Insight into the Volatile Profiles and Key Odorants of Rizhao Green Tea by Application of SBSE-GC-MS, OAVs and GC-O Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Samples
2.2. Chemicals
2.3. SBSE Procedure
2.4. Thermal Desorption
2.5. GC-MS Analysis
2.6. Identification and Qualification of the Volatile Compounds in RZT Samples
2.7. OAVs Calculation
2.8. GC-O Analysis
2.9. Statistical Analysis
3. Results and Discussion
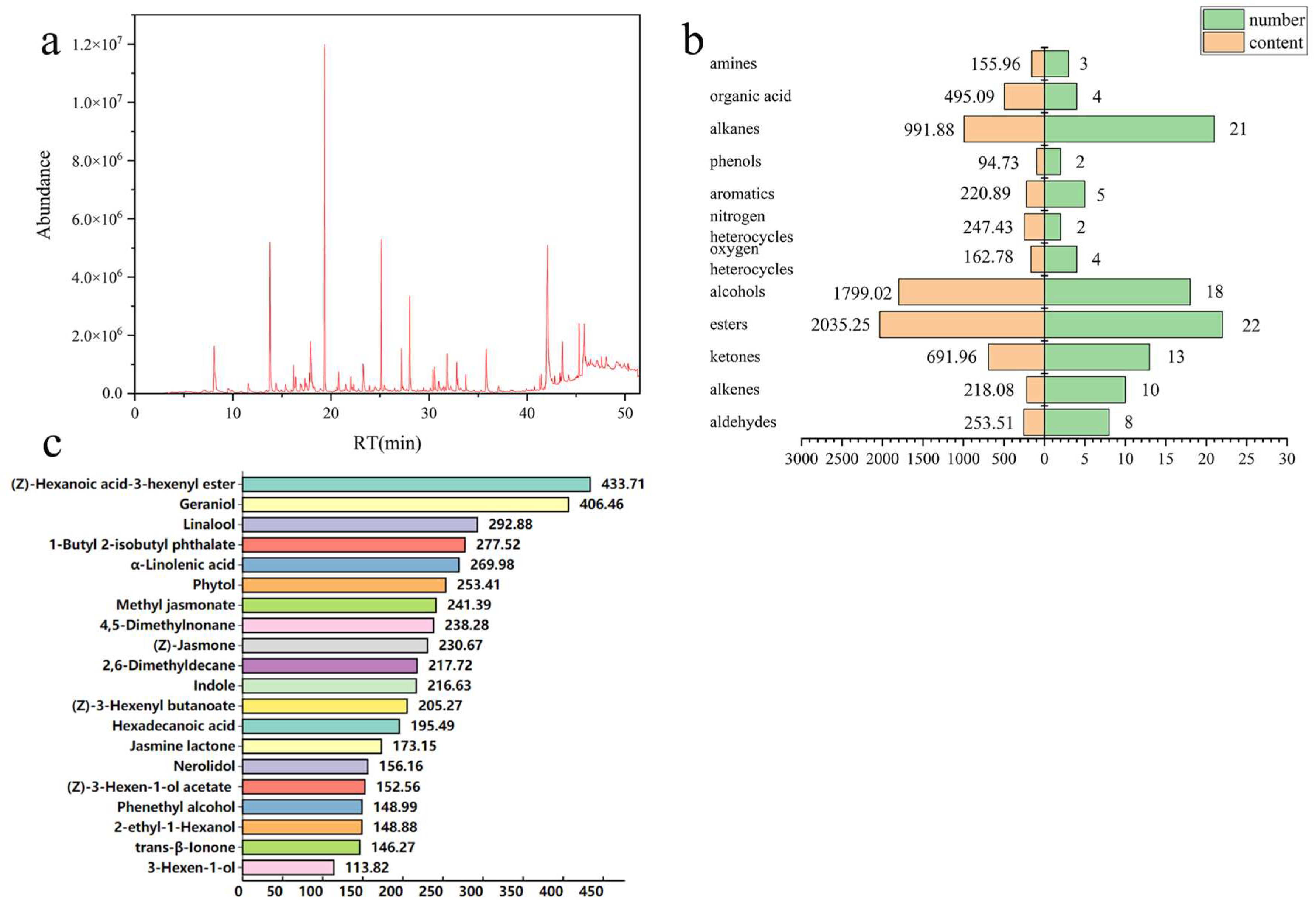
3.1. Characterization of Aroma Compounds in RZT Identified by SBSE-GC-MS
3.2. Key Odorants in RZT Identified by OAV Analysis
3.3. Key Odorants in RZT Identified by GC-O Analysis
3.4. Comparison of the Key Odorants in RZT Screened by OAV and GC-O
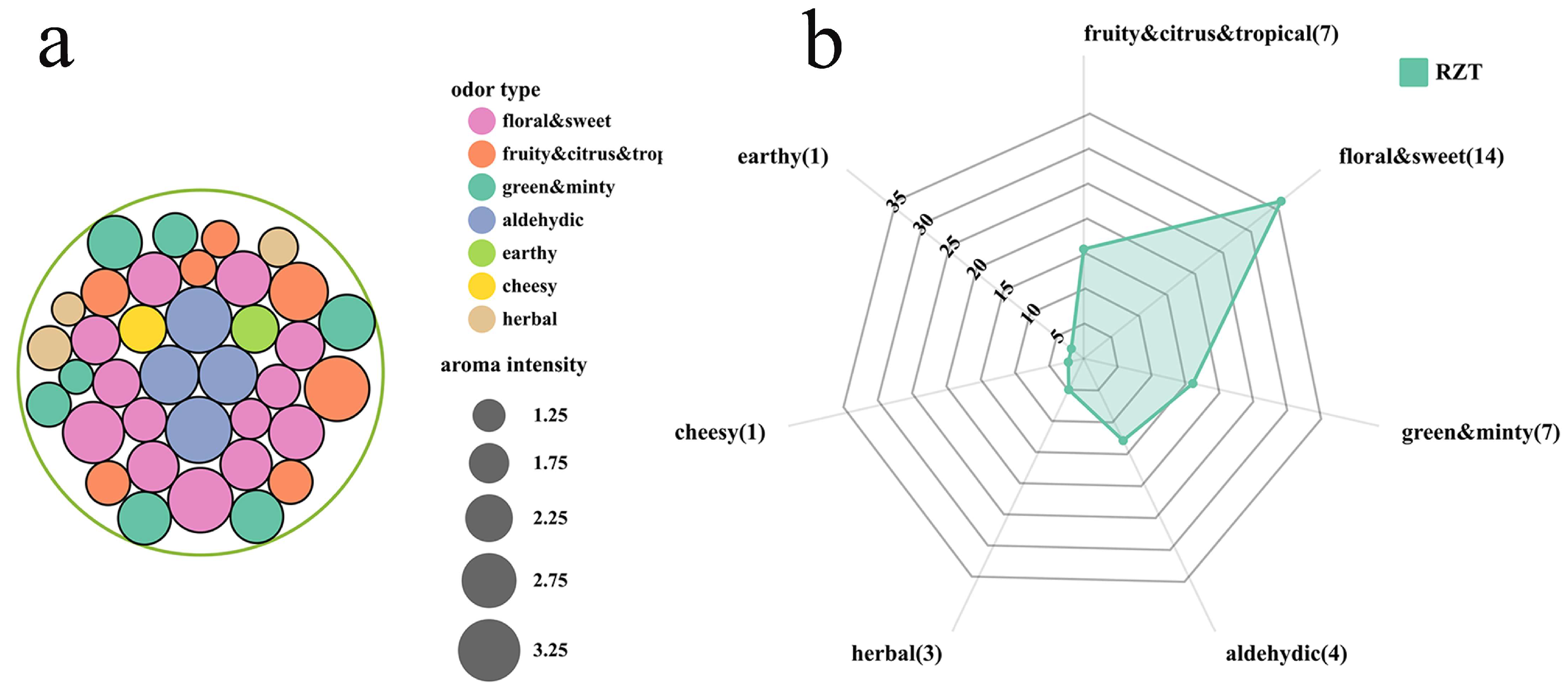
3.5. Establishment of Molecule Aroma Wheel of RZT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Tender | Company | Tea Quality Grade |
|---|---|---|---|
| 1. | One bud and two leaves | GuanQing Tea Technology Co., Ltd. | Super Class |
| 2. | One bud and two leaves | HengShan Tianhu Tea Co., Ltd. | Super Class |
| 3. | One bud and two leaves | HengShan Tianhu Tea Co., Ltd. | First class |
| 4. | One bud and two leaves | BiBo Tea Co., Ltd. | Super Class |
| 5. | One bud and two leaves | BiBo Tea Co., Ltd. | First class |
| 6. | One bud and two leaves | BiBo Tea Co., Ltd. | First class |
| 7. | One bud and two leaves | Lin Yuan Tea Industry Co., Ltd. | Super Class |
| 8. | One bud and two leaves | Lin Yuan Tea Industry Co., Ltd. | First class |
| 9. | One bud and two leaves | MaLing Spring Tea Co., Ltd. | Super Class |
| 10. | One bud and two leaves | MaLing Spring Tea Co., Ltd. | First class |
| 11. | One bud and two leaves | KanHai (Lechun Family Farm) | Super Class |
| 12. | One bud and two leaves | KanHai (Lechun Family Farm) | First class |
| 13. | One bud and two leaves | ShengGu Mountain tea Co., Ltd. | Super Class |
| 14. | One bud and two leaves | ShengGu Mountain tea Co., Ltd. | First class |
| 15. | One bud and two leaves | Liu YuanChun Ecological Agriculture Co., Ltd. | Super Class |
| 16. | One bud and two leaves | Liu YuanChun Ecological Agriculture Co., Ltd. | First class |
| 17. | One bud and two leaves | Lukui Tea Industry Co., Ltd. | Super Class |
| 18. | One bud and two leaves | Lukui Tea Industry Co., Ltd. | First class |
| 19. | One bud and two leaves | Lukui Tea Industry Co., Ltd. | First class |
| 20. | One bud and two leaves | FuYuan Spring Tea Co., Ltd. | Super Class |
| 21. | One bud and two leaves | FuYuan Spring Tea Co., Ltd. | First class |
| 22. | One bud and two leaves | FuLaiQing Group Co., Ltd. | Super Class |
| 23. | One bud and two leaves | RiZhao Royal Bay Tea Expo Garden Co., Ltd. | Super Class |
| 24. | One bud and two leaves | RiZhao Royal Bay Tea Expo Garden Co., Ltd. | First class |
| 25. | One bud and two leaves | RiZhao SongChen Tea Industry Trade Co., Ltd. | Super Class |
| 26. | One bud and two leaves | RiZhao SongChen Tea Industry Trade Co., Ltd. | First class |
| 27. | One bud and two leaves | Rizhao Zhulongshan Green Tea Co., Ltd. | First class |
| 28. | One bud and two leaves | Rizhao Zhongtai Tea Co., Ltd. | First class |
| 29. | One bud and two leaves | RiZhao XinTian Tea Leaf Professional Cooperative | Super Class |
| 30. | One bud and two leaves | RiZhao ShengYe Tea Co., Ltd. | First class |
| 31. | One bud and two leaves | RiZhao City JingYang Green Tea Garden | First class |
References
- Lee, J.E.; Lee, B.J.; Chung, J.O.; Hwang, J.A.; Lee, S.J.; Lee, C.H.; Hong, Y.S. Geographical and climatic dependencies of green tea (Camellia sinensis) metabolites: A 1H NMR-based metabolomics study. J. Agric. Food Chem. 2010, 58, 10582–10589. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.W.; Yin, X.L.; Peng, T.Q.; Pan, Y.; Cui, H.N.; Li, Z.Q.; Sun, W.; Ding, B.; Hu, X.-C.; Zhang, Z.-H.; et al. Geographical origin identification and chemical markers screening of Chinese green tea using two-dimensional fingerprints technique coupled with multivariate chemometric methods. Food Control 2022, 135, 108795. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, D.; Huang, G.; Jiang, X.; Fang, K.; Wang, Q.; Ni, E.; Li, B.; Pan, C.; Li, H.; et al. Identification and characterization of the key volatile flavor compounds in black teas from distinct regions worldwide. J. Food Sci. 2022, 87, 3433–3446. [Google Scholar] [CrossRef]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.; Dai, W.; Guo, L.; Tan, J.; Zhang, Y.; Yu, F.; Shao, C.; Peng, Q.; Lin, Z. Separation of aroma components in Xihu Longjing tea using simultaneous distillation extraction with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Sep. Purif. Technol. 2016, 164, 146–154. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 3, 585–599. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, Y.; Ma, S.; Shi, J.; Yan, H.; Lin, Z.; Lv, H. Aroma characterisation of Liu-pao tea based on volatile fingerprint and aroma wheel using SBSE-GC–MS. Food Chem. 2023, 414, 135739. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Wesselink, W.; de Jong, C. Comparison of the sensitivity of different aroma extraction techniques in combination with gas chromatography–mass spectrometry to detect minor aroma compounds in wine. J. Chromatogr. A 2013, 1272, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Madrera, R.R.; Valles, B.S. Determination of volatile compounds in apple pomace by stir bar sorptive extraction and gas chromatography-mass spectrometry (SBSE-GC-MS). J. Food Sci. 2011, 76, C1326–C1334. [Google Scholar] [CrossRef]
- GB/T 23776–2018; Methodology for Sensory Evaluation of Tea. Standardization Administration of China: Beijing, China, 2018.
- GB/T 14487–2017; Tea Vocabulary for Sensory Evaluation. Standardization Administration of China: Beijing, China, 2017.
- Shi, Y.; Zhu, Y.; Ma, W.; Lin, Z.; Lv, H. Characterisation of the volatile compounds profile of Chinese pan-fried green tea in comparison with baked green tea, steamed green tea, and sun-dried green tea using approaches of molecular sensory science. Cur. Res. Food Sci. 2022, 5, 1098–1107. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Z.; Shao, C.Y.; Zhang, Y.; Lv, H.; Zhang, Z.; Zeng, J.; Peng, Q.; Zhu, Y.; Lin, Z. Aromatic profiles and enantiomeric distributions of chiral odorants in baked green teas with different picking tenderness. Food Chem. 2022, 15, 388. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.P.; Shao, C.Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Cui, J.; Zhai, X.; Guo, D.; Du, W.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C. Characterization of key odorants in xinyang maojian green tea and their changes during the manufacturing process. J. Agric. Food Chem. 2022, 70, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Chen, X.; Wang, W.; Li, F.; Ma, Y. Effects of leaf acetate on physiology and biochemistry of cold tolerance of tea plant under low temperature stress. Jiangsu Agric. Sci. 2021, 49, 127–132. [Google Scholar] [CrossRef]
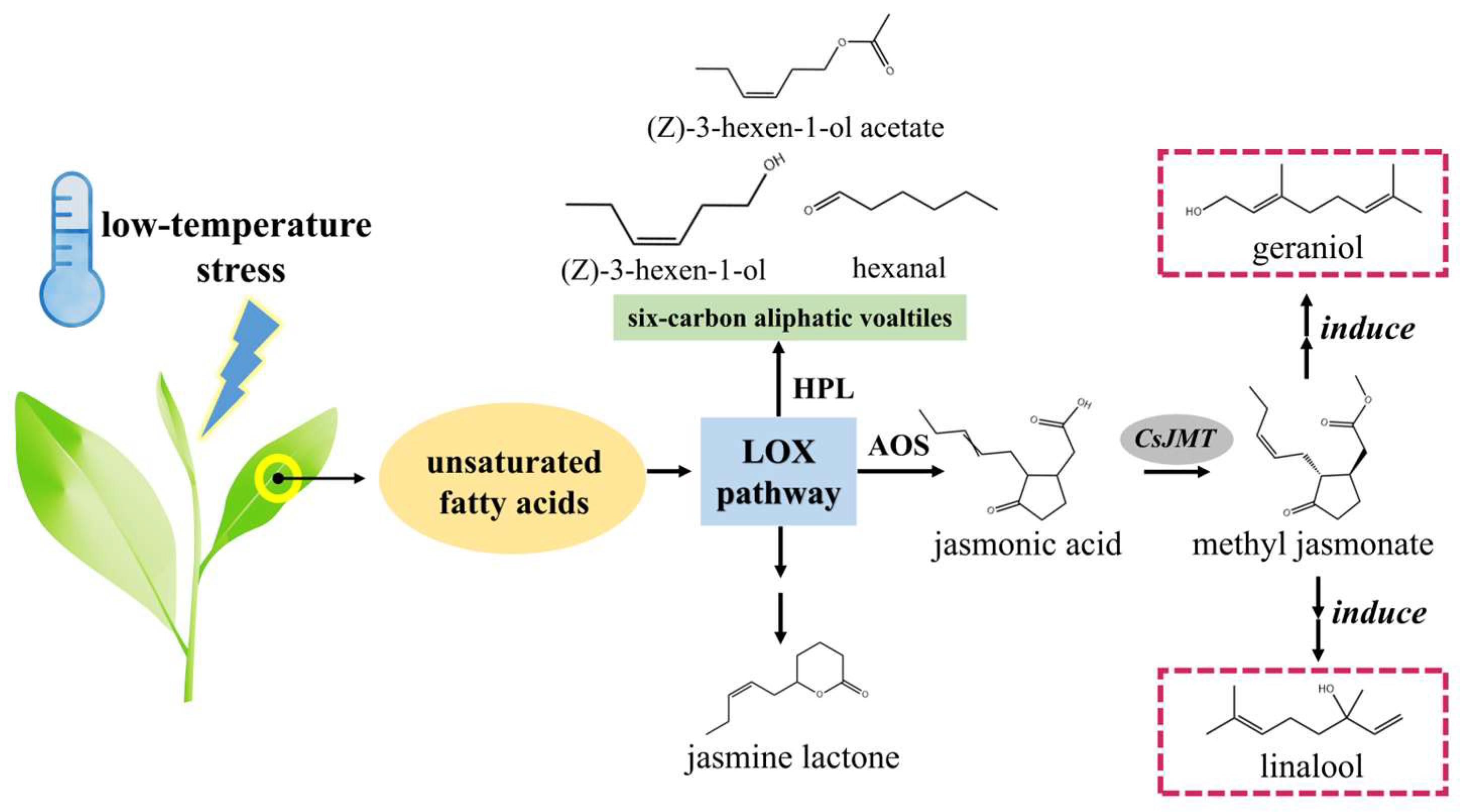
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef]
- Lee, J.; Chambers, D.H.; Chambers, E., IV; Adhikari, K.; Yoon, Y. Volatile aroma compounds in various brewed green teas. Molecules 2013, 18, 10024–10041. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Yan, J.; Wang, B.; Meng, Q.; Zhang, L.; Tong, H. Identification of key odorants responsible for cooked corn-like aroma of green teas made by tea cultivar ‘Zhonghuang 1’. Food Res. Int. 2020, 136, 109355. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, J.; Zhang, J.; Zhang, N.; Lei, L.; Gao, T.; Jing, T.; Zhang, S.; Wu, B.; et al. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. J. Int. Plant Biol. 2020, 62, 1461–1468. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, S.; Wang, C.; Gao, X.; Li, J.; Meng, Q. Comparative analysis of volatiles difference of Yunnan sun-dried Pu-erh green tea from different tea mountains: Jingmai and Wuliang mountain by chemical fingerprint similarity combined with principal component analysis and cluster analysis. Chem. Cent. J. 2016, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Dai, Y.; Guo, Y.N.; Xu, H.R.; Wang, X.C. Volatile profile analysis and quality prediction of Longjing tea (Camellia sinensis) by HS-SPME/GC-MS. J. Zhejiang Uni. Sci. B. 2012, 13, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC×GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef]
- Qian, X.; Xu, X.Q.; Yu, K.J.; Zhu, B.Q.; Lan, Y.B.; Duan, C.Q.; Pan, Q.H. Varietal dependence of GLVs accumulation and LOX-HPL pathway gene expression in four Vitis vinifera wine grapes. Int. J. Mol. Sci. 2016, 17, 1924. [Google Scholar] [CrossRef]
- De Domenico, S.; Bonsegna, S.; Horres, R.; Pastor, V.; Taurino, M.; Poltronieri, P.; Muhammad, I.; Gunter, K.; Victor, F.; Peter, W.; et al. Transcriptomic analysis of oxylipin biosynthesis genes and chemical profiling reveal an early induction of jasmonates in chickpea roots under drought stress. Plant Physiol. Biochem. 2012, 61, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhou, Q.; Chen, W.; Zhang, G.; He, G.; Gu, D.; Zhang, W. Involvement of jasmonate-signaling pathway in the herbivore-induced rice plant defense. Chin. Sci. Bull. 2003, 48, 1982–1987. [Google Scholar] [CrossRef]
- Qian, X.; Sun, L.; Xu, X.Q.; Zhu, B.Q.; Xu, H.Y. Differential expression of VvLOXA diversifies C6 volatile profiles in some Vitis vinifera table grape cultivars. Int. J. Mol. Sci. 2017, 18, 2705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, Y.; Zhang, J.; Qian, X.; Li, X.; Sun, X. Recent progress regarding jasmonates in tea plants: Biosynthesis, signaling, and function in stress responses. Int. J. Mol. Sci. 2024, 25, 1079. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, J.; Lv, H.; Peng, Q.; Schreiner, M.; Baldermann, S.; Lin, Z. Integrated proteomic and metabolomic analyses reveal the importance of aroma precursor accumulation and storage in methyl jasmonate-primed tea leaves. Hortic. Res. 2021, 8, 95. [Google Scholar] [CrossRef]
- Shi, J.; Ma, C.; Qi, D.; Lv, H.; Yang, T.; Peng, Q.; Chen, Z.; Lin, Z. Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. BMC Plant Biol. 2015, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, C.; Zhang, H.; Duan, Y.; Zou, Z.; Zhou, L.; Zhu, X.; Fang, W.; Ma, Y. CsMYB transcription factors participate in jasmonic acid signal transduction in response to cold stress in tea plant (Camellia sinensis). Plants 2022, 11, 2869. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Liu, X.; Gui, J.; Mei, X.; Fu, X.; Dong, F.; Tang, J.; Zhang, L.; Yang, Z. Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem. 2017, 231, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Meng, Q.; Xiao, L.; Li, R.; Peng, C.; Liao, X.; Tong, H. Characterization of aroma compounds of Pu-erh ripen tea using solvent assisted flavor evaporation coupled with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Sci. Hum. Wellness 2022, 11, 618–626. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, L.; Huang, Y.; Jia, L.; Wang, J. Characteristic flavor compounds in Guizhou green tea and the environmental factors influencing their formation: Investigation using stable isotopes, electronic nose, and headspace-gas chromatography ion migration spectrometry. LWT 2024, 196, 115887. [Google Scholar] [CrossRef]
- Morini, G.; Maga, J.A. Volatile compounds in roasted and boiled Chinese chestnuts (Castanea molissima). LWT—Food Sci. Technol. 1995, 28, 638–640. [Google Scholar] [CrossRef]
- Cui, Y.H.; Xu, F.; Wang, F.J.; Wang, J.Z.; Ouyang, J. Effect of processing technology on volatile components of Chinese chestnut. Food Ferment. Ind. 2012, 38, 99–103. (In Chinese) [Google Scholar]
- Ye, G.Z.; Jiang, Y.W.; Yin, J.F.; Yuan, H.B.; Zhang, R.L.; Wang, Z.L.; Shen, D.Y.; Wang, F.; Chen, J.X. Study on the characteristic of aroma components in green tea with chestnut-like aroma. J. Tea Sci. 2009, 29, 385–394. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Shi, J.; Zhu, Y.; Ma, W.; Yan, H.; Shao, C.; Wang, M.; Zhang, Y.; Peng, Q.; Chen, Y.; et al. Insights into crucial odourants dominating the characteristic flavour of citrus-white teas prepared from citrus reticulata Blanco ‘Chachiensis’ and Camellia sinensis ‘Fudingdabai’. Food Chem. 2022, 377, 132048. [Google Scholar] [CrossRef]
- You, Q.; Shi, Y.; Zhu, Y.; Yang, G.; Yan, H.; Lin, Z.; Lv, H. Effect of Different Processing Technologies on the Key Aroma-Active Compounds of Green Tea. Food Sci. 2023, 44, 7. (In Chinese) [Google Scholar] [CrossRef]
- Shu, C.; She, Y.; Xiao, Z.; Xu, L.; Niu, Y.; Zhu, J. Invetigation on the aroma active compounds in fresh and aged Longjing tea by SPME/GC-MS/GC-O/OAV. Food Ind. 2016, 37, 279–285. (In Chinese) [Google Scholar]
- Liu, R.; Ding, L.; Liang, Q.; Ding, X.; Chen, Z. Comparison of aroma components between Rizhao pelleted green tea and Rizhao curly green tea. Sci. Technol. Food Ind. 2015, 36, 323–326. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.; Grosch, W. Odorants of virgin olive oils with different flavor profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
- Benkwitz, F.; Nicolau, L.; Lund, C.; Beresford, M.; Wohlers, M.; Kilmartin, P.A. Evaluation of key odorants in Sauvignon blanc wines using three different methodologies. J. Agric. Food Chem. 2012, 60, 6293–6302. [Google Scholar] [CrossRef]
- Ma, L.; Gao, M.; Zhang, L.; Qiao, Y.; Li, J.; Du, L.; Zhang, H.; Wang, H. Characterization of the key aroma-active compounds in high-grade Dianhong tea using GC-MS and GC-O combined with sensory-directed flavor analysis. Food Chem. 2022, 378, 132058. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Campo, E.; Malfeito-Ferreira, M.; Loureiro, V.; Cacho, J.; Ferreira, V. Analytical and sensorial characterization of the aroma of wines produced with sour rotten grapes using GC-O and GC-MS: Identification of key aroma compounds. J. Agric. Food Chem. 2011, 59, 2543–2553. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, J.; Niu, Y.; Chen, F. Characterization of the key odorants of fennel essential oils of different regions using GC–MS and GC–O combined with partial least squares regression. J. Chromatogr. B 2017, 1063, 226–234. [Google Scholar] [CrossRef]
- Wardencki, W.; Chmiel, T.; Dymerski, T. Gas chromatography-olfactometry (GC-O), electronic noses (e-noses) and electronic tongues (e-tongues) for in vivo food flavour measurement. In Instrumental Assessment of Food Sensory Quality; Woodhead Publishing: Amsterdam, The Netherlands, 2013; pp. 195–229. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.; Jiang, H.; Sun, H.; Zhang, C.; Zhao, H.; Li, X.; Yan, F.; Chen, C.; Xu, Z. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography–mass spectrometry and olfactometry and sensory analysis. Food Chem. 2019, 274, 130–136. [Google Scholar] [CrossRef]
- Niu, Y.; Ma, Y.; Xiao, Z.; Zhu, J.; Xiong, W.; Chen, F. Characterization of the key aroma compounds of three kinds of Chinese representative black tea and elucidation of the perceptual interactions of methyl salicylate and floral odorants. Molecules 2022, 27, 1631. [Google Scholar] [CrossRef]
- Sun, A.; Li, C.; Lv, S.; Gao, J.; Xia, Y.; Geng, Y. Study on the Effects of Processes on Aroma Compounds in Rizhao Green Tea Based on the Gas Chromatography-Ion Mobility Spectrometry. J. Food Process. Preserv. 2023, 3046129. [Google Scholar] [CrossRef]
- Vilar, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. A chemometric approach to characterize the aroma of selected brown and red edible seaweeds/extracts. J. Sci. Food Agric. 2021, 101, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Amano, Y.; Wada, Y.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of Matcha by gas chromatography Olfactometry techniques. J. Agric. Food Chem. 2017, 65, 2984–2989. [Google Scholar] [CrossRef]
- Tan, H.R.; Lau, H.; Liu, S.Q.; Tan, L.P.; Sakumoto, S.; Lassabliere, B.; Leong, K.; Sun, J.; Yu, B. Characterisation of key odourants in Japanese green tea using gas chromatography-olfactometry and gas chromatography-mass spectrometry. LWT 2019, 108, 221–232. [Google Scholar] [CrossRef]
- De Alencar Diniz, J.; Rocha, S.; Pires-Cavalcante, K.; Freitas, J.; Nagano, C.; Sampaio, A.; Saker-Sampaio, S. Chemical composition of volatile compounds in two red seaweeds, Pterocladiella capillacea and Osmundaria obtusiloba, using static headspace gas chromatography mass spectrometry. J. Appl. Phycol. 2017, 29, 1571–1576. [Google Scholar] [CrossRef]
- Rinanda, S.D.; Pratama, R.I.; Riyantini, I.; Rostini, I. Volatile Flavor Compounds Composition of Steamed Squid (Loligo sp.). Asian J. Fish Aquat. Res. 2021, 13, 1–7. [Google Scholar] [CrossRef]
- Wu, N.; Gu, S.; Tao, N.; Wang, X.; Ji, S. Characterization of important odorants in steamed male Chinese mitten crab (Eriocheir sinensis) using gas chromatography-mass spectrometry-olfactometry. J. Food Sci. 2014, 79, C1250–C1259. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, G.; Tu, Q.; Zhou, H.; Li, Y.; Shi, H.; Wu, X.; Ren, H.; Huang, K.; He, X.; et al. Evolution analysis of flavor-active compounds during artificial fermentation of Pu-erh tea. Food Chem. 2021, 357, 129783. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Tufariello, M.; Siciliano, P. Analytical characterisation of Negroamaro red wines by “Aroma Wheels”. Food Chem. 2013, 141, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Sage, E.; Velez, M.; Guinard, J.X. Using single free sorting and multivariate exploratory methods to design a new coffee taster’s flavor wheel. J. Food Sci. 2016, 81, S2997–S3005. [Google Scholar] [CrossRef]
- Imamura, M. Descriptive terminology for the sensory evaluation of soy sauce. J. Sens. Stud. 2016, 31, 393–407. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, X.; Lu, C.Y. Study on primitive morpheme in sensory terminology and flavor wheel construction of Chinese tea. J. Tea Sci. 2019, 39, 474–483. [Google Scholar] [CrossRef]
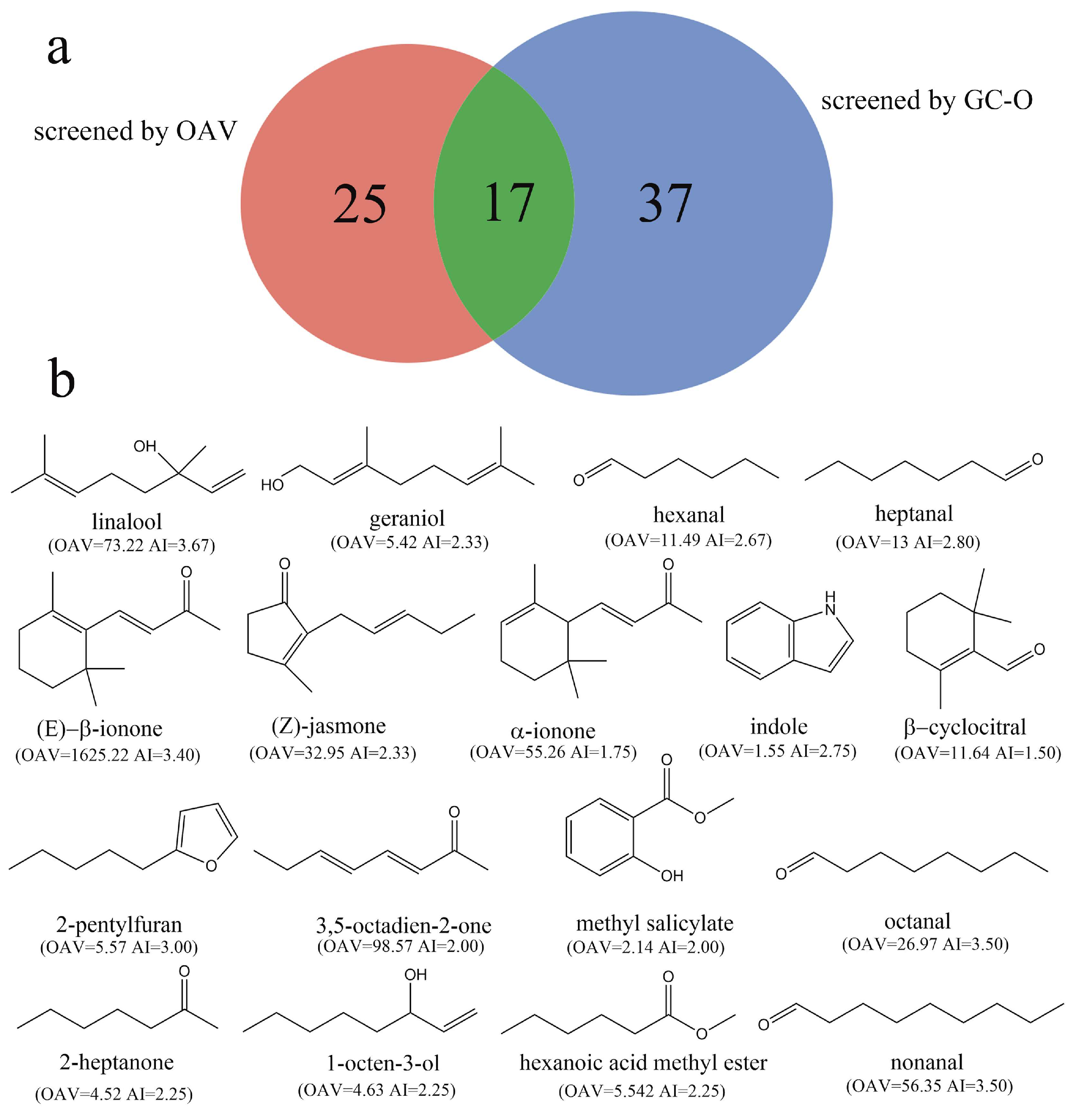
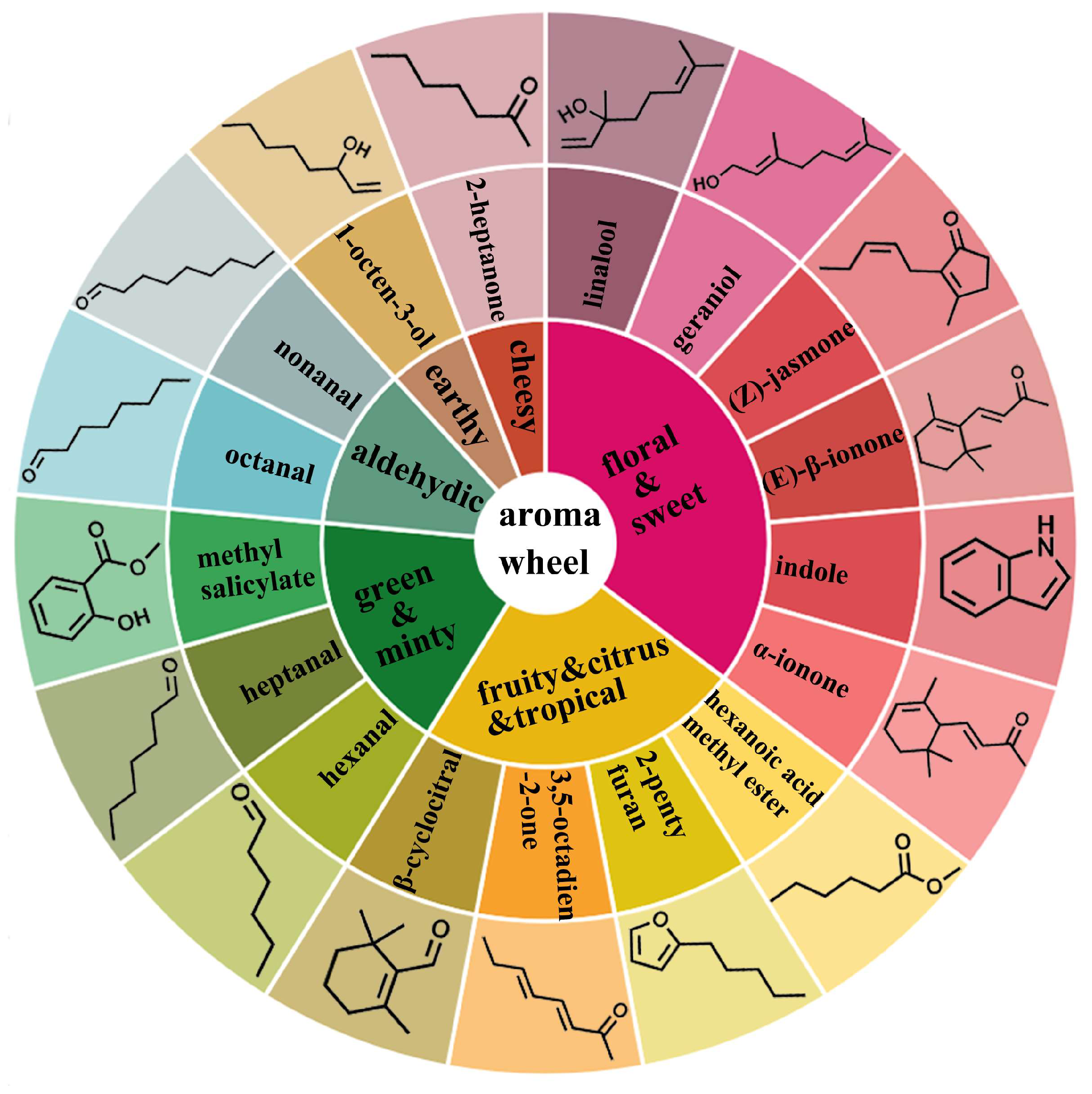
| No | RT a | Compounds | CAS | RI b | Mean Content of RZT (μg/kg) | Content Range of RZT (μg/kg) | OT c (μg/kg) | OAV | Odor Type |
|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||
| 1 | 7.74 | Hexanal | 66-25-1 | 800 | 51.70 | 47.62–55.80 | 4.5 | 11.49 | Grass |
| 2 | 10.79 | Heptanal | 111-71-7 | 903 | 39.00 | 34.72–44.76 | 3 | 13.00 | Green |
| 3 | 14.33 | Octanal | 124-13-0 | 1005 | 18.88 | 17.24–20.26 | 0.7 | 26.97 | Aldehydic |
| 4 | 16.14 | 2,6-Dimethyl-5-heptenal | 106-72-9 | 1052 | 12.50 | 12.30–12.60 | 10 | 1.25 | Melon |
| 5 | 17.96 | Nonanal | 124-19-6 | 1106 | 56.35 | 49.11–61.05 | 1 | 56.35 | Aldehydic |
| 6 | 21.51 | Decanal | 112-31-2 | 1208 | 16.75 | 15.19–18.75 | 0.1 | 167.53 | Aldehydic |
| 7 | 22.18 | β-Cyclocitral | 432-25-7 | 1223 | 34.93 | 33.36–36.99 | 3 | 11.64 | Tropical |
| 8 | 23.83 | Citral | 5392-40-5 | 1276 | 23.40 | 21.65–24.42 | 62 | <1 | Citrus |
| SUM | 253.51 | ||||||||
| Alkenes | |||||||||
| 1 | 8.81 | 2,4-Dimethylhept-1-ene | 19549-87-2 | 843 | 20.12 | 18.71–23.94 | NF | - | - |
| 2 | 11.90 | α-Pinene | 80-56-8 | 940 | 16.58 | 14.94–19.14 | 180 | <1 | Herbal |
| 3 | 13.87 | α-Myrcene | 123-35-3 | 993 | 22.65 | 20.78–24.60 | 44.5 | <1 | Spicy |
| 4 | 14.10 | (+)-4-Carene | 29050-33-7 | 1002 | 11.39 | 9.94–13.04 | NF | - | - |
| 5 | 15.32 | D-Limonene | 138-86-3 | 1032 | 34.82 | 30.67–39.75 | 4 | 8.71 | Fruity |
| 6 | 15.94 | (E)-β-Ocimene | 3779-61-1 | 1051 | 22.03 | 20.48–23.30 | 18.7 | 1.18 | Floral |
| 7 | 17.55 | 4-Methyl-1-undecene | 74630-39-0 | 1085 | 37.78 | 29.61–46.95 | NF | - | - |
| 8 | 18.37 | (E)-4,8-Dimethylnona-1,3,7-triene | 19945-61-0 | 1117 | 27.01 | 24.21–28.85 | NF | - | - |
| 9 | 26.40 | α-Cubebene | 17699-14-8 | 1353 | 13.52 | 12.99–14.64 | NF | - | Herbal |
| 10 | 27.72 | β-cubebene | 13744-15-5 | 1391 | 12.18 | 11.71–12.85 | NF | - | Citrus |
| SUM | 218.08 | ||||||||
| Ketones | |||||||||
| 1 | 7.81 | 4-methyl-3-Penten-2-one | 141-79-7 | 802 | 50.31 | 46.90–54.60 | NF | - | Vegetable |
| 2 | 10.57 | 2-Heptanone | 110-43-0 | 893 | 30.74 | 30.15–31.49 | 140 | 4.52 | Cheesy |
| 3 | 13.31 | 2,2-Dimethyl-3-heptanone | 19078-97-8 | 967 | 22.53 | 19.36–29.29 | NF | - | - |
| 4 | 15.52 | 2,2,6-Trimethyl-cyclohexanone | 2408-37-9 | 1041 | 28.45 | 27.17–29.90 | NF | - | Spicy Cedar |
| 5 | 17.02 | 3,5-Octadien-2-one | 38284-27-4 | 1090 | 49.28 | 47.86–50.74 | 0.5 | 98.57 | Fruity |
| 6 | 22.93 | 2-Isopropyl-5-methyl-2-cyclohexen-1-one | 5113-66-6 | 1251 | 9.98 | 9.74–10.40 | NF | - | - |
| 7 | 24.46 | 2-Undecanone | 112-12-9 | 1294 | 23.43 | 21.09–24.67 | 7 | 3.35 | Fruity |
| 8 | 28.02 | (Z)-Jasmone | 488-10-8 | 1397 | 230.67 | 219.63–246.44 | 7 | 32.95 | Floral |
| 9 | 28.82 | α-Ionone | 127-41-3 | 1430 | 22.10 | 20.54–23.58 | 0.4 | 55.26 | Woody, Floral |
| 10 | 29.45 | Geranylacetone | 3796-70-1 | 1455 | 43.78 | 38.65–47.43 | 60 | <1 | Floral |
| 11 | 30.52 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | 1203-08-3 | 1485 | 17.29 | 16.80–17.94 | NF | - | - |
| 12 | 30.59 | (E)-β-Ionone | 79-77-6 | 1490 | 146.27 | 138.46–159.99 | 0.09 | 1625.22 | Floral |
| 13 | 30.74 | 2-Tridecanone | 593-08-8 | 1501 | 17.13 | 16.11–17.80 | 10,000 | <1 | Waxy |
| SUM | 691.96 | ||||||||
| Esters | |||||||||
| 1 | 11.61 | Hexanoic acid, methyl ester | 106-70-7 | 928 | 22.16 | 20.11–23.88 | 4 | 5.54 | Fruity |
| 2 | 14.40 | 3(Z)-Hexen-1-ol acetate, | 3681-71-8 | 1007 | 152.56 | 144.25–163.41 | NF | - | Green |
| 3 | 20.76 | (Z)-3-Hexenyl butanoate | 16491-36-4 | 1189 | 205.27 | 186.02–219.25 | 20,000 | <1 | Green |
| 4 | 21.05 | (E)-Butanoic acid- 2-hexenyl ester, | 53398-83-7 | 1196 | 19.15 | 17.63–19.98 | NF | - | Green |
| 5 | 21.47 | Methyl salicylate | 119-36-8 | 1194 | 85.68 | 77.20–93.51 | 40 | 2.14 | Minty |
| 6 | 22.32 | (Z)-3-Hexenyl 2-methylbutanoate | 53398-85-9 | 1234 | 76.61 | 69.87–82.52 | NF | - | Green |
| 7 | 26.48 | Propanoic acid, 2-methyl-, 2-ethyl-3-hydroxyhexyl ester | 74367-31-0 | 1373 | 37.78 | 29.61–46.95 | NF | - | - |
| 8 | 27.17 | Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | 77-68-9 | 1380 | 68.00 | 54.05–88.04 | NF | - | - |
| 9 | 27.19 | (Z)-Hexanoic acid-3-hexenyl ester, | 31501-11-8 | 1383 | 433.71 | 425.88–438.43 | NF | - | Green |
| 10 | 27.33 | Hexanoic acid, hexyl ester | 6378-65-0 | 1386 | 19.49 | 17.90–20.15 | 6400 | <1 | Green |
| 11 | 27.34 | cis-3-Hexenyl cis-3-hexenoate | 61444-38-0 | 1389 | 35.96 | 27.91–52.94 | NF | - | Green |
| 12 | 27.43 | (E)-Hexanoic acid-2-hexenyl ester | 53398-86-0 | 1391 | 21.68 | 20.33–22.65 | NF | - | Waxy |
| 13 | 29.23 | β-Phenylethyl butyrate | 103-52-6 | 1450 | 16.30 | 15.35–17.04 | NF | - | Floral |
| 14 | 30.99 | Jasmine lactone | 25524-95-2 | 1518 | 173.15 | 163.00–188.20 | 2 | 86.58 | Creamy |
| 15 | 32.22 | Dihydroactinidiolide | 17092-92-1 | 1542 | 61.41 | 58.56–62.81 | NF | - | Musk Coumarin |
| 16 | 33.04 | Acetaminophen | 1068-90-2 | 1602 | 21.46 | 19.72–22.63 | NF | - | - |
| 17 | 35.83 | Methyl jasmonate | 1211-29-6 | 1655 | 241.39 | 230.21–252.00 | 5700 | <1 | Floral |
| 18 | 40.18 | Isoamyl laurate | 6309-51-9 | 1848 | 11.33 | 10.91–11.65 | NF | - | Waxy |
| 19 | 41.92 | 1-Butyl 2-isobutyl phthalate | 17851-53-5 | 1933 | 277.52 | 240.18–320.20 | NF | - | - |
| 20 | 42.80 | Hexadecanoic acid, 15-methyl-, methyl ester | 6929-04-0 | 1984 | 29.11 | 19.01–43.86 | NF | - | - |
| 21 | 45.07 | 9,12-Octadecenoic acid, methyl ester | 2462-85-3 | 2091 | 11.48 | 10.61–12.22 | NF | - | - |
| 22 | 45.15 | Linolenic acid, methyl ester | 301-00-8 | 2095 | 14.05 | 12.28–15.49 | NF | - | - |
| SUM | 2035.25 | ||||||||
| Alcohols | |||||||||
| 1 | 9.62 | (Z-)3-Hexen-1-ol | 928-96-1 | 857 | 111.82 | 103.84–123.67 | NF | - | Green |
| 2 | 10.06 | 1-Hexanol | 111-27-3 | 872 | 17.14 | 15.73–20.01 | 200 | <1 | Green |
| 3 | 13.63 | 1-Octen-3-ol | 3391-86-4 | 982 | 64.78 | 61.84–67.61 | 14 | 4.63 | Earthy |
| 4 | 15.34 | 2-Ethyl-1-hexanol | 104-76-7 | 1033 | 148.88 | 142.35–158.97 | 270,000 | <1 | Citrus |
| 5 | 16.89 | 2-Furanmethanol, 5-ethenyltetrahydro-5-trimethyl-, cis- | 104188-13-8 | 1066 | 69.08 | 64.60–73.70 | NF | - | - |
| 6 | 17.92 | Linalool | 78-70-6 | 1101 | 292.88 | 281.05–305.56 | 4 | 73.22 | Floral |
| 7 | 18.89 | Phenethyl alcohol | 60-12-8 | 1121 | 148.99 | 142.27–155.66 | 1200 | <1 | Floral |
| 8 | 19.85 | (Z)-3-Nonen-1-ol | 10340-23-5 | 1159 | 12.02 | 11.69–12.50 | NF | - | Waxy |
| 9 | 20.44 | 1-Nonanol | 143-08-8 | 1175 | 16.47 | 16.31–16.62 | 50 | <1 | Floral, Citrus |
| 10 | 21.30 | α-Terpineol | 98-55-5 | 1191 | 21.69 | 20.63–23.67 | 280 | <1 | Terpenic |
| 11 | 23.28 | Geraniol | 106-24-1 | 1258 | 406.46 | 402.09–415.01 | 75 | 5.42 | Floral |
| 12 | 30.15 | 1-Dodecanol | 112-53-8 | 1477 | 15.23 | 13.75–16.69 | 73 | <1 | Waxy |
| 13 | 31.02 | (3S,3aR,3bR,4S,7R,7aR)-4-Isopropyl-3,7-dimethyloctahydro-1H | 23445-02-5 | 1515 | 21.85 | 20.26–23.35 | NF | - | Spicy |
| 14 | 31.63 | Bicyclo [3.1.0]hexan-2-ol, 5-[(1R)-1,5-dimethyl-4-hexen-1-yl]-2-methyl | 58319-05-4 | 1540 | 11.84 | 11.10–12.57 | NF | - | |
| 15 | 32.82 | Nerolidol | 7212-44-4 | 1568 | 156.16 | 146.93–169.98 | 10 | 15.62 | Floral |
| 16 | 34.58 | (-)-Torreyol | 19435-97-3 | 1642 | 17.85 | 16.55–19.40 | NF | - | Herbal |
| 17 | 35.31 | α-Cadinol | 481-34-5 | 1653 | 12.47 | 12.31–12.73 | NF | - | Herbal |
| 18 | 45.31 | Phytol | 150-86-7 | 2109 | 253.41 | 220.29–292.77 | 0.64 | 395.95 | Floral |
| SUM | 1799.02 | ||||||||
| Oxygen heterocycles | |||||||||
| 1 | 20.60 | (E)-linalool oxide (pyranoid) | 39,028-58-5 | 1180 | 48.98 | 45.76–51.61 | 3000 | <1 | Woody |
| 2 | 13.93 | 2-Pentyl-furan | 3777-69-3 | 995 | 33.43 | 23.28–37.09 | 6 | 5.57 | Fruity |
| 3 | 13.94 | (2R,5R)-2-Methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran | 54750-70-8 | 994 | 17.46 | 16.20–19.83 | NF | - | - |
| 4 | 17.46 | (E)-Linalool oxide (furanoid) | 34995-77-2 | 1091 | 62.91 | 58.10–70.62 | 190 | <1 | Woody, Floral |
| SUM | 162.78 | ||||||||
| Nitrogen heterocycles | |||||||||
| 1 | 16.27 | Tea pyrrole | 2167-14-8 | 1053 | 30.80 | 29.06–32.38 | 65,000 | <1 | Roasted |
| 2 | 25.35 | Indole | 120-72-9 | 1302 | 216.63 | 200.18–237.29 | 140 | 1.55 | Floral |
| SUM | 247.43 | ||||||||
| Aromatics | |||||||||
| 1 | 7.01 | Toluene | 108-88-3 | 771 | 82.69 | 75.05–94.57 | NF | - | - |
| 2 | 9.88 | 1,3-Dimethyl-benzene | 108-38-3 | 873 | 65.94 | 59.29–82.55 | 41 | 1.61 | Benzene-like |
| 3 | 15.19 | 1-Methyl-3-(1-methylethyl)-benzene | 535-77-3 | 1029 | 23.16 | 19.86–27.59 | NF | - | - |
| 4 | 31.34 | Butylated Hydroxytoluene | 128-37-0 | 1518 | 34.99 | 31.34–39.13 | NF | - | Phenolic |
| 5 | 28.49 | 1,6-Dimethylnaphthalene | 575-43-9 | 1428 | 14.11 | 12.82–15.34 | NF | - | - |
| SUM | 220.89 | ||||||||
| Phenols | |||||||||
| 1 | 31.46 | 2,4-Bis(1,1-dimethylethyl)-phenol | 96-76-4 | 1523 | 83.33 | 74.51–92.86 | NF | - | - |
| 2 | 36.42 | Juniper camphor | 473-04-1 | 1700 | 11.40 | 10.27–12.14 | NF | - | - |
| SUM | 94.73 | ||||||||
| Alkanes | |||||||||
| 1 | 8.22 | 2,4-Dimethyl-heptane | 2213-23-2 | 822 | 90.53 | 85.76–98.94 | NF | - | - |
| 2 | 9.25 | 2,3-dimethyl-heptane | 3074-71-3 | 856 | 15.57 | 14.41–19.23 | NF | - | - |
| 3 | 9.48 | 4-Methyl-octane | 2216-34-4 | 864 | 59.15 | 54.37–67.28 | NF | - | - |
| 4 | 14.93 | 2,6-Dimethyl-nonane | 17302-28-2 | 1020 | 31.60 | 23.80–42.38 | NF | - | - |
| 5 | 16.20 | 4,5-Dimethylnonane | 17302-23-7 | 1057 | 238.28 | 220.13–254.99 | NF | - | - |
| 6 | 18.01 | 2,6-Dimethyldecane | 13,150-81-7 | 1112 | 217.72 | 209.81–227.89 | NF | - | - |
| 7 | 18.71 | 3,7-Dimethyl-decane, | 17312-54-8 | 1126 | 13.93 | 12.86–16.16 | NF | - | - |
| 8 | 21.24 | 4,7-Dimethyl-undecane, | 17301-32-5 | 1208 | 33.16 | 25.34–41.45 | NF | - | - |
| 9 | 23.91 | 2,6,11-Trimethyl-dodecane | 31295-56-4 | 1275 | 47.10 | 35.96–59.10 | NF | - | - |
| 10 | 24.64 | 2,3,5,8-Tetramethyl-decane, | 192823-15-7 | 1318 | 21.34 | 17.30–25.02 | NF | - | - |
| 11 | 25.42 | 4,6-Dimethyl-dodecane, | 61141-72-8 | 1325 | 35.34 | 28.16–46.36 | NF | - | - |
| 12 | 25.75 | 2,2,4,4,6,8,8-Heptamethylnonane | 4390-04-9 | 1326 | 22.78 | 19.32–27.97 | NF | - | - |
| 13 | 26.08 | 1-Cyclohexylheptane | 5617-41-4 | 1346 | 11.48 | 11.00–12.06 | NF | - | - |
| 14 | 26.30 | 2,6,10-Trimethyl-dodecane, | 3891-98-3 | 1364 | 13.60 | 11.79–15.46 | NF | - | - |
| 15 | 26.87 | 3-Methyl-tridecane, | 6418-41-3 | 1372 | 12.97 | 11.87–14.27 | NF | - | - |
| 16 | 27.78 | 6-Ethyl-2-methyldecane | 62108-21-8 | 1390 | 24.05 | 18.99–28.36 | NF | - | - |
| 17 | 30.79 | 5-Propyl-tridecane, | 55045-11-9 | 1502 | 20.56 | 18.50–22.65 | NF | - | - |
| 18 | 32.40 | Nonylcyclohexane | 2883-02-5 | 1558 | 15.46 | 14.25–16.47 | NF | - | - |
| 19 | 37.66 | Phytane | 638-36-8 | 1789 | 16.75 | 16.07–17.58 | NF | - | - |
| 20 | 40.48 | 2,6,10,14-Tetramethylheptadecane | 18344-37-1 | 1872 | 17.79 | 15.65–21.63 | NF | - | - |
| 21 | 41.30 | 1,2-Epoxyoctadecane | 7390-81-0 | 1900 | 32.72 | 23.14–43.23 | NF | - | - |
| SUM | 991.88 | ||||||||
| Organic acid | |||||||||
| 1 | 26.26 | 2-Methoxy-, methyl ester benzoic acid, | 579-75-9 | 1347 | 11.93 | 11.68–12.28 | NF | - | - |
| 2 | 39.93 | Pentadecylic acid | 1002-84-2 | 1861 | 17.69 | 13.95–21.24 | NF | - | Waxy |
| 3 | 43.66 | Hexadecanoic acid | 57-10-3 | 1975 | 195.49 | 173.03–210.17 | 10,000 | <1 | Waxy |
| 4 | 45.87 | α-Linolenic acid | 463-40-1 | 2119 | 269.98 | 241.30–291.66 | NF | - | Fatty |
| SUM | 495.09 | ||||||||
| Amines | |||||||||
| 1 | 24.97 | N,N-dibutyl-formamide | 761-65-9 | 1310 | 51.24 | 47.65–56.54 | NF | - | - |
| 2 | 42.25 | 5-Methyl-2-benzylhydrazide-3-isoxazolecarboxylic acid, | 59-63-2 | 1945 | 14.81 | 14.00–15.23 | NF | - | - |
| 3 | 47.14 | Hexadecanamide | 629-54-9 | 2182 | 89.91 | 80.21–98.10 | NF | - | - |
| SUM | 155.96 | ||||||||
| No. | TR (min) d | Aroma Compounds | Odor Descriptors | AI e | IM f |
|---|---|---|---|---|---|
| 1 | 7.72–8.01 | Hexanal | Grass, leafy | 2.67 | MS g, RI h, A i, O j |
| 2 | 9.63–9.77 | 3-Hexen-1-ol | Green, grassy | 1.33 | MS, RI, A, O |
| 3 | 10.45–10.57 | 2-Heptanone | Cheese, coconut | 2.25 | MS, RI, A |
| 4 | 10.80–11.23 | Heptanal | Fresh, green | 2.80 | MS, RI, A, O |
| 5 | 11.50–11.97 | Hexanoic acid, methyl ester | Fruity, sweet | 2.25 | MS, RI, A, O |
| 6 | 13.45–13.78 | 1-Octen-3-ol | Green, metallic | 2.25 | MS, RI, A, O |
| 7 | 13.75–14.07 | 2-Pentyl-furan | Fruity, green | 3.00 | MS, RI, A, O |
| 8 | 14.32–14.57 | Octanal | Aldhydic, waxy | 3.50 | MS, RI, A, O |
| 9 | 15.04–15.15 | 2-Ethyl-1-hexanol | Sweet, herbal, green | 1.50 | MS, RI, A, O |
| 10 | 16.19–16.29 | (E)-β-Ocimene | Sweet, floral | 2.00 | MS, RI, A, O |
| 11 | 16.95–17.23 | 3,5-Octadien-2-one | Fruity, green | 2.00 | MS, RI, A, O |
| 12 | 17.46–17.83 | (E)-Linalool oxide (furanoid) | Floral, coffee, baked | 2.67 | MS, RI, A |
| 13 | 17.53–18.14 | Linalool | Floral, sweet | 3.67 | MS, RI, A, O |
| 14 | 17.89–18.14 | Nonanal | Waxy aldehydic | 3.50 | MS, RI, A, O |
| 15 | 18.89–19.12 | Phenethyl alcohol | Sweet, floral | 2.50 | MS, RI, A, O |
| 16 | 19.85–20.13 | (Z)-3-Nonen-1-ol | Mushroom, green, spicy | 3.00 | MS, RI, A, O |
| 17 | 20.49–20.72 | (E)-Linalool oxide (pyranoid) | Fresh, woody | 3.17 | MS, RI, A |
| 18 | 21.41–21.45 | Methyl salicylate | Wintergreen, minty | 2.00 | MS, RI, A, O |
| 19 | 22.12–22.46 | β-Cyclocitral | Fresh, clean, citrus | 1.50 | MS, RI, A, O |
| 20 | 22.38–22.99 | (Z)-3-Hexenyl 2-methylbutanoate | Green, apple, sweet | 2.00 | MS, RI, A |
| 21 | 23.28–23.49 | Geraniol | floral, sweet | 2.33 | MS, RI, A, O |
| 22 | 23.93–24.09 | Citral | Sweet, citrus, fruity | 2.00 | MS, RI, A, O |
| 23 | 25.34–25.56 | Indole | Floral, sweet, plastic | 2.75 | MS, RI, A, O |
| 24 | 26.50–26.66 | α-Cubebene | herbal | 2.00 | MS, RI, A, |
| 25 | 26.79–27.05 | (Z)-3-Hexenyl hexanoate | Citrus, fruity, | 2.60 | MS, RI, A, O |
| 26 | 27.44–27.76 | Hexanoic acid, hexyl ester | Fruity, green, sweet | 2.60 | MS, RI, A, O |
| 27 | 28.04–28.38 | (Z)-Jasmone | Floral, sour, sweet | 2.33 | MS, RI, A, O |
| 28 | 28.85–29.04 | α-Ionone | Floral, violet | 1.75 | MS, RI, A, O |
| 29 | 29.25–29.75 | Geranylacetone | Magnolia, sweet | 2.00 | MS, RI, A, O |
| 30 | 29.98–30.29 | 1-Dodecanol | Fatty, waxy, sweet | 3.00 | MS, RI, A |
| 31 | 30.51–30.79 | (E)-β-Ionone | Floral, orris | 3.40 | MS, RI, A, O |
| 32 | 31.06–31.53 | Unknown 1 | coconut | 3.40 | - |
| 33 | 31.87–32.25 | Dihydroactinidiolide | Floral, sweet, fresh | 2.50 | MS, RI, A, O |
| 34 | 34.46–34.70 | (-)-Torreyol | Fresh, sweet, herbal | 1.25 | MS, RI, A |
| 35 | 35.33–35.61 | α-Cadinol | Herbal. green, mild sweet | 1.67 | MS, RI, A |
| 36 | 35.83–36.11 | Methyl jasmonate | Floral, sweet, milk | 2.25 | MS, RI, A, O |
| 37 | 37.11–37.43 | Unknown 2 | Floral | 2.75 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Song, D.; Yin, H.; Fang, F.; Shi, Y.; Wang, H.; Li, J.; Wang, K.; Zhu, Y.; Lv, H.; et al. Insight into the Volatile Profiles and Key Odorants of Rizhao Green Tea by Application of SBSE-GC-MS, OAVs and GC-O Analysis. Foods 2025, 14, 458. https://doi.org/10.3390/foods14030458
Wang M, Song D, Yin H, Fang F, Shi Y, Wang H, Li J, Wang K, Zhu Y, Lv H, et al. Insight into the Volatile Profiles and Key Odorants of Rizhao Green Tea by Application of SBSE-GC-MS, OAVs and GC-O Analysis. Foods. 2025; 14(3):458. https://doi.org/10.3390/foods14030458
Chicago/Turabian StyleWang, Mengqi, Dapeng Song, Hongxu Yin, Fengxiang Fang, Yali Shi, Hui Wang, Jiyan Li, Kunpeng Wang, Yin Zhu, Haipeng Lv, and et al. 2025. "Insight into the Volatile Profiles and Key Odorants of Rizhao Green Tea by Application of SBSE-GC-MS, OAVs and GC-O Analysis" Foods 14, no. 3: 458. https://doi.org/10.3390/foods14030458
APA StyleWang, M., Song, D., Yin, H., Fang, F., Shi, Y., Wang, H., Li, J., Wang, K., Zhu, Y., Lv, H., & Ding, S. (2025). Insight into the Volatile Profiles and Key Odorants of Rizhao Green Tea by Application of SBSE-GC-MS, OAVs and GC-O Analysis. Foods, 14(3), 458. https://doi.org/10.3390/foods14030458







