Abstract
Phytohormones are key signaling molecules that regulate plant growth, stress adaptation, and fruit ripening. However, their low abundance and structural diversity complicate accurate quantification in food matrices. This study presents a validated LC–MS/MS method for the simultaneous detection of seven phytohormones in tomato fruit, including two synthetic analogs that mimic natural auxins and cytokinins. Method optimization focused on extraction efficiency, solid-phase cleanup, and mobile phase composition, achieving high recovery (85–95%) and reduced matrix effects. Chromatographic separation was performed on a C18 column, with detection by triple quadrupole mass spectrometry in MRM mode. The method demonstrated excellent linearity (R2 > 0.98), precision, and robustness, with detection limits as low as 0.05 ng/mL for abscisic acid and 6-benzylaminopurine. Validation followed US-FDA and EC 2021/808 guidelines, ensuring regulatory compliance and analytical reliability. Analysis of tomato samples from five geographic origins revealed significant differences in phytohormone profiles, particularly in abscisic and salicylic acids, highlighting the method’s ability to capture biologically and agriculturally relevant variation. This workflow offers a sensitive, transferable platform for monitoring bioactive compounds in tomatoes and other food crops, supporting post-harvest quality assessment and food metabolomics research.
1. Background
Phytohormones, critical regulators of plant growth and development, have been extensively studied in recent years due to their vital roles in mediating responses to environmental stimuli and controlling key physiological processes [1,2]. Phytohormones, also known as plant hormones, are signaling molecules that regulate various physiological processes in plants. These small molecules act as messengers, facilitating communication within the plant and in response to external factors. The intricate balance and precise regulation of phytohormone levels are fundamental for the overall well-being of plants [3,4]. Among the diverse classes of phytohormones, cytokinins, auxins, gibberellins, abscisic acid, ethylene, and brassinosteroids, along with strigolactones, jasmonates (including jasmonic and salicylic acids), nitric oxide, and systemin peptides, play vital roles in plant growth, development, and stress adaptation [3,5,6]. Tomato (Solanum lycopersicum) is one of the most important globally due to its high consumption rate and economic significance [7,8]. Recent advances in phytohormone research in tomatoes have highlighted their roles in fruit development, stress responses, and yield optimization [9,10,11,12,13]. Understanding the dynamics of phytohormones in tomatoes is crucial for optimizing crop yield, quality, and resilience. Additionally, since the tomato plant is considered a model organism, insights obtained from tomato studies can be applied to other plants in the Solanaceae family, which comprises approximately 3000–4000 species across nearly 90 genera. This highly diverse family includes perennial trees and herbaceous animals that thrive in a wide range of terrestrial habitats, from deserts to rainforests. Members of Solanaceae hold great importance in human civilization, serving as food crops (e.g., potato, tomato, pepper, eggplant, pepino, naranjilla, and tamarillo), ornamentals (e.g., Petunia, Datura, Schizanthus, and some Solanum species), and medicinal plants (e.g., Tobacco, Atropa, Hyoscyamus, and Mandragora) [14,15,16]. Thus, a comprehensive analysis of phytohormones in plants provides valuable insights into agricultural practices and crop improvement.
Plants, lacking a nervous system, utilize alternative mechanisms for signaling to regulate organ development and growth [17,18]. Environmental signals such as light, temperature, and moisture directly affect the synthesis, catabolism, and translocation of plant hormones [19]. Quantifying multiple plant hormones simultaneously is necessary to gain a comprehensive understanding of plant physiology [20,21]. Phytohormones are categorized into various groups, including auxins, cytokinins (CK), gibberellins (GA), salicylates (SA), Abscisic acid (ABA), brassinosteroids (BR), ethylene (ETH), jasmonates (JA), and strigolactones (SL) [22,23,24,25]. These classes are complemented by additional signaling molecules such as nitric oxide (NO), systemin peptides, and karrikins, which function synergistically with classical phytohormones to regulate growth and stress responses [6,26]. Auxins are derivatives of indole, CKs are derivatives of adenine, ABA is a sesquiterpene, BRs are polyhydroxy steroids, ETH is a simple alkene, GAs are tetracyclic diterpenoid acids, SLs are terpenoid lactones derivatives of carotenoids, and JA is a fatty acid derived from linolenic acid [27,28,29,30,31]. Similarly, salicylic acid and jasmonates serve as central regulators of plant defense and signaling cascades [32]. Some other known identified plant growth regulators include plant-derived peptide hormones such as Systemin and CLE peptides [33,34], karrikins [35,36], nitric oxide (NO) [37], and polyamines [38,39].
This study focuses on analyzing five essential phytohormones: Indole-3-acetic acid, Isopentenyl adenine, Gibberellic acid, Salicylic acid, and Abscisic acid, along with two synthetic molecules 2-Naphthalene acetic acid and 6-benzylaminopurine. While plants do not naturally synthesize 2-Naphthalene acetic acid and 6-benzylaminopurine, these compounds mimic the functions of natural auxins and cytokinins, respectively, and are widely used in agricultural research to better understand plant growth and development. 2-NAA promotes cell elongation and root initiation, whereas 6-BAP has been shown to enhance plant resistance to abiotic stresses and improve seedling growth under adverse environmental conditions [40,41,42]. The method developed in this study aims to address concerns regarding the presence of phytohormones, including synthetic molecules, in tomato samples and to assess whether these compounds are inadvertently introduced during cultivation.
Several methods are available for quantifying phytohormones in plants, including biological approaches such as bioassays and immunoassays [43], as well as chemical approaches like gas chromatography/mass spectrometry (GC-MS) and N-doped carbon nanotube-reinforced hollow fiber solid-phase microextraction (N-doped CNTs-HF-SPME) [44,45,46]. Other methods include a UV detector which is a chromatography detector used to measure the visible or ultraviolet light absorbed by a sample, or a Photodiode array (PDA) detector, which utilizes reverse optics and makes it more versatile since it uses all wavelengths of the spectrum [47,48,49]. Other methods include Fluorescence detectors (FLD) that work by passing a beam of light through the sample which excites electrons causing the emission of light from the sample [50,51]. All these methods are considered cheap, accessible, and accurate options. However, they have higher limits of detection and quantification compared to LC-MS.
Analytical techniques for the quantification of phytohormones have evolved over the years. The coupling of liquid chromatography with tandem mass spectrometry (LC-MS/MS) has emerged as a superior analytical technique for quantifying phytohormones [46,47,48,52,53]. The integration of LC with mass spectrometry provides an extremely sensitive and quantitative tool for detecting and identifying phytohormones, overcoming the limitations of other techniques like GC-MS. The introduction of MS-based analytical techniques has significantly improved the sensitivity, accuracy, and practicality of plant hormone measurements. LC-MS/MS, in particular, allows for high separation performance and the detection of trace-level plant hormones in complex matrices without the need for derivatization. This study aims to fill existing gaps in the understanding of phytohormonal dynamics in tomatoes by developing and optimizing a method for the simultaneous quantification of multiple phytohormones using LC-MS/MS. This method not only enables the detection of both natural and synthetic hormones with high sensitivity and specificity but also addresses the challenge of quantifying phytohormones in complex plant matrices. Although previous research has focused on the quantification of individual phytohormones or small sets of hormones [8,20,52,54,55], these studies often fail to capture the complex interactions and synergies between multiple phytohormones during fruit development. This study presents an optimized method for the simultaneous analysis of seven phytohormones, including two synthetic molecules (2-Naphthalene acetic acid and 6-Benzyl aminopurine), using LC-MS/MS. To our knowledge, no previous studies have investigated the comprehensive analysis of phytohormones in tomatoes using this method. The development, validation, and application of this LC-MS/MS method aim to provide a more complete understanding of the role of phytohormones in the shelf life and stability of tomatoes, with potential applications in improving agricultural practices and crop management.
2. Materials and Methods
2.1. Chemicals & Reagents
LC-MS grade methanol, formic acid, and acetic acid were obtained from Supelco (Darmstadt, Germany) and Fluka (Buchs, Switzerland). Milli-Q-Water was obtained from In-house (UAE University, Al Ain, United Arab Emirates). Indole-3-acetic acid (100%), Isopentenyl adenine (98.5%), 2-Naphthalene acetic acid (95%), 6-Benzyl aminopurine (99%), Gibberellic acid (90%), Salicylic acid (99%), Abscisic acid (98%), Salicylic acid (99%) and Salicylic acid d4 (internal standard) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of Standard Solution
The preparation of standard solutions is a critical step in ensuring precision and accuracy in phytohormone analysis. Phytohormone standard solutions were prepared by dissolving approximately 5 mg of each compound in 5 mL of LC-MS/MS grade methanol to produce a stock solution of 1 mg/mL. Methanol was chosen as the solvent to ensure the solubility and stability of the phytohormones, providing a reliable foundation for LC-MS/MS analysis. Calibration standards were prepared by diluting the stock solution with a 50:50 methanol-water diluent for linearity analysis. This process followed established methodologies for LC-MS/MS analysis, which have been widely applied in phytohormone quantification studies [56,57]. This standardized approach ensures consistent and reliable quantification of phytohormones. Methanol’s use as the solvent further enhances the stability of phytohormones, forming the basis for robust analytical performance.
2.3. Tomato Plant Cultivation, Collection, and Sample Preparation
To investigate the phytohormonal profiles in tomatoes, a diverse range of tomato samples was sourced from local markets across different geographic regions, including the United Arab Emirates (UAE), Malaysia, Iran, India, and Holland. This broad geographic distribution of market-sourced tomatoes allowed for a comparative analysis of phytohormonal profiles across various growing environments and agricultural practices. By employing market-based sampling, our study ensures a comprehensive analysis of phytohormonal dynamics across a wide array of tomato varieties and growing conditions. The sample preparation process began with the careful removal of the tomato fruit skin, followed by the extraction of flesh, excluding seeds. To enhance the efficiency of the subsequent steps, the obtained material was finely ground using a mortar and pestle, with liquid nitrogen introduced during grinding to preserve the sample’s integrity and facilitate the creation of a solid form. The prepared homogenized material was weighed immediately after grinding under liquid nitrogen to approximately 1 g fresh weight (FW) for extraction. The frozen powder was transferred directly into extraction tubes while maintaining cryogenic conditions to prevent analyte degradation. Because the samples were obtained as fresh market fruits, the results were normalized to a fresh-weight basis (ng/g).
For the extraction of phytohormones, 0.05 mL of Salicylic acid D4 (500 ng/mL) was added as an internal standard, and the mixture was vortexed to ensure thorough mixing. Salicylic acid D4 was selected due to its structural similarity to the target analytes. Although not all phytohormones share the exact structural features of Salicylic acid, the D4-labeled form was effective in compensating for sample matrix effects, thus aiding in the reliable quantification of the targeted phytohormones.
A comparative analysis was conducted to evaluate the effectiveness of the sample preparation method. This approach involved an initial extraction with acetonitrile containing 1% acetic acid, followed by dilution with a mobile phase of methanol and water (35:65). Acetonitrile was used in the extraction to facilitate protein precipitation and enhance the extraction of phytohormones from the samples. This optimized method resulted in extraction efficiencies ranging from 85% to 95%, consistent with high recoveries reported for acidified organic extraction workflows and higher than several earlier single-step or less-optimized protocols in comparable plant matrices, where recoveries for some analytes can be appreciably lower (e.g., ~60–70% depending on matrix and cleanup) [21,55,58]. Additionally, this approach reduces matrix effects and supports improved LC–MS/MS sensitivity, in line with prior SPE-based phytohormone methods [52,54]. The deliberate exclusion of acetonitrile from the mobile phase minimized ion suppression, enhancing the ionization efficiency of target phytohormones, particularly gibberellic acid and abscisic acid. Moreover, the simplicity of this method reduces both sample handling complexity and analytical runtime, facilitating efficient high-throughput analysis. To further enhance the reliability of the extraction process, Solid-Phase Extraction (SPE) was incorporated using a C18 cartridge. This clean-up step effectively removed non-polar interferences, concentrating the target analytes to ensure maximum sensitivity and specificity during LC-MS/MS analysis. The resulting solution was subjected to centrifugation at 3000× g for 10 min at 4 °C to separate the components. After centrifugation, 1 mL of the supernatant was carefully extracted and mixed with 1 mL of the mobile phase. This step was crucial for optimizing chromatographic separation and minimizing matrix interferences. This extraction method, though simple, has been validated for accurate phytohormonal quantification in tomatoes and provides a robust alternative to more complex extraction techniques [59,60].
2.4. LC-MS/MS Analysis and Method Development
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was performed using a SHIMADZU Nexera X2 system comprising an LC-30AD binary pump and an LC-MS 8060 Shimadzu mass spectrometer (Shimadzu Corporation, Kyoto, Japan). Chromatographic separation was achieved using a ZORBAX Eclipse Plus C18 column (4.6 × 100 mm, 3.5 µm, Agilent Technologies, Santa Clara, CA, USA). The mass spectrometer operated in Electrospray Ionization (ESI) mode, utilizing both positive and negative ionization. The mobile phases consisted of formic acid (0.01%, v/v) in water and methanol (35:65, v/v). The mobile phase flow rate was set to 0.5 mL/min, and the injection volume was 10 µL. The autosampler temperature was maintained at 5 °C, while the column temperature was carefully controlled at 30 °C. Prior to validation, method optimization was conducted to enhance chromatographic resolution and sensitivity. The mobile phase composition was optimized by varying the methanol-water ratio (20:80 to 80:20, v/v), with a 35:65 ratio yielding the best separation and minimal ion suppression. Column temperature (tested at 25 °C, 30 °C, and 35 °C) was set at 30 °C to ensure optimal resolution, while flow rate (0.5 mL/min) provided the best balance of sensitivity and resolution. Collision energies (CE) for each analyte were optimized individually to maximize ionization efficiency, resulting in a 20–30% improvement in signal intensity. These optimizations ensured robust and reliable performance across all analytes, making the method suitable for high-throughput analysis of phytohormones in tomato matrices.
2.5. MS Tuning Procedure and Parameters
The MS parameters were optimized by injecting diluted standards in full scan followed by selecting the best multiple reaction monitoring (MRM), which means the best precursor and product ions for the determination of phytohormones. The triple quadrupole instrument utilized argon as the collision gas in the collision cell, supplied at high purity and regulated internally by the system to ensure reproducible fragmentation efficiency. The main stock solution of plant hormones was used for the preparation of tuning solution by combining the phytohormone solutions with a 50:50 mixture of methanol and water, resulting in a 100 ng/mL concentration. This solution is then subjected to injection into an LCMS/MS system. The LCMS/MS analysis is carried out using MRM to optimize the Q1, Q3, and fragmentation parameters. MRM enables precise monitoring of specific mass transitions, enhancing the accuracy and sensitivity of the analysis. The optimized final CE values and tuning parameters are listed in Table 1. The results were used as one quantifier to quantify the analyte, and the remaining results were qualifiers to confirm the presence of the compound.

Table 1.
The operating parameters and conditions of Mass Spectrometry.
2.6. Method Validation
The validation of the LC-MS/MS method was carried out following established guidelines from the US Food and Drug Administration (US-FDA) to ensure the robustness and reliability of the method [61]. Several critical validation parameters were assessed, including linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, and recovery. Quality control (QC) samples were prepared at three concentrations: low (10 ng/mL), medium (50 ng/mL), and high (200 ng/mL) to evaluate intra- and inter-day accuracy and precision. Acceptance of the inter-day precision accuracy happened when the experimental concentrations were obtained around 15% of the actual concentration, while acceptance of the lower limit of quantification (LLOQ) occurred after obtaining results within a 20% limit range. The quality control data that was obtained after analysis was used to calculate the inter/intra-day precision (% CV) data using the following equation:
The inter/intra-day accuracy (% recovery) was calculated using following equation:
Whereas Nominal value is calculated concentration. It is determined by:
The importance of the validation process lies in its capacity to demonstrate that the analytical method is relevant for the intended purpose and to ensure that the obtained values are close to the analyte content present in actual samples [62,63]. As part of the method validation, we also evaluated the ion ratio for each phytohormone by comparing the intensity of the quantifier (primary) to qualifier (secondary) product ions monitored in multiple reaction monitoring (MRM) mode. This parameter is used as an additional identity-confirmation criterion: for each phytohormone, the ion ratio observed in sample extracts must agree within ±30% of the ratio obtained for the corresponding calibration standard, as recommended by the US-FDA and EC 2021/808 validation guidelines [64]. This evaluation ensures the robustness of the method and its compliance with the required standards for analyte confirmation and selectivity. To evaluate robustness, several key parameters were varied to assess their impact on method performance. The column temperature was adjusted from 30 °C to 35 °C, and recovery remained within 80–120%, indicating the method’s stability under temperature variations. The flow rate varied by ±0.05 mL/min from the optimized flow rate (0.5 mL/min), with recovery and precision remaining within acceptable limits. Additionally, the mobile phase composition was slightly altered (±5% methanol-water ratio), and recovery remained within the desired range, confirming method accuracy. The injection volume varied by ±10 µL from the optimal 10 µL, with stable recovery values. Lastly, sample preparation conditions were modified by adjusting the extraction volume (±1 mL), and recovery remained consistent. These variations showed that the method is robust and reliable for phytohormone quantification, even with minor experimental adjustments.
3. Results and Discussion
3.1. Method Validation Results
The method employed for the analysis of phytohormones involved measuring their retention time and relative abundance (intensity). The results in Table 2 display the method validation parameters, including the linearity range, R2 values, LOD, LOQ, intra-day and inter-day accuracy, and precision data for each analyte. The outstanding regression value (R2 = 0.99) suggests a high degree of correlation between the concentration of phytohormones and their corresponding signal intensities. This is a crucial aspect of method validation, ensuring accurate and reliable quantification across a range of concentrations. Spiking was performed using 10 µL of each phytohormone standard at a concentration of 100 ng/mL, ensuring consistency across all samples. The quality control concentrations (Low quality control (LQC), Medium quality control (MQC), and High quality control (HQC)) help evaluate how matrix components influence the analyte signal. By comparing the observed concentration in spiked samples to the nominal concentration, the method accounts for potential matrix interferences that could suppress or enhance the analyte signals. Accuracy was assessed by comparing the spiked concentrations with the observed concentrations, while precision was evaluated using the relative standard deviation. Both accuracy and precision were assessed through interday and intraday analyses, with results found to be satisfactory (accuracy within 80–120% and precision <20%). The findings indicate that the method is highly sensitive, accurate, and precise, providing valuable insights.

Table 2.
Method validation parameters including Linearity range, Correlation coefficient (R2), Limit of Detection (LOD), Limit of Quantification (LOQ), Intra-day and Inter-day Accuracy and Precision.
Figure 1 displays chromatogram that visually represent the phytohormones along with the internal standard. Each hormone is depicted as a well-defined peak at its specific retention time. The clear separation of peaks is essential for accurate identification and quantification of individual compounds. Matrix peaks, which could arise from other substances in the sample, are appropriately excluded from the analysis. Each compound was accurately identified and quantified through its specific MRM transition, ensuring reliable distinction from potential interferences. Selectivity in this method therefore refers primarily to the ability of the LC–MS/MS system to distinguish and quantify the target analytes in the presence of matrix components through mass-spectrometric selectivity, rather than complete chromatographic resolution.
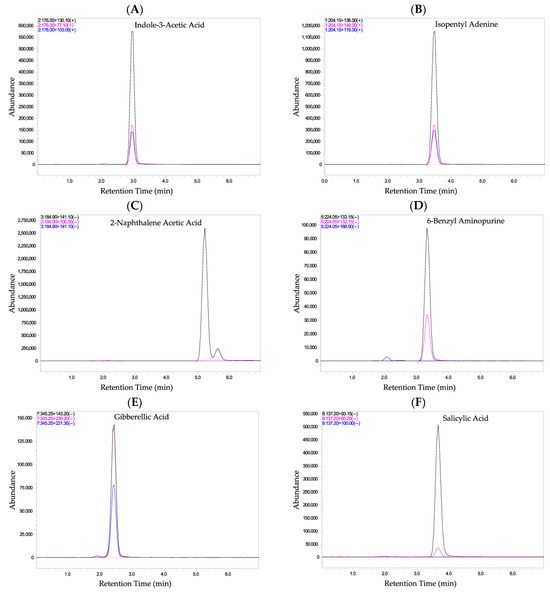
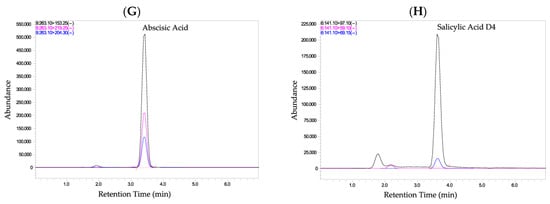
Figure 1.
Individual Chromatograms showing the detection of phytohormones and internal standard. (A) Indole-3-acetic acid; (B) Isopentenyl adenine; (C) 2-Naphthaleneacetic acid; (D) 6-Benzylaminopurine; (E) Gibberellic acid; (F) Salicylic acid; (G) Abscisic acid; and (H) Salicylic acid-D4 (internal standard). Compound identification and quantification were confirmed by their specific MRM transitions, demonstrating overall method selectivity and accuracy.
To further confirm the selectivity, the method was validated by analyzing blank samples. The absence of significant signals in blank samples assures that the peaks observed in the chromatograms are indeed associated with the phytohormones and not interference from the matrix. Additionally, the response in blank samples was compared to that of spiked LLOQ samples which were prepared from linearity solution, and the difference was within the satisfactory range (less than 30%). This further validates the method’s ability to accurately quantify phytohormones in the presence of potential sample matrix effects. The combination of HPLC and MS/MS components provides a powerful tool for detecting and confirming the presence of hormones in matrices (such as tomato fruit). This technique offers high specificity and sensitivity, and it is widely applied for the simultaneous identification of phytohormones and other metabolites.
The term “chromatographic conditions” refers to the specific set of parameters and techniques employed during the chromatographic analysis. The chromatographic conditions facilitated the separation, identification, and quantitation of phytohormones in tomato fruit samples. Using chromatographic separation, this method could effectively separate the phytohormones during the analysis of tomato samples, as a difference was observed between them in terms of their retention times, as shown in Figure 2 below. Different phytohormones have distinct chemical properties, leading to variations in their retention times. The chromatograms (Figure 2) visually represent these differences, allowing for the easy identification of individual phytohormones based on their elution times.
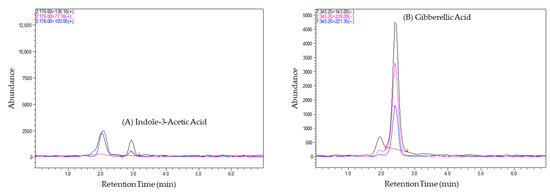
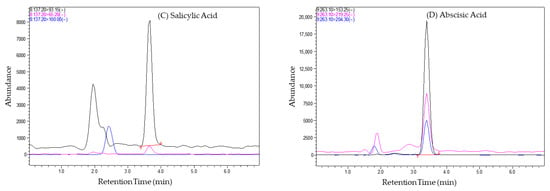
Figure 2.
Chromatograms of tomato samples showing the separation of phytohormones (A) Indole 3-acetic acid, (B) Gibberellic acid, (C) Salicylic acid, (D) Abscisic acid, with distinct retention times, enabling their identification and quantification. Black represents the precursor ion (Q1), and blue and pink represent the product ions (Q3).
3.2. Sample Analysis
The validated LC-MS/MS method was employed to analyze the phytohormonal content in tomato samples from each of the five countries. The results, as shown in Figure 3, revealed the presence of four phytohormones in all samples: Indole-3-acetic acid, Gibberellic acid, Salicylic acid, and Abscisic acid. Each of these hormones play a specific role in plant growth, development, and response to environmental stimuli. The concentrations of these phytohormones varied among the samples, with Abscisic acid consistently found in the highest concentrations across all samples, suggesting its prominent role during fruit maturation and ripening. On the other hand, Indole-3-acetic acid had the lowest concentration among the detected phytohormones indicating its limited presence during the final stages of tomato ripening. The results are expressed in nanograms per gram (ng/g) of fresh weight, as this unit accurately represents the concentration range observed in tomato matrices and ensures readability, consistent with previous LC–MS/MS studies on phytohormone quantification in fruits [65,66]. The plot in Figure 3 exhibits notable variation in concentrations of detected phytohormones among tomato samples. This variability could be due to several factors such as genetic differences among the tomato plants, environmental conditions, or other external factors. All data points were visualized using bar plots with overlaid individual values and error bars (Figure 3 and Figure 4), allowing the identification of any potential extreme values. All data points were reviewed for consistency, and no exclusions were made unless justified by confirmed technical error. Statistical analysis, including one-way ANOVA, confirmed that the differences in phytohormone concentrations among samples were statistically significant (p < 0.05), further underscoring the influence of external factors on phytohormonal levels. The existence of significant variation aligns with findings that have previously been reported in the literature [52].
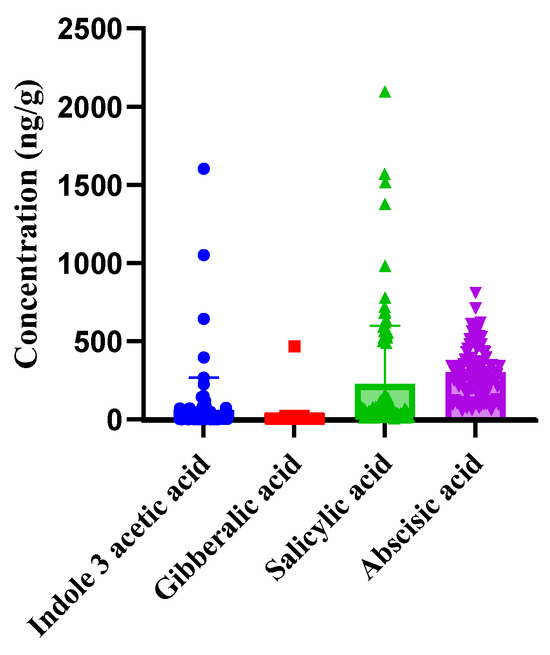
Figure 3.
The plot shows the overall concentrations of detected phytohormones in tomato samples (= 5 countries), with error bars indicating standard deviations, highlighting the significant variation in phytohormone levels across samples.
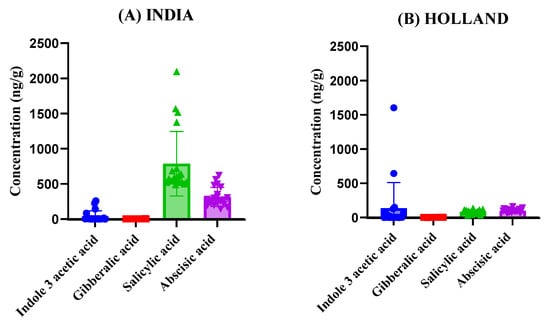
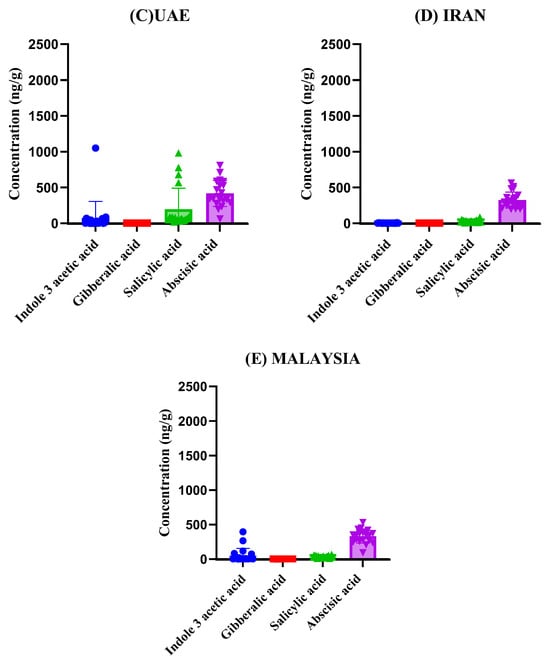
Figure 4.
Comparison of phytohormone concentrations across tomato samples from different countries (A) India, (B) Holland, (C) United Arab Emirates, (D) Iran, (E) Malaysia. Subfigures (A–E) show the variation in Indole-3-acetic acid, Gibberellic acid, Salicylic acid, and Abscisic acid levels among the samples.
The graphs presented in Figure 4 provide a detailed comparison of phytohormone concentrations across tomato samples from each of the five countries. By presenting the results side by side, we can observe variation in phytohormonal levels among samples from different geographical origins, which may reflect general differences in local growing environments, tomato cultivars, or post-harvest handling. As the tomatoes were market-sourced, detailed information regarding specific agricultural practices, genetic subspecies, nutrient management, or pesticide use was not available; therefore, these factors are discussed in a general, interpretative sense based on existing literature rather than from direct measurement. The results reveal substantial variation in the concentrations of Indole-3-acetic acid, Gibberellic acid, Salicylic acid, and Abscisic acid among the different countries. For instance, tomatoes from United Arab Emirates exhibited the highest levels of Abscisic acid on average followed by India, suggesting that tomatoes cultivated in these regions may undergo more significant stress responses or ripening processes compared to those from other regions. In contrast, tomatoes from Holland displayed relatively lower concentrations of Abscisic acid, potentially indicating differences in storage conditions or the timing of harvest. Salicylic acid concentrations showed notable fluctuations across samples, with tomatoes from India displaying the highest average concentrations. This could reflect differences in post-harvest handling or the specific cultivars used. Gibberellic acid levels were comparatively lower in tomatoes from Malaysia, indicating a less pronounced role in their ripening process compared to the samples from Iran, where higher levels were recorded (Figure 4). Although the developed LC–MS/MS method was optimized and validated for the simultaneous analysis of seven phytohormones (including two synthetic analogs, 2-Naphthalene acetic acid and 6-Benzylaminopurine), only four compounds were detected above the quantification limit in the analyzed tomato samples. The remaining three analytes were either absent or present below the method’s limit of detection (LOD). This outcome likely reflects the natural hormonal composition of mature tomato fruit rather than a limitation of the analytical method. The developed method remains fully capable of detecting all seven hormones in matrices or developmental stages where they occur within quantifiable ranges.
Statistical analysis confirmed significant differences in phytohormone concentrations among the countries (p < 0.01), suggesting that regional origin may be associated with measurable variation in hormonal composition. However, as the tomato samples were commercially obtained, specific details regarding genotype, cultivation practices, planting schedules, or climatic parameters were unavailable. Thus, the observed differences are interpreted as region-associated trends rather than causally linked outcomes, with genetic and agronomic factors also likely contributing to the observed variability. These findings not only highlight the robustness of the LC-MS/MS method in detecting and quantifying phytohormonal levels but also contribute to the growing body of literature on how external factors influence tomato ripening and shelf life.
4. Conclusions
In this study, we successfully developed, optimized, and validated an LC-MS/MS method for the precise quantification of key phytohormones in tomatoes. By optimizing the phase composition, we achieved improved separation and resolution, and the method adhered to stringent US Food and Drug Administration (FDA) guidelines, ensuring precision, accuracy, specificity, and linearity. The MS tuning process optimized compound detection and established ideal conditions for quantification. This validated method demonstrated high sensitivity with reduced sample run times and simplified extraction procedures, contributing to its potential for more efficient analysis in various laboratory settings. Chromatograms confirmed the presence of phytohormones, showing distinct peaks at specific retention times. The analysis of tomato samples from various geographic origins revealed significant variations in phytohormone levels, with Abscisic acid generally present at higher concentrations and Indole-3-acetic acid at the lowest. An exception was observed in tomatoes from India, which displayed comparatively elevated Salicylic acid levels and slightly lower Abscisic acid concentrations, potentially reflecting heat- and stress-related physiological responses commonly associated with tropical growing conditions. These findings indicate that such variability may be influenced by general factors such as cultivar characteristics, growing climate, and post-harvest physiology. Previous studies have shown that Abscisic acid and ethylene act as key maturity and senescence-regulating hormones, with ABA known to promote ripening by enhancing ethylene biosynthesis and activating ripening-related gene expression [67,68,69]. Although these processes were not directly monitored here, the observed variation among countries aligns with such known post-harvest physiological mechanisms. This validated LC–MS/MS method not only enabled accurate quantification of endogenous phytohormones and revealed distinct compositional differences among samples from different regions but also confirmed the absence of the two synthetic phytohormone analogs, 2-Naphthalene acetic acid and 6-Benzylaminopurine, in all analyzed tomato samples. This outcome supports the method’s sensitivity and reinforces its applicability for monitoring potential exogenous hormone residues during cultivation or post-harvest handling. This study provides a methodological and analytical insight into the roles of key phytohormones in fruit ripening and post-harvest quality. The developed method offers a valuable tool for further research into phytohormonal interactions and their effects on agricultural products. Additionally, this approach can be adapted to quantify bioactive compounds in other plant species, potentially advancing the study of plant growth regulators in agricultural science.
5. Future Prospects
This study has successfully developed and validated an LC-MS/MS method for quantifying key phytohormones in tomatoes. However, there are several avenues for future research to further enhance the understanding and application of this method. The developed technique can be adapted to quantify phytohormones in a wider range of agricultural crops. Expanding its use to other fruits and vegetables will enable comparisons across different species and provide deeper insights into plant growth regulation in diverse agricultural settings. Additionally, while the current sample preparation method is effective, there is potential for further optimization to minimize sample volume and improve throughput, which would enhance the scalability of the method for large-scale analyses.
Further research could involve investigating phytohormone dynamics over the entire growth cycle of tomatoes, from seedling to post-harvest, to better understand how these hormones influence various stages of development and ripening. Moreover, integrating this method with other analytical techniques, such as transcriptomics or proteomics, could provide a more comprehensive understanding of the molecular mechanisms that regulate phytohormonal activity in tomatoes and other plants. Additionally, future studies could explore the environmental and agricultural factors that influence phytohormone concentrations, such as soil composition, irrigation practices, and the impact of climate change on plant growth and fruit quality. Studying the effect of post-harvest handling and storage conditions on phytohormonal content could also help optimize post-harvest management strategies and improve shelf life and product quality.
Author Contributions
Conceptualization, M.K.H. and I.S.; methodology, M.K.H. and I.S.; validation, H.A. (Haneen Abufarajallah), G.A. and H.A. (Hind Alneyadi); formal analysis, M.K.H. and M.A.; investigation, M.K.H., H.A. (Haneen Abufarajallah), M.A., and G.A.; resources, I.S.; data curation, M.K.H., H.A. (Hind Alneyadi), and G.A.; writing original draft preparation, M.K.H. and I.S.; writing review and editing, all authors; visualization, M.K.H.; supervision, I.S.; project administration, I.S.; funding acquisition, I.S. All authors helped with the re-visions. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by UAEU SURE-Plus grant, UAE University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We offer our Special thanks to United Arab Emirates University for sponsoring this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pal, P.; Ansari, S.A.; Jalil, S.U.; Ansari, M.I. Regulatory Role of Phytohormones in Plant Growth and Development. In Plant Hormones in Crop Improvement; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–13. [Google Scholar]
- Asif, R.; Yasmin, R.; Mustafa, M.; Ambreen, A.; Mazhar, M.; Rehman, A.; Umbreen, S.; Ahmad, M. Phytohormones as Plant Growth Regulators and Safe Protectors against Biotic and Abiotic Stress. In Plant Hormones—Recent Advances, New Perspectives and Applications; IntechOpen: Rijeka, Croatia, 2022; pp. 115–130. [Google Scholar]
- Peres, A.L.G.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the Sixth Class of Phytohormones: A Molecular View from the Discovery to Hormonal Interactions in Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of Phytohormones and Their Signaling Pathways in Leaf Development and Stress Responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.; Nath, M.; Sharma, M.; Bhatt, M.D.; Bisht, D.S.; Butani, N.V. Role of Growth Regulators and Phytohormones in Overcoming Environmental Stress. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; Academic Press: Cambridge, MA, USA, 2020; pp. 254–279. [Google Scholar]
- Bhatla, S.C.; Lal, M.A. Novel Plant Growth Regulators and Gaseous Signaling Molecules. In Plant Physiology, Development and Metabolism; Bhatla, S.C., Lal, M.A., Eds.; Springer Nature: Singapore, 2023; pp. 479–515. ISBN 978-981-99-5736-1. [Google Scholar]
- Kimura, S.; Sinha, N. Tomato (Solanum lycopersicum): A Model Fruit-Bearing Crop. Cold Spring Harb. Protoc. 2008, 2008, pdb-emo105. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, M.K.; Elangovan, S.; Rafi, M.; George, S.; Shah, I.; Amiri, K.M.A. Advancing Antibiotic Residue Analysis: LC-MS/MS Methodology for Ticarcillin Degradation Products in Tomato Leaves. Antibiotics 2024, 13, 133. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, M.; Li, J.; Cui, B.; Liu, Z.; Fu, D.; Zhou, H.; Zhou, A. Comparative Transcriptome Analysis Reveals the Function of SlPRE2 in Multiple Phytohormones Biosynthesis, Signal Transduction and Stomatal Development in Tomato. Plant Cell Rep. 2023, 42, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, J.; Wei, S.; Hu, D.; Liu, Y.; Feng, L.; Li, C.; Qi, N.; Wang, C.; Liao, W. Nitric Oxide Enhanced Salt Stress Tolerance in Tomato Seedlings, Involving Phytohormone Equilibrium and Photosynthesis. Int. J. Mol. Sci. 2022, 23, 4539. [Google Scholar] [CrossRef]
- Waseem, M.; Aslam, M.M.; Shaheen, I. The DUF221 Domain-Containing (DDP) Genes Identification and Expression Analysis in Tomato under Abiotic and Phytohormone Stress. GM Crops Food 2021, 12, 586–599. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Ntatsi, G.; Kläring, H.-P.; Schwarz, D. Growth, Yield, and Metabolic Responses of Temperature-Stressed Tomato to Grafting onto Rootstocks Differing in Cold Tolerance. J. Amer. Soc. Hort. Sci. 2014, 39, 230–243. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Druege, U.; Schwarz, D. Contribution of Phytohormones in Alleviating the Impact of Sub-Optimal Temperature Stress on Grafted Tomato. Sci. Hortic. 2013, 149, 28–38. [Google Scholar] [CrossRef]
- Saleh, M.O.; Al-Bayati, A.H.I. Comparative Chemical Classification of Selected Species of the Solanaceae Family Growing Wild in Anbar Governorate. IOP Conf. Ser. Earth Environ. Sci. 2025, 1538, 012057. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Merecz-Sadowska, A.; Rijo, P.; Mori, M.; Hatziantoniou, S.; Górski, K.; Szemraj, J.; Piekarski, J.; Śliwiński, T.; Bijak, M.; et al. Hidden in Plants—A Review of the Anticancer Potential of the Solanaceae Family in In Vitro and In Vivo Studies. Cancers 2022, 14, 1455. [Google Scholar] [CrossRef]
- Motti, R. The Solanaceae Family: Botanical Features and Diversity. In The Wild Solanums Genomes; Carputo, D., Aversano, R., Ercolano, M.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–9. ISBN 978-3-030-30343-3. [Google Scholar]
- Li, J.; Li, C.; Smith, S.M. Hormone Metabolism and Signaling in Plants; Academic Press: Cambridge, MA, USA, 2017; ISBN 0-12-811563-7. [Google Scholar]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Balcke, G.U.; Handrick, V.; Bergau, N.; Fichtner, M.; Henning, A.; Stellmach, H.; Tissier, A.; Hause, B.; Frolov, A. An UPLC-MS/MS Method for Highly Sensitive High-Throughput Analysis of Phytohormones in Plant Tissues. Plant Methods 2012, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Almeida Trapp, M.; De Souza, G.D.; Rodrigues-Filho, E.; Boland, W.; Mithöfer, A. Validated Method for Phytohormone Quantification in Plants. Front. Plant Sci. 2014, 5, 417. [Google Scholar] [CrossRef]
- Su, Y.; Xia, S.; Wang, R.; Xiao, L. Phytohormonal Quantification Based on Biological Principles. Horm. Metab. Signal. Plants 2017, 13, 431–470. [Google Scholar]
- Sabagh, A.E.; Mbarki, S.; Hossain, A.; Iqbal, M.A.; Islam, M.S.; Raza, A.; Llanes, A.; Reginato, M.; Rahman, M.A.; Mahboob, W.; et al. Potential Role of Plant Growth Regulators in Administering Crucial Processes Against Abiotic Stresses. Front. Agron. 2021, 3, 648694. [Google Scholar] [CrossRef]
- Vaishnav, D.; Chowdhury, P. Types and Function of Phytohormone and Their Role in Stress. In Plant Abiotic Stress Responses and Tolerance Mechanisms; IntechOpen: Rijeka, Croatia, 2023; ISBN 1-80356-957-3. [Google Scholar]
- Mohanta, T.K.; Mohanta, Y.K.; Yadav, D.; Hashem, A.; Abd_Allah, E.F.; Al-Harrasi, A. Global Trends in Phytohormone Research: Google Trends Analysis Revealed African Countries Have Higher Demand for Phytohormone Information. Plants 2020, 9, 1248. [Google Scholar] [CrossRef]
- Ahmad, B.; Qadir, S.U.; Dar, T.A.; Alam, P.; Yousuf, P.Y.; Ahmad, P. Karrikins: Smoke-Derived Phytohormones from Stress Alleviation to Signaling. J. Plant Growth Regul. 2023, 42, 4784–4796. [Google Scholar] [CrossRef]
- Sun, P.; Huang, Y.; Yang, X.; Liao, A.; Wu, J. The Role of Indole Derivative in the Growth of Plants: A Review. Front. Plant Sci. 2023, 13, 1120613. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Q.; Jiang, W.; Xiao, N.; Zeng, F.; Chen, G.; Mak, M.; Chen, Z.-H.; Deng, F. Molecular Regulation and Evolution of Cytokinin Signaling in Plant Abiotic Stresses. Plant Cell Physiol. 2022, 63, 1787–1805. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Biosynthetic Pathways of Hormones in Plants. Metabolites 2023, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone Signaling and Crosstalk in Regulating Drought Stress Response in Plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Secondary Metabolites. In Plant Physiology, Development and Metabolism; Springer: Berlin/Heidelberg, Germany, 2023; pp. 765–808. [Google Scholar]
- Hou, S.; Tsuda, K. Salicylic Acid and Jasmonic Acid Crosstalk in Plant Immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [CrossRef]
- Lindsey, K. Plant Peptide Hormones: The Long and the Short of It. Curr. Biol. 2001, 11, R741–R743. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Small Peptide Modulates Stomatal Control via Abscisic Acid in Long-Distance Signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Chiwocha, S.D.S.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Riseborough, J.-A.M.; Smith, S.M.; Stevens, J.C. Karrikins: A New Family of Plant Growth Regulators in Smoke. Plant Sci. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Chu, H.D.; Ha, C.V.; Watanabe, Y.; Osakabe, Y.; Leyva-González, M.A.; Sato, M.; Toyooka, K.; Voges, L.; et al. The Karrikin Receptor KAI2 Promotes Drought Resistance in Arabidopsis Thaliana. PLoS Genet. 2017, 13, e1007076. [Google Scholar] [CrossRef]
- Shapiro, A.D. Nitric Oxide Signaling in Plants. In Vitamins & Hormones; Litwack, G., Ed.; Plant Hormones; Academic Press: Cambridge, MA, USA, 2005; Volume 72, pp. 339–398. [Google Scholar]
- Pandey, S.; Ranade, S.A.; Nagar, P.K.; Kumar, N. Role of Polyamines and Ethylene as Modulators of Plant Senescence. J. Biosci. 2000, 25, 291–299. [Google Scholar] [CrossRef]
- Moschou, P.N.; Roubelakis-Angelakis, K.A. Polyamines and Programmed Cell Death. J. Exp. Bot. 2014, 65, 1285–1296. [Google Scholar] [CrossRef]
- Suliman, A.A.; Elkhawaga, F.A.; Zargar, M.; Bayat, M.; Pakina, E.; Abdelkader, M. Boosting Resilience and Efficiency of Tomato Fields to Heat Stress Tolerance Using Cytokinin (6-Benzylaminopurine). Horticulturae 2024, 10, 170. [Google Scholar] [CrossRef]
- Narendar, M.; Sreenu, P.; Mustafa, M.; Shailaja, K. High Efficiency Callus-Mediated Regeneration in Corynandra Felina: A Medicinal Plant Endemic to Peninsular India. In Vegetos; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Zarea, M.J.; Karimi, N. Grain Yield and Quality of Wheat Are Improved through Post-Flowering Foliar Application of Zinc and 6-Benzylaminopurine under Water Deficit Condition. Front. Plant Sci. 2023, 13, 1068649. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zou, Y.; Kaw, H.Y.; Wang, G.; Sun, H.; Cai, L.; Li, C.; Meng, L.-Y.; Li, D. Recent Developments and Emerging Trends of Mass Spectrometric Methods in Plant Hormone Analysis: A Review. Plant Methods 2020, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-F.; Chen, J.; Shi, Y.-P. N-Doped Carbon Nanotubes-Reinforced Hollow Fiber Solid-Phase Microextraction Coupled with High Performance Liquid Chromatography for the Determination of Phytohormones in Tomatoes. Talanta 2018, 185, 132–140. [Google Scholar] [CrossRef]
- Rawlinson, C.; Kamphuis, L.G.; Gummer, J.P.; Singh, K.B.; Trengove, R.D. A Rapid Method for Profiling of Volatile and Semi-Volatile Phytohormones Using Methyl Chloroformate Derivatisation and GC–MS. Metabolomics 2015, 11, 1922–1933. [Google Scholar] [CrossRef]
- Chu, J.; Fang, S.; Xin, P.; Guo, Z.; Chen, Y. Quantitative Analysis of Plant Hormones Based on LC-MS/MS. In Hormone Metabolism and Signaling in Plants; Academic Press: Cambridge, MA, USA, 2017; pp. 471–537. [Google Scholar]
- Yalçın, S.; Şükran Okudan, E.; Karakaş, Ö.; Önem, A.N.; Sözgen Başkan, K. Identification and Quantification of Some Phytohormones in Seaweeds Using UPLC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 475–484. [Google Scholar] [CrossRef]
- Taher, M.A.; Moniruzzaman, M.; Kama, S.; Jahan, F.; Khan, M. Development and Validation of Reverse Phase Liquid Chromatography Method for Quantifying Gibberellic Acid in Fertilizer Matrix. Available online: https://dx.doi.org/10.2139/ssrn.4549842 (accessed on 22 May 2025).
- Maoka, T. Carotenoids: Distribution, Function in Nature, and Analysis Using LC-Photodiode Array Detector (DAD)-MS and MS/MS System. Mass Spectrom. 2023, 12, A0133. [Google Scholar] [CrossRef]
- Balcerowicz, M.; Shetty, K.N.; Jones, A.M. Fluorescent Biosensors Illuminating Plant Hormone Research. Plant Physiol. 2021, 187, 590–602. [Google Scholar] [CrossRef]
- Chen, Y.; He, B.; Hu, M.; Bao, J.; Yan, W.; Han, X.; Ye, Y. Fluorescent Probes for Imaging and Detection of Plant Hormones and Their Receptors. Adv. Agrochem 2023, 3, 83–98. [Google Scholar] [CrossRef]
- Jon, C.-S.; Zou, Y.; Zhao, J.; Ri, H.-C.; Wang, L.; Kaw, H.Y.; Meng, L.-Y.; Shang, H.; Li, D. Simultaneous Determination of Multiple Phytohormones in Tomato by Ionic Liquid-Functionalized Carbon Fibers-Based Solid-Phase Microextraction Coupled with Liquid Chromatography-Mass Spectrometry. Anal. Chim. Acta 2020, 1137, 143–155. [Google Scholar] [CrossRef]
- Hakeem, M.K.; Maraqa, M.; Elangovan, S.K.; Saeed, E.E.; Mishra, A.K.; Hazzouri, K.M.; Shah, I.; Amiri, K.M.A. Innovative Determination of Phytohormones in Aloe Vera. Front. Chem. 2025, 12, 1490639. [Google Scholar] [CrossRef]
- Fu, J.; Chu, J.; Sun, X.; Wang, J.; Yan, C. Simple, Rapid, and Simultaneous Assay of Multiple Carboxyl Containing Phytohormones in Wounded Tomatoes by UPLC-MS/MS Using Single SPE Purification and Isotope Dilution. Anal. Sci. 2012, 28, 1081–1087. [Google Scholar] [CrossRef]
- Delatorre, C.; Rodríguez, A.; Rodríguez, L.; Majada, J.P.; Ordás, R.J.; Feito, I. Hormonal Profiling: Development of a Simple Method to Extract and Quantify Phytohormones in Complex Matrices by UHPLC–MS/MS. J. Chromatogr. B 2017, 1040, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Khedr, T.; Ryad, L.; Youssef, A.O. Testing the Validity of Reference Standard Materials and Stock Solutions of Veterinary Drugs Using LC-MS/MS. Food Addit. Contam. Part A 2019, 36, 405–412. [Google Scholar] [CrossRef]
- Ito, S.; Tsukada, K. Matrix Effect and Correction by Standard Addition in Quantitative Liquid Chromatographic–Mass Spectrometric Analysis of Diarrhetic Shellfish Poisoning Toxins. J. Chromatogr. A 2002, 943, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xia, N.; Meng, X.; Duan, C.; Pan, Q. A One-Step Polyphenol Removal Approach for Detection of Multiple Phytohormones from Grape Berry. Horticulturae 2022, 8, 548. [Google Scholar] [CrossRef]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.E.; Strnad, M.; Ljung, K.; Novák, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef]
- Jeong, M.-J.; Ko, B.J.; Kim, J.Y. Mass Spectrometry-Based Metabolomics Study for Delay Tomato Fruit Ripening by Sound Waves. J Anal Sci Technol 2023, 14, 27. [Google Scholar] [CrossRef]
- Zimmer, D. New US FDA Draft Guidance on Bioanalytical Method Validation versus Current FDA and EMA Guidelines: Chromatographic Methods and ISR. Bioanalysis 2014, 6, 13–19. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Bioanalytical Method Validation. In Guidance for Industry; US Food and Drug Administration: Silver Spring, MD, USA, 2001. [Google Scholar]
- Sargent, M. Guide to Achieving Reliable Quantitative LC-MS Measurements; RSC Analytical Methods Committee: London, UK, 2013; p. 68. [Google Scholar]
- Commission Implementing Regulation (EU) 2021/808 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC.|FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC203999/ (accessed on 22 May 2025).
- Song, Y.; Dong, M.; Gu, J.; Jin, X.; Hu, Y.; Chen, F.; Qiao, Z.; Zhang, T. Synergistic Effects of Combined Foliar Fertilizers on Growth, Stress Tolerance, and Yield of High-Quality Japonica Rice. Int. J. Plant Prod. 2025, 19, 255–268. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Wang, M.; Wu, Y.; Shi, Y.; Chen, Y.; Feng, R.; Yang, X.; Chen, X.; Wang, B. High-Performance Liquid Chromatographic Quantification of the Plant Hormone Abscisic Acid at Ppb Levels in Plant Samples after a Single Immunoaffinity Column Cleanup. J. Agric. Food Chem. 2024, 72, 11794–11803. [Google Scholar] [CrossRef]
- Kou, X.; Zhou, J.; Wu, C.E.; Yang, S.; Liu, Y.; Chai, L.; Xue, Z. The Interplay between ABA/Ethylene and NAC TFs in Tomato Fruit Ripening: A Review. Plant Mol. Biol. 2021, 106, 223–238. [Google Scholar] [CrossRef]
- Gupta, K.; Wani, S.H.; Razzaq, A.; Skalicky, M.; Samantara, K.; Gupta, S.; Pandita, D.; Goel, S.; Grewal, S.; Hejnak, V.; et al. Abscisic Acid: Role in Fruit Development and Ripening. Front. Plant Sci. 2022, 13, 817500. [Google Scholar] [CrossRef]
- Tipu, M.M.H.; Sherif, S.M. Ethylene and Its Crosstalk with Hormonal Pathways in Fruit Ripening: Mechanisms, Modulation, and Commercial Exploitation. Front. Plant Sci. 2024, 15, 1475496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).