolated from Italian rye-grass (Lolium multiflorum) forage. Anaerobe 2015, 32, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, N.; Gupta, D.; Lee, H.-J.; Park, Y.-S. Comparative genome analysis of four Leuconostoc strains with a focus on carbohydrate-active enzymes and oligosaccharide utilization pathways. Comput. Struct. Biotechnol. J. 2022, 20, 4771–4785. [Google Scholar] [CrossRef]
- Ogier, J.-C.; Casalta, E.; Farrokh, C.; Saïhi, A. Safety assessment of dairy microorganisms: The Leuconostoc genus. Int. J. Food Microbiol. 2008, 126, 286–290. [Google Scholar] [CrossRef]
- Rao, W.; Fang, Z.; Chen, Z.; Wu, J.; Fang, X. Antibacterial mechanism of metabolites of Leuconostoc mesenteroides against Serratia liquefaciens. LWT 2023, 187, 115335. [Google Scholar] [CrossRef]
- Toushik, S.H.; Park, J.-H.; Kim, K.; Ashrafudoulla, M.; Ulrich, M.S.I.; Mizan, M.F.R.; Roy, P.K.; Shim, W.-B.; Kim, Y.-M.; Park, S.H. Antibiofilm efficacy of Leuconostoc mesenteroides J. 27-derived postbiotic and food-grade essential oils against Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Escherichia coli alone and in combination, and their application as a green preservative in the seafood industry. Food Res. Int. 2022, 156, 111163. [Google Scholar] [CrossRef]
- Su, H.; Guo, Y.; Cheng, H.; Hu, S.; Zhang, P.; Yang, Z. Probiotic and fermentation properties of Leuconostoc mesenteroides strain I1/53 from sugarcane juice by a multi-omics approach. LWT 2024, 211, 116897. [Google Scholar] [CrossRef]
- Diana, C.-R.; Humberto, H.-S.; Jorge, Y.F. Probiotic properties of Leuconostoc mesenteroides isolated from aguamiel of Agave salmiana. Probiotics Antimicrob. Proteins 2015, 7, 107–117. [Google Scholar] [CrossRef]
- Chakchouk-Mtibaa, A.; Mechri, S.; Cheffi Azabou, M.; Triki, M.A.; Smaoui, S.; Mellouli, L. The novel bacteriocin BacYB1 produced by Leuconostoc mesenteroides YB1: From recent analytical characterization to biocontrol Verticillium dahliae and Agrobacterium tumefaciens. Microb. Pathog. 2024, 192, 106680. [Google Scholar] [CrossRef]
- Sabri, M.; Habbadi, K.; Achbani, E.H.; Benkirane, R.; El Handi, K.; Ou-Zine, M.; Benali, T.; Elbeaino, T. Antagonistic effect of Leuconostoc mesenteroides on grapevine crown gall and fire blight. J. Crop Improv. 2023, 37, 431–446. [Google Scholar] [CrossRef]
- Muthusamy, K.; Han, H.-S.; Soundharrajan, I.; Jung, J.-S.; Valan Arasu, M.; Choi, K.-C. A Novel Strain of Probiotic Leuconostoc citreum Inhibits Infection-Causing Bacterial Pathogens. Microorganisms 2023, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-s.; Wu, H.-c.; Kuo, C.-y.; Chen, Y.-w.; Ho, S.; Yanagida, F. Leucocin C-607, a Novel Bacteriocin from the Multiple-Bacteriocin-Producing Leuconostoc pseudomesenteroides 607 Isolated from Persimmon. Probiotics Antimicrob. Proteins 2018, 10, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Montemurro, M.; Chieffi, D.; Cho, G.-S.; Franz, C.M.; Dell’Aquila, A.; Rizzello, C.G.; Fusco, V. Novel insights into the phylogeny and biotechnological potential of Weissella species. Front. Microbiol. 2022, 13, 914036. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Montemurro, M.; Rizzello, C.G.; Franz, C.M. The Weissella and Periweissella genera: Up-to-date taxonomy, ecology, safety, biotechnological, and probiotic potential. Front. Microbiol. 2023, 14, 1289937. [Google Scholar] [CrossRef]
- Pabari, K.; Pithva, S.; Kothari, C.; Purama, R.K.; Kondepudi, K.K.; Vyas, B.R.M.; Kothari, R.; Ambalam, P. Evaluation of probiotic properties and prebiotic utilization potential of Weissella paramesenteroides isolated from fruits. Probiotics Antimicrob. Proteins 2020, 12, 1126–1138. [Google Scholar] [CrossRef]
- Micallef, S.A.; Goldstein, R.E.R.; George, A.; Ewing, L.; Tall, B.D.; Boyer, M.S.; Joseph, S.W.; Sapkota, A.R. Diversity, distribution and antibiotic resistance of Enterococcus spp. recovered from tomatoes, leaves, water and soil on US Mid-Atlantic farms. Food Microbiol. 2013, 36, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns—An update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Choeisoongnern, T.; Sirilun, S.; Waditee-Sirisattha, R.; Pintha, K.; Peerajan, S.; Chaiyasut, C. Potential Probiotic Enterococcus faecium OV3-6 and Its Bioactive Peptide as Alternative Bio-Preservation. Foods 2021, 10, 2264. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Wachsman, M.B.; Knoetze, H.; Meincken, M.; Dicks, L.M. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soya beans. Int J Antimicrob Agents 2005, 25, 508–513. [Google Scholar] [CrossRef]
- Fidan, A.; Ugras, S. First isolation of a probiotic candidate Enterococcus mundtii from Herniaria glabra L. and evaluation of its wound healing activity. FEMS Microbiol. Lett. 2023, 370, fnad083. [Google Scholar] [CrossRef]
- Nawaz, F.; Khan, M.N.; Javed, A.; Ahmed, I.; Ali, N.; Ali, M.I.; Bakhtiar, S.M.; Imran, M. Genomic and functional characterization of Enterococcus mundtii QAUEM2808, isolated from artisanal fermented milk product dahi. Front. Microbiol. 2019, 10, 434. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, E.; Peña-Ramos, E.A.; Juneja, V.K.; Martínez-Téllez, M.Á.; González-Ríos, H.; Paredes-Aguilar, M.d.l.C.; Valenzuela-Melendres, M.; Aispuro-Hernández, E. Antagonistic Activity of Bacteriocin-like Inhibitory Substances from Enterococcus lactis Isolated from the Surface of Jalapeno Pepper against Foodborne Pathogens. Microbiol. Res. 2024, 15, 889–899. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Huycke, M.M. Risks associated with enterococci as probiotics. Food Res. Int. 2020, 129, 108788. [Google Scholar] [CrossRef] [PubMed]
- Oruc, O.; Cetin, O.; Onal Darilmaz, D.; Yüsekdag, Z.N. Determination of the biosafety of potential probiotic Enterococcus faecalis and Enterococcus faecium strains isolated from traditional white cheeses. LWT 2021, 148, 111741. [Google Scholar] [CrossRef]
- Rungsirivanich, P.; Supandee, W.; Futui, W.; Chumsai-Na-Ayudhya, V.; Yodsombat, C.; Thongwai, N. Culturable bacterial community on leaves of Assam tea (Camellia sinensis var. assamica) in Thailand and human probiotic potential of isolated Bacillus spp. Microorganisms 2020, 8, 1585. [Google Scholar] [CrossRef]
- Tenea, G.N.; Gonzalez, G.L.; Moreno, J.L. Probiotic Characteristics and Antimicrobial Potential of a Native Bacillus subtilis Strain Fa17.2 Rescued from Wild Bromelia sp. Flowers. Microorganisms 2022, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Jadhav, A.; Patil, U. Functional characterization and in vitro screening of Fructobacillus fructosus MCC 3996 isolated from Butea monosperma flower for probiotic potential. Lett. Appl. Microbiol. 2020, 70, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczuk-Szczyrba, A.; Wojtczak, A.; Kieliszek, M.; Sokołowska, B. Characteristics and in vitro properties of potential probiotic strain Fructobacillus tropaeoli KKP 3032 isolated from orange juice. Folia Microbiol. 2024, 1–18. [Google Scholar] [CrossRef]
- Kotb, E. Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK-07 isolated from kishk, a traditional Egyptian fermented food. Appl. Biochem. Microbiol. 2015, 51, 34–43. [Google Scholar] [CrossRef]
- Payne, J.; Bellmer, D.; Jadeja, R.; Muriana, P. The Potential of Bacillus Species as Probiotics in the Food Industry: A Review. Foods 2024, 13, 2444. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Y.; Ismail, B.B.; Muhammad, A.I.; Li, G.; Liu, D. Bacillus spore germination: Mechanisms, identification, and antibacterial strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 11146–11160. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-K.; Kim, W.-S.; Paik, H.-D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Shobharani, P.; Padmaja, R.J.; Halami, P.M. Diversity in the antibacterial potential of probiotic cultures Bacillus licheniformis MCC2514 and Bacillus licheniformis MCC2512. Res. Microbiol. 2015, 166, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wong-Chew, R.M.; de Castro, J.-A.A.; Morelli, L.; Perez, M.; Ozen, M. Gut immune homeostasis: The immunomodulatory role of Bacillus clausii, from basic to clinical evidence. Expert Rev. Clin. Immunol. 2022, 18, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Freedman, K.E.; Hill, J.L.; Wei, Y.; Vazquez, A.R.; Grubb, D.S.; Trotter, R.E.; Wrigley, S.D.; Johnson, S.A.; Foster, M.T.; Weir, T.L. Examining the gastrointestinal and immunomodulatory effects of the novel probiotic Bacillus subtilis DE111. Int. J. Mol. Sci. 2021, 22, 2453. [Google Scholar] [CrossRef] [PubMed]
- Safronova, L.S.; Skorochod, I.A.; Ilyash, V.M. Antioxidant and Antiradical Properties of Probiotic Strains Bacillus amyloliquefaciens ssp. plantarum. Probiotics Antimicrob. Proteins 2021, 13, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yu, T.; Yan, F. Probiotic role and application of thermophilic Bacillus as novel food materials. Trends Food Sci. Technol. 2023, 138, 1–15. [Google Scholar] [CrossRef]
- Endo, A.; Tanizawa, Y.; Tanaka, N.; Maeno, S.; Kumar, H.; Shiwa, Y.; Okada, S.; Yoshikawa, H.; Dicks, L.; Nakagawa, J.; et al. Comparative genomics of Fructobacillus spp. and Leuconostoc spp. reveals niche-specific evolution of Fructobacillus spp. BMC Genom. 2015, 16, 1117. [Google Scholar] [CrossRef]
- Endo, A.; Maeno, S.; Tanizawa, Y.; Kneifel, W.; Arita, M.; Dicks, L.; Salminen, S. Fructophilic Lactic Acid Bacteria, a Unique Group of Fructose-Fermenting Microbes. Appl. Environ. Microbiol. 2018, 84, e01290-18. [Google Scholar] [CrossRef]
- Endo, A.; Okada, S. Reclassification of the genus Leuconostoc and proposals of Fructobacillus fructosus gen. nov., comb. nov., Fructobacillus durionis comb. nov., Fructobacillus ficulneus comb. nov. and Fructobacillus pseudoficulneus comb. nov. Int. J. Syst. Evol. Microbiol. 2008, 58, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Dicks, L.M. The genus Fructobacillus. In Lactic Acid Bacteria: Biodiversity and Taxonomy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 381–389. [Google Scholar]
- Samedi, L.; Charles, A.L. Isolation and characterization of potential probiotic Lactobacilli from leaves of food plants for possible additives in pellet feeding. Ann. Agric. Sci. 2019, 64, 55–62. [Google Scholar] [CrossRef]
- dos Santos Leandro, E.; Ginani, V.C.; de Alencar, E.R.; Pereira, O.G.; Rose, E.C.P.; do Vale, H.M.M.; Pratesi, R.; Hecht, M.M.; Cavalcanti, M.H.; Tavares, C.S.O. Isolation, identification, and screening of lactic acid bacteria with probiotic potential in silage of different species of forage plants, cocoa beans, and artisanal salami. Probiotics Antimicrob. Proteins 2021, 13, 173–186. [Google Scholar] [CrossRef]
- Divyashree, S.; Shruthi, B.; Vanitha, P.R.; Sreenivasa, M.Y. Probiotics and their postbiotics for the control of opportunistic fungal pathogens: A review. Biotechnol. Rep. 2023, 38, e00800. [Google Scholar] [CrossRef] [PubMed]
- Taroub, B.; Salma, L.; Manel, Z.; Ouzari, H.-I.; Hamdi, Z.; Moktar, H. Isolation of lactic acid bacteria from grape fruit: Antifungal activities, probiotic properties, and in vitro detoxification of ochratoxin A. Ann. Microbiol. 2019, 69, 17–27. [Google Scholar] [CrossRef]
- Ding, L.; Han, M.; Wang, X.; Guo, Y. Ochratoxin A: Overview of Prevention, Removal, and Detoxification Methods. Toxins 2023, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- Obafemi, B.A.; Adedara, I.A.; Rocha, J.B.T. Neurotoxicity of ochratoxin A: Molecular mechanisms and neurotherapeutic strategies. Toxicology 2023, 497–498, 153630. [Google Scholar] [CrossRef] [PubMed]
- Junnarkar, M.; Pawar, S.; Gaikwad, S.; Mandal, A.; Jass, J.; Nawani, N. Probiotic potential of lactic acid bacteria from fresh vegetables: Application in food preservation. Indian J. Exp. Biol. 2019, 57, 825–838. [Google Scholar]
- Jabeen, I.; Islam, S.; Hassan, A.I.; Tasnim, Z.; Shuvo, S.R. A brief insight into Citrobacter species-a growing threat to public health. Front. Antibiot. 2023, 2, 1276982. [Google Scholar] [CrossRef] [PubMed]
- Sakandar, H.A.; Kubow, S.; Sadiq, F.A. Isolation and in-vitro probiotic characterization of fructophilic lactic acid bacteria from Chinese fruits and flowers. LWT 2019, 104, 70–75. [Google Scholar] [CrossRef]
- Li, D.; Ni, K.; Pang, H.; Wang, Y.; Cai, Y.; Jin, Q. Identification and antimicrobial activity detection of lactic acid bacteria isolated from corn stover silage. Asian-Australas. J. Anim. Sci. 2015, 28, 620. [Google Scholar] [CrossRef]
- González-Pérez, C.; Vargas-Arispuro, I.; Aispuro-Hernández, E.; Aguilar-Gil, C.; Aguirre-Guzmán, Y.; Castillo, A.; Hernández-Mendoza, A.; Ayala-Zavala, J.; Martínez-Téllez, M. Potential control of foodborne pathogenic bacteria by Pediococcus pentosaceus and Lactobacillus graminis isolated from fresh vegetables. Microbiol. Biotechnol. Lett. 2019, 47, 183–194. [Google Scholar] [CrossRef]
- Dinoto, A.; Susilo, A.R.P.; Julistiono, H. Isolation, identification and antimicrobial activities of Lactic Acid Bacteria from fruits of wild plants in Tambrauw Forest, West Papua, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 3391–3397. [Google Scholar] [CrossRef]
- Linares-Morales, J.R.; Cuellar-Nevárez, G.E.; Rivera-Chavira, B.E.; Gutiérrez-Méndez, N.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Selection of lactic acid bacteria isolated from fresh fruits and vegetables based on their antimicrobial and enzymatic activities. Foods 2020, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Abe Sato, S.T.; Marques, J.M.; da Luz de Freitas, A.; Sanches Progenio, R.C.; Nunes, M.R.T.; Mota de Vasconcelos Massafra, J.; Gomes Moura, F.; Rogez, H. Isolation and genetic identification of endophytic lactic acid bacteria from the Amazonian açai fruits: Probiotics features of selected strains and their potential to inhibit pathogens. Front. Microbiol. 2021, 11, 610524. [Google Scholar] [CrossRef]
- de Amorim Trindade, D.P.; Barbosa, J.P.; Martins, E.M.F.; Tette, P.A.S. Isolation and identification of lactic acid bacteria in fruit processing residues from the Brazilian Cerrado and its probiotic potential. Food Biosci. 2022, 48, 101739. [Google Scholar] [CrossRef]
- Alameri, F.; Tarique, M.; Osaili, T.; Obaid, R.; Abdalla, A.; Masad, R.; Al-Sbiei, A.; Fernandez-Cabezudo, M.; Liu, S.-Q.; Al-Ramadi, B.; et al. Lactic Acid Bacteria Isolated from Fresh Vegetable Products: Potential Probiotic and Postbiotic Characteristics Including Immunomodulatory Effects. Microorganisms 2022, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, L.; Luo, D.; Soni, V.; Rosenn, E.H.; Wang, Z. Mycobacterium smegmatis, a Promising Vaccine Vector for Preventing TB and Other Diseases: Vaccinomics Insights and Applications. Vaccines 2023, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.A.; Poluektova, E.U.; Belkina, T.V.; Danilenko, V.N. Lactobacilli: Legal Regulation and Prospects for New Generation Drugs. Appl. Biochem. Microbiol. 2022, 58, 652–664. [Google Scholar] [CrossRef]
- Tóth, A.G.; Judge, M.F.; Nagy, S.Á.; Papp, M.; Solymosi, N. A survey on antimicrobial resistance genes of frequently used probiotic bacteria, 1901 to 2022. Eurosurveillance 2023, 28, 2200272. [Google Scholar] [CrossRef]
- Das, D.J.; Shankar, A.; Johnson, J.B.; Thomas, S. Critical insights into antibiotic resistance transferability in probiotic Lactobacillus. Nutrition 2020, 69, 110567. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Hosseini, S.; Pourjafar, H. Postbiotics as dynamic biological molecules for antimicrobial activity: A mini-review. Biointerface Res. Appl. Chem 2022, 12, 6543–6556. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.-M. Nanotechnology: Current uses and future applications in the food industry. 3 Biotech 2018, 8, 74. [Google Scholar] [CrossRef]
- Holkem, A.T.; Silva, M.P.d.; Favaro-Trindade, C.S. Probiotics and plant extracts: A promising synergy and delivery systems. Crit. Rev. Food Sci. Nutr. 2023, 63, 9561–9579. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kitabatake, M.; Kayano, S.-i.; Ito, T. Dietary phenolic compounds: Their health benefits and association with the gut microbiota. Antioxidants 2023, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, S.; Adeyemi, D.E.; Choi, I.Y.; Sultan, G.; Madar, I.H.; Park, M.-K. Comprehensive in silico analysis of lactic acid bacteria for the selection of desirable probiotics. LWT 2020, 130, 109617. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Lee, H.-J. Antimicrobial Activity of Probiotic Bacteria Isolated from Plants: A Review. Foods 2025, 14, 495. https://doi.org/10.3390/foods14030495
Sharma A, Lee H-J. Antimicrobial Activity of Probiotic Bacteria Isolated from Plants: A Review. Foods. 2025; 14(3):495. https://doi.org/10.3390/foods14030495
Chicago/Turabian StyleSharma, Anshul, and Hae-Jeung Lee. 2025. "Antimicrobial Activity of Probiotic Bacteria Isolated from Plants: A Review" Foods 14, no. 3: 495. https://doi.org/10.3390/foods14030495
APA StyleSharma, A., & Lee, H.-J. (2025). Antimicrobial Activity of Probiotic Bacteria Isolated from Plants: A Review. Foods, 14(3), 495. https://doi.org/10.3390/foods14030495







