Enhancing Rice Bran Soluble Dietary Fiber Yield Through Sequential Ultrasound–Xylanase Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Pretreatments
2.3. RBDF Extraction
2.4. Single Modification of RBDF Samples
2.4.1. Enzymatic Treatment
2.4.2. Ultrasound Treatment
2.4.3. AMF Treatment
2.5. Combined Modifications of RBDF Samples
2.5.1. Ultrasound-Assisted Enzyme Treatment
2.5.2. AMF-Assisted Enzyme Treatment
2.6. Response Surface Analysis Method (RSM) Design
2.7. Structural Characterization
2.7.1. Scanning Electron Microscopy (SEM)
2.7.2. Dynamic Light Scattering (DLS) Analysis
2.7.3. X-Ray Diffraction (XRD) Spectroscopy
2.7.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.8. Hydration Properties
2.8.1. Water-Holding Capacity (WHC)
2.8.2. Oil-Holding Capacity (OHC)
2.8.3. Water Solubility (WS)
2.9. Statistical Analysis
3. Results and Discussion
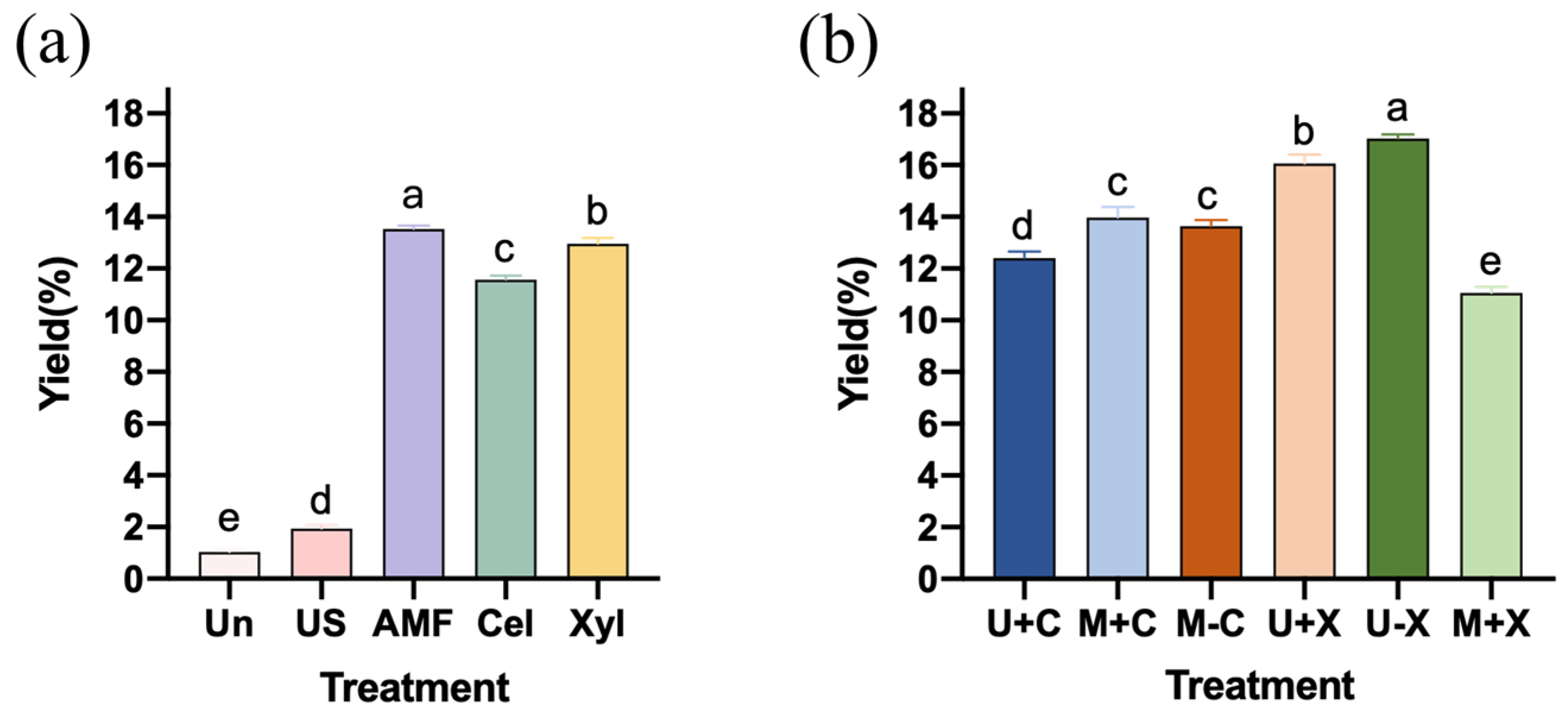
3.1. Effects of Different Treatments on SDF Yield
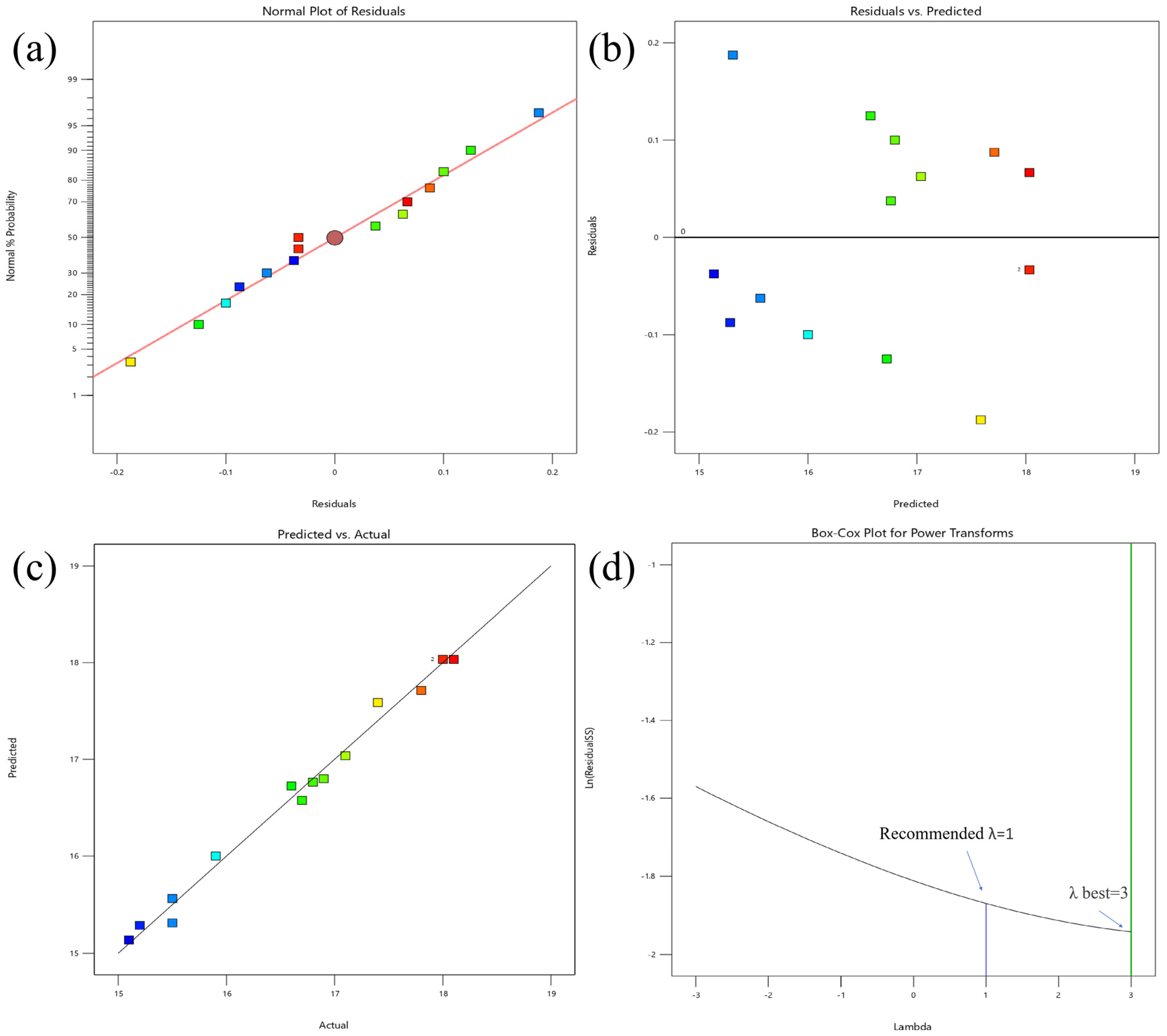
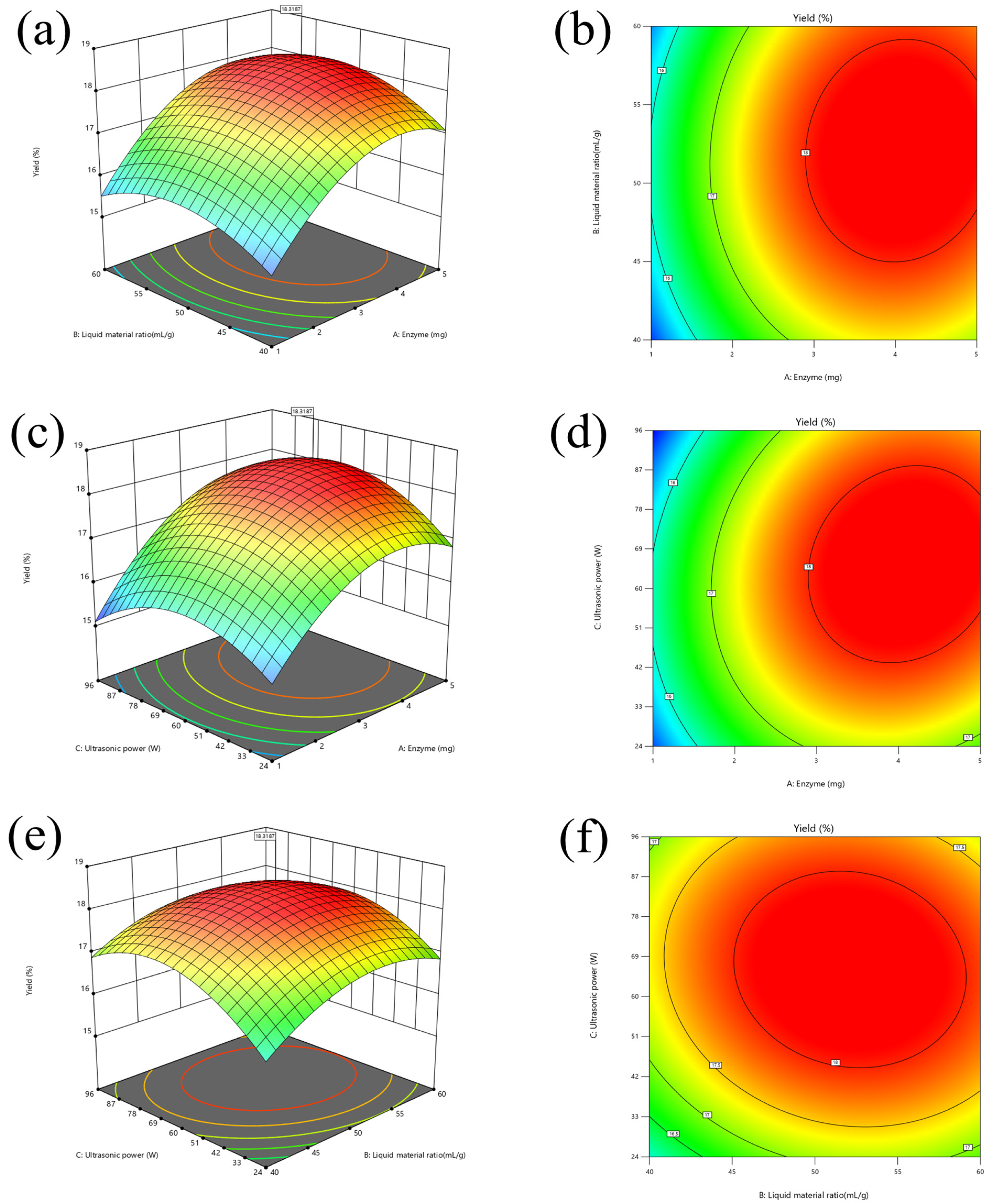
3.2. Optimization of Modification Conditions
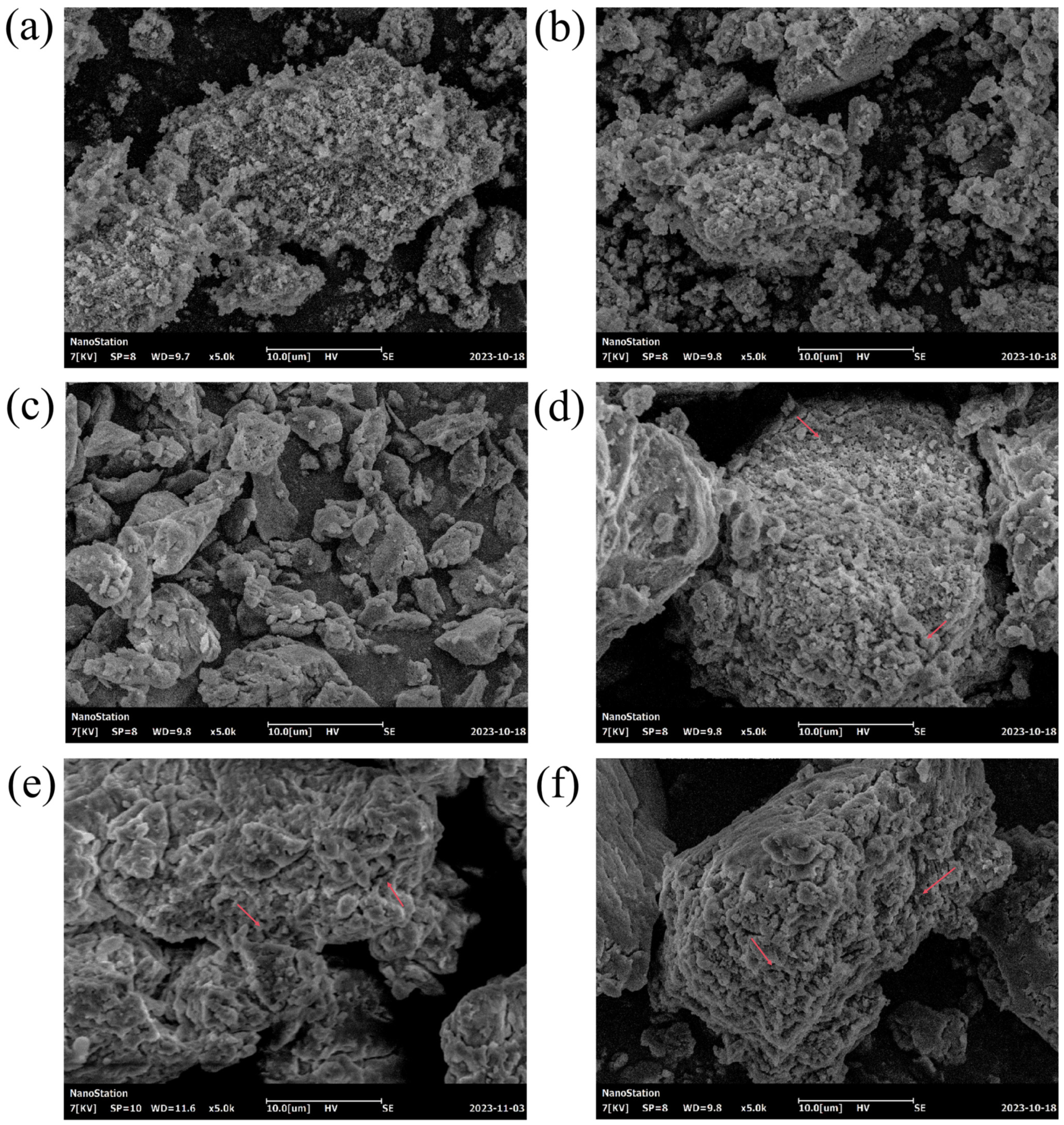
3.3. SEM Analysis
3.4. Particle Size Analysis
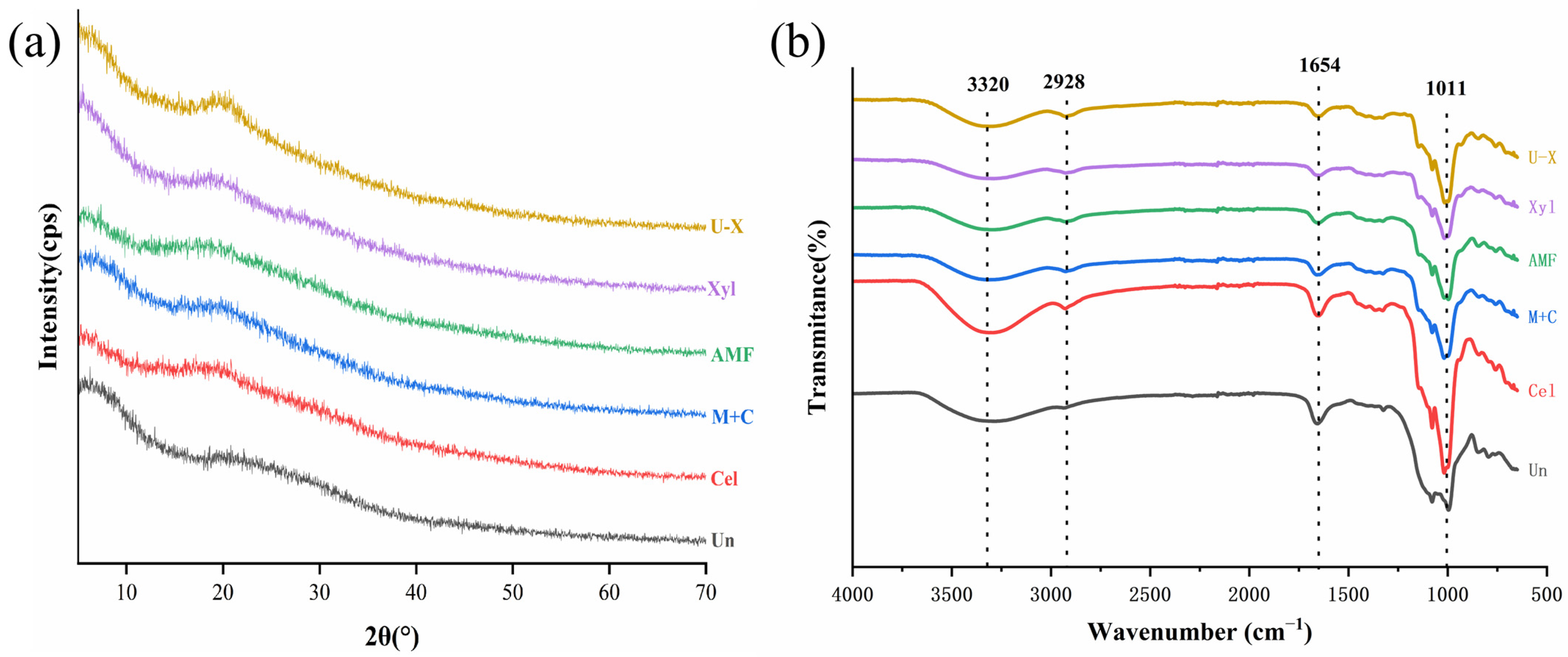
3.5. XRD Analysis
3.6. FT-IR Analysis
3.7. WHC, OHC, and WS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yılmaz Tuncel, N. Stabilization of Rice Bran: A Review. Foods 2023, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Tirpanalan-Staben, Ö.; Franke, K. Modification of Dietary Fibers to Valorize the By-Products of Cereal, Fruit and Vegetable Industry—A Review on Treatment Methods. Plants 2022, 11, 3466. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmad, A.B.; Sen, S.; Deka, B.; Hazarika, Q.K.; Kapil, M.J.; Pachuau, L. Recent Advances in Food, Nutrition & Agriculture. Recent Adv. Food Nutr. Agric. 2023, 14, 26–40. [Google Scholar] [PubMed]
- Zhang, H.; Wang, H.; Cao, X.; Wang, J. Preparation and modification of high dietary fiber flour: A review. Food Res. Int. 2018, 113, 24–35. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Tang, W.; Lin, X.; Walayat, N.; Liu, J.; Zhao, P. Dietary fiber modification: Structure, physicochemical properties, bioactivities, and application—A review. Crit. Rev. Food Sci. Nutr. 2023, 64, 7895–7915. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, S.; Ye, T.; Leng, Y.; Alomgir Hossen, M.; Sameen, D.E.; Dai, J.; Li, S.; Qin, W. Effects of ultrasonic treatment and homogenization on physicochemical properties of okara dietary fibers for 3D printing cookies. Ultrason. Sonochem. 2021, 77, 105693. [Google Scholar] [CrossRef] [PubMed]
- Bader Ul Ain, H.; Saeed, F.; Khan, M.A.; Niaz, B.; Khan, S.G.; Anjum, F.M.; Tufail, T.; Hussain, S. Comparative study of chemical treatments in combination with extrusion for the partial conversion of wheat and sorghum insoluble fiber into soluble. Food Sci. Nutr. 2019, 7, 2059–2067. [Google Scholar] [CrossRef]
- Yang, C.; Si, J.; Chen, Y.; Xie, J.; Tian, S.; Cheng, Y.; Hu, X.; Yu, Q. Physicochemical structure and functional properties of soluble dietary fibers obtained by different modification methods from Mesona chinensis Benth. residue. Food Res. Int. 2022, 157, 111489. [Google Scholar] [CrossRef]
- Wei, C.; Ge, Y.; Liu, D.; Zhao, S.; Wei, M.; Jiliu, J.; Hu, X.; Quan, Z.; Wu, Y.; Su, Y.; et al. Effects of High-Temperature, High-Pressure, and Ultrasonic Treatment on the Physicochemical Properties and Structure of Soluble Dietary Fibers of Millet Bran. Front. Nutr. 2022, 8, 820715. [Google Scholar] [CrossRef]
- Jia, F.; Liu, X.; Gong, Z.; Cui, W.; Wang, Y.; Wang, W. Extraction, modification, and property characterization of dietary fiber from Agrocybe cylindracea. Food Sci. Nutr. 2020, 8, 6131–6143. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, J.; Chen, Y.; Xie, J.; Liu, S.; Sun, N.; Hu, X.; Yu, Q. Modification of tea residue dietary fiber by high-temperature cooking assisted enzymatic method: Structural, physicochemical and functional properties. LWT 2021, 145, 111314. [Google Scholar] [CrossRef]
- Dong, R.; Liao, W.; Xie, J.; Chen, Y.; Peng, G.; Xie, J.; Sun, N.; Liu, S.; Yu, C.; Yu, Q. Enrichment of yogurt with carrot soluble dietary fiber prepared by three physical modified treatments: Microstructure, rheology and storage stability. Innov. Food Sci. Emerg. Technol. 2022, 75, 102901. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, W.; Cao, J.; He, K.; Liu, Y.; Bai, X. Microstructure characteristics of tea seed dietary fibre and its effect on cholesterol, glucose and nitrite ion adsorption capacities in vitro: A comparison study among different modifications. Int. J. Food Sci. Technol. 2019, 55, 1781–1791. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Wang, X.; Guo, M.; Cheng, C.; Zhang, Y. Effects of three biological combined with chemical methods on the microstructure, physicochemical properties and antioxidant activity of millet bran dietary fibre. Food Chem. 2023, 411, 135503. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Gouseti, O.; Lovegrove, A.; Kosik, O.; Fryer, P.J.; Mills, C.; Gates, F.; Tucker, G.; Latty, C.; Shewry, P.; Bakalis, S. Exploring the Role of Cereal Dietary Fiber in Digestion. J. Agric. Food Chem. 2019, 67, 8419–8424. [Google Scholar] [CrossRef]
- Wang, C.; Song, R.; Wei, S.; Wang, W.; Li, F.; Tang, X.; Li, N. Modification of insoluble dietary fiber from ginger residue through enzymatic treatments to improve its bioactive properties. LWT 2020, 125, 109220. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Liao, A.-M.; Thakur, K.; Huang, J.-H.; Zhang, J.-G.; Wei, Z.-J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Chen, J.; Liu, X.; Xie, M.; Nie, S.; Chen, Y.; Xie, J.; Yu, Q. Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation. Food Hydrocoll. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Zhu, Y.; He, C.; Fan, H.; Lu, Z.; Lu, F.; Zhao, H. Modification of foxtail millet (Setaria italica) bran dietary fiber by xylanase-catalyzed hydrolysis improves its cholesterol-binding capacity. LWT 2019, 101, 463–468. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Lei, D.; Huang, Y.; Cheng, S.; Zhu, Z.; Wu, Z.; Cravotto, G. Impact of ultrasound, microwaves and high-pressure processing on food components and their interactions. Trends Food Sci. Technol. 2021, 109, 1–15. [Google Scholar] [CrossRef]
- Fan, X.; Chang, H.; Lin, Y.; Zhao, X.; Zhang, A.; Li, S.; Feng, Z.; Chen, X. Effects of ultrasound-assisted enzyme hydrolysis on the microstructure and physicochemical properties of okara fibers. Ultrason. Sonochem. 2020, 69, 105247. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Xu, B.; Gao, M. Effect of alternating magnetic field treatments on enzymatic parameters of cellulase. J. Sci. Food Agric. 2012, 92, 1384–1388. [Google Scholar] [CrossRef]
- Armenia, I.; Grazú Bonavia, M.V.; De Matteis, L.; Ivanchenko, P.; Martra, G.; Gornati, R.; de la Fuente, J.M.; Bernardini, G. Enzyme activation by alternating magnetic field: Importance of the bioconjugation methodology. J. Colloid Interface Sci. 2019, 537, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Prosky, L.; Vries, J.W.D. Determination of total, soluble, and insoluble dietary fiber in foods—Enzymatic-gravimetric method, MES-TRIS buffer: Collaborative study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.-N.; Zhou, X.-j.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y.-l. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.). LWT 2021, 145, 111313. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Zhang, J.; Wen, C.; Zhou, J.; Yao, H.; He, Y.; Ma, H.; Duan, Y. Optimization, characterization, rheological study and immune activities of polysaccharide from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 246, 116595. [Google Scholar] [CrossRef]
- Hussain, S.; Sharma, M.; Bhat, R. Valorisation of Sea Buckthorn Pomace by Optimization of Ultrasonic-Assisted Extraction of Soluble Dietary Fibre Using Response Surface Methodology. Foods 2021, 10, 1330. [Google Scholar] [CrossRef]
- Yun, C.; Ji, X.; Chen, Y.; Zhao, Z.; Gao, Y.; Gu, L.; She, D.; Ri, I.; Wang, W.; Wang, H. Ultrasound-assisted enzymatic extraction of Scutellaria baicalensis root polysaccharide and its hypoglycemic and immunomodulatory activities. Int. J. Biol. Macromol. 2023, 227, 134–145. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, Y.; Jia, Y.; Wang, Y.; Lu, Y.; Chen, H.; Zhang, M. Structure, thermal and rheological properties of different soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler residues. Food Hydrocoll. 2019, 95, 10–18. [Google Scholar] [CrossRef]
- Wang, H.o.; Liu, S.h.; Zhou, X.j.; Yang, X.y.; Gao, Q.; Tanokura, M.; Xue, Y.l. Treatment with hydrogen peroxide improves the physicochemical properties of dietary fibres from Chinese yam peel. Int. J. Food Sci. Technol. 2019, 55, 1289–1297. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Tan, C.; Cai, N.; Ameer, K.; Feng, X. Modification of Ginseng Insoluble Dietary Fiber by Enzymatic Method: Structural, Rheological, Thermal and Functional Properties. Foods 2023, 12, 2809. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Benito, M.J.; Martín, A.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Casquete, R. Improve the functional properties of dietary fibre isolated from broccoli by-products by using different technologies. Innov. Food Sci. Emerg. Technol. 2022, 80, 103075. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, J.; Liu, S.; Liu, X.; Tang, X.; Lv, X. Characterisation of alkaline and enzymatic modified insoluble dietary fibre from Undaria pinnatifida. Int. J. Food Sci. Technol. 2020, 55, 3533–3541. [Google Scholar] [CrossRef]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Lu, H.; Yang, K.; Zhan, L.; Lu, T.; Chen, X.; Cai, X.; Zhou, C.; Li, H.; Qian, L.; Lv, G.; et al. Optimization of Flavonoid Extraction in Dendrobium officinale Leaves and Their Inhibitory Effects on Tyrosinase Activity. Int. J. Anal. Chem. 2019, 2019, 7849198. [Google Scholar] [CrossRef]
- Devi, S.R.; Kumari, T.; Deka, S.C. Extraction of dietary fiber and phytochemicals from bottle gourd seeds (Lagenaria siceraria), its physicochemical properties and application in food model. Food Chem. Adv. 2023, 2, 100252. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Kamasani, J.R.; Siddaiah, C.; Gupta, V.K.; Krishna, K.; Mudili, V. Combinational Inhibitory Action of Hedychium spicatum L. Essential Oil and γ-Radiation on Growth Rate and Mycotoxins Content of Fusarium graminearum in Maize: Response Surface Methodology. Front. Microbiol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Tahmasebi Boldaji, R.; Rajabi Kuyakhi, H.; Tahmasebi Boldaji, N. Prediction of 1-butanol and diesel fuel blend heat capacity by response surface methodology. Pet. Sci. Technol. 2020, 38, 737–743. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, K.; Tian, X.; Sui, W.; Wu, T.; Wang, S.; Jin, Y.; Zhu, Q.; Meng, J.; Zhang, M. Combined Modification of Soluble Dietary Fibers from Apple Pomace by Steam Explosion and Enzymatic Hydrolysis to Improve its Structural, Physicochemical and Functional Properties. Waste Biomass Valorization 2022, 13, 4869–4879. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, Y.; Zhang, C.; Xie, P.; Huang, L. Characterization of Enzymatic Modified Soluble Dietary Fiber from Rhodomyrtus tomentosa fruits: A Potential Ingredient in Reducing AGEs Accumulation. Food Bioprocess. Technol. 2022, 16, 232–246. [Google Scholar] [CrossRef]
- Tang, C.; Yang, J.; Zhang, F.; Kan, J.; Wu, L.; Zheng, J. Insight into the physicochemical, structural, and in vitro hypoglycemic properties of bamboo shoot dietary fibre: Comparison of physical modification methods. Int. J. Food Sci. Technol. 2022, 57, 4998–5010. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Huang, J.; Yuan, X.; Wang, Q.; Ma, L. Dietary fiber extracted from pomelo fruitlets promotes intestinal functions, both In Vitro and In Vivo. Carbohydr. Polym. 2021, 252, 117186. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, Z.; Wang, W.; Mu, J.; Liu, Y.; Wang, J.; Stipkovits, L.; Hui, X.; Wu, G.; Sun, J. The effects of enzymatic modification on the functional ingredient—Dietary fiber extracted from potato residue. LWT 2022, 153, 112511. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Li, L.; Liu, H. Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification. Foods 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Hormozi Jangi, S.R.; Akhond, M.; Dehghani, Z. High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochem. 2020, 90, 102–112. [Google Scholar] [CrossRef]
- Hormozi Jangi, S.R.; Akhond, M. Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochem. 2022, 120, 138–155. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef]
- Hormozi Jangi, S.R.; Akhond, M. Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sens. Actuators B Chem. 2021, 339, 129901. [Google Scholar] [CrossRef]
- Dehghani, Z.; Akhond, M.; Hormozi Jangi, S.R.; Absalan, G. Highly sensitive enantioselective spectrofluorimetric determination of R-/S-mandelic acid using l-tryptophan-modified amino-functional silica-coated N-doped carbon dots as novel high-throughput chiral nanoprobes. Talanta 2024, 266, 124977. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef]
- Zadeike, D.; Vaitkeviciene, R.; Degutyte, R.; Bendoraitiene, J.; Rukuiziene, Z.; Cernauskas, D.; Svazas, M.; Juodeikiene, G. A comparative study on the structural and functional properties of water-soluble and alkali-soluble dietary fibres from rice bran after hot-water, ultrasound, hydrolysis by cellulase, and combined pre-treatments. Int. J. Food Sci. Technol. 2021, 57, 1137–1149. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef] [PubMed]
- Sezer, D.B.; Ahmed, J.; Sumnu, G.; Sahin, S. Green processing of sour cherry (Prunus cerasus L.) pomace: Process optimization for the modification of dietary fibers and property measurements. J. Food Meas. Charact. 2021, 15, 3015–3025. [Google Scholar] [CrossRef]
- Gu, M.; Fang, H.; Gao, Y.; Su, T.; Niu, Y.; Yu, L. Characterization of enzymatic modified soluble dietary fiber from tomato peels with high release of lycopene. Food Hydrocoll. 2020, 99, 105321. [Google Scholar] [CrossRef]

| Run | X1 | X2 | X3 | Yield |
|---|---|---|---|---|
| 1 | 1 | 50 | 96 | 15.1 |
| 2 | 1 | 60 | 60 | 15.5 |
| 3 | 3 | 60 | 96 | 16.9 |
| 4 | 3 | 40 | 96 | 16.7 |
| 5 | 3 | 50 | 60 | 18.0 |
| 6 | 5 | 60 | 60 | 17.8 |
| 7 | 3 | 40 | 24 | 15.9 |
| 8 | 1 | 50 | 24 | 15.5 |
| 9 | 1 | 40 | 60 | 15.2 |
| 10 | 5 | 50 | 96 | 17.4 |
| 11 | 5 | 50 | 24 | 16.8 |
| 12 | 3 | 50 | 60 | 18.1 |
| 13 | 5 | 40 | 60 | 17.1 |
| 14 | 3 | 60 | 24 | 16.6 |
| 15 | 3 | 50 | 60 | 18.0 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 15.44 | 9 | 1.72 | 55.62 | 0.0002 | ** |
| A-enzyme | 7.6 | 1 | 7.6 | 246.65 | <0.0001 | ** |
| B-liquid material ratio | 0.4512 | 1 | 0.4512 | 14.64 | 0.0123 | * |
| C-ultrasonic power | 0.2112 | 1 | 0.2112 | 6.85 | 0.0472 | * |
| AB | 0.04 | 1 | 0.04 | 1.3 | 0.3063 | |
| AC | 0.25 | 1 | 0.25 | 8.11 | 0.0359 | * |
| BC | 0.0625 | 1 | 0.0625 | 2.03 | 0.2138 | |
| A2 | 3.54 | 1 | 3.54 | 114.81 | 0.0001 | ** |
| B2 | 1.58 | 1 | 1.58 | 51.25 | 0.0008 | ** |
| C2 | 2.69 | 1 | 2.69 | 87.37 | 0.0002 | ** |
| Residual | 0.1542 | 5 | 0.0308 | |||
| Lack of Fit | 0.1475 | 3 | 0.0492 | 14.75 | 0.0642 | not significant |
| Pure Error | 0.0067 | 2 | 0.0033 | |||
| Cor Total | 15.59 | 14 |
| Un-SDF | Cel-SDF | C+M-SDF | AMF-SDF | Xyl-SDF | U-X-SDF | |
|---|---|---|---|---|---|---|
| Z-average size (d.nm) | 879.8 ± 61.79 a | 627.9 ± 17.67 b | 590.2 ± 78.32 b | 459.2 ± 37.65 c | 559.4 ± 48.84 b | 342.8 ± 44.7 d |
| CI | 18.50% | 7.43% | 5.82% | 1.96% | 12.94% | 4.39% |
| Un-SDF | Cel-SDF | C+M-SDF | AMF-SDF | Xyl-SDF | U-X-SDF | |
|---|---|---|---|---|---|---|
| WHC (g/g) | 2.28 ± 0.31 c | 3.29 ± 0.35 b | 4.20 ± 0.5 a | 3.85 ± 0.35 ab | 4.25 ± 0.59 a | 3.25 ± 0.46 b |
| WS (g/g) | 0.62 ± 0.04 b | 0.68 ± 0.03 b | 0.75 ± 0.06 a | 0.78 ± 0.03 a | 0.76 ± 0.02 a | 0.81 ± 0.06 a |
| OHC (g/g) | 1.64 ± 0.14 a | 1.44 ± 0.19 ab | 1.15 ± 0.11 c | 1.19 ± 0.13 bc | 1.02 ± 0.13 c | 1.17 ± 0.16 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zhang, S.; Huang, Y.; Yang, S.; Zhou, A.; Zhang, W.; Wu, Z. Enhancing Rice Bran Soluble Dietary Fiber Yield Through Sequential Ultrasound–Xylanase Treatment. Foods 2025, 14, 388. https://doi.org/10.3390/foods14030388
Lin Y, Zhang S, Huang Y, Yang S, Zhou A, Zhang W, Wu Z. Enhancing Rice Bran Soluble Dietary Fiber Yield Through Sequential Ultrasound–Xylanase Treatment. Foods. 2025; 14(3):388. https://doi.org/10.3390/foods14030388
Chicago/Turabian StyleLin, Yanting, Siling Zhang, Yifei Huang, Shuyuan Yang, An Zhou, Wencheng Zhang, and Zeyu Wu. 2025. "Enhancing Rice Bran Soluble Dietary Fiber Yield Through Sequential Ultrasound–Xylanase Treatment" Foods 14, no. 3: 388. https://doi.org/10.3390/foods14030388
APA StyleLin, Y., Zhang, S., Huang, Y., Yang, S., Zhou, A., Zhang, W., & Wu, Z. (2025). Enhancing Rice Bran Soluble Dietary Fiber Yield Through Sequential Ultrasound–Xylanase Treatment. Foods, 14(3), 388. https://doi.org/10.3390/foods14030388







