Integrative Multi-Omics Reveals Quality Markers and Metabolic Pathways Across Genotype and Ripening Gradients in High-Altitude Malus
Abstract
1. Introduction
2. Materials and Methods
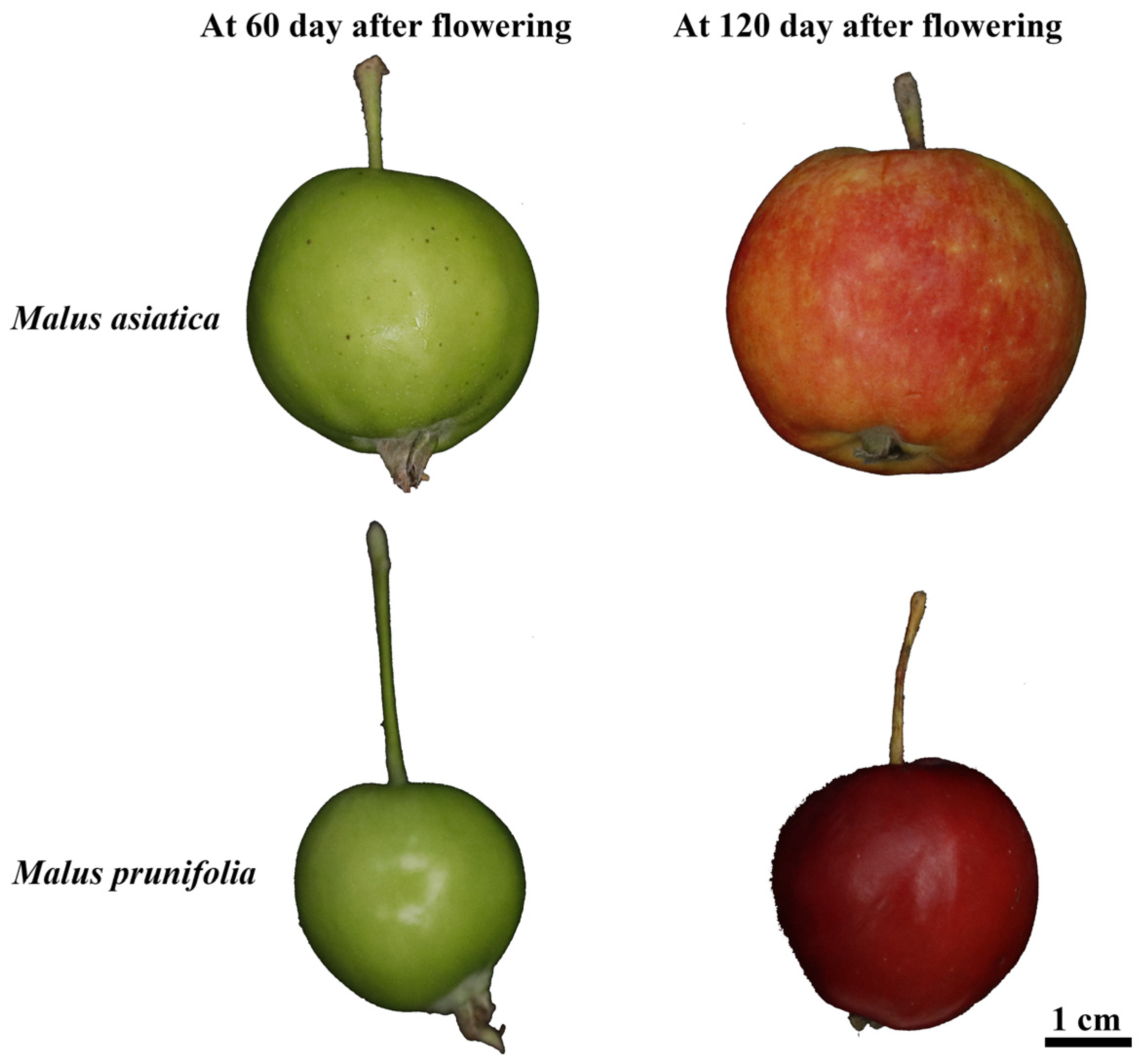
2.1. Plant Materials
2.2. Organic-Acid Components Assay
2.2.1. Sample Pretreatment
2.2.2. Liquid Chromatography–Mass Spectrometry Conditions
2.2.3. Quantification of Organic-Acid Concentration
2.3. Sugars Components Assay
2.3.1. Sample Pretreatment
2.3.2. Gas Chromatography–Mass Spectrometry Conditions
2.3.3. Quantification of Sugar Concentration
2.4. RNA Extraction, Detection, and Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analyses
3. Results and Discussion
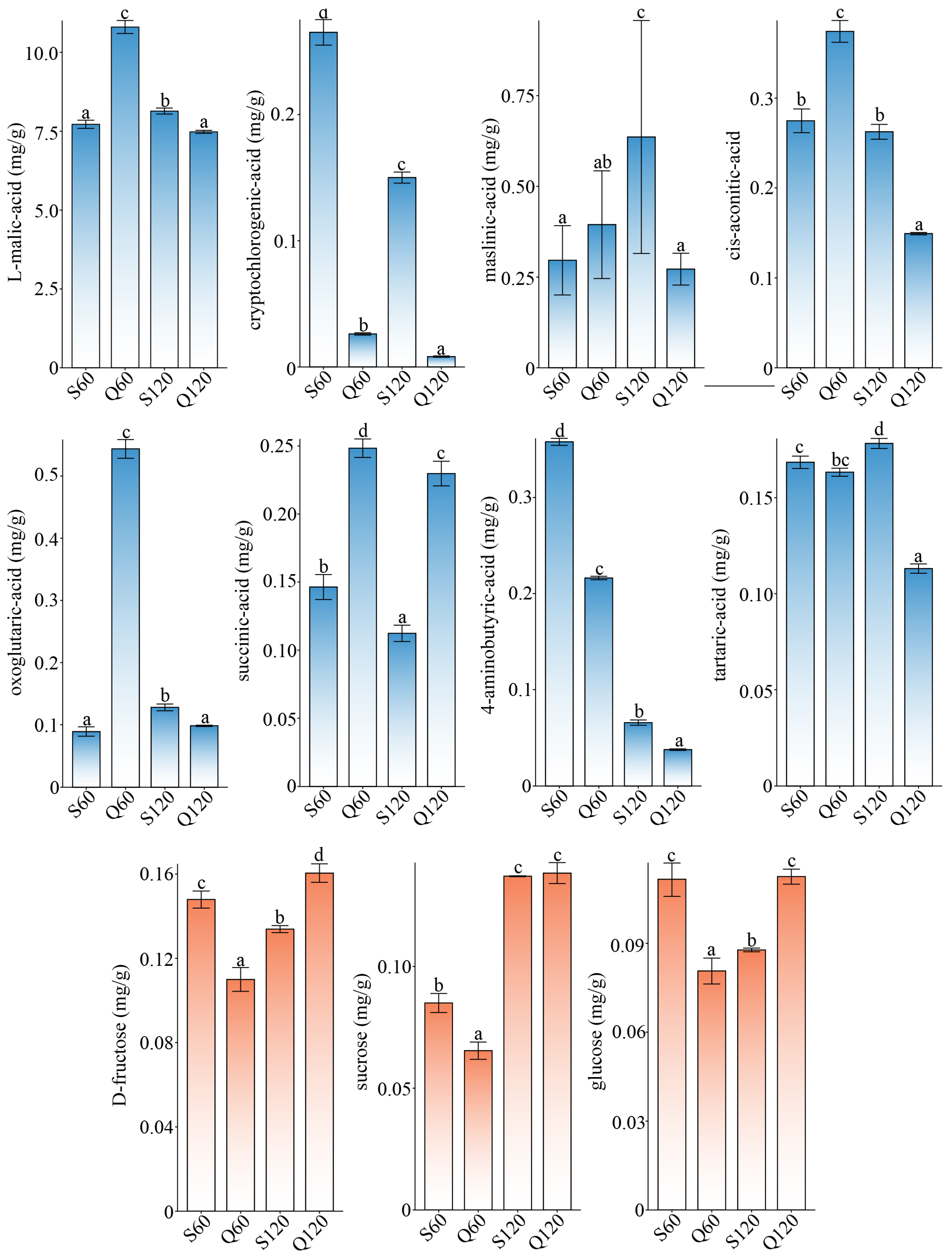
3.1. Differential Analysis of Dominant Organic Acids and Sugars in Malus Fruits
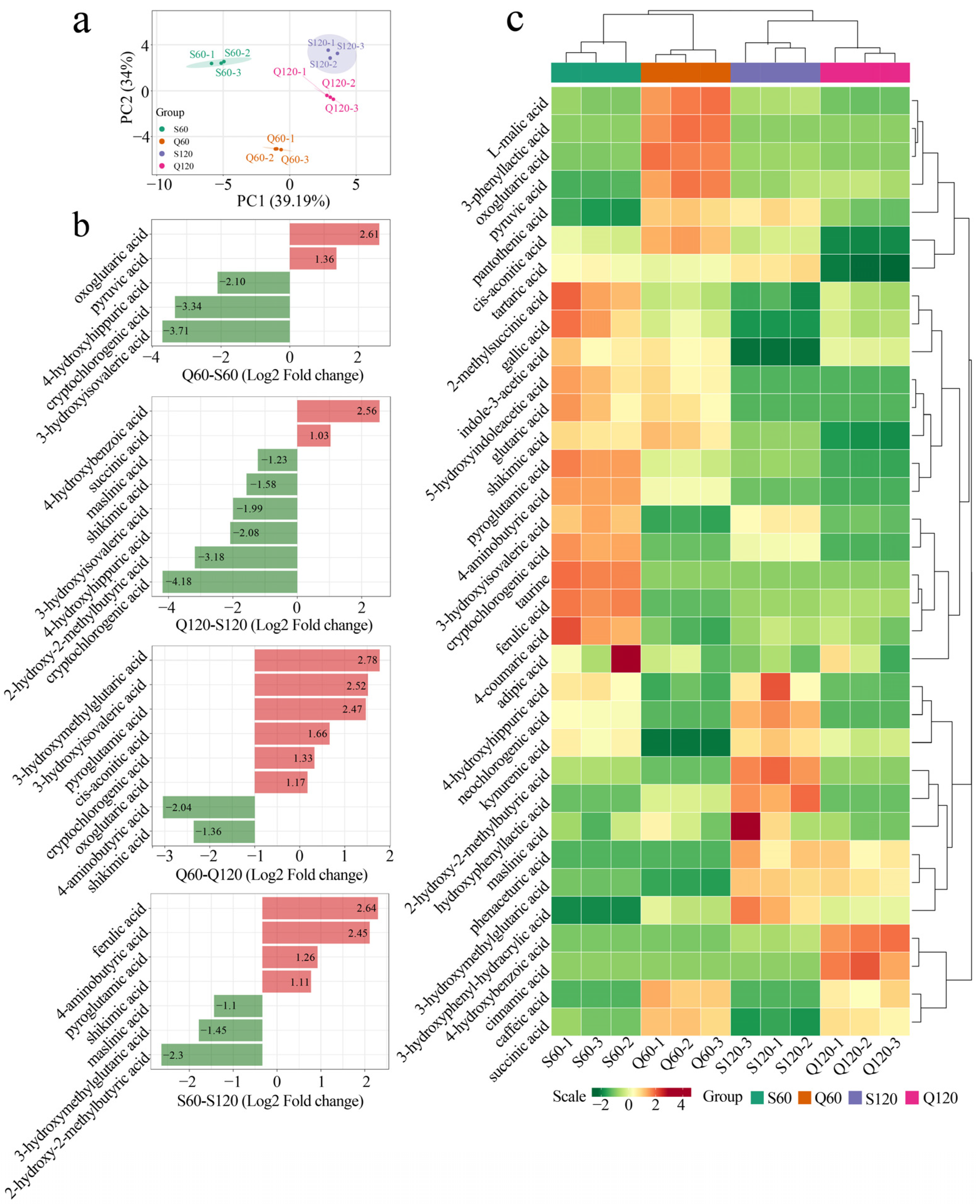
3.2. Varietal and Maturity Effects on Common Organic-Acid Profiles in Malus Fruits
3.3. Metabolic Pathways Mediating Organic-Acid Differences
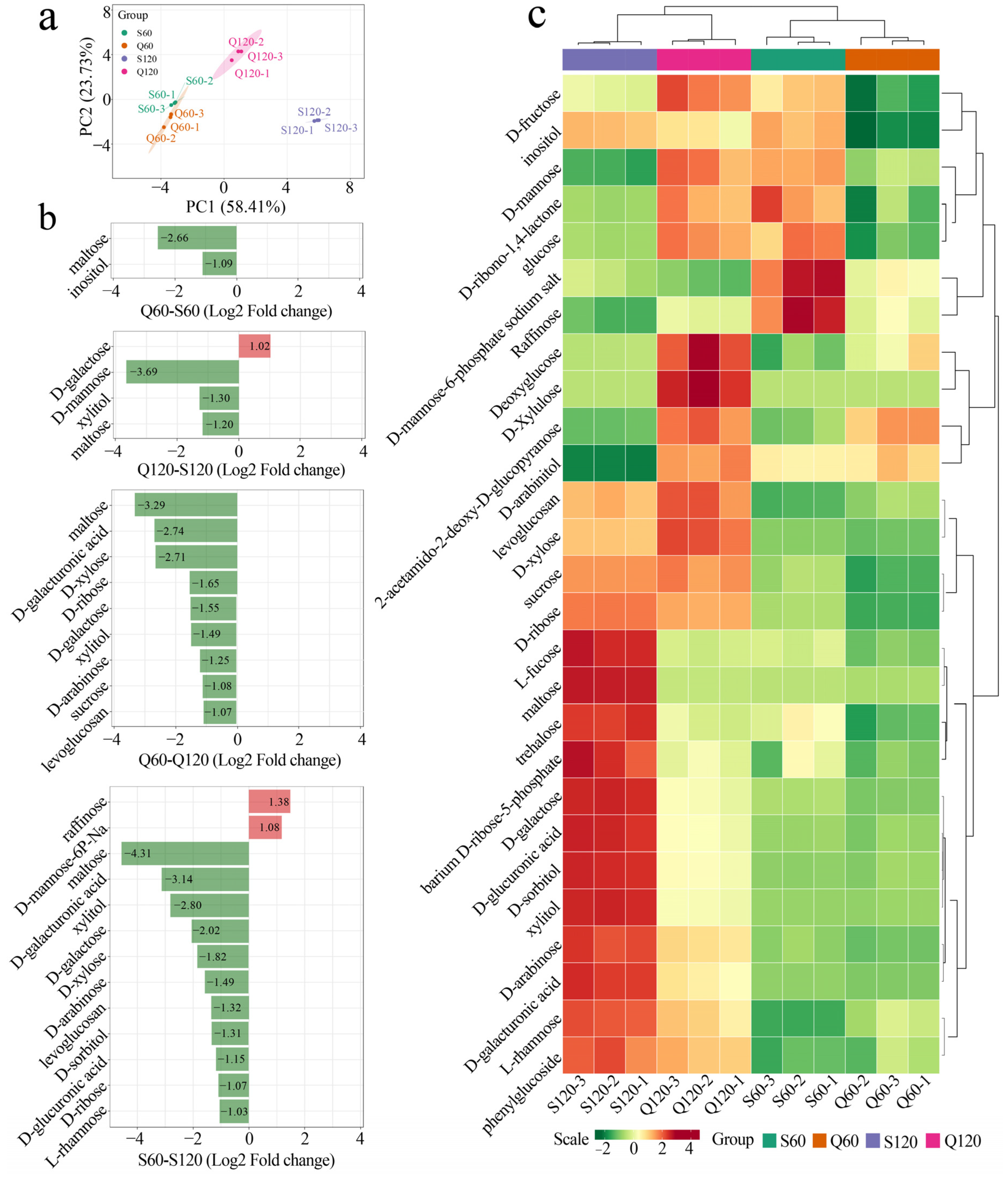
3.4. Varietal and Maturity Effects on Common Sugar Profiles in Malus Fruits
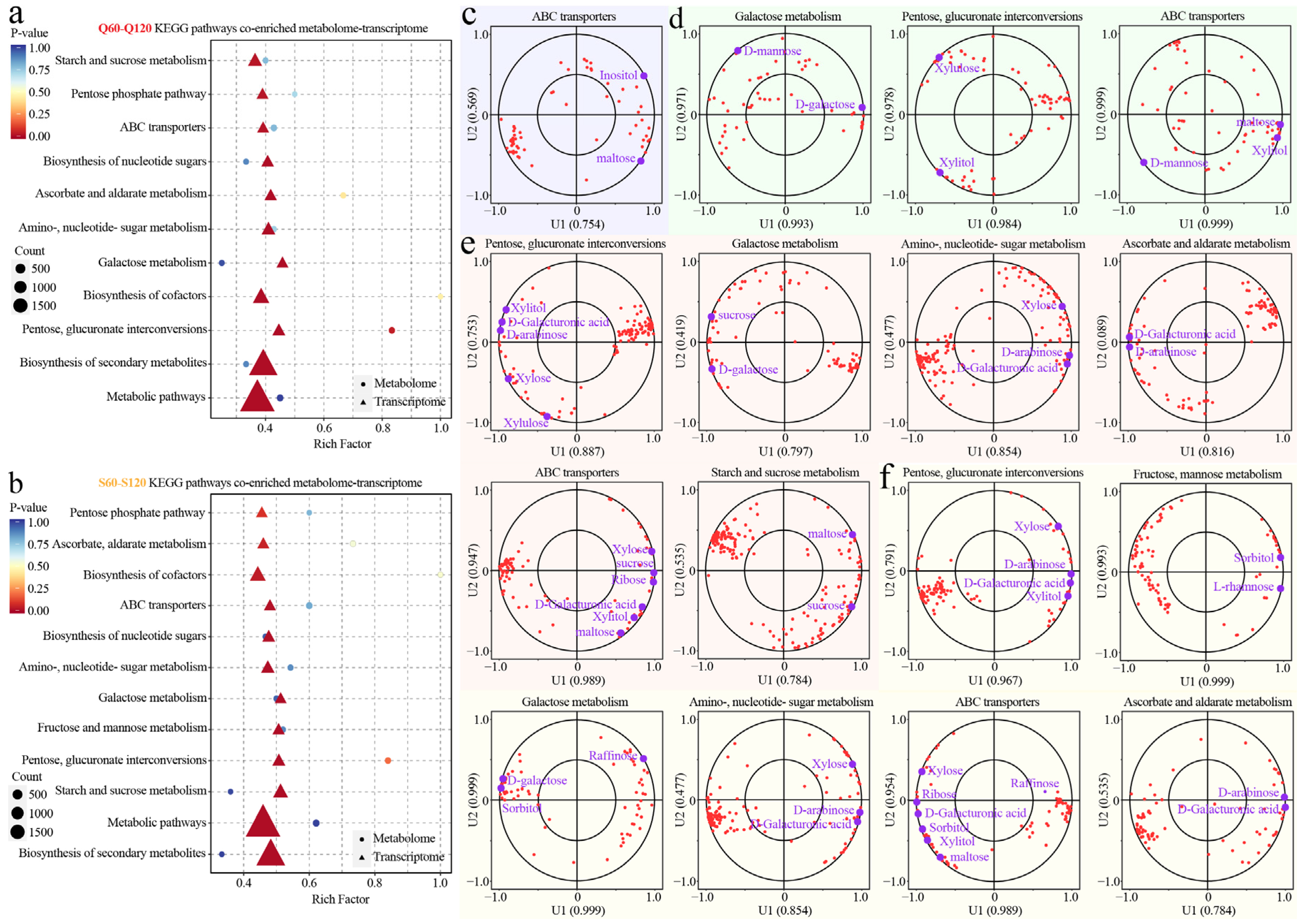
3.5. Metabolic Pathways Mediating Sugar Differences
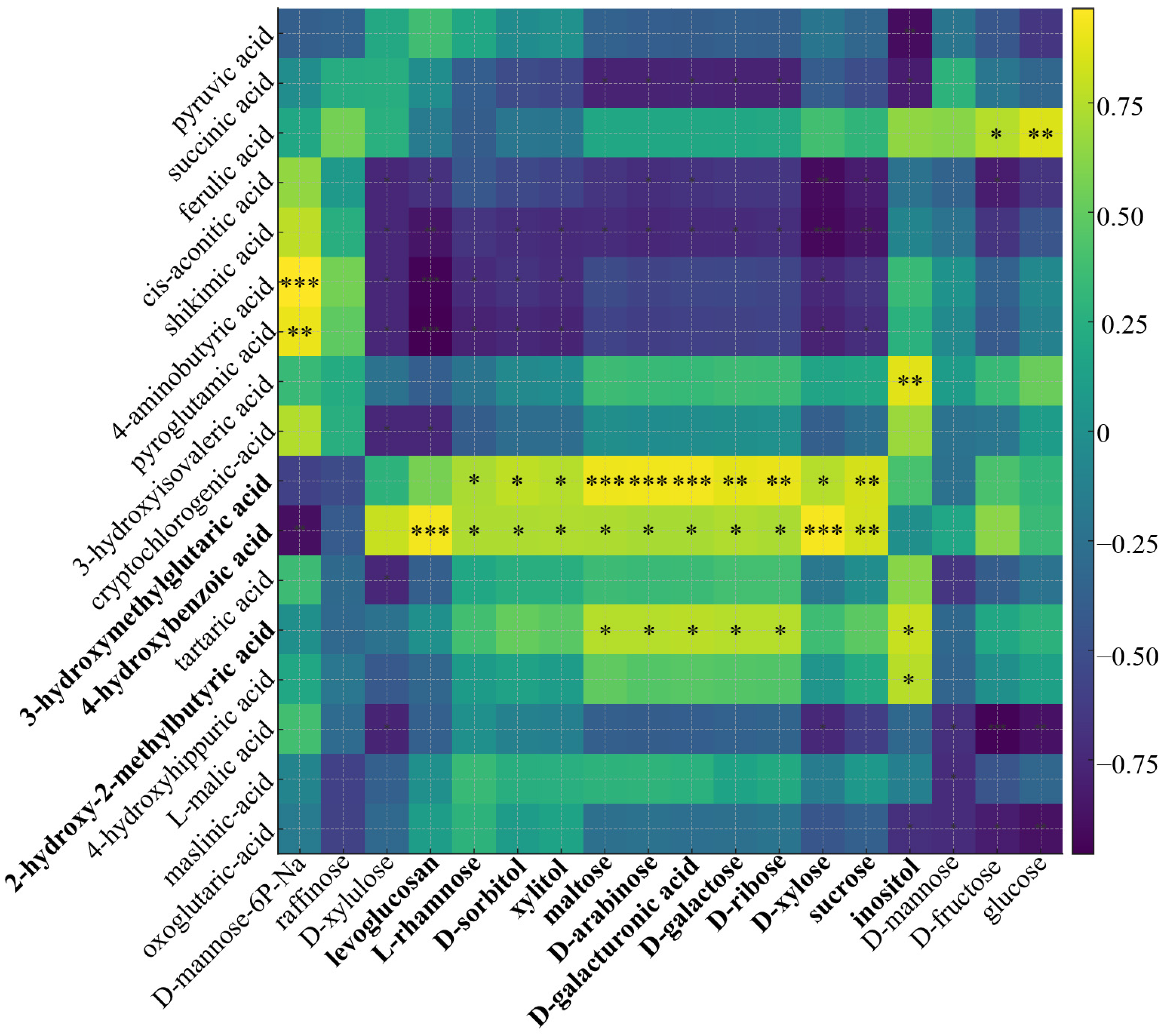
3.6. Correlation Analysis of Key Organic Acids and Sugar Interconversions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Zhang, M.; Zhang, G.; Li, P.; Ma, F. Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. Int. J. Mol. Sci. 2019, 20, 6060. [Google Scholar] [CrossRef]
- Sun, J.Q.; Chen, Q.; Liu, F.G.; Zhou, Q. Reconstruction of prehistoric cropland spatial patterns in Hehuang Valley. Appl. Ecol. Environ. Res. 2025, 23, 4715–4733. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Wang, K.; Sun, S.; Tian, W.; Li, Z.; Wang, G.; Lu, X.; Liu, Z.; Li, Q.; et al. Genetic Structure and Molecular Identities of 46 Apple Landraces (Malus Mill.) in China. Agronomy 2023, 13, 1262. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, X.; van Nocker, S.; Gong, X.; Ma, F. Overexpression of a protein kinase gene MpSnRK2.10 from Malus prunifolia confers tolerance to drought stress in transgenic Arabidopsis thaliana and apple. Gene 2019, 692, 26–34. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wang, X.; Wang, X.; Zhang, H.; Ji, Y.; Ren, D.; Lu, J. An efficient method using ultrasound to accelerate aging in crabapple (Malus asiatica) vinegar produced from fresh fruit and its influencing mechanism investigation. Ultrason. Sonochem. 2021, 72, 105464. [Google Scholar] [CrossRef] [PubMed]
- Mignard, P.; Beguería, S.; Giménez, R.; Font i Forcada, C.; Reig, G.; Moreno, M.Á. Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles. Agronomy 2022, 12, 827. [Google Scholar] [CrossRef]
- Ropelewska, E.; Szwejda-Grzybowska, J.; Lewandowski, M.; Mieszczakowska-Frąc, M. The Estimation of Phenolic Compounds, Sugars, and Acids of the Cultivar and Clones of Red-Fleshed Apples Based on Image Features. Foods 2025, 14, 1138. [Google Scholar] [CrossRef]
- Tijero, V.; Girardi, F.; Botton, A. Fruit Development and Primary Metabolism in Apple. Agronomy 2021, 11, 1160. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, J.; Qin, S.; Yang, W.; Ma, X.; Qiao, X.; Yang, B. Effects of Genetic Background and Altitude on Sugars, Malic Acid and Ascorbic Acid in Fruits of Wild and Cultivated Apples (Malus sp.). Foods 2021, 10, 2950. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, X.-Y.; Zhao, Y.-W.; Wang, C.-K.; Sun, Q.; Hu, D.-G. Optimization of apple fruit flavor by MdVHP1-2 via modulation of soluble sugar and organic acid accumulation. Plant Physiol. Biochem. 2024, 206, 108227. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, W.; Liu, C.; Chen, P.; Zhou, L. Study on the accumulation pattern of anthocyanins, sugars and organic acids in medicinal Vitis vinifera ‘SuoSuo’ during ripening. Food Chem. 2024, 433, 137294. [Google Scholar] [CrossRef]
- Lin, X.; Huang, S.; Zhang, Q.; Zhu, S.; Dong, X. Changes in the Primary Metabolites of ‘Fengtang’ Plums during Storage Detected by Widely Targeted Metabolomics. Foods 2022, 11, 2830. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Gao, Z.; Lin, M.; Zhang, X.; Ning, X.; Gong, X.; Lu, Y.; Chen, L.; Wang, X. Targeted multi-platform metabolome analysis and enzyme activity analysis of kiwifruit during postharvest ripening. Front. Plant Sci. 2023, 14, 1120166. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Q.; Yuan, C.; Fang, S.; Pan, K.; Long, L. Differences in taste characteristics and antioxidant properties of four substitute teas based on a targeted metabolomics approach. Beverage Plant Res. 2023, 3, 33. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; He, C.; Liu, Z.; Liang, J. Effect of puffing treatment on volatile components of green tea explored by gas chromatography–mass spectrometry and gas chromatography-olfactometry. Food Chem. X 2024, 23, 101746. [Google Scholar] [CrossRef]
- Póliska, S.; Fareh, C.; Lengyel, A.; Göczi, L.; Tőzsér, J.; Szatmari, I. Comparative transcriptomic analysis of Illumina and MGI next-generation sequencing platforms using RUNX3- and ZBTB46-instructed embryonic stem cells. Front. Genet. 2024, 14, 1275383. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Ma, Y.; Jiang, G.; Mei, X.; Li, X.; Yang, Q.; Kan, J.; Xu, Y.; Yang, T.; et al. WGCNA analysis revealing molecular mechanism that bio-organic fertilizer improves pear fruit quality by increasing sucrose accumulation and reducing citric acid metabolism. Front. Plant Sci. 2022, 13, 1039671. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef]
- Nosarzewski, M.; Archbold, D.D. Tissue-specific expression of SORBITOL DEHYDROGENASE in apple fruit during early development. J. Exp. Bot. 2007, 58, 1863–1872. [Google Scholar] [CrossRef]
- Horvacki, N.M.; Jakanovski, M.V.; Krstić, Đ.D.; Nedić, J.M.; Dramićanin, A.M.; Fotirić-Akšić, M.M.; Milojković-Opsenica, D.M. Evaluation of Sugar and Organic Acid Composition of Apple Cultivars (Malus domestica Borkh.) Grown in Serbia. Processes 2025, 13, 3093. [Google Scholar] [CrossRef]
- Jing, S.; Malladi, A. Higher growth of the apple (Malus × domestica Borkh.) fruit cortex is supported by resource intensive metabolism during early development. BMC Plant Biol. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Basile, L.A.; Zalguizuri, A.; Briones, G.; Lepek, V.C. Two Rieske Fe/S Proteins and TAT System in Mesorhizobium loti MAFF303099: Differential Regulation and Roles on Nodulation. Front. Plant Sci. 2018, 9, 1686. [Google Scholar] [CrossRef] [PubMed]
- Boeckx, J.; Pols, S.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Regulation of the Central Carbon Metabolism in Apple Fruit Exposed to Postharvest Low-Oxygen Stress. Front. Plant Sci. 2019, 10, 1384. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Jia, R.; Yang, N.; Jin, L.; Zhu, L.; Ma, B.; Yao, Y.-X.; Ma, F.; Li, M. Malate metabolism mediated by the cytoplasmic malate dehydrogenase gene MdcyMDH affects sucrose synthesis in apple fruit. Hortic. Res. 2022, 9, uhac194. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, B.; Wang, C.; Chen, X.; Ruan, Y.-L.; Yuan, Y.; Ma, F.; Li, M. MdWRKY126 modulates malate accumulation in apple fruit by regulating cytosolic malate dehydrogenase (MdMDH5). Plant Physiol. 2022, 188, 2059–2072. [Google Scholar] [CrossRef]
- Li, C.; Dougherty, L.; Coluccio, A.E.; Meng, D.; El-Sharkawy, I.; Borejsza-Wysocka, E.; Liang, D.; Piñeros, M.A.; Xu, K.; Cheng, L. Apple ALMT9 Requires a Conserved C-Terminal Domain for Malate Transport Underlying Fruit Acidity. Plant Physiol. 2019, 182, 992–1006. [Google Scholar] [CrossRef]
- Takayama, M.; Ezura, H. How and why does tomato accumulate a large amount of GABA in the fruit? Front. Plant Sci. 2015, 6, 612. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression Patterns of Genes Involved in Sugar Metabolism and Accumulation during Apple Fruit Development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, W.; Xiao, Z.; Xiang, X.; Zhong, Y. Metabolomics Unveiled the Accumulation Characteristics of Taste Compounds During the Development and Maturation of Litchi Fruit. Foods 2025, 14, 144. [Google Scholar] [CrossRef]
- Michaeli, S.; Fromm, H. Closing the Loop on the GABA Shunt in Plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef]
- Olmedo, P.; Zepeda, B.; Delgado-Rioseco, J.; Leiva, C.; Moreno, A.A.; Sagredo, K.; Blanco-Herrera, F.; Pedreschi, R.; Infante, R.; Meneses, C.; et al. Metabolite Profiling Reveals the Effect of Cold Storage on Primary Metabolism in Nectarine Varieties with Contrasting Mealiness. Plants 2023, 12, 766. [Google Scholar] [CrossRef]
- Gutschker, S.; Ruescher, D.; Rabbi, I.Y.; Rosado-Souza, L.; Pommerrenig, B.; Pauly, M.; Robertz, S.; van Doorn, A.M.; Schlereth, A.; Neuhaus, H.E.; et al. Carbon usage in yellow-fleshed Manihot esculenta storage roots shifts from starch biosynthesis to cell wall and raffinose biosynthesis via the myo-inositol pathway. Plant J. 2024, 119, 2045–2062. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.; Orellana, A. Nucleotide Sugar Transporters: Orchestrating Luminal Glycosylation in Plants. Annu. Rev. Plant Biol. 2025, 76, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, Z.; Wang, X.; Zhang, C.; Ma, F.; Li, M. Research advances in genetic quality of sugar content in apples. Fruit Res. 2023, 3, 13. [Google Scholar] [CrossRef]
- Le, X.H.; Millar, A.H. The diversity of substrates for plant respiration and how to optimize their use. Plant Physiol. 2023, 191, 2133–2149. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Guo, T.; Wei, C.; Tian, J.; Hou, X.; Li, Y. Integrative Multi-Omics Reveals Quality Markers and Metabolic Pathways Across Genotype and Ripening Gradients in High-Altitude Malus. Foods 2025, 14, 4039. https://doi.org/10.3390/foods14234039
Shi H, Guo T, Wei C, Tian J, Hou X, Li Y. Integrative Multi-Omics Reveals Quality Markers and Metabolic Pathways Across Genotype and Ripening Gradients in High-Altitude Malus. Foods. 2025; 14(23):4039. https://doi.org/10.3390/foods14234039
Chicago/Turabian StyleShi, Huiqin, Ting Guo, Chenlong Wei, Jie Tian, Xiaoqing Hou, and Yi Li. 2025. "Integrative Multi-Omics Reveals Quality Markers and Metabolic Pathways Across Genotype and Ripening Gradients in High-Altitude Malus" Foods 14, no. 23: 4039. https://doi.org/10.3390/foods14234039
APA StyleShi, H., Guo, T., Wei, C., Tian, J., Hou, X., & Li, Y. (2025). Integrative Multi-Omics Reveals Quality Markers and Metabolic Pathways Across Genotype and Ripening Gradients in High-Altitude Malus. Foods, 14(23), 4039. https://doi.org/10.3390/foods14234039





