Comparison of the Thermal Behavior and Chemical Composition of Milk Powders of Animal and Plant Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Description
2.3. Thermal Analysis
2.4. Lipid Extraction from Milk Powder and Gas Chromatography Analysis
2.5. Lipids Nutritional Quality Index
2.6. Volatiles Analysis from Milk Powder Varieties by GC-MS
2.7. Determination of Carbohydrates from Milk Powder
2.8. Determination of Reduced Sugars in Milk Powder
2.9. Statistical Analysis
3. Results and Discussion
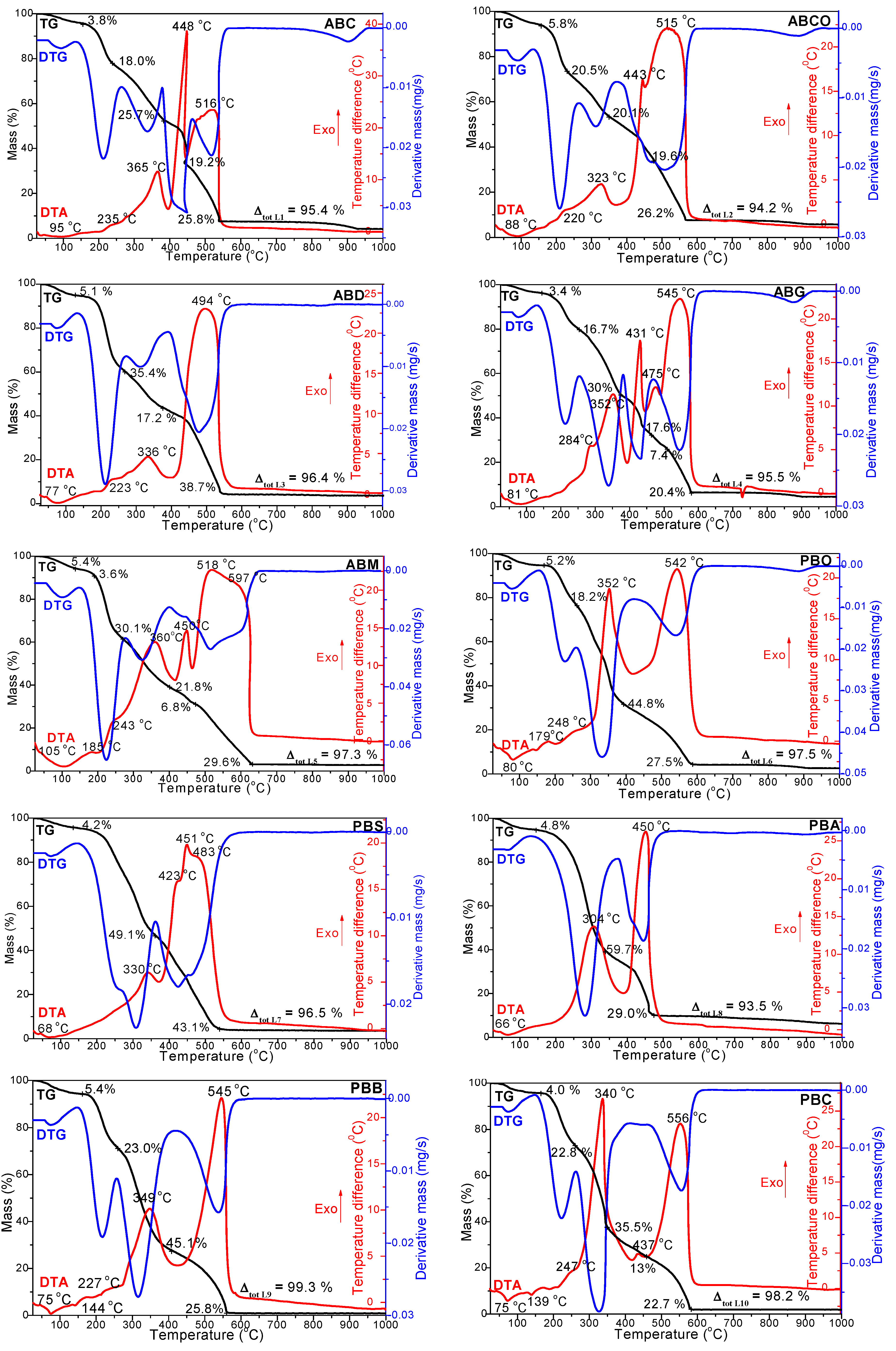
3.1. Thermal Analysis of Milk Powder Varieties
3.2. HS-SPME GC-MS Analysis of Volatile Organic Compounds
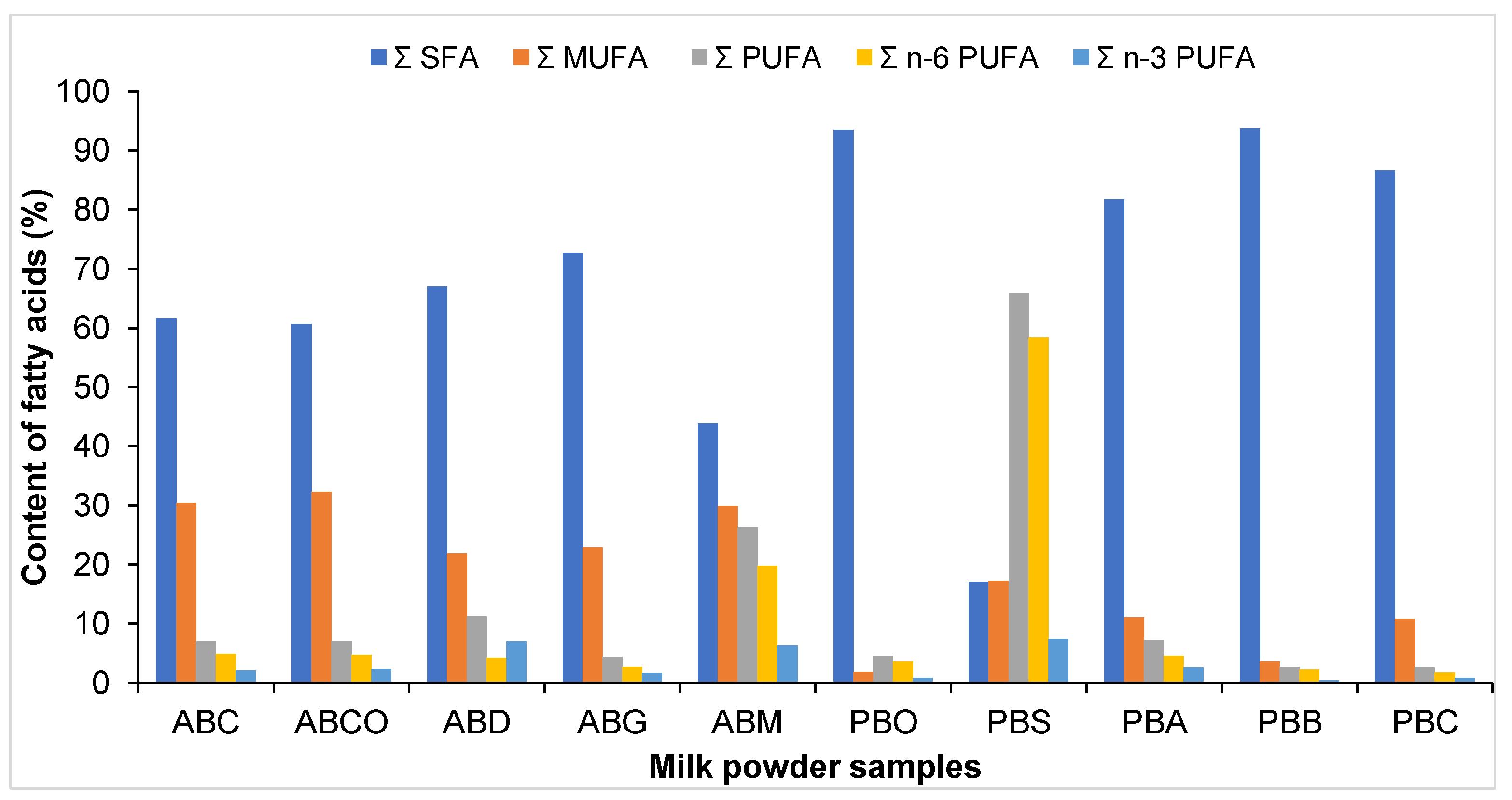
3.3. GC-FID Analysis of FAMEs Content
3.4. Reduced Sugars and Carbohydrates from Milk Powder
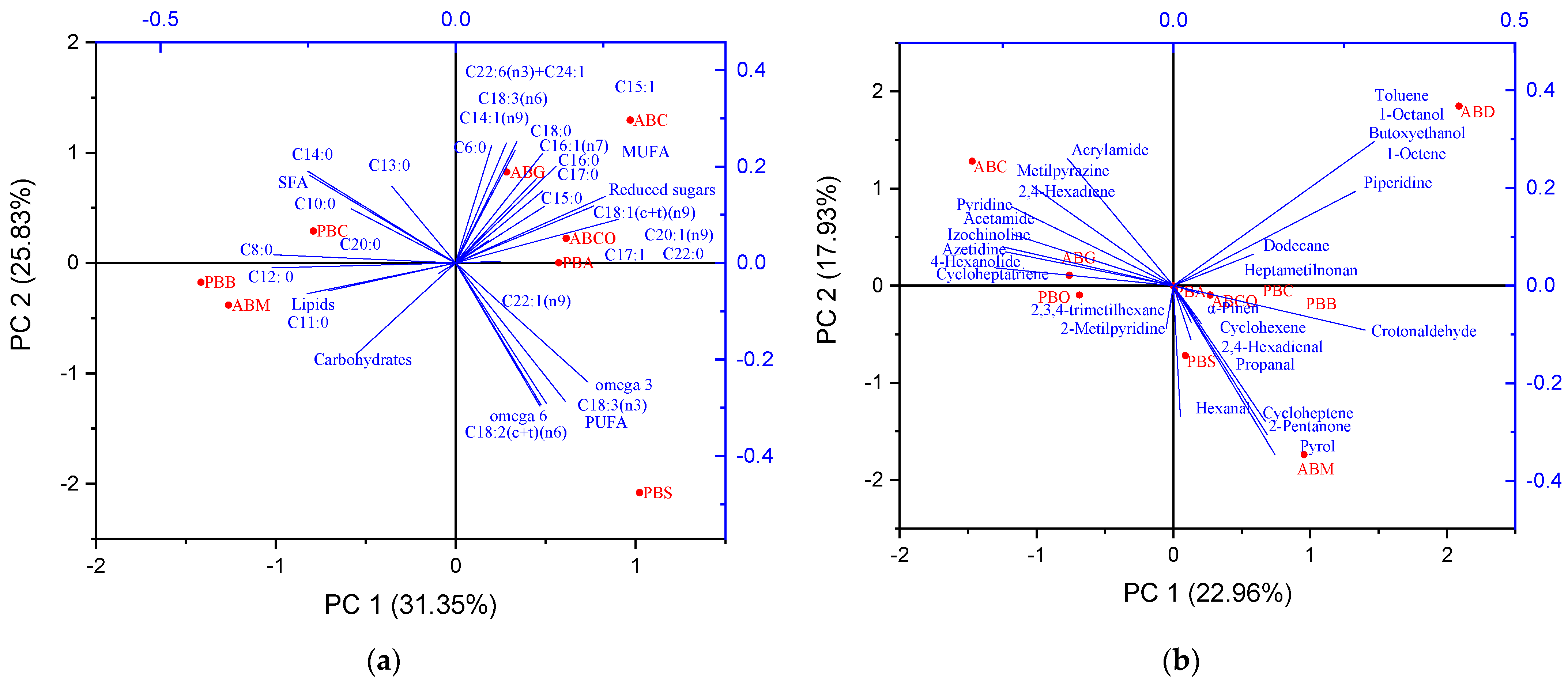
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrasco, I.M.; Carbonero-Aguilar, P.; Dahiri, B.; Moreno, I.M.; Hinojosa, M. Comparison between pollutants found in breast milk and infant formula in the last decade: A review. Sci. Total Environ. 2023, 875, 162461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.M.; Chauhan, O.; Rajani, C.; Sabikhi, L. Effect of coconut milk, skim milk powder, and banana pulp on sensory and functional properties of coconut curd and its applicability as a carrier for probiotic microorganisms. J. Food Proc. Preserv. 2017, 42, e13460. [Google Scholar]
- Chen, Q.; Yin, Q.; Xie, Q.; Evivie, S.E.; Zhao, X.; Li, B.; Huo, G. Elucidating the physicochemical properties and surface composition of goat milk based infant formula powders. Food Chem. 2022, 377, 131936. [Google Scholar] [CrossRef]
- Bakshi, S.; Paswan, V.K.; Yadav, S.P.; Bhinchhar, B.K.; Kharkwal, S.; Rose, H.; Kanetkar, P.; Kumar, V.; Al-Zamani, Z.A.S.; Buncar, S.D. A comprehensive review on infant formula: Nutritional and functional constituents, recent trends in processing and its impact on infants’ gut microbiota. Front. Nutr. 2023, 10, 1194679. [Google Scholar] [CrossRef]
- Vashisht, P.; Sharma, A.; Awasti, N.; Wason, S.; Singh, L.; Sharma, S.; Charles, A.P.R.; Sharma, S.; Gill, A.; Khattra, A.K. Comparative review of nutri-functional and sensorial properties, health benefits and environmental impact of dairy (bovine milk) and plant-based milk (soy, almond, and oat milk). Food Humanit. 2024, 2, 100301. [Google Scholar] [CrossRef]
- Mudgil, P.; Baba, W.N.; Alneyadi, M.; Redha, A.; Maqsood, S. Production, characterization, and bioactivity of novel camel milk-based infant formula in comparison to bovine and commercial sources. LWT 2023, 153, 112813. [Google Scholar] [CrossRef]
- Bakry, A.I.; Wei, W.; Farag, A.M.; Korma, S.A.; Khalifa, I.; Ziedan, N.I.; Mahdi, H.K.; Jin, J.; Wang, X. How does camel milk fat profile compare with that of human milk fat to serve as a substitute for human milk. Int. Dairy J. 2023, 146, 105738. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Sancho, A.I.; Nielsen, S.D.; Larsen, L.B.; Gao, Y.; Bøgh, K.L.; Hansen, E.B. Enzymatic hydrolysis and extensive heat treatment induce distinct modifications of cow’s and camel milk proteins of relevance for production of infant formula. LWT 2024, 191, 115591. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Solah, V.A.; Hunt, W.; Daubney, S.; Meek, J.; Gray, N.; Du, X. Fatty acid profiling of Western Australian pasteurised milk using gas chromatography-mass spectrometry. Int. Food Res. 2024, 180, 114050. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Carpenter, E.; Prosser, C.; Singh, H. Dynamic in vitro gastric digestion of infant formulae made with goat milk and cow milk: Influence of protein composition. Int. Dairy J. 2019, 97, 76–85. [Google Scholar] [CrossRef]
- Fotschki, J.; Szyc, A.M.; Laparra, J.M.; Wróblewska, B. Mare’s and cow’s milk: Promote similarmetabolic effects and expression of innate markers in Caco-2 cells? Int. Food Res. 2015, 72, 184–190. [Google Scholar] [CrossRef]
- Li, N.; Li, B.; Guan, J.; Shi, J.; Evivie, S.E.; Zhao, L.; Huo, G.; Wang, S. Distinct Effects of Milks from Various Animal Types on Infant Fecal Microbiota Through in vitro Fermentations. Front. Microbiol. 2020, 11, 580931. [Google Scholar] [CrossRef] [PubMed]
- Souroullas, K.; Aspri, M.; Papademas, P. Donkey milk as a supplement in infant formula: Benefits and technological challenges. Food Res. Int. 2018, 109, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.K.; Lee, S.W.; Kim, J.H.; Shim, S.; Kim, T.H.; Song, J.Y.; Koh, H.Y.; Shin, Y.H.; Han, M.Y. Neurodevelopmental Outcomes in Infants Fed with Soy Formula: A Retrospective, National Population-Based Observational Cohort Study. J. Nutr. 2021, 151, 3045–3052. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Zhang, J.; Li, X.; Wang, J.; Sun, B. Oat milk analogue versus traditional milk: Comprehensive evaluation of scientific evidence for processing techniques and health effects. Food Chem. 2023, 19, 100859. [Google Scholar] [CrossRef]
- Olias, R.; Delgado-Andrade, C.; Padial, M.; Marin-Manzano, M.C.; Clemente, A. An Updated Review of Soy-Derived Beverages: Nutrition, Processing, and Bioactivity. Foods 2023, 12, 2665. [Google Scholar] [CrossRef]
- Deswal, A.; Deora, N.S.; Mishra, H.N. Effect of concentration and temperature on the rheological properties of oat milk. Food Bioprocess. Technol. 2014, 7, 2451–2459. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Hou, B.; Li, B.; De Souza, C.; Ramachandran, M.; Yi, H.; Zhang, L.; Lin, K. Characterization and comparison of phospholipid fatty acids and molecular species in different animal milk. J. Food Compos. Anal. 2023, 121, 105420. [Google Scholar] [CrossRef]
- Bai, Y.P.; Zhou, H.M. Impact of aqueous ozone mixing on microbiological, quality and physicochemical characteristics of semi-dried buckwheat noodles. Food Biosci. 2023, 53, 102537. [Google Scholar] [CrossRef]
- Hadidi, M.; Palacios, J.C.O.; McClements, D.J.; Mahfouzi, M.; Moreno, A. Alfalfa as a sustainable source of plant-based food proteins. Trends Food Sci. Technol. 2023, 135, 202–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Chen, Y.; Pandiselvam, R.; Liu, Y. Surface free fat bridging contributes to the stickiness of powdered infant formula milk pasteurized by radio frequency dry heat treatment. J. Food Eng. 2022, 323, 111001. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M.; Luo, R.; Hunag, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2021, 105, 1687–1700. [Google Scholar] [CrossRef]
- Zafisah, N.; Yusof, Y.; Ali, M.; Roslan, N.; Tahir, A.; Aziz, M.; Chin, N. Processing of raw coconut milk for its value addition using spray and freeze drying techniques. J. Food Proc. Eng. 2017, 41, e12602. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2006; Volume 2. [Google Scholar]
- Fox, P.F.; McSweeney, P.L.H. Advanced Dairy Chemistry-Proteins; Springer: Berlin/Heidelberg, Germany, 2003; Volume 1. [Google Scholar]
- Tang, L.; Zhang, Y.; Jin, Y.; Yu, M.; Song, H. Switchable GC/GC × GC–olfactometry–mass spectrometry system for the analysis of aroma components of infant formula milk-based on cow and goat milk. J. Food Compos. Anal. 2022, 112, 104709. [Google Scholar] [CrossRef]
- Koletzo, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global Standard for the Composition of Infant Formula-Recommendations of an ESPGHAN Coordinated International Expert Group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 567–689. [Google Scholar]
- Cui, L.; Jia, Q.; Zhao, J.; Hou, D.; Zhou, S. A comprehensive review on oat milk: From oat nutrients and phytochemicals to its processing technologies, product features, and potential applications. Food Funct. 2023, 13, 5858–5869. [Google Scholar] [CrossRef]
- Romeu-Nadal, M.; Chavez-Servin, J.L.; Castellote, A.I.; Rivero, M.; Lopez-Sabater, M.C. Oxidation stability of the lipid fraction in milk powder formulas. Food Chem. 2007, 100, 756–763. [Google Scholar] [CrossRef]
- Conceiçao, D.G.; Reis, L.C.C.; Virgens, E.S.; Jesus, J.C.; Bauer, L.C.; Chaves, M.A.; Bonomo, R.C.F.; Santos, L.S.; Ferrao, S.P.B. Thermal, structural and spectroscopic properties of powder sheep milk obtained by different drying methods. Int. Dairy J. 2024, 150, 105845. [Google Scholar] [CrossRef]
- Dippong, T.; Goga, F. Tehnici Avansate de analiză Instrumentală. Metode Termice; Editura Risoprint: Cluj, Napoca, 2016. (In Romanian) [Google Scholar]
- Rahman, M.M.S.; Al-Hakmani, H.; Al-Alawi, A.; Al Marhubi, I. Thermal characteristics of freeze-dried camel milk and its major components. Thermochim. Acta 2012, 549, 116–123. [Google Scholar] [CrossRef]
- Malec, L.S.; Gonzales, A.S.P.; Naranjo, G.B.; Vigo, M.S. Influence of water activity and storage temperature on lysine availability of a milk like system. Food Res Int. 2002, 35, 849–853. [Google Scholar] [CrossRef]
- Schmitz, I.; Gianfrancesco, A.; Kulozik, U.; Foerst, P. Chair for Food Process Engineering and Dairy Technology; Technische Universität München: Weihenstephaner Berg, Germany, 2011. [Google Scholar]
- Senila, M.; Resz, A.M.; Torok, I.; Senila, L. Nutritional composition and health risk of toxic metals of some edible wild mushrooms growing in a mining area of Apuseni Mountains, Western Carpathians. J. Food Compos. Anal. 2024, 128, 106061. [Google Scholar] [CrossRef]
- Dongmo, Y.K.M.; Tali, M.B.T.; Dize, D.; Mbouna, C.D.J.; Fotsing, S.K.; Ngouana, V.; Pinlap, B.R.; Menkem, E.Z.; Tchokouaha, L.R.Y.; Wabo, G.F.; et al. Anti-Shigella and antioxidant-based screening of some Cameroonian medicinal plants, UHPLC-LIT-MS/MS fingerprints, and prediction of pharmacokinetic and drug-likeness properties of identified chemicals. J. Ethnopharmacol. 2024, 324, 117788. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Melnikova, E.; Bogdanova, E.; Paveleva, D.; Saranov, I. Sucrose, Lactose, Thermogravimetry, and Differential Thermal Analysis: The Estimation of the Moisture Bond Types in Lactose Containing Ingredients for Confectionery Products with Reduced Glycemic Index. Int. J. Food Sci. 2023, 2023, 8835418. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.J. Thermal and mechanical interfacial behaviors of graphene oxide-reinforced epoxy composites cured by thermal latent catalyst. Materials 2019, 12, 1354. [Google Scholar] [CrossRef]
- Dippong, T.; Dan, M.; Kovacs, M.H.; Kovacs, E.D.; Levei, E.A.; Cadar, O. Analysis of Volatile Compounds, Composition, and Thermal Behavior of Coffee Beans According to Variety and Roasting Intensity. Foods 2022, 11, 3146. [Google Scholar] [CrossRef]
- Dippong, T.; Senila, L.; Muresan, L.M. Preparation and Characterization of the Composition of Volatile Compounds, Fatty Acids and Thermal Behavior of Paprika. Foods 2023, 12, 2041. [Google Scholar] [CrossRef]
- Dippong, T.; Cadar, O.; Kovacs, M.H.; Dan, M.; Senila, L. Chemical Analysis of Various Tea Samples Concerning Volatile Compounds, Fatty Acids, Minerals and Assessment of Their Thermal Behavior. Foods 2023, 12, 3063. [Google Scholar] [CrossRef]
- Sunooj, K.V.; George, J.; Sajeev Kumar, V.A.; Radhakrishna, K.; Bawa, A.S. Thermal Degradation and Descomposition Kinetics of Freeze Dried Cow and Camel Milk as Their Constituents. J. Food Sci. Eng. 2011, 1, 77–84. [Google Scholar]
- Chen, X.; Yu, H.; Bai, X.; Wu, C.; Yang, M. Proteomics and phosphoproteomics differences between human and cow milk fat globule membrane: An innovative guide for infant formula. Food Chem. 2025, 472, 142890. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 6, pp. 929’930, 1078’1079. [Google Scholar]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; Volume 4, pp. 498–544. [Google Scholar]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry; Oxford University Press: Oxford, UK, 2012; Volume I. [Google Scholar]
- Li, Y.; Li, R.; Hu, X.; Jiani, L.J.; Liu, G.; Gao, L.; Zhang, Y.; Wang, H.; Zhu, B. Changes of the volatile compounds and odors in one-stage and three-stage infant formulas during their secondary shelf-life. Curr. Res. Food Sci. 2024, 8, 100693. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.G.; Zheng, C.D.; Li, T.; Song, H.L.; Wang, L.J.; Zhang, W.; Sun, H.; Xie, O.G.; Jiang, S.L. Comparison of aroma properties of infant formulas: Differences in key aroma compounds and their possible origins in processing. J. Dairy Sci. 2023, 106, 5970–5987. [Google Scholar] [CrossRef] [PubMed]
- Colmenar, I.; Martin, P.; Cabanas, B.; Salgado, S.; Martinez, E. Analysis of reaction products formed in the gas phase reaction of E,E-2,4-hexadienal with atmospheric oxidants: Reaction mechanisms and atmospheric implications. Atmos. Environ. 2018, 176, 188–200. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, H.; Pan, M.; Wang, C.; Sun, B.; Ai, N. Unraveling volatilomics profiles of milk products from diverse regions in China. Food Res. Int. 2024, 179, 114006. [Google Scholar] [CrossRef]
- Leparmarai, P.T.; Kunz, C.; Mwangi, D.M.; Gluecks, I.; Kreuzer, M.; Marquardt, S. Camels and cattle respond differently in milk phenol excretion and milk fatty acid profile to free ranging conditions in East-African rangelands. Sci. Afr. 2021, 13, e00896. [Google Scholar] [CrossRef]
- Ediriweera, M.K. Analyzing the fatty acid composition of goat milk and its HDAC inhibitory effects: A rational approach towards understanding the epigenetic impacts of goat milk. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Alvarez-Hess, P.S.; Thompson, A.L.; Williams, S.R.O.; Taylor, C.; Singh, T.; MRoque, B.; Neachtain, A.S.O.; Kinley, R.D.; Jacobs, J.L. The influence of feeding canola oil steeped Asparagopsis armata on resulting fatty acid profile and dairy processing properties of cow’s milk. Anim. Feed Sci. Technol. 2024, 310, 115924. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, H.; Ma, N.; Xu, H.; Wang, M.; Chen, P.; Guo, G.; Cao, Y.; Gao, Y.; Li, J. Effects of dietary palmitic acid and oleic acid ratio on milk production, nutrient digestibility, blood metabolites and milk fatty acids profile of lactating dairy cows. J. Dairy Sci. 2024, 107, 4370–4380. [Google Scholar] [CrossRef]
- Rindsig, R.B.; Schultz, L.H. Effect of Feeding Lauric Acid to Lactating Cows on Milk Composition, Rumen Fermentation, and Blood Lipids. J. Dairy Sci. 1974, 57, 1414–1419. [Google Scholar] [CrossRef]
- Dongmo, F.F.D.; Fogang Mba, A.R.; Ngamga, F.H.N.; Asongni, W.D.; Zokou, R.; Noutsa, N.; Hagbe, D.N.; Tchuenbou-Magaia, F.L.; Etame, R.M.E. An overview of fatty acids-based nutritional quality indices of fish oils from Cameroon: Impact of fish pre-treatment and preservation methods. J. Food Compos. Anal. 2024, 131, 106250. [Google Scholar] [CrossRef]
- Liu, T.; Zhen, X.; Li, J.; Wang, Y.; Gou, D.; Zhao, J. Investigating the physicochemical characteristics and importance of insoluble dietary fiber extracted from legumes: An in-depth study on its biological functions. Food Chem. X 2024, 22, 101424. [Google Scholar] [CrossRef] [PubMed]
- Tulashie, S.K.; Amenakpor, J.; Atisey, S.; Odai, R.; Akpari, E.E.A. Production of coconut milk: A sustainable alternative plant-based milk. Case Stud. Chem. Environ. Eng. 2022, 6, 100206. [Google Scholar] [CrossRef]

| VOC | Group | Odor | ABC | ABCO | ABD | ABG | ABM | PBO | PBS | PBA | PBB | PBC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetamide | amide | pungent | 4.3 ± 0.3 b | 0.2 ± 0.01 ef | 0.1 ± 0.01 f | <0.1 | 0.1 ± 0.01 f | 5.1 ± 0.4 a | 0.8 ± 0.07 de | 1.7 ± 0.1 c | <0.1 | 1.2 ± 0.1 cd |
| Acrylamide | amide | pungent | 30.3 ± 2.5 a | 0.7 ± 0.08 c | 6.6 ± 0.5 b | 2.7 ± 0.2 c | 0.3 ± 0.03 c | 0.7 ± 0.06 c | 2.6 ± 0.21 c | 2.3 ± 0.2 c | <0.1 | <0.1 |
| Azetidine | heterocyclic | pungent | 31.2 ± 2.1 b | 0.5 ± 0.04 e | 1.4 ± 0.2 e | 52.2 ± 5.1 a | 0.4 ± 0.04 e | 20.5 ± 2.1 c | 10.1 ± 1.1 d | 24.5 ± 1.8 c | 3.6 ± 0.3 de | 6.8 ± 0.4 de |
| Butoxyethanol | alcohol | sweet | <0.1 | <0.1 | 28.6 ± 2.3 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Crotonaldehyde | aldehyde | sweet | <0.1 | <0.1 | 0.3 ± 0.03 b | <0.1 | 0.5 ± 0.04 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Cycloheptene | hydrocarbon | sweet | <0.1 | <0.1 | <0.1 | <0.1 | 17.7 ± 1.5 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Cycloheptatriene | hydrocarbon | pungent | 9.3 ± 0.8 c | 5.6 ± 0.5 d | <0.1 | 29.0 ± 2.5 a | 1.5 ± 0.1 ef | 16.0 ± 1.8 b | 2.2 ± 0.2 def | 9.2 ± 0.8 c | 4.5 ± 0.3 de | 17.5 ± 1.2 b |
| Cyclohexene | hydrocarbon | sweet | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 3.5 ± 0.3 a | <0.1 | <0.1 |
| 1-Octanol | alcohol | green | <0.1 | <0.1 | 7.8 ± 0.7 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Dodecane | hydrocarbon | gasoline | <0.1 | 86.9 ± 5.6 a | 23.7 ± 2.0 b | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Heptametilnonan | hydrocarbon | green | <0.1 | 5.7 ± 0.4 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 2,4-Hexadienal | aldehyde | green | <0.1 | <0.1 | 1.0 ± 0.01 bc | <0.1 | <0.1 | <0.1 | 2.8 ± 0.2 b | 23.2 ± 2.1 a | <0.1 | <0.1 |
| 2,4-Hexadiene | hydrocarbon | sweet | 4.6 ± 0.3 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Hexanal | aldehyde | green | <0.1 | <0.1 | <0.1 | 6.4 ± 0.5 e | 37.6 ± 2.8 c | 46.8 ± 3.2 b | 57.5 ± 4.5 a | 15.2 ± 1.4 d | 0.6 ± 0.05 e | 2.7 ± 0.2 e |
| 4-Hexanolide | ketone | sweet | 4.1 ± 0.21 b | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 60.4 ± 5.2 a |
| Izochinoline | heterocycle | sweet | 3.3 ± 0.25 d | <0.1 | 0.1 ± 0.01 e | 8.4 ± 0.7 b | <0.1 | 9.5 ± 0.8 a | <0.1 | 0.5 ± 0.04 e | <0.1 | 4.7 ± 0.3 c |
| Metilpyrazine | heterocycle | green | 4.3 ± 0.24 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 2-Metilpyridine | heterocyclic | sweet | <0.1 | <0.1 | 0.1 ± 0.01 c | 1.3 ± 0.1 c | <0.1 | 0.7 ± 0.06 c | 6.3 ± 0.5 b | <0.1 | 17.6 ± 1.3 a | <0.1 |
| 1-Octene | hydrocarbon | gasoline | <0.1 | <0.1 | 20.9 ± 1.8 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 2-Pentanone | ketone | green | <0.1 | <0.1 | <0.1 | <0.1 | 40.6 ± 3.4 a | <0.1 | 9.6 ± 0.8 b | <0.1 | <0.1 | <0.1 |
| α-Pinen | hydrocarbon | sweet | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 12.3 ± 1.0 a | <0.1 |
| Piperidine | heterocycle | sweet | <0.1 | <0.1 | 5.7 ± 0.4 a | <0.1 | <0.1 | <0.1 | 0.4 ± 0.04 c | 4.4 ± 0.3 b | <0.1 | <0.1 |
| Propanal | aldehyde | sweet | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.6 ± 0.05 c | 4.6 ± 0.31 b | 14.7 ± 1.2 a | <0.1 | <0.1 |
| Pyridine | heterocycle | pungent | 8.7 ± 0.7 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 2.1 ± 0.2 c | <0.1 | 2.5 ± 0.2 c | 6.8 ± 0.4 b |
| Pyrol | heterocycle | sweet | <0.1 | 0.2 ± 0.01 c | <0.1 | <0.1 | 1.3 ± 0.1 a | <0.1 | 0.6 ± 0.05 b | 0.6 ± 0.04 b | <0.1 | <0.1 |
| Toluene | hydrocarbon | sweet | <0.1 | <0.1 | 2.3 ± 0.2 a | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 2,3,4-trimetilhexane | hydrocarbon | green | <0.1 | <0.1 | 1.4 ± 0.1 b | <0.1 | 0.1 ± 0.01 b | <0.1 | 0.4 ± 0.03 b | 0.2 ± 0.01 b | 58.9 ± 4.2 a | <0.1 |
| Chemical Class | Animal Milk Powder (%) | Plant-Based Milk Powder (%) |
|---|---|---|
| Hydrocarbons | 41.7 | 24.9 |
| Heterocycles | 23.8 | 24.4 |
| Aldehydes | 9.2 | 33.8 |
| Ketones | 8.9 | 14.0 |
| Amides | 9.1 | 2.9 |
| Alcohols | 7.3 | - |
| Total | 100 | 100 |
| Flavor Profile | Animal Milk Powder (%) | Plant-Based Milk Powder (%) |
|---|---|---|
| Pungent | 37.0 | 28.1 |
| Green | 21.0 | 43.6 |
| Sweet | 15.7 | 28.3 |
| Gasoline | 26.3 | - |
| Total | 100 | 100 |
| Flavor | Sweet (%) | Green (%) | Astringent (%) | Creamy (%) | |
|---|---|---|---|---|---|
| Milk | |||||
| ABC | 5.43 | 1.32 | 25.74 | - | |
| ABCO | 0.14 | 1.77 | 2.16 | 66.09 | |
| ABD | 16.86 | 3.17 | 2.48 | 33.91 | |
| ABG | 4.41 | 1.98 | 25.75 | - | |
| ABM | 8.85 | 24.25 | 0.69 | - | |
| PBO | 4.93 | 14.50 | 12.99 | - | |
| PBS | 5.40 | 21.78 | 5.46 | - | |
| PBA | 10.78 | 11.96 | 11.58 | - | |
| PBB | 13.60 | 18.43 | 3.25 | - | |
| PBC | 29.60 | 0.84 | 9.90 | - | |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | |
| Acid Type | ABC | ABCO | ABD | ABG | ABM | PBO | PBS | PBA | PBB | PBC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| caproic acid | C6:0 | nd | 3.57 ± 0.25 a | nd | 0.98 ± 0.08 b | nd | nd | nd | nd | 1.29 ± 0.1 b | nd |
| caprylic acid | C8:0 | 0.46 ± 0.03 e | 3.79 ± 0.25 c | 6.77 ± 0.51 b | 3.19 ± 0.24 cd | 1.64 ± 0.1 de | 9.15 ± 0.85 a | 0.12 ± 0.01 e | 3.79 ± 0.2 c | 8.20 ± 0.6 ab | 7.75 ± 0.6 ab |
| capric acid | C10:0 | 0.48 ± 0.03 d | 4.34 ± 0.3 c | 11.32 ± 1.23 a | 10.63 ± 1.23 a | 4.48 ± 0.3 c | 6.54 ± 0.5 b | nd | nd | 5.79 ± 0.4 bc | 5.45 ± 0.5 bc |
| undecanoic acid | C11:0 | nd | nd | nd | nd | nd | 0.75 ± 0.04 a | nd | nd | 0.45 ± 0.04 b | nd |
| lauric acid | C12: 0 | 1.11 ± 0.09 c | 4.64 ± 0.25 bc | 10.47 ± 1.02 b | 5.34 ± 0.41 bc | 5.65 ± 0.42 bc | 40.3 ± 2.5 a | nd | 4.52 ± 0.3 bc | 38.27 ± 2.8 a | 39.3 ±2.8 a |
| tridecanoic acid | C13:0 | 0.25 ± 0.01 b | nd | nd | 0.18 ± 0.01 c | 0.25 ± 0.01 b | nd | nd | nd | 0.37 ± 0.04 | 0.13 ± 0.01 |
| myristic acid | C14:0 | 11.37 ± 1.2 c | 9.74 ± 0.8 c | 8.67 ± 0.75 c | 11.58 ± 1.25 bc | 10.42 ± 1.3 c | 15.49 ± 1.2 a | 1.66 ± 0.1 d | 4.90 ± 0.4 d | 14.72 ± 1.2 a | 14.96 ± 1.2 d |
| myristoleic acid | C14:1(n9) | 0.22 ± 0.02 b | nd | nd | 0.15 ± 0.01 b | 0.40 ± 0.03 a | nd | nd | nd | nd | 0.16 ± 0.01 b |
| pentadecanoic acid | C15:0 | 1.46 ± 0.1 c | 3.42 ± 0.21 b | 1.32 ± 0.15 e | 1.29 ± 0.13 c | 0.64 ± 0.05 d | nd | nd | 4.22 ± 0.4 a | 0.59 ± 0.04 d | 0.29 ± 0.01 de |
| pentadesenoic acid | C15:1 | 0.39 ± 0.02 b | nd | 1.16 ± 0.13 a | 0.11 ± 0.01 d | 0.24 ± 0.01 c | nd | nd | nd | nd | 0.14 ± 0.01 cd |
| palmitic acid | C16:0 | 31.67 ± 2.5 b | 15.92 ± 1.2 cde | 21.2 ± 2.2 c | 28.28 ± 2.4 b | 17.80 ± 1.23 cde | 18.56 ± 1.6 cd | 11.7 ± 1.2 e | 40.38 ± 3.8 a | 13.48 ± 1.2 de | 11.14 ± 1.1 e |
| palmitoleic acid | C16:1(n7) | 9.80 ± 0.85 c | 2.51 ± 0.18 bc | 3.26 ± 0.28 d | 0.65 ± 0.05 b | 4.23 ± 0.3 d | 0.1 ± 0.01 d | 0.32 ± 0.03 c | 2.35 ± 0.18 d | 0.42 ± 0.05 d | 0.12 ± 0.01 d |
| heptadecanoic acid | C17:0 | 1.93 ± 0.1 b | 2.94 ± 0.16 a | 2.08 ± 0.2 b | 0.84 ± 0.07 c | 0.45 ± 0.03 cde | 0.83 ± 0.07 c | 0.05 ± 0.002 e | 2.76 ± 0.15 a | 0.51 ± 0.04 cd | 0.14 ± 0.02 de |
| cis-10-hepta-decenoic acid | C17:1 | 0.75 ± 0.06 d | 3.96 ± 0.2 a | 1.31 ± 0.15 c | 0.44 ± 0.03 de | 0.13 ± 0.01 ef | nd | 0.55 ± 0.04 de | nd | 2.13 ± 0.21 b | 0.15 ± 0.02 ef |
| stearic acid | C18:0 | 12.89 ± 1.1 a | 12.09 ± 1.3 ab | 5.14 ± 0.42 ef | 9.87 ± 0.56 bc | 2.40 ± 0.17 gh | nd | 3.41 ± 0.21 fg | 12.53 ± 0.15 a | 9.16 ± 0.84 cd | 7.01 ± 0.6 de |
| oleic acid | C18:1 (n9) | 19.0 ± 1.2 bc | 17.69 ± 1.5 bc | 15.08 ± 1.3 c | 20.53 ± 1.8 ab | 24.45 ± 2.2 a | 1.89 ± 0.12 e | 15.7 ± 1.2 c | 8.72 ± 0.8 d | 1.13 ± 0.15 e | 6.80 ± 0.52 d |
| linoleic acid | C18:2 (n6) | 4.24 ± 0.3 c | 4.69 ± 0.3 c | 4.25 ± 0.32 c | 2.29 ± 0.16 c | 19.80 ± 1.5 b | 3.75 ± 0.2 c | 58.37 ± 4.2 a | 4.60 ± 0.42 c | 2.28 ± 0.26 c | 1.60 ± 0.02 c |
| γ-linolenic acid | C18:3(n6) | 0.68 ± 0.05 a | nd | nd | 0.44 ± 0.02 b | nd | nd | nd | nd | nd | 0.24 ± 0.02 c |
| α-linolenic acid | C18:3(n3) | 0.84 ± 0.04 d | 2.40 ± 0.16 c | 2.52 ± 0.15 c | 0.43 ± 0.03 ef | 6.41 ± 0.4 b | 0.82 ± 0.07 d | 7.39 ± 0.62 a | 2.65 ± 0.26 c | 0.38 ± 0.03 de | 0.15 ± 0.02 f |
| arachidic acid | C20:0 | nd | 0.20 ± 0.01 e | nd | 0.38 ± 0.02 d | 0.16 ± 0.01 e | 1.94 ± 0.2 b | 0.12 ± 0.01 e | 4.48 ± 0.5 a | 0.82 ± 0.07 c | 0.36 ± 0.03 d |
| gondoic acid | C20:1(n9) | 0.24 ± 0.01 e | 8.12 ± 0.67 a | 1.02 ± 0.13 c | 1.01 ± 0.12 c | 0.24 ± 0.02 e | nd | 0.65 ± 0.05 d | nd | nd | 3.46 ± 0.21 b |
| behenic acid | C22:0 | nd | nd | nd | 0.11 ± 0.01 b | nd | nd | nd | 4.10 ± 0.51 a | nd | nd |
| erucic acid | C22:1(n9) | nd | nd | nd | nd | 0.20 ± 0.02 a | nd | nd | nd | nd | nd |
| docosahexanoic + nervonic acid | C22:6(n3) + C24:1 | 1.25 ± 0.1 b | nd | 4.44 ± 0.34 a | 1.27 ± 0.01 b | nd | nd | nd | nd | nd | 0.62 ± 0.05 c |
| Index | ABC | ABCO | ABD | ABG | ABM | PBO | PBS | PBA | PBB | PBC |
|---|---|---|---|---|---|---|---|---|---|---|
| MUFA/SFA | 0.49 | 0.53 | 0.33 | 0.31 | 0.68 | 0.02 | 1.01 | 0.14 | 0.04 | 0.13 |
| PUFA/SFA | 0.11 | 0.12 | 0.17 | 0.06 | 0.60 | 0.05 | 3.87 | 0.09 | 0.03 | 0.03 |
| 0.42 | 0.51 | 1.64 | 0.62 | 0.32 | 0.22 | 0.13 | 0.58 | 0.17 | 0.42 | |
| AI | 2.09 | 1.51 | 2.01 | 2.93 | 1.16 | 18.42 | 0.22 | 3.52 | 17.45 | 8.21 |
| TI | 1.70 | 1.14 | 1.28 | 2.30 | 0.36 | 2.37 | 0.08 | 2.60 | 3.74 | 2.70 |
| h/H | 0.59 | 0.82 | 0.65 | 0.55 | 1.50 | 0.09 | 6.12 | 0.32 | 0.06 | 0.14 |
| HPI | 0.85 | 1.30 | 0.82 | 0.60 | 1.66 | 0.09 | 6.23 | 0.37 | 0.10 | 0.21 |
| NVI | 3.3 | 11.9 | 6.2 | 46.8 | 6.3 | 18.9 | 59.6 | 9.0 | 24.5 | 115.1 |
| PI | 0.04 | nd | 0.21 | 0.04 | nd | nd | nd | nd | nd | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippong, T.; Muresan, L.E.; Senila, L. Comparison of the Thermal Behavior and Chemical Composition of Milk Powders of Animal and Plant Origin. Foods 2025, 14, 389. https://doi.org/10.3390/foods14030389
Dippong T, Muresan LE, Senila L. Comparison of the Thermal Behavior and Chemical Composition of Milk Powders of Animal and Plant Origin. Foods. 2025; 14(3):389. https://doi.org/10.3390/foods14030389
Chicago/Turabian StyleDippong, Thomas, Laura Elena Muresan, and Lacrimioara Senila. 2025. "Comparison of the Thermal Behavior and Chemical Composition of Milk Powders of Animal and Plant Origin" Foods 14, no. 3: 389. https://doi.org/10.3390/foods14030389
APA StyleDippong, T., Muresan, L. E., & Senila, L. (2025). Comparison of the Thermal Behavior and Chemical Composition of Milk Powders of Animal and Plant Origin. Foods, 14(3), 389. https://doi.org/10.3390/foods14030389







