Effects of Conjugation with Basil Seed Gum on Physicochemical, Functional, Foaming, and Emulsifying Properties of Albumin, Whey Protein Isolate and Soy Protein Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BSG Conjugates
2.3. FT-IR Spectroscopy
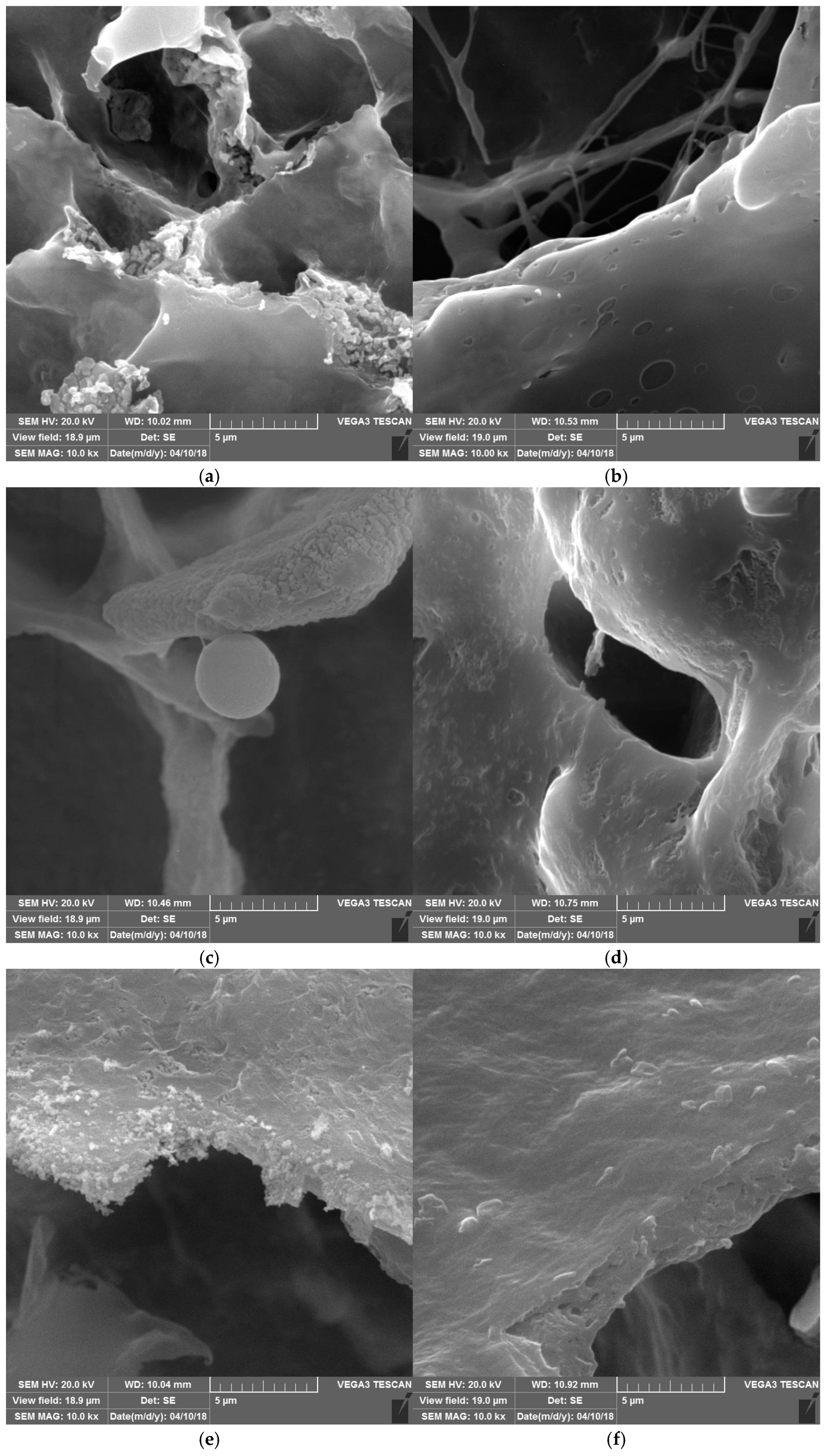
2.4. Microstructure (SEM)
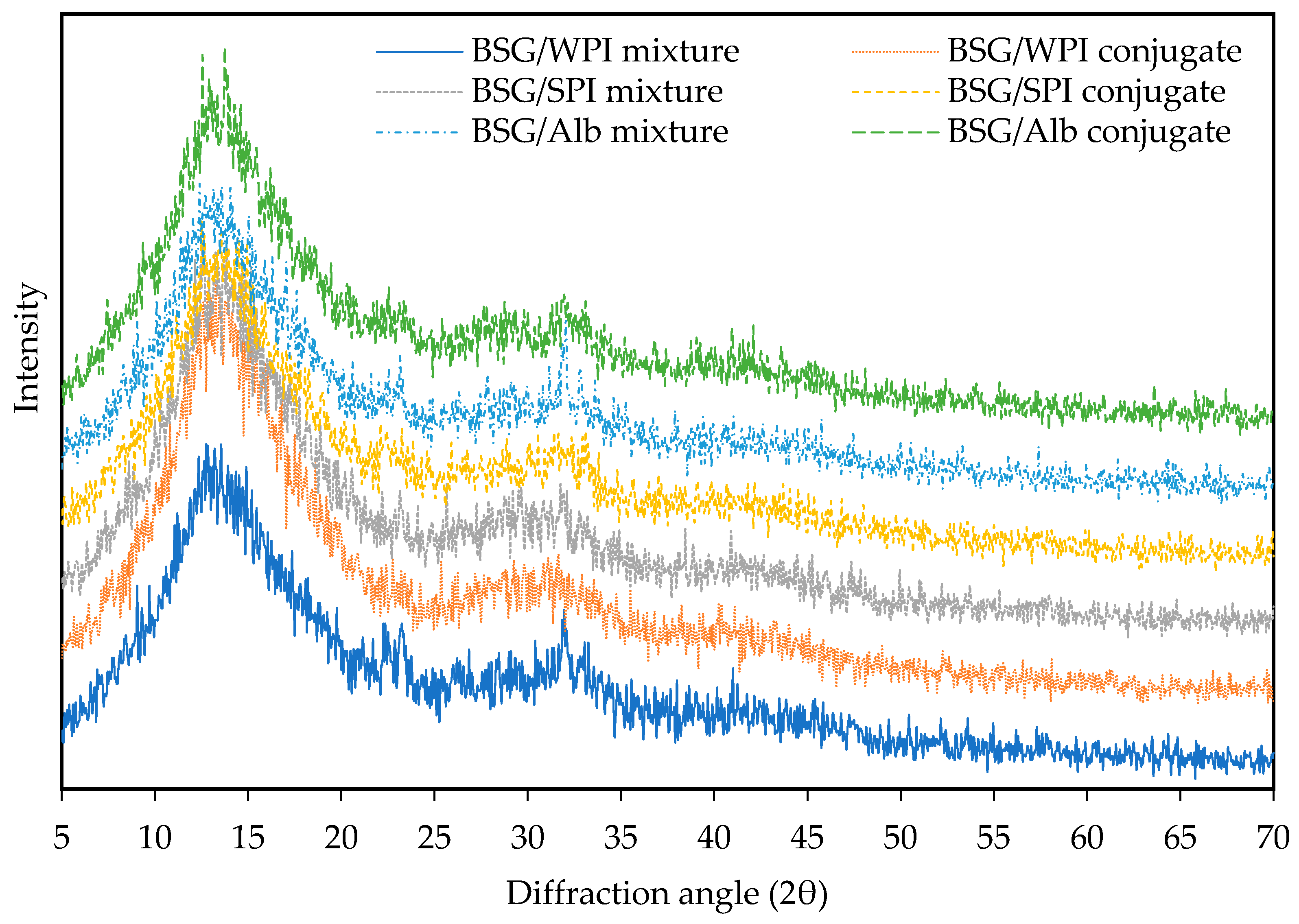
2.5. X-Ray Diffraction
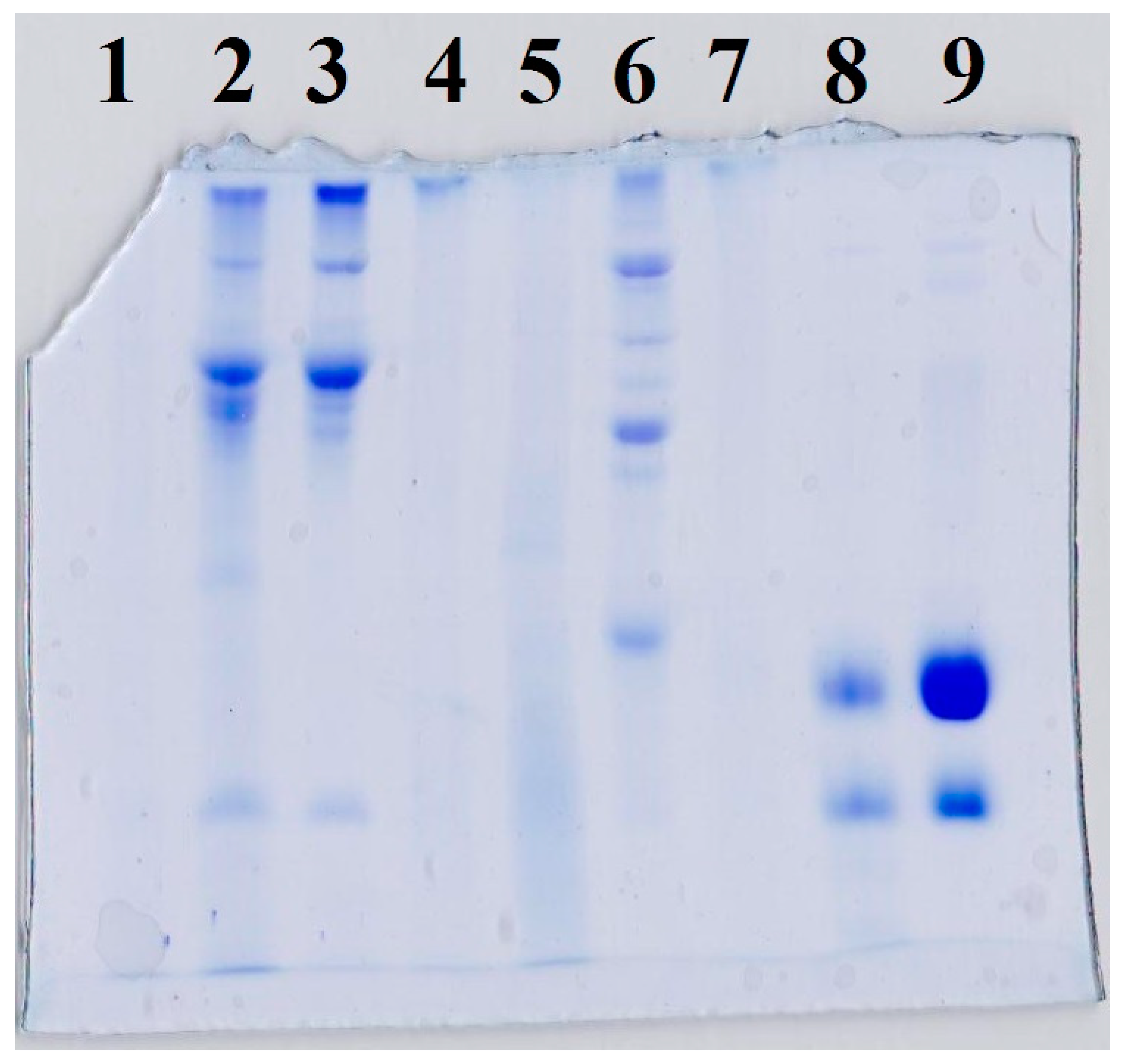
2.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Zeta Potential
2.8. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.9. Temperature Effects on Viscosity
2.10. Apparent Viscosity Measurement
2.11. Emulsion Preparation
2.11.1. Emulsion Activity
2.11.2. Emulsion Stability
2.11.3. Droplet Size
2.11.4. Zeta Potential
2.12. Determination of Foaming Capacity and Foam Stability
2.13. Statistical Analysis
3. Results and Discussion
3.1. FT-IR Analysis
3.2. Microstructure Properties
3.3. X-Ray Diffraction
3.4. Protein Analysis (SDS-PAGE)
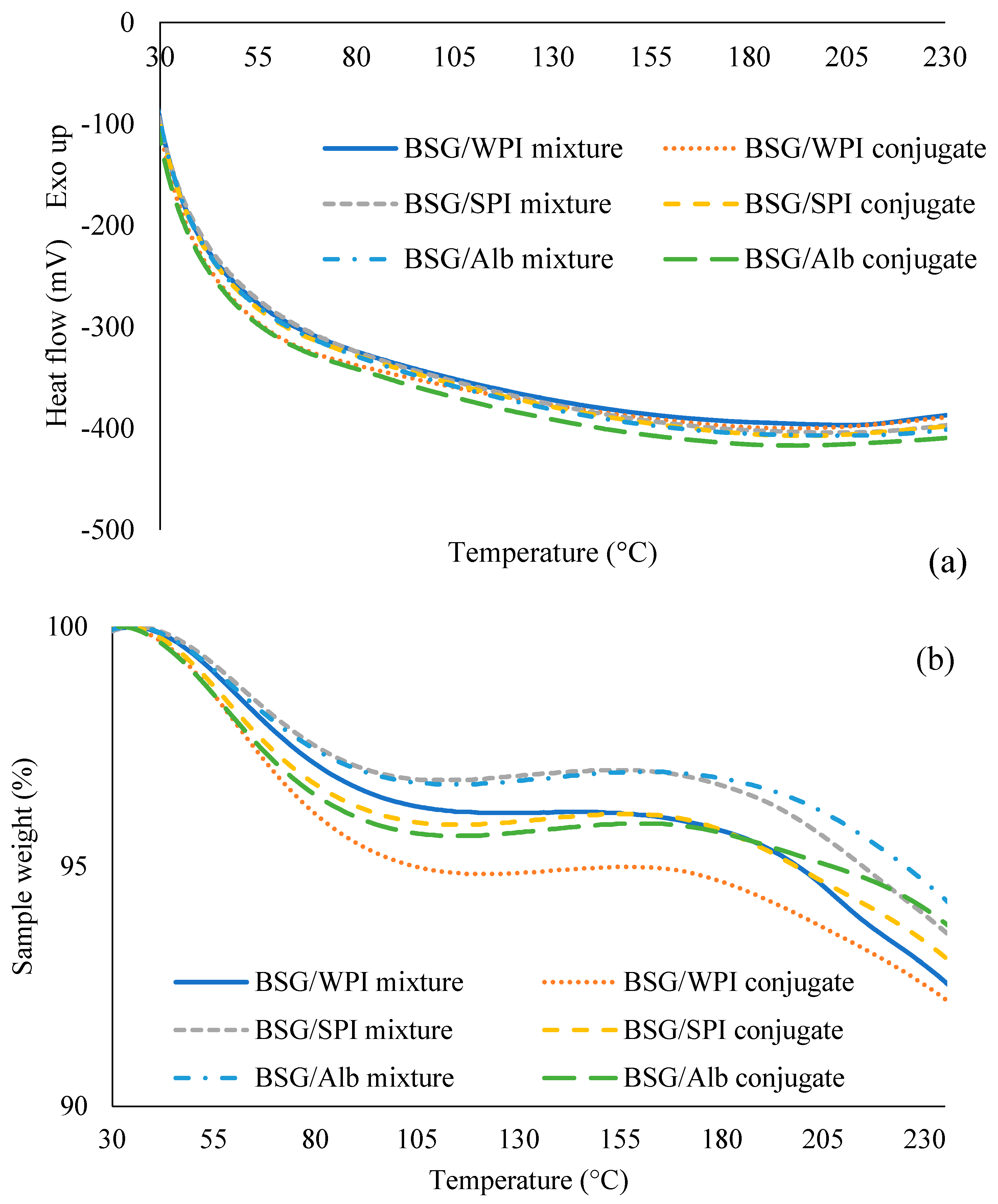
3.5. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
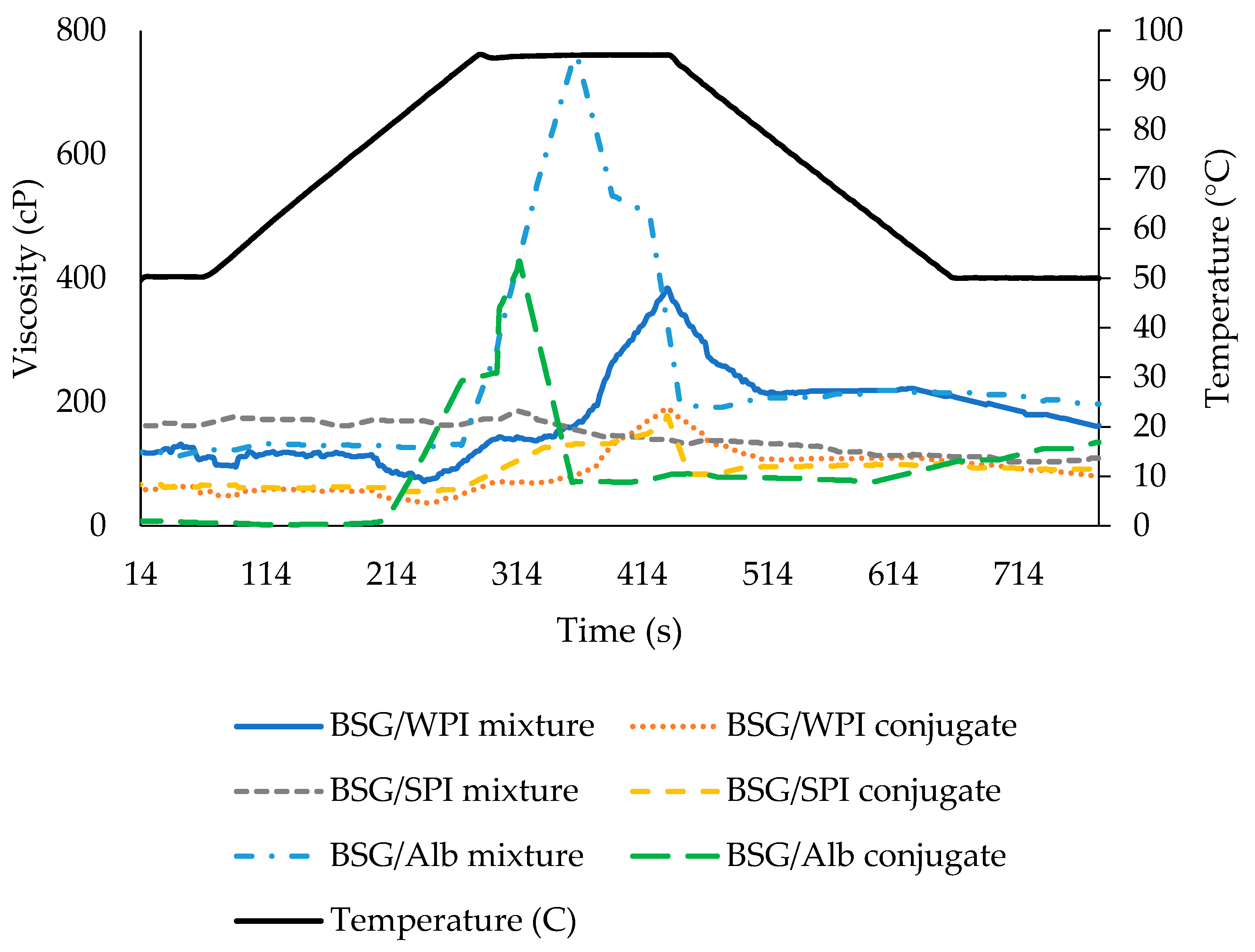
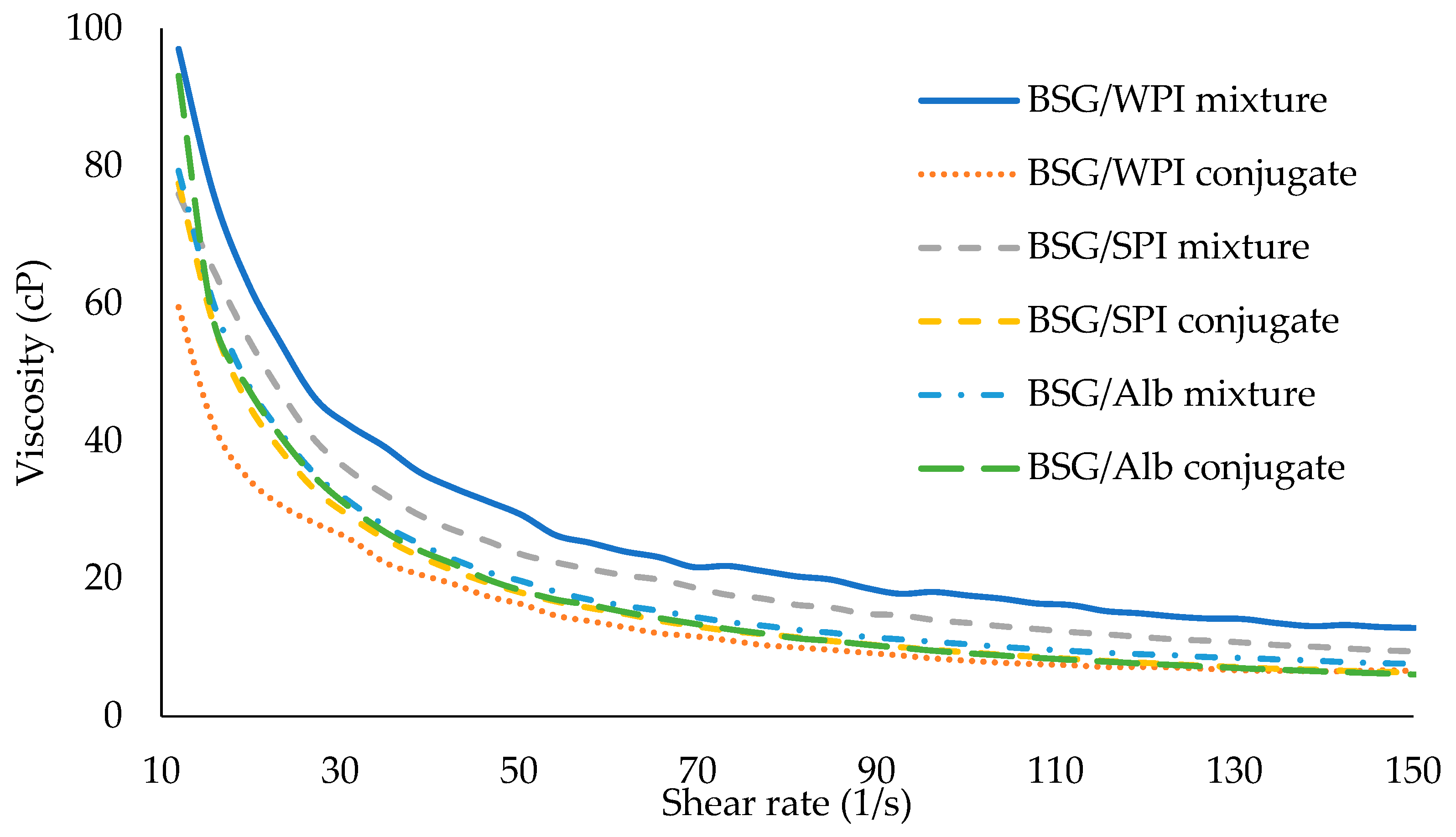
3.6. Rheological Results
3.7. Zeta Potential
3.8. Emulsion Properties
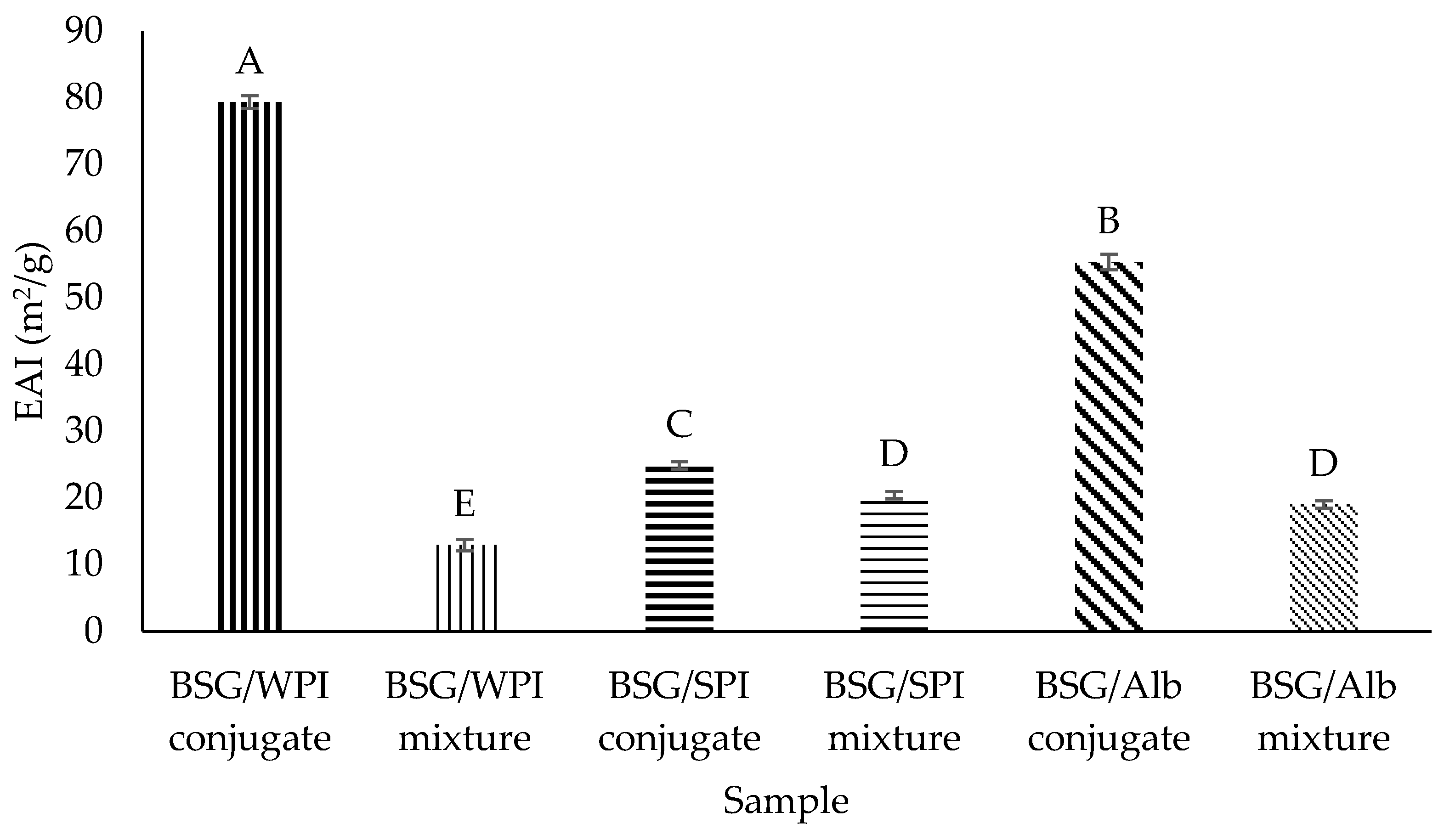
3.8.1. Emulsifying Activity Index (EAI) and Emulsion Stability (ES)
3.8.2. Droplet Size Distribution
3.8.3. Zeta Potential
3.9. Foaming Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malakar, M.; Mandal, D. Ocimum spp.(basil): An incredible plant. In Advances in Medicinal and Aromatic Plants; Apple Academic Press: Cambridge, MA, USA, 2024; pp. vol1: 89–vol81: 164. [Google Scholar]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil seeds as a novel food, source of nutrients and functional ingredients with beneficial properties: A review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B. New studies on basil (Ocimum bacilicum L.) seed gum: Part i–fractionation, physicochemical and surface activity characterization. Food Hydrocoll. 2016, 52, 350–358. [Google Scholar] [CrossRef]
- Hosseini-Parvar, S.; Matia-Merino, L.; Goh, K.; Razavi, S.M.A.; Mortazavi, S.A. Steady shear flow behavior of gum extracted from Ocimum basilicum L. Seed: Effect of concentration and temperature. J. Food Eng. 2010, 101, 236–243. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. New studies on basil (Ocimum bacilicum L.) seed gum: Part ii—Emulsifying and foaming characterization. Carbohydr. Polym. 2016, 149, 140–150. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Gumus, C.E.; Davidov-Pardo, G.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Impact of maillard conjugation on physicochemical stability and gastrointestinal fate. Food Hydrocoll. 2016, 60, 38–49. [Google Scholar] [CrossRef]
- Cai, B.; Saito, A.; Ikeda, S. Maillard conjugation of sodium alginate to whey protein for enhanced resistance to surfactant-induced competitive displacement from air–water interfaces. J. Agric. Food Chem. 2018, 66, 704–710. [Google Scholar] [CrossRef]
- Bi, B.; Yang, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Characterization and emulsifying properties of β-lactoglobulin-gum acacia seyal conjugates prepared via the maillard reaction. Food Chem. 2017, 214, 614–621. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Shin, W.-S. Functional improvements in bovine serum albumin–fucoidan conjugate through the maillard reaction. Food Chem. 2016, 190, 974–981. [Google Scholar] [CrossRef]
- Koshani, R.; Aminlari, M.; Niakosari, M.; Farahnaky, A.; Mesbahi, G. Production and properties of tragacanthin-conjugated lysozyme as a new multifunctional biopolymer. Food Hydrocoll. 2015, 47, 69–78. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Aminlari, M.; Moosavinasab, M. Preparation of and studies on the functional properties and bactericidal activity of the lysozyme–xanthan gum conjugate. LWT-Food Sci. Technol. 2014, 57, 594–602. [Google Scholar] [CrossRef]
- Zhao, M.; He, H.; Ma, A.; Hou, T. Sources, chemical synthesis, functional improvement and applications of food-derived protein/peptide-saccharide covalent conjugates: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5985–6004. [Google Scholar] [CrossRef] [PubMed]
- Aziznia, S.; Askari, G.; Emamdjomeh, Z.; Salami, M. Effect of ultrasonic assisted grafting on the structural and functional properties of mung bean protein isolate conjugated with maltodextrin through maillard reaction. Int. J. Biol. Macromol. 2024, 254, 127616. [Google Scholar] [CrossRef]
- Ali, N.; Saeidy, S. Maillard modification. In Physicochemical and Enzymatic Modification of Gums: Synthesis, Characterization and Application; Springer: Berlin/Heidelberg, Germany, 2022; pp. 77–98. [Google Scholar]
- Hashemi, M.M.; Aminlari, M.; Forouzan, M.M.; Moghimi, E.; Tavana, M.; Shekarforoush, S.; Mohammadifar, M.A. Production and application of lysozyme-gum arabic conjugate in mayonnaise as a natural preservative and emulsifier. Pol. J. Food Nutr. Sci. 2018, 68, 33–43. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E. Whey protein–maltodextrin conjugates as emulsifying agents: An alternative to gum arabic. Food Hydrocoll. 2007, 21, 607–616. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.; Qin, Y.; Zhu, S.; Gao, Q.; Liu, D. The synthesis, biological activities and applications of protein–polysaccharide conjugates in food system: A review. Food Qual. Saf. 2023, 7, fyad006. [Google Scholar] [CrossRef]
- Siddiquy, M.; JiaoJiao, Y.; Rahman, M.H.; Iqbal, M.W.; Al-Maqtari, Q.A.; Easdani, M.; Yiasmin, M.N.; Ashraf, W.; Hussain, A.; Zhang, L. Advances of protein functionalities through conjugation of protein and polysaccharide. Food Bioprocess Technol. 2024, 17, 2077–2097. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Wang, N.; Luo, J.; Li, M.; Li, S.; Hemar, Y. Conjugation of chitopentaose with β-lactoglobulin using maillard reaction, and its effect on the allergic desensitization in vivo. Int. J. Biol. Macromol. 2024, 258, 128913. [Google Scholar] [CrossRef]
- Urango, A.C.M.; Meireles, M.A.A.; Silva, E.K. Maillard conjugates produced from proteins and prebiotic dietary fibers: Technological properties, health benefits and challenges. Trends Food Sci. Technol. 2024, 147, 104438. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, Z.; Li, Y.; Qi, B. Effects of polysaccharide type on the structure, interface behavior, and foam properties of soybean protein isolate hydrolysate-polysaccharide maillard conjugates. Food Hydrocoll. 2024, 151, 109801. [Google Scholar] [CrossRef]
- Tao, L.; Wang, H.; Wang, J.; Zhang, J.; Yu, L.; Song, S. Characterization and emulsion stability of soybean protein isolate/soybean peptide and ginseng polysaccharide conjugates. LWT 2024, 196, 115860. [Google Scholar] [CrossRef]
- Amiratashani, F.; Yarmand, M.S.; Kiani, H.; Askari, G.; Naeini, K.K.; Parandi, E. Comprehensive structural and functional characterization of a new protein-polysaccharide conjugate between grass pea protein (Lathyrus sativus) and xanthan gum produced by wet heating. Int. J. Biol. Macromol. 2024, 254, 127283. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Hu, Y.; Huang, Y.; Fan, X.; Chen, G.; Ye, H.; Zeng, X. Characterization of whey protein isolate-gum Arabic Maillard conjugate and evaluation of the effects of conjugate-stabilized emulsion on microbiota of human fecal cultures. Food Hydrocoll. 2023, 134, 108060. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Chen, B.; Chen, L.; Li, C.; Huang, W.; Zhao, Y.; Ai, C.; Teng, H. Ultrasound-assisted glycosylation of ovalbumin and dextran conjugate carrier for anthocyanins and their stability evaluation. Ultrason. Sonochem. 2024, 109, 107024. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Khalesi, M.; Van der Meeren, P.; Hosseini, S.M.H. Rheological and interfacial properties of basil seed gum modified with octenyl succinic anhydride. Food Hydrocoll. 2020, 101, 105489. [Google Scholar] [CrossRef]
- Kheynoor, N.; Hosseini, S.M.H.; Yousefi, G.-H.; Gahruie, H.H.; Mesbahi, G.-R. Encapsulation of vitamin c in a rebaudioside-sweetened model beverage using water in oil in water double emulsions. LWT 2018, 96, 419–425. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of basil seed gum-based edible films incorporated with zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Gahruie, H.H.; Razmjooie, M.; Sepeidnameh, M.; Rastehmanfard, M.; Tatar, M.; Naghibalhossaini, F.; Van der Meeren, P. Effects of novel and conventional thermal treatments on the physicochemical properties of iron-loaded double emulsions. Food Chem. 2019, 270, 70–77. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Zhang, S.; Dongye, Z.; Kang, M.; Li, Z.; Chen, C.; Qian, Y.; Ren, Y. The rheological/interfacial behavior and stability properties of nanoemulsions prepared using whey protein-carboxymethyl chitosan conjugates. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130924. [Google Scholar] [CrossRef]
- Al-Lafi, A.G.; Isam, A.-N. Application of 2d-cos-ftir spectroscopic analysis to milk powder adulteration: Detection of melamine. J. Food Compos. Anal. 2022, 113, 104720. [Google Scholar] [CrossRef]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Moremedi, T.; Katata-Seru, L.; Sardar, S.; Bandyopadhyay, A.; Makhado, E.; Hato, M.J. Application of synthetic monomers grafted xanthan gum for rhodamine b removal in aqueous solution. Int. J. Mater. Metall. Eng. 2020, 14, 123–131. [Google Scholar]
- Tayel, A.A.; Ebaid, A.M.; Otian, A.M.; Mahrous, H.; El Rabey, H.A.; Salem, M.F. Application of edible nanocomposites from chitosan/fenugreek seed mucilage/selenium nanoparticles for protecting lemon from green mold. Int. J. Biol. Macromol. 2024, 273, 133109. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghorbani, M.; Sadeghi Mahoonak, A.; Jafari, S.M. Improving the oxidative stability of purslane seed oil via emulsions stabilized by whey protein isolate-inulin mixtures and conjugates. Innov. Food Technol. 2024, 11, 226–248. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Niu, L.Y.; Jiang, S.T.; Pan, L.J.; Zhai, Y.S. Characteristics and functional properties of wheat germ protein glycated with saccharides through maillard reaction. Int. J. Food Sci. Technol. 2011, 46, 2197–2203. [Google Scholar] [CrossRef]
- Yadav, M.P.; Strahan, G.D.; Mukhopadhyay, S.; Hotchkiss, A.T.; Hicks, K.B. Formation of corn fiber gum–milk protein conjugates and their molecular characterization. Food Hydrocoll. 2012, 26, 326–333. [Google Scholar] [CrossRef]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S.; Jasniewski, J. Structural properties of canola protein isolate-gum arabic maillard conjugate in an aqueous model system. Food Hydrocoll. 2018, 79, 228–234. [Google Scholar] [CrossRef]
- Diftis, N.; Kiosseoglou, V. Improvement of emulsifying properties of soybean protein isolate by conjugation with carboxymethyl cellulose. Food Chem. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Nakamura, S.; Gohya, Y.; Losso, J.N.; Nakai, S.; Kato, A. Protective effect of lysozyme-galactomannan or lysozyme-palmitic acid conjugates against Edwardsiella tarda infection in carp, Cyprinus carpio L. FEBS Lett. 1996, 383, 251–254. [Google Scholar] [CrossRef]
- Kasran, M.; Cui, S.W.; Goff, H.D. Covalent attachment of fenugreek gum to soy whey protein isolate through natural maillard reaction for improved emulsion stability. Food Hydrocoll. 2013, 30, 552–558. [Google Scholar] [CrossRef]
- Johnson, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ibanoglu, E.; Erçelebi, E.A. Thermal denaturation and functional properties of egg proteins in the presence of hydrocolloid gums. Food Chem. 2007, 101, 626–633. [Google Scholar] [CrossRef]
- Boye, J.I.; Alli, I.; Ismail, A.A. Interactions involved in the gelation of bovine serum albumin. J. Agric. Food Chem. 1996, 44, 996–1004. [Google Scholar] [CrossRef]
- Takahashi, K.; Lou, X.-F.; Ishii, Y.; Hattori, M. Lysozyme-glucose stearic acid monoester conjugate formed through the maillard reaction as an antibacterial emulsifier. J. Agric. Food Chem. 2000, 48, 2044–2049. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Mirzapour, A.; Ghiasi, F.; Eskandari, M.H.; Moosavi-Nasab, M.; Hosseini, S.M.H. Development and characterization of gelatin and persian gum composite edible films through complex coacervation. LWT 2022, 153, 112422. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Ikeda, S. Isolation and characterization of hydrocolloids from monoi (Cissampelos pareira) leaves. Food Hydrocoll. 2006, 20, 885–891. [Google Scholar] [CrossRef]
- Sadar, L.N. Rheological and Textural Characteristics of Copolymerized Hydrocolloidal Solutions Containing Curdlan Gum. Master’s Thesis, University of Maryland, College Park, MD, USA, 2004. [Google Scholar]
- Liang, R.-H.; Wang, L.-H.; Chen, J.; Liu, W.; Liu, C.-M. Alkylated pectin: Synthesis, characterization, viscosity and emulsifying properties. Food Hydrocoll. 2015, 50, 65–73. [Google Scholar] [CrossRef]
- Wang, W.-D.; Li, C.; Chen, C.; Fu, X.; Liu, R.H. Effect of chitosan oligosaccharide glycosylation on the emulsifying property of lactoferrin. Int. J. Biol. Macromol. 2022, 209, 93–106. [Google Scholar] [CrossRef]
- Kan, X.; Chen, G.; Zhou, W.; Zeng, X. Application of protein-polysaccharide maillard conjugates as emulsifiers: Source, preparation and functional properties. Food Res. Int. 2021, 150, 110740. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Zareie, Z.; Alkobeisi, F. Behavior of protein-polysaccharide conjugate-stabilized food emulsions under various destabilization conditions. Food Chem. X 2023, 18, 100725. [Google Scholar] [CrossRef] [PubMed]
- Lavaei, Y.; Varidi, M.; Nooshkam, M. Gellan gum conjugation with soy protein via maillard-driven molecular interactions and subsequent clustering lead to conjugates with tuned technological functionality. Food Chem. X 2022, 15, 100408. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, Y. Acylation modification and/or guar gum conjugation enhanced functional properties of pea protein isolate. Food Hydrocoll. 2021, 117, 106686. [Google Scholar] [CrossRef]
- Ghayour, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.-R.; Gahruie, H.H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- Márquez, A.L.; Palazolo, G.G.; Wagner, J.R. Water in oil (w/o) and double (w/o/w) emulsions prepared with spans: Microstructure, stability, and rheology. Colloid Polym. Sci. 2007, 285, 1119–1128. [Google Scholar] [CrossRef]
- Wang, H.; Williams, P.A.; Senan, C. Synthesis, characterization and emulsification properties of dodecenyl succinic anhydride derivatives of gum arabic. Food Hydrocoll. 2014, 37, 143–148. [Google Scholar] [CrossRef]
- Niakousari, M.; Damyeh, M.S.; Gahruie, H.H.; Bekhit, A.E.D.A.; Greiner, R.; Roohinejad, S. Conventional emulsions. In Emulsion-Based Systems for Delivery of Food Active Compounds: Formation, Application, Health and Safety; Wiley: Hoboken, NJ, USA, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Chen, H.; Ji, A.; Qiu, S.; Liu, Y.; Zhu, Q.; Yin, L. Covalent conjugation of bovine serum album and sugar beet pectin through maillard reaction/laccase catalysis to improve the emulsifying properties. Food Hydrocoll. 2018, 76, 173–183. [Google Scholar] [CrossRef]
- Feng, S.; Guo, Y.; Liu, F.; Li, Z.; Chen, K.; Handa, A.; Zhang, Y. The impacts of complexation and glycated conjugation on the performance of soy protein isolate-gum Arabic composites at the o/w interface for emulsion-based delivery systems. Food Hydrocoll. 2023, 135, 108168. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A.; Siddiqi, R.A. Effect of guar gum conjugation on functional, antioxidant and antimicrobial activity of egg white lysozyme. Food Chem. 2018, 240, 1201–1209. [Google Scholar] [CrossRef]
- Naji, S.; Razavi, S.M.; Karazhiyan, H. Effect of thermal treatments on functional properties of cress seed (Lepidium sativum) and xanthan gums: A comparative study. Food Hydrocoll. 2012, 28, 75–81. [Google Scholar] [CrossRef]
- Jahanbin, K.; Moini, S.; Gohari, A.R.; Emam-Djomeh, Z.; Masi, P. Isolation, purification and characterization of a new gum from Acanthophyllum bracteatum roots. Food Hydrocoll. 2012, 27, 14–21. [Google Scholar] [CrossRef]
- Koocheki, A.; Taherian, A.R.; Bostan, A. Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Res. Int. 2013, 50, 446–456. [Google Scholar] [CrossRef]
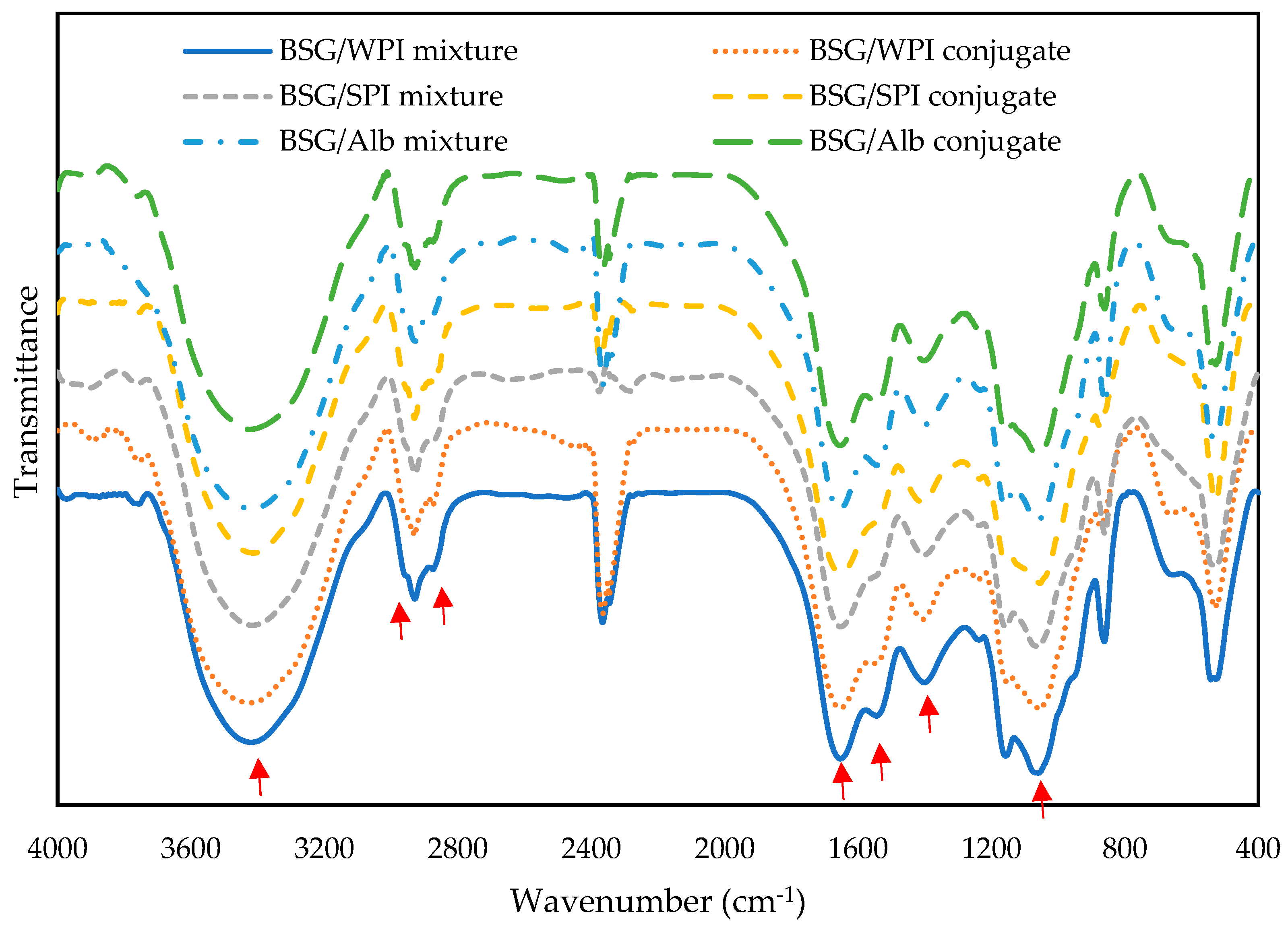

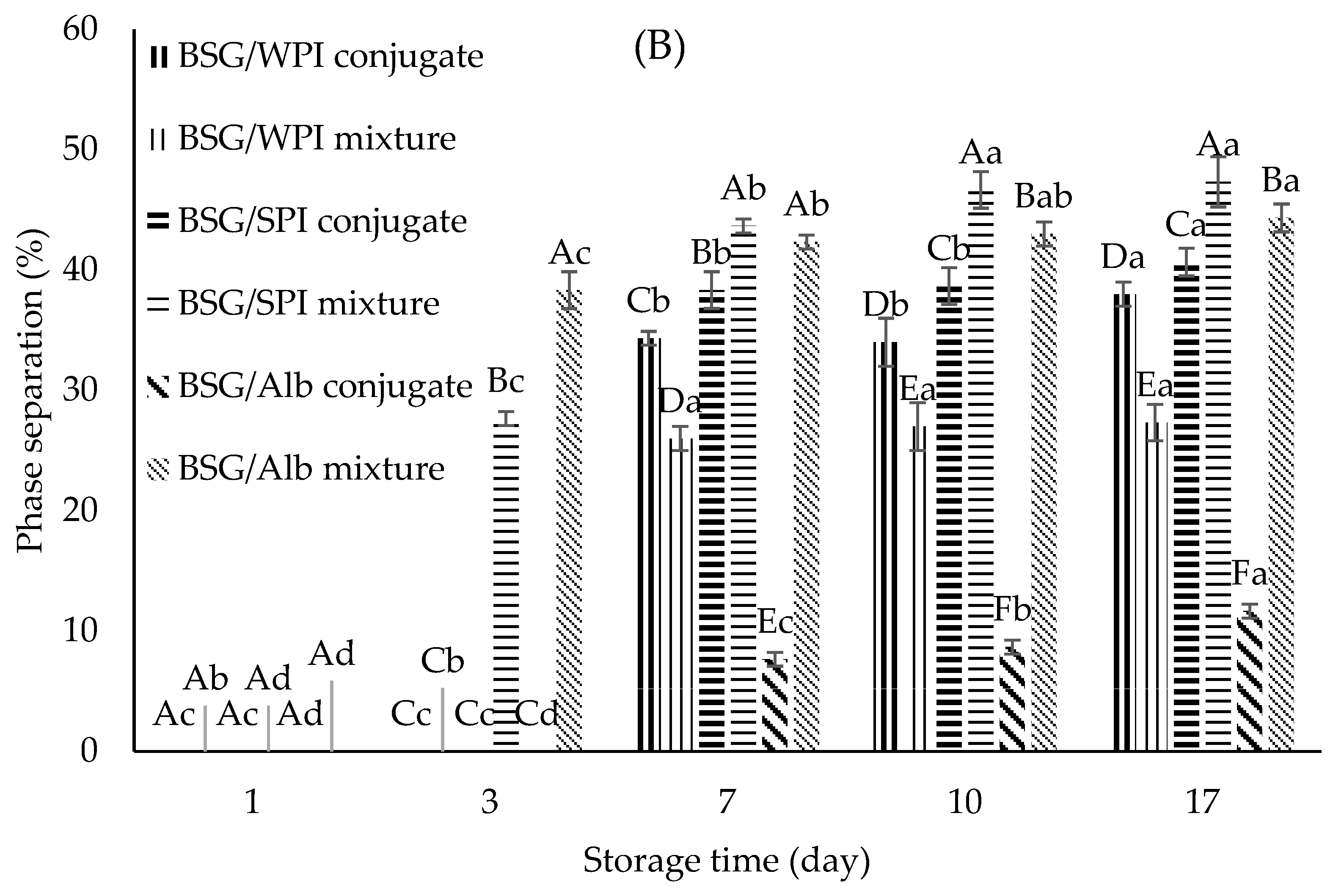
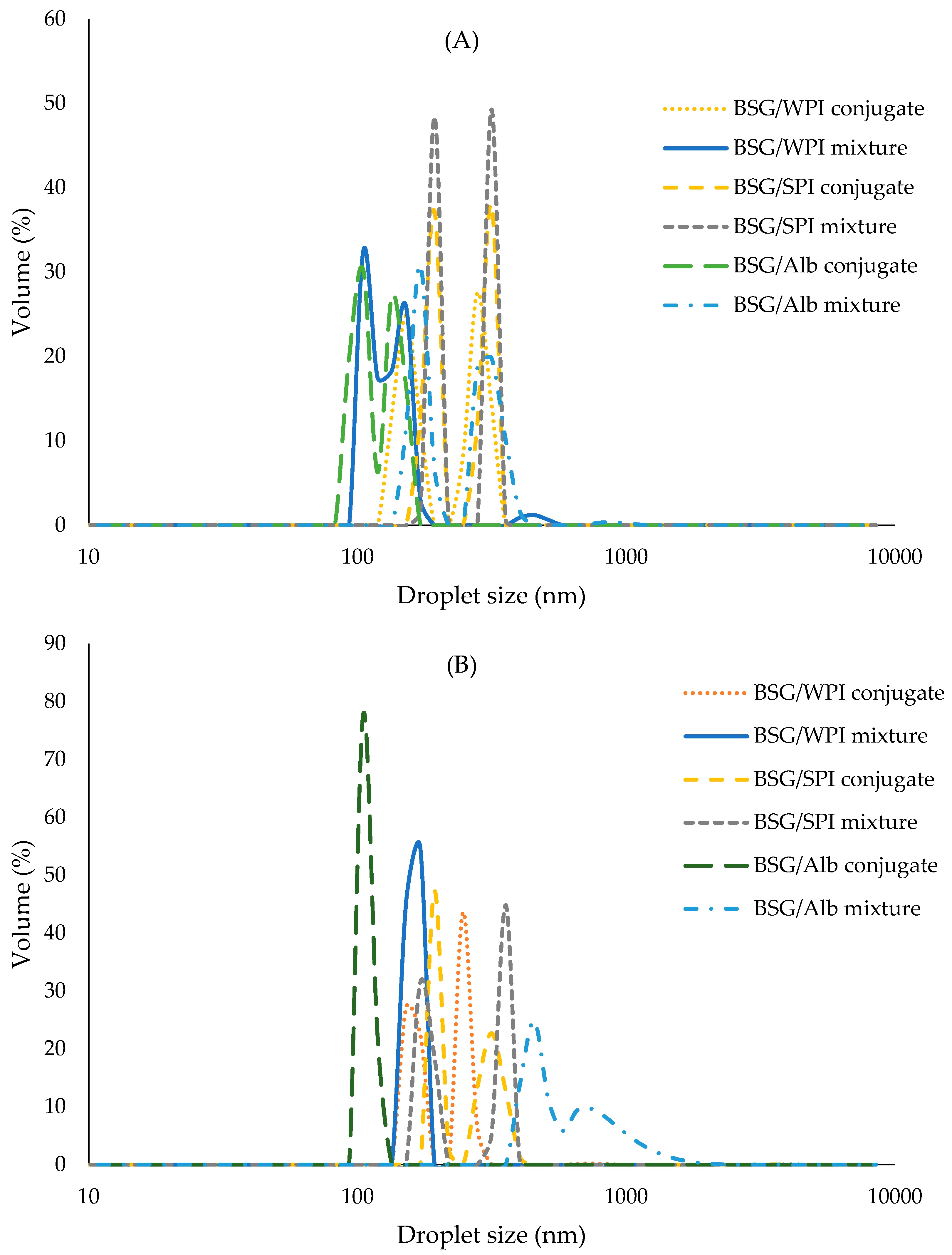
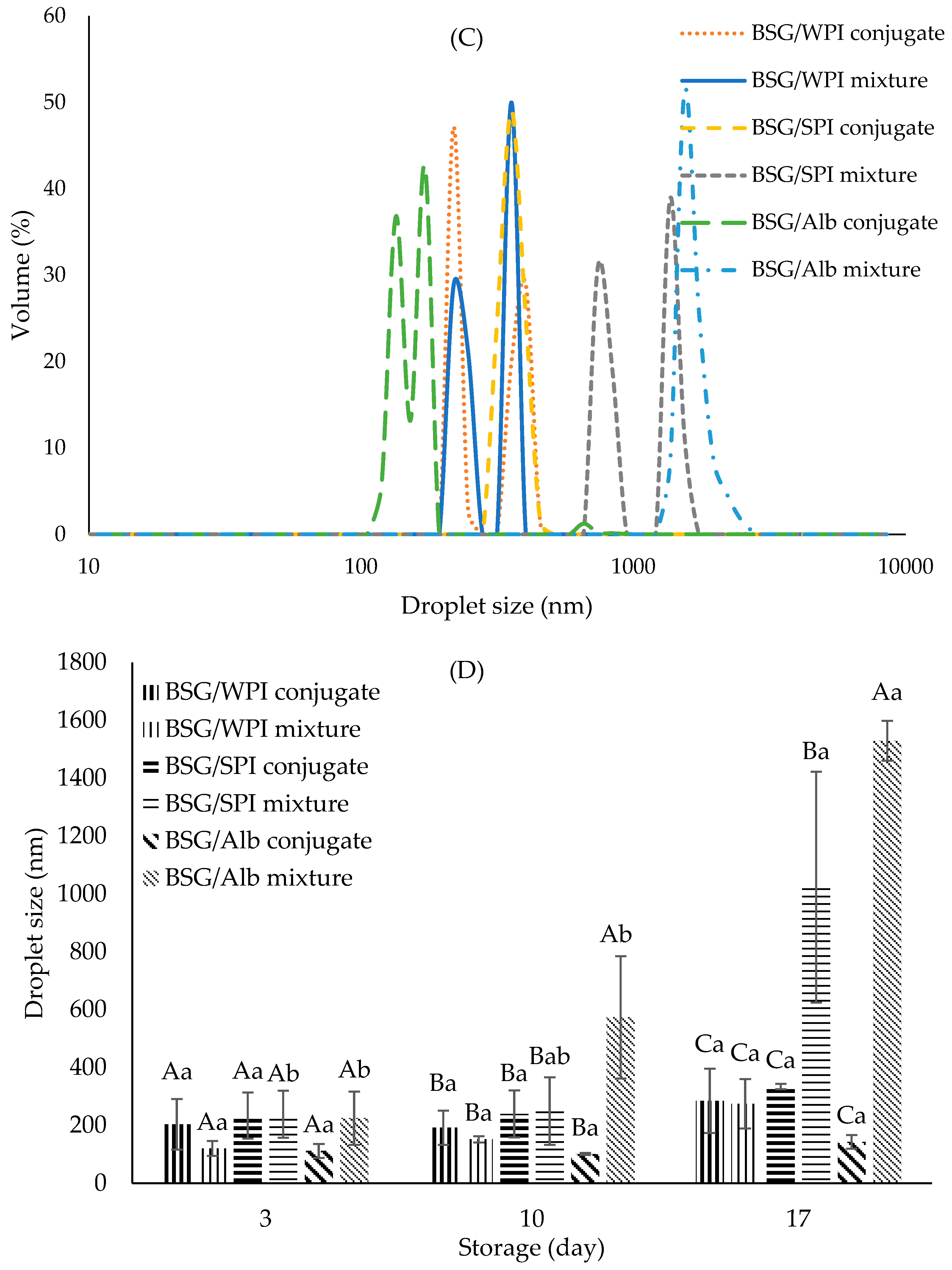


| Samples’ Wavenumber (cm−1) | ||||||
|---|---|---|---|---|---|---|
| Assignment | BSG/WPI Mixture | BSG/WPI Conjugate | BSG/SPI Mixture | BSG/SPI Conjugate | BSG/Alb Mixture | BSG/Alb Conjugate |
| -OH stretching vibrations | 3420 | 3424 | 3417 | 3412 | 3418 | 3425 |
| -CH2- and >CH- stretching and bending vibrations | 2929 | 2929 | 2928 | 2931 | 2928 | 2928 |
| C-H absorption | 2874 | 2874 | 2878 | 2873 | 2878 | 2870 |
| C=O stretching as amide I | 1653 | 1654 | 1653 | 1654 | 1655 | 1654 |
| -NH twisting (amide II) | 1545 | 1542 | 1540 | 1544 | 1544 | 1545 |
| C=N stretching vibrations | 1402 | 1406 | 1402 | 1408 | 1400 | 1402 |
| -OH bending vibration | 1238 | 1240 | 1238 | 1236 | 1236 | 1235 |
| C-O-C stretching vibrations of glycosidic bonds | 1060 | 1055 | 1060 | 1055 | 1060 | 1055 |
| Samples | Denaturation Temperature (°C) | |||
|---|---|---|---|---|
| Toneset | Tg | Tendset | ∆H (J/g) | |
| BSG/WPI conjugate | 198.12 | 210.21 | 222.60 | −52.47 |
| BSG/WPI mixture | 164.06 | 190.30 | 217.35 | −203.36 |
| BSG/SPI conjugate | 182.18 | 207.21 | 226.73 | −124.66 |
| BSG/SPI mixture | 162.76 | 192.91 | 222.52 | −336.31 |
| BSG/Alb conjugate | 185.65 | 210.90 | 224.86 | −67.07 |
| BSG/Alb mixture | 176.77 | 196.66 | 219.57 | −80.82 |
| Bingham | Casson | Power Law | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vis (cP) at 50.3 1/s | K (Pa sn) | τ0 (Pa) | R2 | K (Pa sn) | τ0 (Pa) | R2 | K (mPa sn) | n | R2 | |
| BSG/WPI mixture | 29.27 ± 3.67 * | 4.95 ± 0.72 | 10.24 ± 2.56 | 96.73 ± 1.86 | 1.28 ± 0.17 | 7.80 ± 2.28 | 98.73 ± 0.42 | 536.10 ± 185.12 | 0.23 ± 0.04 | 97.60 ± 0.56 |
| BSG/WPI conjugate | 16.30 ± 1.57 | 2.14 ± 0.90 | 7.06 ± 2.30 | 96.83 ± 3.50 | 0.69 ± 0.01 | 3.36 ± 1.16 | 98.50 ± 1.68 | 449.00 ± 352.26 | 0.26 ± 0.16 | 96.97 ± 3.50 |
| BSG/SPI mixture | 23.53 ± 1.83 | 2.96 ± 0.18 | 15.38 ± 8.70 | 95.70 ± 2.95 | 0.53 ± 0.09 | 8.64 ± 0.53 | 98.20 ± 1.25 | 646.20 ± 4.67 | 0.17 ± 0.02 | 96.67 ± 1.76 |
| BSG/SPI conjugate | 17.97 ± 2.88 | 0.38 ± 0.11 | 7.42 ± 2.83 | 99.13 ± 0.29 | 0.02 ± 0.01 | 7.21 ± 2.85 | 99.57 ± 0.15 | 686.97 ± 287.90 | 0.03 ± 0.02 | 99.07 ± 0.32 |
| BSG/Alb mixture | 19.64 ± 2.25 | 1.41 ± 0.62 | 9.16 ± 1.26 | 99.00 ± 0.40 | 0.07 ± 0.03 | 8.44 ± 1.35 | 99.27 ± 0.35 | 746.20 ± 147.77 | 0.08 ± 0.04 | 98.00 ± 0.95 |
| BSG/Alb conjugate | 18.28 ± 6.59 | 0.05 ± 0.05 | 9.43 ± 3.46 | 99.07 ± 0.38 | 0.01 ± 0.01 | 9.51 ± 3.61 | 99.50 ± 0.26 | 967.87 ± 390.47 | 0.01 ± 0.01 | 98.90 ± 0.56 |
| BSG/WPI Mixture | BSG/WPI Conjugate | BSG/SPI Mixture | BSG/SPI Conjugate | BSG/Alb Mixture | BSG/Alb Conjugate | |
|---|---|---|---|---|---|---|
| Zeta potential | −39.50 ± 3.54 A | −45.43 ± 2.06 B | −41.60 ± 1.05 AB | −45.30 ± 0.66 B | −43.83 ± 4.15 AB | −51.97 ± 0.81 C |
| Storage Time (day) | ||||
|---|---|---|---|---|
| Sample | 3 | 7 | 10 | 17 |
| BSG/WPI conjugate | −61.50 ± 0.61 Ab | −58.53 ± 2.47 Bab | −57.70 ± 0.14 BCa | −57.60 ± 1.37 CDa |
| BSG/WPI mixture | −60.23 ± 1.61 Aa | −58.33 ± 2.64 Ba | −58.40 ± 1.51 BCa | −57.83 ± 1.48 CDa |
| BSG/SPI conjugate | −61.70 ± 1.35 Ab | −57.77 ± 1.60 ABa | −56.13 ± 0.81 Ba | −56.17 ± 2.01 BCa |
| BSG/SPI mixture | −60.60 ± 1.45 Ab | −54.23 ± 2.47 Aa | −53.67 ± 1.00 Aa | −54.13 ± 0.68 ABa |
| BSG/Alb conjugate | −73.23 ± 2.97 Bc | −67.13 ± 2.25 Cb | −59.77 ± 2.03 Ca | −58.90 ± 1.57 Da |
| BSG/Alb mixture | −61.37 ± 1.80 Ab | −54.77 ± 0.84 ABa | −53.77 ± 0.49 Aa | −53.13 ± 0.61 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi, H.; Eskandari, M.H.; Khalesi, M.; Golmakani, M.-T.; Niakousari, M.; Hosseini, S.M.H. Effects of Conjugation with Basil Seed Gum on Physicochemical, Functional, Foaming, and Emulsifying Properties of Albumin, Whey Protein Isolate and Soy Protein Isolate. Foods 2025, 14, 390. https://doi.org/10.3390/foods14030390
Hashemi H, Eskandari MH, Khalesi M, Golmakani M-T, Niakousari M, Hosseini SMH. Effects of Conjugation with Basil Seed Gum on Physicochemical, Functional, Foaming, and Emulsifying Properties of Albumin, Whey Protein Isolate and Soy Protein Isolate. Foods. 2025; 14(3):390. https://doi.org/10.3390/foods14030390
Chicago/Turabian StyleHashemi, Hadi, Mohammad Hadi Eskandari, Mohammadreza Khalesi, Mohammad-Taghi Golmakani, Mehrdad Niakousari, and Seyed Mohammad Hashem Hosseini. 2025. "Effects of Conjugation with Basil Seed Gum on Physicochemical, Functional, Foaming, and Emulsifying Properties of Albumin, Whey Protein Isolate and Soy Protein Isolate" Foods 14, no. 3: 390. https://doi.org/10.3390/foods14030390
APA StyleHashemi, H., Eskandari, M. H., Khalesi, M., Golmakani, M.-T., Niakousari, M., & Hosseini, S. M. H. (2025). Effects of Conjugation with Basil Seed Gum on Physicochemical, Functional, Foaming, and Emulsifying Properties of Albumin, Whey Protein Isolate and Soy Protein Isolate. Foods, 14(3), 390. https://doi.org/10.3390/foods14030390









