Distinctive Lipogenic Gene Expression Patterns in the Mammary Glands of Dairy Cows Are Associated with the Unique Fatty Acid Composition of Bovine Milk Fat
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Data Analysis
3. Results
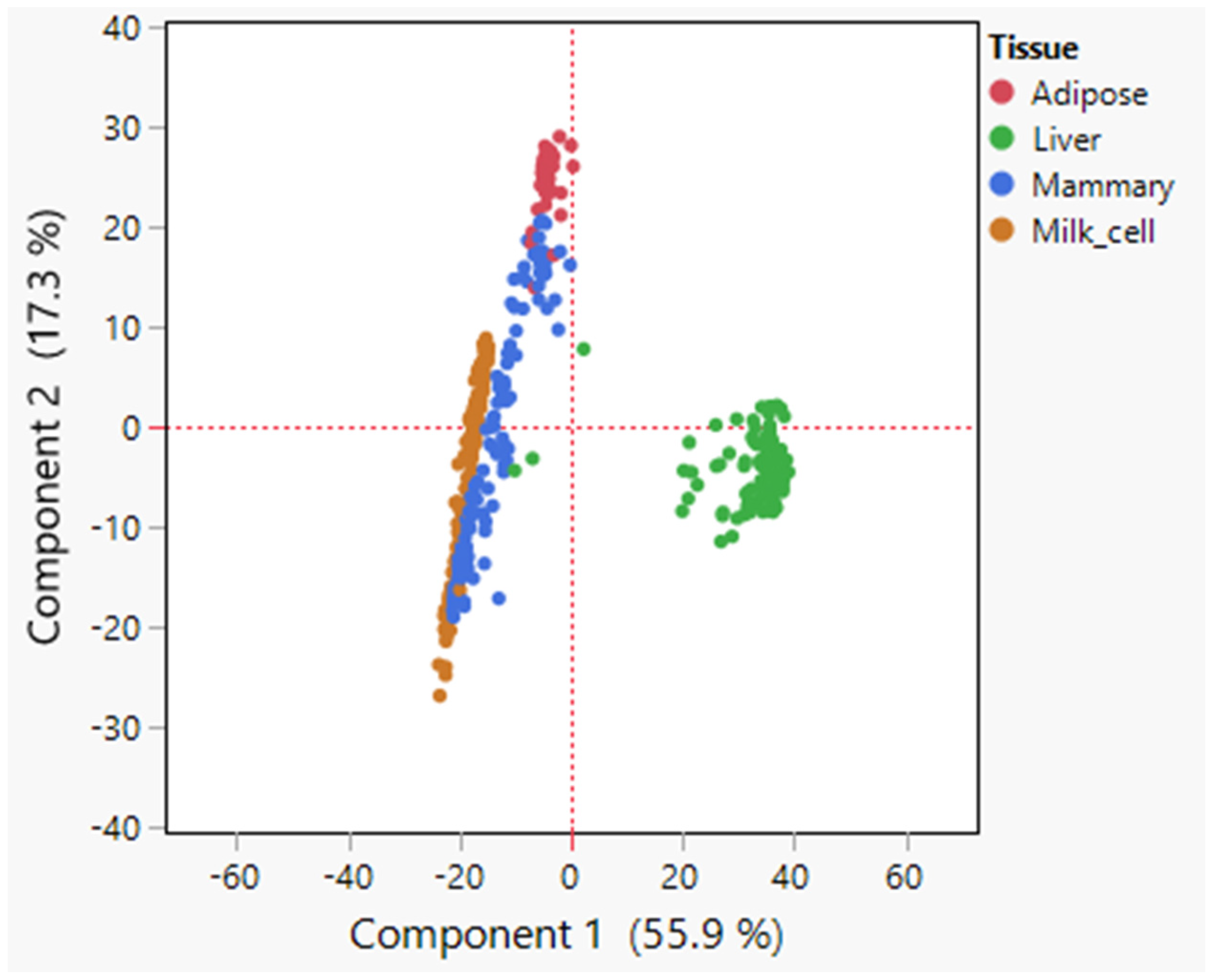
3.1. Overview of Lipid Metabolism Genes in Analyzed Tissues
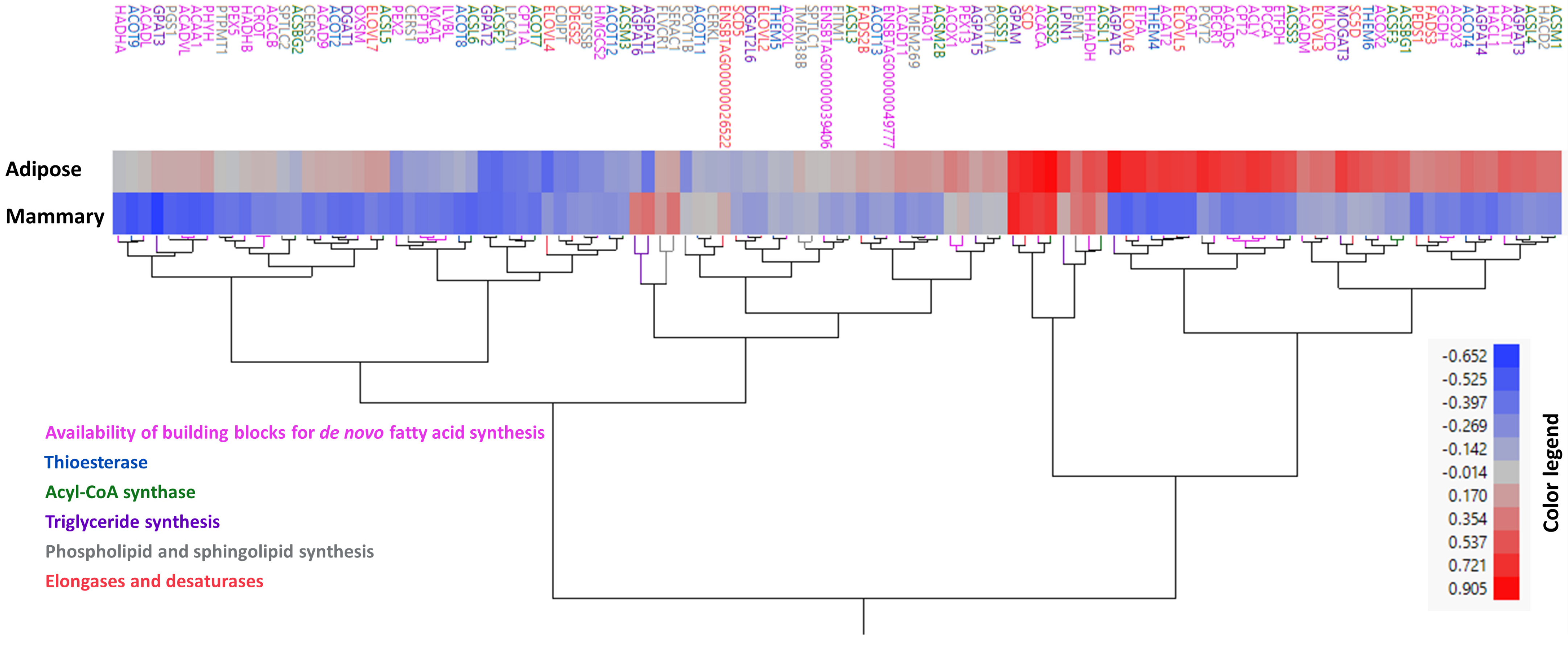
3.2. Correlation Patterns with FASN Gene Expression
3.3. Analysis of Expression for Specific Genes
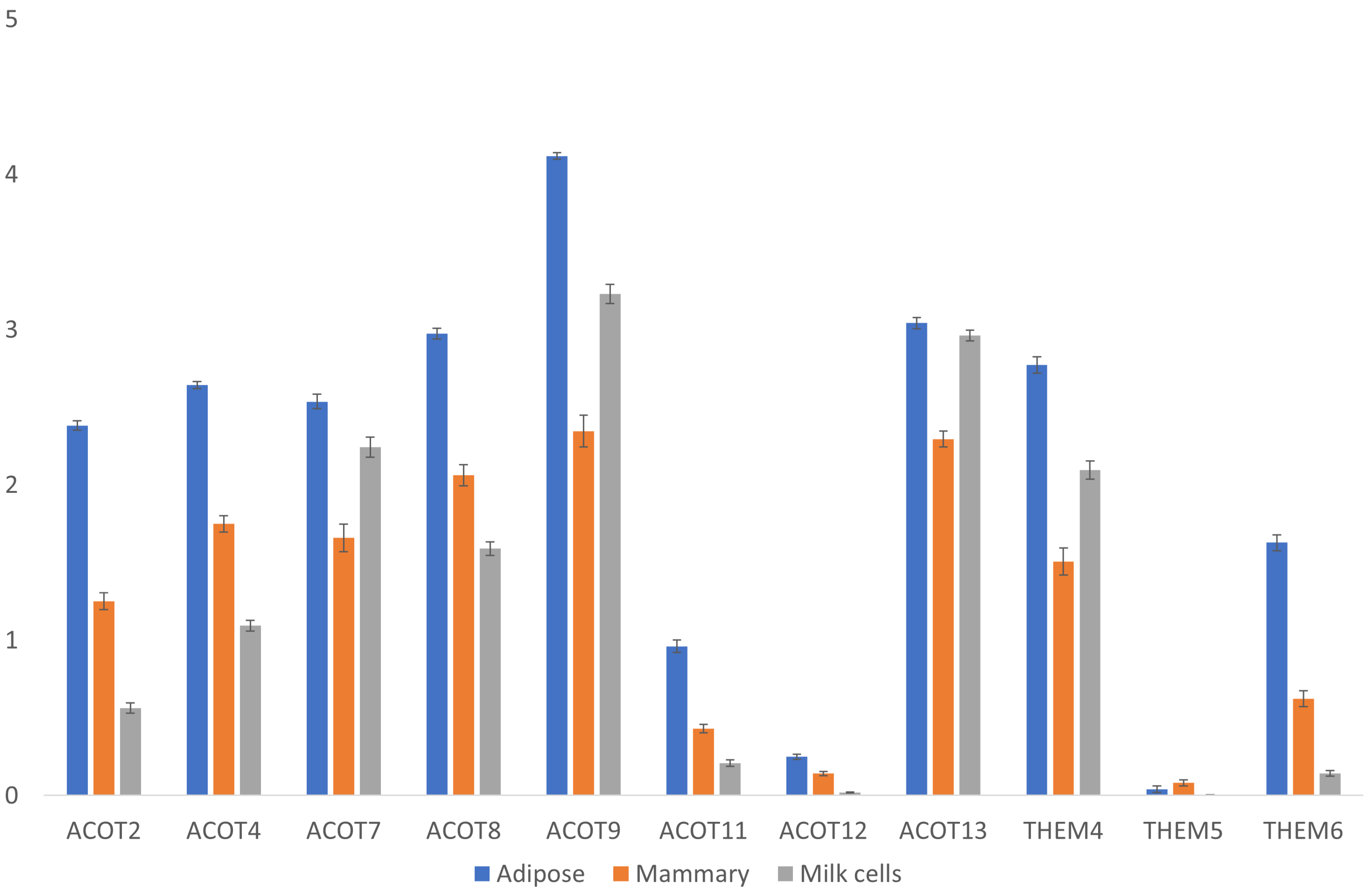
3.3.1. Thioesterase Genes
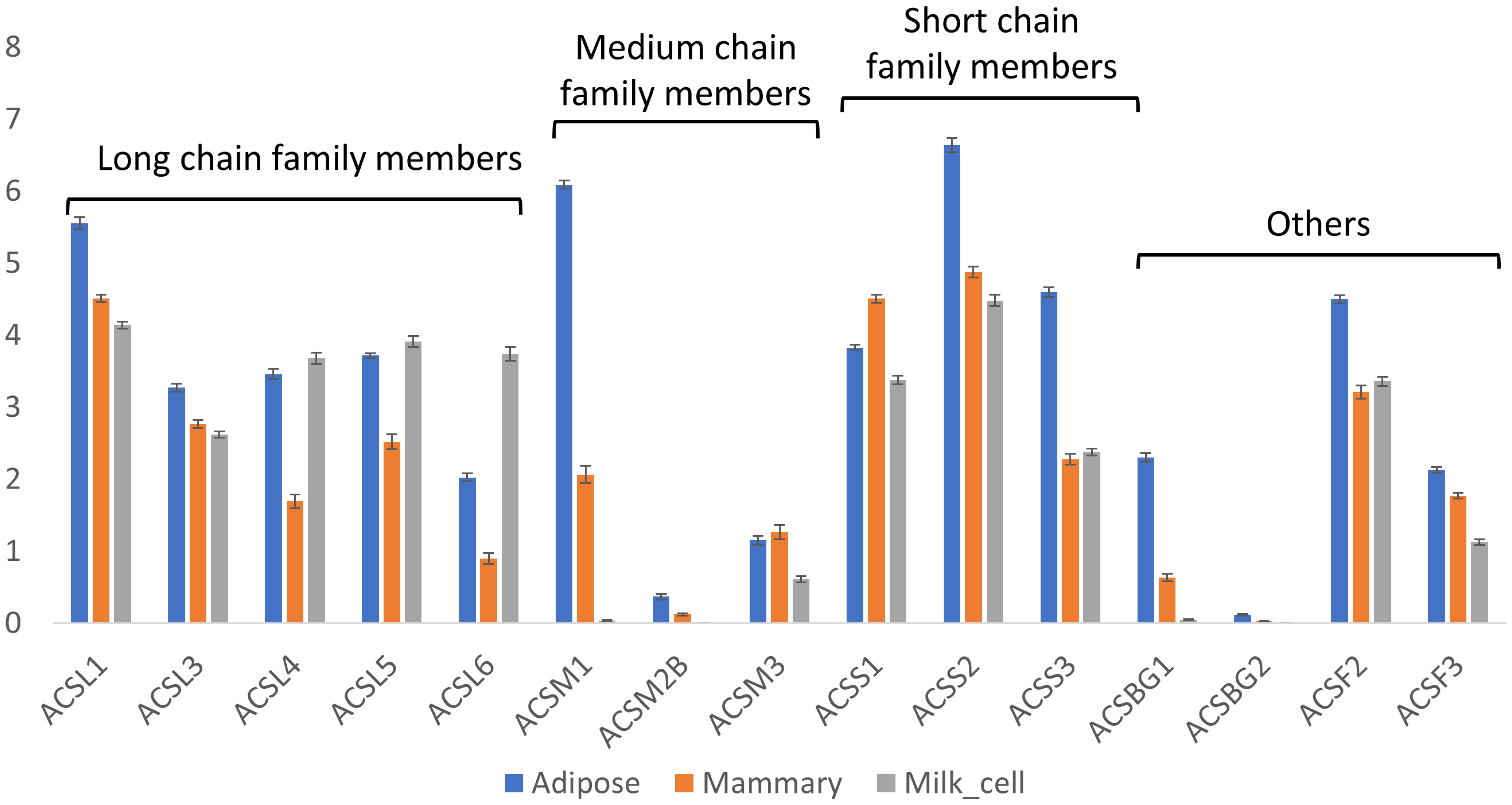
3.3.2. Acyl-CoA Synthase Genes
3.3.3. Triglyceride Synthesis Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J. The Composition of Milk Fat1. J. Dairy Sci. 1991, 74, 3228–3243. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Kęszycka, M.; Czyżak-Runowska, G.; Lipińska, P.; Wójtowski, J. Fatty Acid Profile of Milk-a Review. Bull. Vet. Inst. Pulawy 2013, 57, 135–139. [Google Scholar] [CrossRef]
- MacGibbon, A. Composition and Structure of Bovine Milk Lipids. In Advanced Dairy Chemistry: Lipids; McSweeney, P.L.H., Fox, P.F., O’Mahony, J.A., Eds.; Springer: New York, NY, USA, 2020; Volume 2, pp. 1–32. [Google Scholar]
- Bornaz, S.; Fanni, J.; Parmentier, M. Limit of the Solid Fat Content Modification of Butter. J. Am. Oil Chem. Soc. 1994, 71, 1373–1380. [Google Scholar] [CrossRef]
- Arnold, R.; Shahani, K.; Dwivedi, B. Application of Lipolytic Enzymes to Flavor Development in Dairy Products. J. Dairy Sci. 1975, 58, 1127–1143. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Meyer, A.S.; Arnau, J. The Next Food Revolution Is Here: Recombinant Microbial Production of Milk and Egg Proteins by Precision Fermentation. Annu. Rev. Food Sci. Technol. 2024, 15, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.; Davis, C.; Larson, L.; Smith, V. Biosynthesis of Milk Fat. Lact. A Compr. Treatise 2013, 2, 31–75. [Google Scholar]
- Smith, S. The Animal Fatty Acid Synthase: One Gene, One Polypeptide, Seven Enzymes. FASEB J. 1994, 8, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Regulation of Fatty Acid Synthesis via Phosphorylation of Acetyl-CoA Carboxylase. Prog. Lipid Res. 1989, 28, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.J.; Stoops, J.K.; Joshi, V.C. Fatty Acid Synthesis and Its Regulation. Annu. Rev. Biochem. 1983, 52, 537–579. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Knudsen, J. Transacylation as a Chain-Termination Mechanism in Fatty Acid Synthesis by Mammalian Fatty Acid Synthetase. Synthesis of Butyrate and Hexanoate by Lactating Cow Mammary Gland Fatty Acid Synthetase. Biochem. J. 1980, 186, 287–294. [Google Scholar] [CrossRef]
- Roy, R.; Taourit, S.; Zaragoza, P.; Eggen, A.; Rodellar, C. Genomic Structure and Alternative Transcript of Bovine Fatty Acid Synthase Gene (FASN): Comparative Analysis of the FASN Gene between Monogastric and Ruminant Species. Cytogenet. Genome Res. 2005, 111, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Link, B.; Bray, R.; Cassens, R.; Kauffman, R. Fatty Acid Composition of Bovine Subcutaneous Adipose Tissue Lipids during Growth. J. Anim. Sci. 1970, 30, 722–725. [Google Scholar] [CrossRef]
- Lindmark Månsson, H. Fatty Acids in Bovine Milk Fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef]
- Cattle Genotype-Tissue Expression Atlas (cGTEx). Available online: https://cgtex.roslin.ed.ac.uk/ (accessed on 1 November 2024).
- Liu, S.; Gao, Y.; Canela-Xandri, O.; Wang, S.; Yu, Y.; Cai, W.; Li, B.; Xiang, R.; Chamberlain, A.J.; Pairo-Castineira, E. A Multi-Tissue Atlas of Regulatory Variants in Cattle. Nat. Genet. 2022, 54, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA Abundance Using RNA-Seq Data: RPKM Measure Is Inconsistent among Samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat.Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Oliveros, J.C.; VENNY. An Interactive Tool for Comparing Lists with Venn Diagrams. 2007. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 1 December 2024).
- Laliotis, G.; Bizelis, I.; Rogdakis, E. Comparative Approach of the de novo Fatty Acid Synthesis (Lipogenesis) between Ruminant and Non Ruminant Mammalian Species: From Biochemical Level to the Main Regulatory Lipogenic Genes. Curr. Genom. 2010, 11, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res. 2023, 51, D933–D941. [Google Scholar] [CrossRef] [PubMed]
- Dehesh, K.; Jones, A.; Knutzon, D.S.; Voelker, T.A. Production of High Levels of 8:0 and 10:0 Fatty Acids in Transgenic Canola by Overexpression of Ch FatB2, a Thioesterase cDNA from Cuphea Hookeriana. Plant J. 1996, 9, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Leber, C.; Da Silva, N.A. Engineering of Saccharomyces Cerevisiae for the Synthesis of Short Chain Fatty Acids. Biotechnol. Bioeng. 2014, 111, 347–358. [Google Scholar] [CrossRef]
- Radakovits, R.; Eduafo, P.M.; Posewitz, M.C. Genetic Engineering of Fatty Acid Chain Length in Phaeodactylum tricornutum. Metab. Eng. 2011, 13, 89–95. [Google Scholar] [CrossRef]
- Becker, D.; Weikard, R.; Hadlich, F.; Kühn, C. Single-Cell RNA Sequencing of Freshly Isolated Bovine Milk Cells and Cultured Primary Mammary Epithelial Cells. Sci. Data 2021, 8, 177. [Google Scholar] [CrossRef]
- Danev, N.; Harman, R.M.; Oliveira, L.; Huntimer, L.; Van de Walle, G.R. Bovine Milk-Derived Cells Express Transcriptome Markers of Pluripotency and Secrete Bioactive Factors with Regenerative and Antimicrobial Activity. Sci. Rep. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Shimano, H. Sterol Regulatory Element-Binding Proteins (SREBPs): Transcriptional Regulators of Lipid Synthetic Genes. Prog. Lipid Res. 2001, 40, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.C.; Monks, J.; Burns, V.; Phistry, M.; Marians, R.; Foote, M.R.; Bauman, D.E.; Anderson, S.M.; Neville, M.C. Sterol Regulatory Element Binding Protein and Dietary Lipid Regulation of Fatty Acid Synthesis in the Mammary Epithelium. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E918–E927. [Google Scholar] [CrossRef][Green Version]
- Lemay, D.G.; Neville, M.C.; Rudolph, M.C.; Pollard, K.S.; German, J.B. Gene Regulatory Networks in Lactation: Identification of Global Principles Using Bioinformatics. BMC Syst. Biol. 2007, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Kuksis, A. Biosynthesis of Triacylglycerols. Prog. Lipid Res. 1996, 35, 169–201. [Google Scholar] [CrossRef]
- Smith, S.J.; Cases, S.; Jensen, D.R.; Chen, H.C.; Sande, E.; Tow, B.; Sanan, D.A.; Raber, J.; Eckel, R.H.; Farese, R.V. Obesity Resistance and Multiple Mechanisms of Triglyceride Synthesis in Mice Lacking Dgat. Nat. Genet. 2000, 25, 87–90. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene Networks Driving Bovine Milk Fat Synthesis during the Lactation Cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Adewuyi, A.A.; Gruys, E.; van Eerdenburg, F.J.C.M. Non Esterified Fatty Acids (NEFA) in Dairy Cattle. A Review. Vet. Q. 2005, 27, 117–126. [Google Scholar] [CrossRef]
- Argov-Argaman, N.; Mesilati-Stahy, R.; Magen, Y.; Moallem, U. Elevated Concentrate-to-Forage Ratio in Dairy Cow Rations Is Associated with a Shift in the Diameter of Milk Fat Globules and Remodeling of Their Membranes. J. Dairy Sci. 2014, 97, 6286–6295. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Nutritional Regulation of Milk Fat Synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Chitraju, C.; Mejhert, N.; Haas, J.T.; Diaz-Ramirez, L.G.; Grueter, C.A.; Imbriglio, J.E.; Pinto, S.; Koliwad, S.K.; Walther, T.C.; Farese, R.V. Triglyceride Synthesis by DGAT1 Protects Adipocytes from Lipid-Induced ER Stress during Lipolysis. Cell Metab. 2017, 26, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Voelker, T. Plant Acyl-ACP Thioesterases: Chain-Length Determining Enzymes in Plant Fatty Acid Biosynthesis. Genet. Eng. Princ. Methods 1996, 18, 111–133. [Google Scholar]
- Smith, S. Medium-Chain Fatty Acyl-s-4’-phosphopantetheine-fatty Acid Synthase Thioester Hydrolase from Lactating Mammary Gland of Rat; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Soupene, E.; Kuypers, F.A. Mammalian Long-Chain Acyl-CoA Synthetases. Exp. Biol. Med. 2008, 233, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Reue, K. Biochemistry, Physiology, and Genetics of GPAT, AGPAT, and Lipin Enzymes in Triglyceride Synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef]
- De Renobales, M.; Rogers, L.; Kolattukudy, P.E. Involvement of a Thioesterase in the Production of Short-Chain Fatty Acids in the Uropygial Glands of Mallard Ducks (Anas platyrhynchos). Arch. Biochem. Biophys. 1980, 205, 464–477. [Google Scholar] [CrossRef]
- Harwood, J.L. Fatty Acid Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 101–138. [Google Scholar] [CrossRef]
- Jones, A.; Davies, H.M.; Voelker, T.A. Palmitoyl-Acyl Carrier Protein (ACP) Thioesterase and the Evolutionary Origin of Plant Acyl-ACP Thioesterases. Plant Cell 1995, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Kondo, J.; Ishikawa, M.; Morikawa, K.; Yamamoto, T.T. Acetyl-CoA Synthetase 2, a Mitochondrial Matrix Enzyme Involved in the Oxidation of Acetate. J. Biol. Chem. 2001, 276, 11420–11426. [Google Scholar] [CrossRef]
- Friggens, N.C.; Emmans, G.C.; Kyriazakis, I.; Oldham, J.D.; Lewis, M. Feed Intake Relative to Stage of Lactation for Dairy Cows Consuming Total Mixed Diets with a High or Low Ratio of Concentrate to Forage. J. Dairy Sci. 1998, 81, 2228–2239. [Google Scholar] [CrossRef]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.-D.G.; Kraft, J. Content and Composition of Branched-Chain Fatty Acids in Bovine Milk Are Affected by Lactation Stage and Breed of Dairy Cow. PLoS ONE 2016, 11, e0150386. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 Are the Most Abundant Isoforms in Bovine Mammary Tissue and Their Expression Is Affected by Stage of Lactation123. J. Nutr. 2008, 138, 1019–1024. [Google Scholar] [CrossRef]
- Marshall, M.O.; Knudsen, J. The Specificity of 1-Acyl-Sn-Glycerol 3-Phosphate Acyltransferase in Microsomal Fractions from Lactating Cow Mammary Gland towards Short, Medium and Long Chain Acyl-CoA Esters. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1977, 489, 236–241. [Google Scholar] [CrossRef]
- Marshall, M.O.; Knudsen, J. Specificity of Diacylglycerol Acyltransferase from Bovine Mammary Gland, Liver and Adipose Tissue towards Acyl-CoA Esters: European Journal of Biochemistry. Eur. J. Biochem. 1979, 94, 93–98. [Google Scholar] [CrossRef] [PubMed]
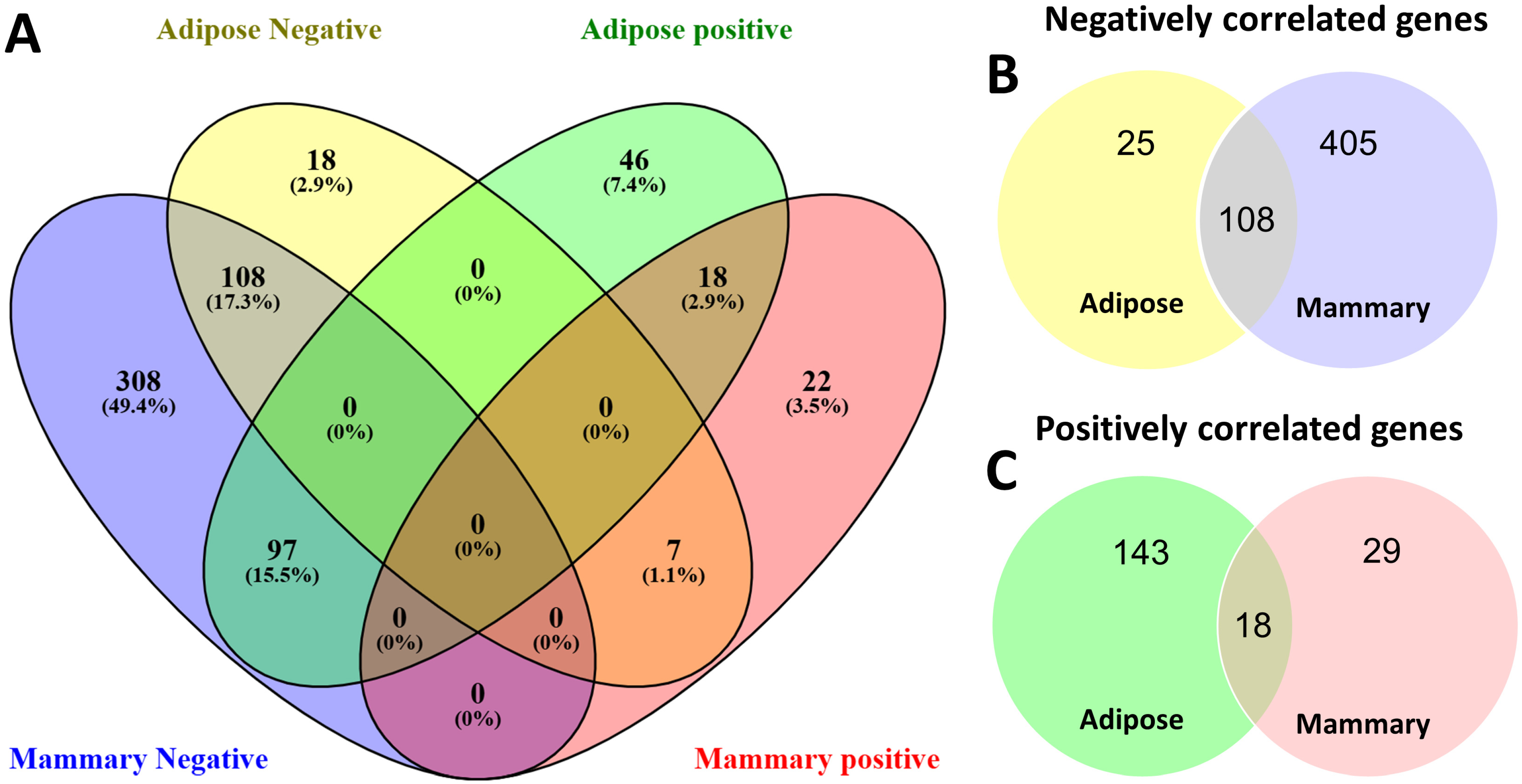

| Category | Mammary | Shared | Adipose |
|---|---|---|---|
| Availability of building blocks for fatty acid de novo synthesis | 2 | 18 | |
| Acyl-CoA thioesterases | 3 | ||
| Elongase/desaturase | 1 | 6 | |
| Acyl-CoA synthase | 2 | 5 | |
| Phospholipid/sphingolipid biosynthesis | 2 | 1 | 2 |
| TAG synthesis | 2 | 1 | 5 |
| Others | 25 | 11 | 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadmor-Levi, R.; Argov-Argaman, N. Distinctive Lipogenic Gene Expression Patterns in the Mammary Glands of Dairy Cows Are Associated with the Unique Fatty Acid Composition of Bovine Milk Fat. Foods 2025, 14, 412. https://doi.org/10.3390/foods14030412
Tadmor-Levi R, Argov-Argaman N. Distinctive Lipogenic Gene Expression Patterns in the Mammary Glands of Dairy Cows Are Associated with the Unique Fatty Acid Composition of Bovine Milk Fat. Foods. 2025; 14(3):412. https://doi.org/10.3390/foods14030412
Chicago/Turabian StyleTadmor-Levi, Roni, and Nurit Argov-Argaman. 2025. "Distinctive Lipogenic Gene Expression Patterns in the Mammary Glands of Dairy Cows Are Associated with the Unique Fatty Acid Composition of Bovine Milk Fat" Foods 14, no. 3: 412. https://doi.org/10.3390/foods14030412
APA StyleTadmor-Levi, R., & Argov-Argaman, N. (2025). Distinctive Lipogenic Gene Expression Patterns in the Mammary Glands of Dairy Cows Are Associated with the Unique Fatty Acid Composition of Bovine Milk Fat. Foods, 14(3), 412. https://doi.org/10.3390/foods14030412






