Polysaccharides from Sea Cucumber (Stichopus japonicus) Synergize with Anti-PD1 Immunotherapy to Reduce MC-38 Tumor Burden in Mice Through Shaping the Gut Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SCPs
2.2. Cell Culture
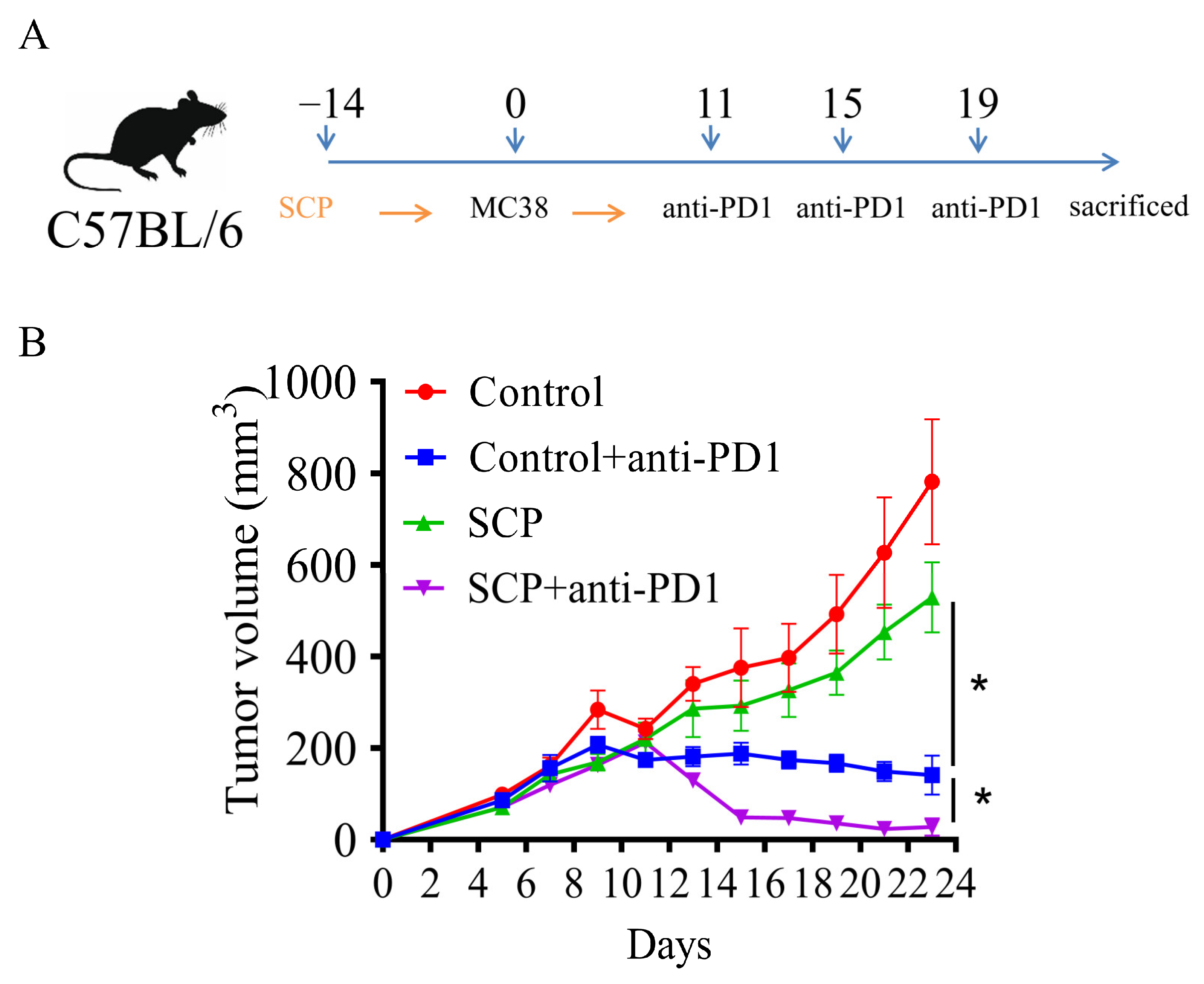
2.3. Mouse Experiments
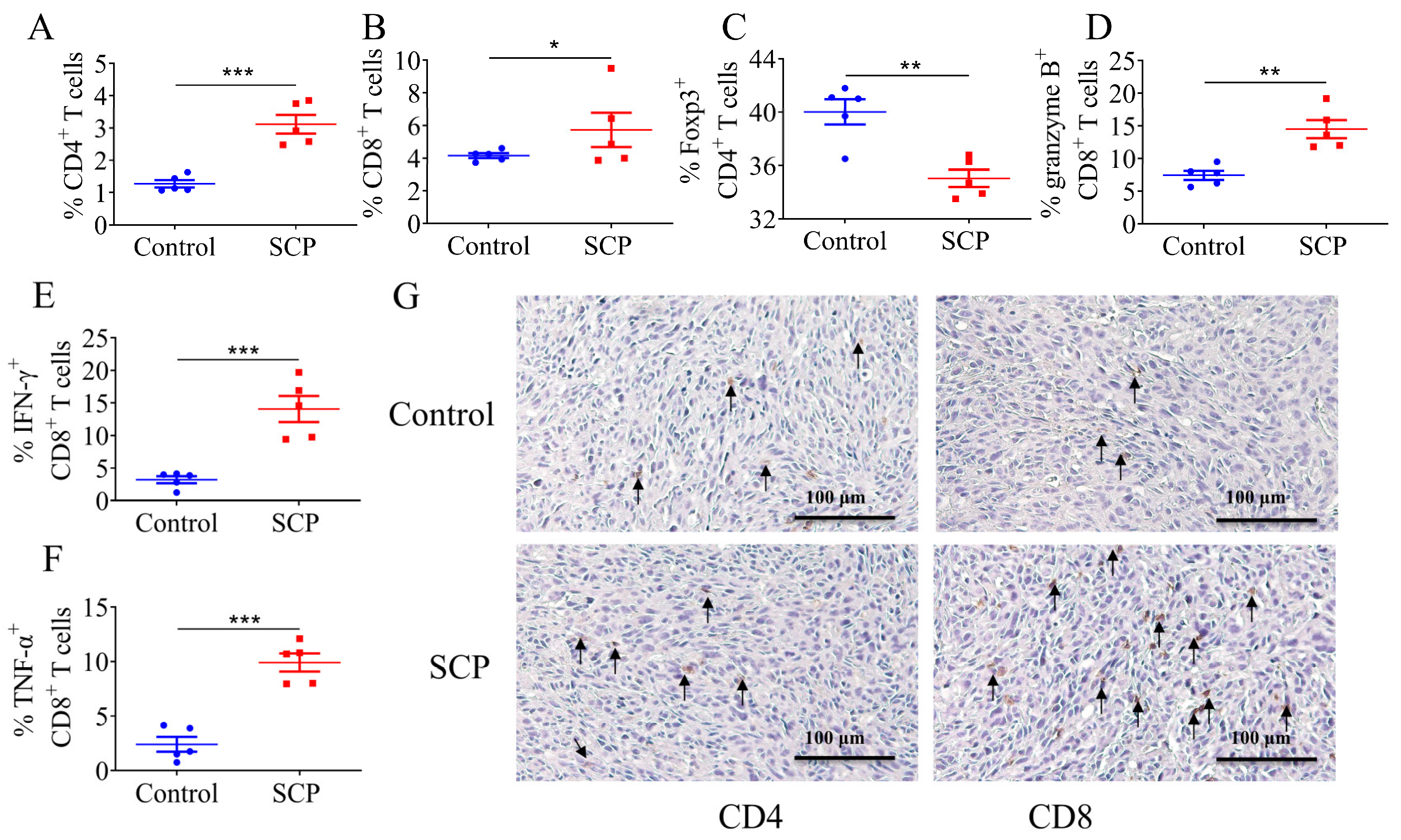
2.4. Flow Cytometry
2.5. Immunohistochemical Staining
2.6. Fecal DNA Extraction and 16S rRNA Sequencing
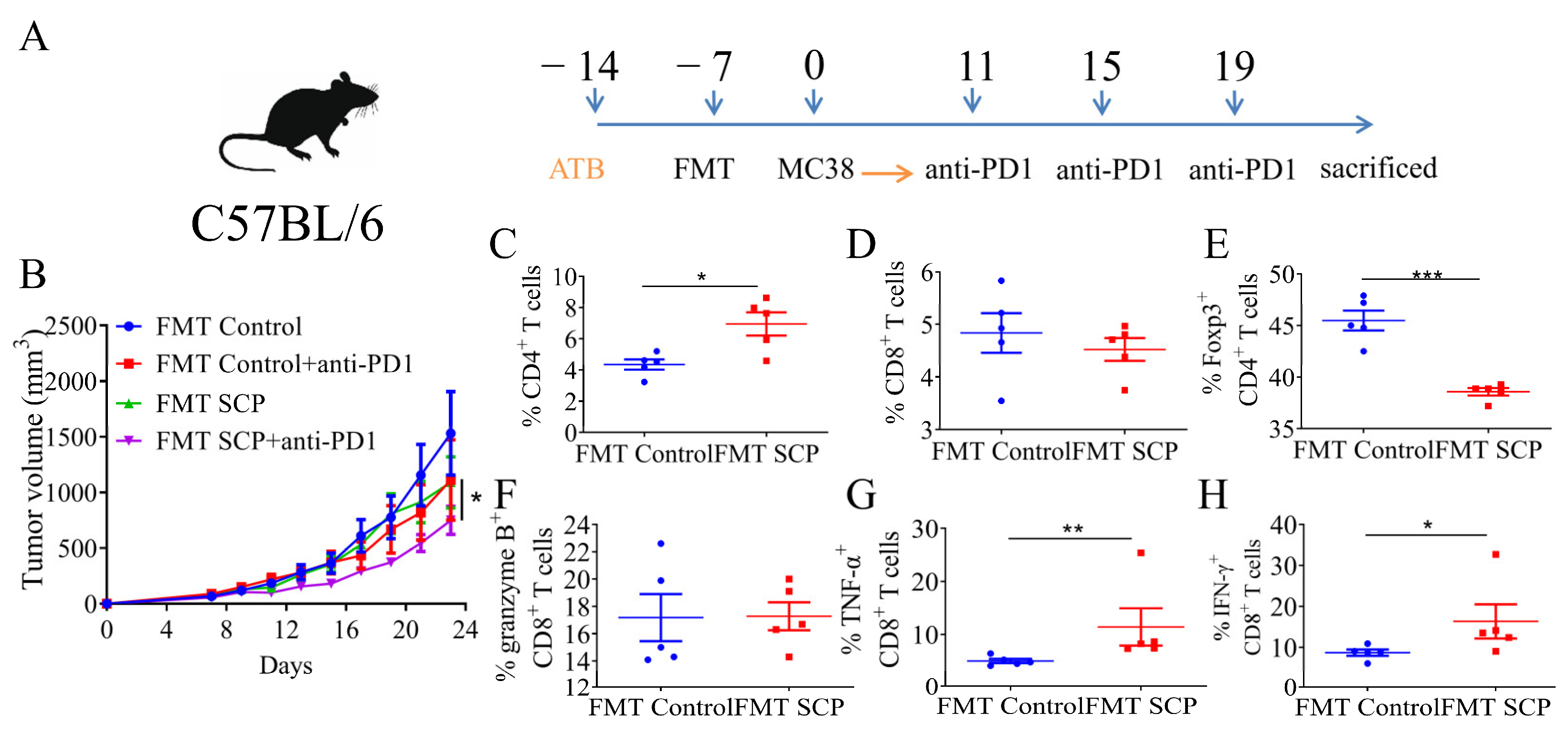
2.7. Fecal Microbiota Transplantation
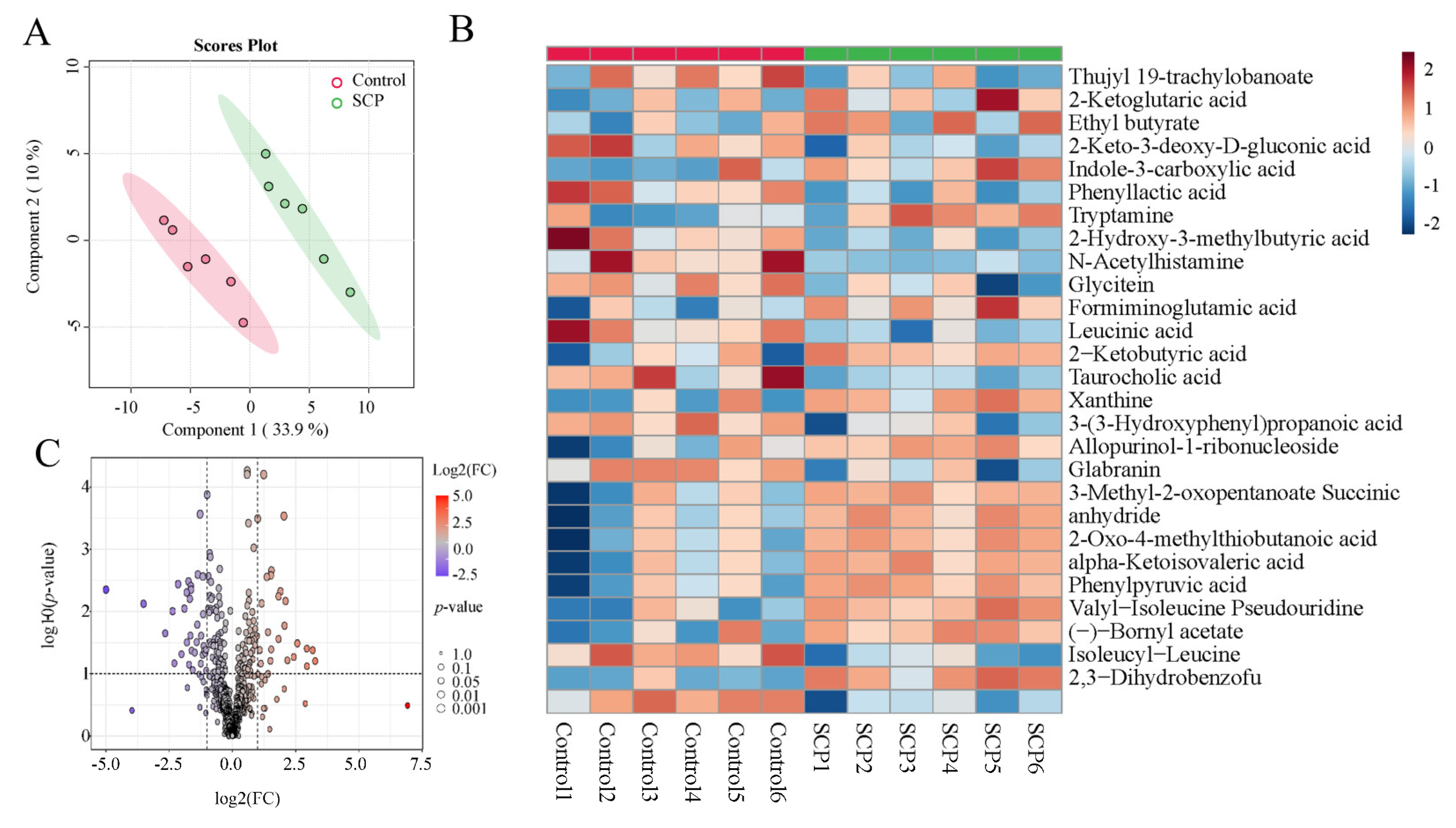
2.8. Non-Targeted Metabolomic Analysis of Feces
2.9. Statistical Analysis
3. Results
3.1. SCPs Augmented Anti-Tumor Efficacy of Anti-PD1 in Syngeneic Mouse Tumor Models
3.2. SCPs Modulated Intratumor T Lymphocyte Profiles
3.3. SCPs Increased Microbiota Diversity and Beneficially Modified the Gut Microbiome Composition
3.4. Gut Microbiota Mediated the Potentiating Function of SCPs on Anti-PD1 Treatment
3.5. SCPs Caused Changes in Fecal Metabolomic Landscape
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Usset, J.; Rosendahl Huber, A.; Andrianova, M.A.; Batlle, E.; Carles, J.; Cuppen, E.; Elez, E.; Felip, E.; Gómez-Rey, M.; Lo Giacco, D.; et al. Five latent factors underlie response to immunotherapy. Nat. Genet. 2024, 56, 2112–2120. [Google Scholar] [CrossRef]
- Xie, J.; Liu, M.; Deng, X.; Tang, Y.; Zheng, S.; Ou, X.; Tang, H.; Xie, X.; Wu, M.; Zou, Y. Gut microbiota reshapes cancer immunotherapy efficacy: Mechanisms and therapeutic strategies. iMeta 2024, 3, e156. [Google Scholar] [CrossRef]
- Lee, K.A.; Thomas, A.M.; Bolte, L.A.; Björk, J.R.; de Ruijter, L.K.; Armanini, F.; Asnicar, F.; Blanco-Miguez, A.; Board, R.; Calbet-Llopart, N.; et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 2022, 28, 535–544. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Jia, D.; Wang, Q.; Qi, Y.; Jiang, Y.; He, J.; Lin, Y.; Sun, Y.; Xu, J.; Chen, W.; Fan, L.; et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 2024, 187, 1651–1665. [Google Scholar] [CrossRef]
- Kang, X.; Lau, H.C.-H.; Yu, J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep. Med. 2024, 5, 101478. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Guo, Y. Polysaccharides influence human health via microbiota-dependent and -independent pathways. Front. Nutr. 2022, 9, 1030063. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Y.; Chen, D.; Zhang, M.; Xu, J.; Xu, C.; Liu, J.; Kan, J.; Jin, C. Natural polysaccharides protect against diet-induced obesity by improving lipid metabolism and regulating the immune system. Food Res. Int. 2023, 172, 113192. [Google Scholar] [CrossRef]
- Sha, W.; Zhao, B.; Wei, H.; Yang, Y.; Yin, H.; Gao, J.; Zhao, W.; Kong, W.; Ge, G.; Lei, T. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarization via potentiating Nrf2/HO-1 signaling pathway. Phytomedicine 2023, 112, 154667. [Google Scholar] [CrossRef]
- Li, H.; Dong, T.; Tao, M.; Zhao, H.; Lan, T.; Yan, S.; Gong, X.; Hou, Q.; Ma, X.; Song, Y. Fucoidan enhances the anti-tumor effect of anti-PD-1 immunotherapy by regulating gut microbiota. Food Funct. 2024, 15, 3463–3478. [Google Scholar] [CrossRef]
- Oh, G.-W.; Ko, S.-C.; Lee, D.H.; Heo, S.-J.; Jung, W.-K. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): A review. Fish. Aquat. Sci. 2017, 20, 28. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, L.; Lin, L.; Cai, Y.; Sun, H.; Zhao, L.; Gao, N.; Yin, R.; Zhao, J. Physicochemical Characteristics and Anticoagulant Activities of the Polysaccharides from Sea Cucumber Pattalus mollis. Mar. Drugs 2019, 17, 198. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Sulfated polysaccharides in sea cucumbers and their biological properties: A review. Int. J. Biol. Macromol. 2023, 253, 127329. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y.; Ai, C.; Wang, L.; Wen, C.; Yang, J.; Song, S.; Liu, X. Sulfated Polysaccharide from Sea Cucumber and its Depolymerized Derivative Prevent Obesity in Association with Modification of Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1800446. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y.; Ai, C.; Wu, S.; Wang, L.; Song, S.; Liu, X. Sulfated polysaccharide from sea cucumber modulates the gut microbiota and its metabolites in normal mice. Int. J. Biol. Macromol. 2018, 120, 502–512. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Pei, J.; Zhang, Y.; Hao, D.; Han, T.; Wang, X.; Song, S.; Huang, L.; Wang, Z. Sea cucumber chondroitin sulfate polysaccharides attenuate OVA-induced food allergy in BALB/c mice associated with gut microbiota metabolism and Treg cell differentiation. Food Funct. 2023, 14, 7375–7386. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, T.; Zhang, X.; Zhao, Q.; Zhu, K.; Cao, J.; Liu, Z.; Shen, X.; Li, C. Holothuria leucospilota Polysaccharides Improve Immunity and the Gut Microbiota in Cyclophosphamide-Treated Immunosuppressed Mice. Mol. Nutr. Food Res. 2022, 67, 2200317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Ai, C.; Wen, C.; Dong, X.; Sun, X.; Cao, C.; Zhang, X.; Zhu, B.; Song, S. Gut microbiota response to sulfated sea cucumber polysaccharides in a differential manner using an in vitro fermentation model. Food Res. Int. 2021, 148, 110562. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Nam, J.; Xu, J.; Sun, X.; Huang, X.; Animasahun, O.; Achreja, A.; Jeon, J.H.; Pursley, B.; Kamada, N.; et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat. Biomed. Eng. 2021, 5, 1377–1388. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, Y.; Yang, Y.; Teng, Y.; Yan, C.; Shan, Z.; Meng, J.; Xia, X. Intestinal linoleic acid contributes to the protective effects of Akkermansia muciniphila against Listeria monocytogenes infection in mice. iMeta 2024, 3, e196. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, X.; Fu, J.; Wang, H. Progress and Challenges in Precise Treatment of Tumors With PD-1/PD-L1 Blockade. Front. Immunol. 2020, 11, 339. [Google Scholar] [CrossRef]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef]
- Leena, H.C.; Vivek, V.; Maddie, M.; Pal, K.; Oliveira, A.F.d. Foods may modify responsiveness to cancer immune checkpoint blockers by altering both the gut microbiota and activation of estrogen receptors in immune cells. Front. Microbiomes 2022, 1, 1049688. [Google Scholar]
- Liu, W.; Li, N.; Hou, J.; Cao, R.; Jia, L.; Guo, Y.; Xu, J. Structure and antitumor activity of a polysaccharide from Rosa roxburghii. Int. J. Biol. Macromol. 2024, 273, 132807. [Google Scholar] [CrossRef]
- Xue, M.; Liang, H.; Tang, Q.; Xue, C.; He, X.; Zhang, L.; Zhang, Z.; Liang, Z.; Bian, K.; Zhang, L.; et al. The Protective and Immunomodulatory Effects of Fucoidan Against 7,12-Dimethyl benz[a]anthracene-Induced Experimental Mammary Carcinogenesis Through the PD1/PDL1 Signaling Pathway in Rats. Nutr. Cancer 2017, 69, 1234–1244. [Google Scholar] [CrossRef]
- Yu, H.; Ding, G.; Gong, Q.; Ma, J.; Zhao, Y.; Wang, Y.; Qiao, X.; Cheng, X. Modulation of PD-L1 by Astragalus polysaccharide attenuates the induction of melanoma stem cell properties and overcomes immune evasion. BMC Cancer 2024, 24, 1034. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Lv, B.; Li, N.; Bian, X.; Chen, L.; Kong, M.; Shen, Y.; Zheng, W.; Zhang, J.; et al. Ganoderma lucidum Polysaccharide Supplementation Significantly Activates T-Cell-Mediated Antitumor Immunity and Enhances Anti-PD-1 Immunotherapy Efficacy in Colorectal Cancer. J. Agric. Food Chem. 2024, 72, 12072–12082. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 2022, 13, 4981. [Google Scholar] [CrossRef]
- Liu, N.; Yan, M.; Lu, C.; Tao, Q.; Wu, J.; Zhou, Z.; Chen, J.; Chen, X.; Peng, C. Eravacycline improves the efficacy of anti-PD1 immunotherapy via AP1/CCL5 mediated M1 macrophage polarization in melanoma. Biomaterials 2024, 314, 122815. [Google Scholar] [CrossRef]
- Wei, Z.; Hae-Bin, P.; Eun-Koung, A.; So-Jung, K.; Dayoung, R.; Dayoung, K.; Daeun, L.; Juyoung, H.; Minseok, K.; SangGuan, Y.; et al. Fucoidan from Durvillaea Antarctica enhances the anti-cancer effect of anti-PD-L1 antibody by activating dendritic cells and T cells. Int. J. Biol. Macromol. 2024, 280, 135922. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Mao, Y.-Q.; Zhang, Z.-Y.; Li, Z.-M.; Kong, C.-Y.; Chen, H.-L.; Cai, P.-R.; Han, B.; Ye, T.; Wang, L.-S. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 2021, 11, 4155–4170. [Google Scholar] [CrossRef]
- Zhu, R.; Yuan, W.; Xia, A.; Sun, X.; Yan, W.; Wu, T.; Wang, G.; Li, Y.; Yin, Q.; Li, Y. Inulin-Based Nanoparticle Modulates Gut Microbiota and Immune Microenvironment for Improving Colorectal Cancer Therapy. Adv. Funct. Mater. 2024, 34, 2407685. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Wang, H.; Rong, X.; Zhao, G.; Zhou, Y.; Xiao, Y.; Ma, D.; Jin, X.; Wu, Y.; Yan, Y.; Yang, H.; et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022, 34, 581–594. [Google Scholar] [CrossRef]
- Ye, G.; Sun, X.; Li, J.; Mai, Y.; Gao, R.; Zhang, J. Secondary metabolites of mulberry leaves exert anti-lung cancer activity through regulating the PD-L1/PD-1 signaling pathway. J. Pharm. Anal. 2023, 14, 100926. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Ji, F.; Liang, W.; Lau, H.C.H.; Kang, X.; Liu, W.; To, K.K.-W.; Zuo, Z.; Li, X.; et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis. Gut 2023, 72, 2272–2285. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jia, J.; Teng, Y.; Wang, X.; Xia, X.; Song, S.; Zhu, B.; Xia, X. Polysaccharides from Sea Cucumber (Stichopus japonicus) Synergize with Anti-PD1 Immunotherapy to Reduce MC-38 Tumor Burden in Mice Through Shaping the Gut Microbiome. Foods 2025, 14, 387. https://doi.org/10.3390/foods14030387
Li J, Jia J, Teng Y, Wang X, Xia X, Song S, Zhu B, Xia X. Polysaccharides from Sea Cucumber (Stichopus japonicus) Synergize with Anti-PD1 Immunotherapy to Reduce MC-38 Tumor Burden in Mice Through Shaping the Gut Microbiome. Foods. 2025; 14(3):387. https://doi.org/10.3390/foods14030387
Chicago/Turabian StyleLi, Jiahui, Jinhui Jia, Yue Teng, Xiaojuan Wang, Xiaojun Xia, Shuang Song, Beiwei Zhu, and Xiaodong Xia. 2025. "Polysaccharides from Sea Cucumber (Stichopus japonicus) Synergize with Anti-PD1 Immunotherapy to Reduce MC-38 Tumor Burden in Mice Through Shaping the Gut Microbiome" Foods 14, no. 3: 387. https://doi.org/10.3390/foods14030387
APA StyleLi, J., Jia, J., Teng, Y., Wang, X., Xia, X., Song, S., Zhu, B., & Xia, X. (2025). Polysaccharides from Sea Cucumber (Stichopus japonicus) Synergize with Anti-PD1 Immunotherapy to Reduce MC-38 Tumor Burden in Mice Through Shaping the Gut Microbiome. Foods, 14(3), 387. https://doi.org/10.3390/foods14030387






