Grains, Cereals, and Legumes: Implications in Glycemic Index and Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Grain Carbohydrate Content Data Collection
2.2. Estimation of Glycemic Index (GI) and Glycemic Load (GL) of the Grains
2.3. Estimation of Carbohydrate Content and Its Fiber Ratios
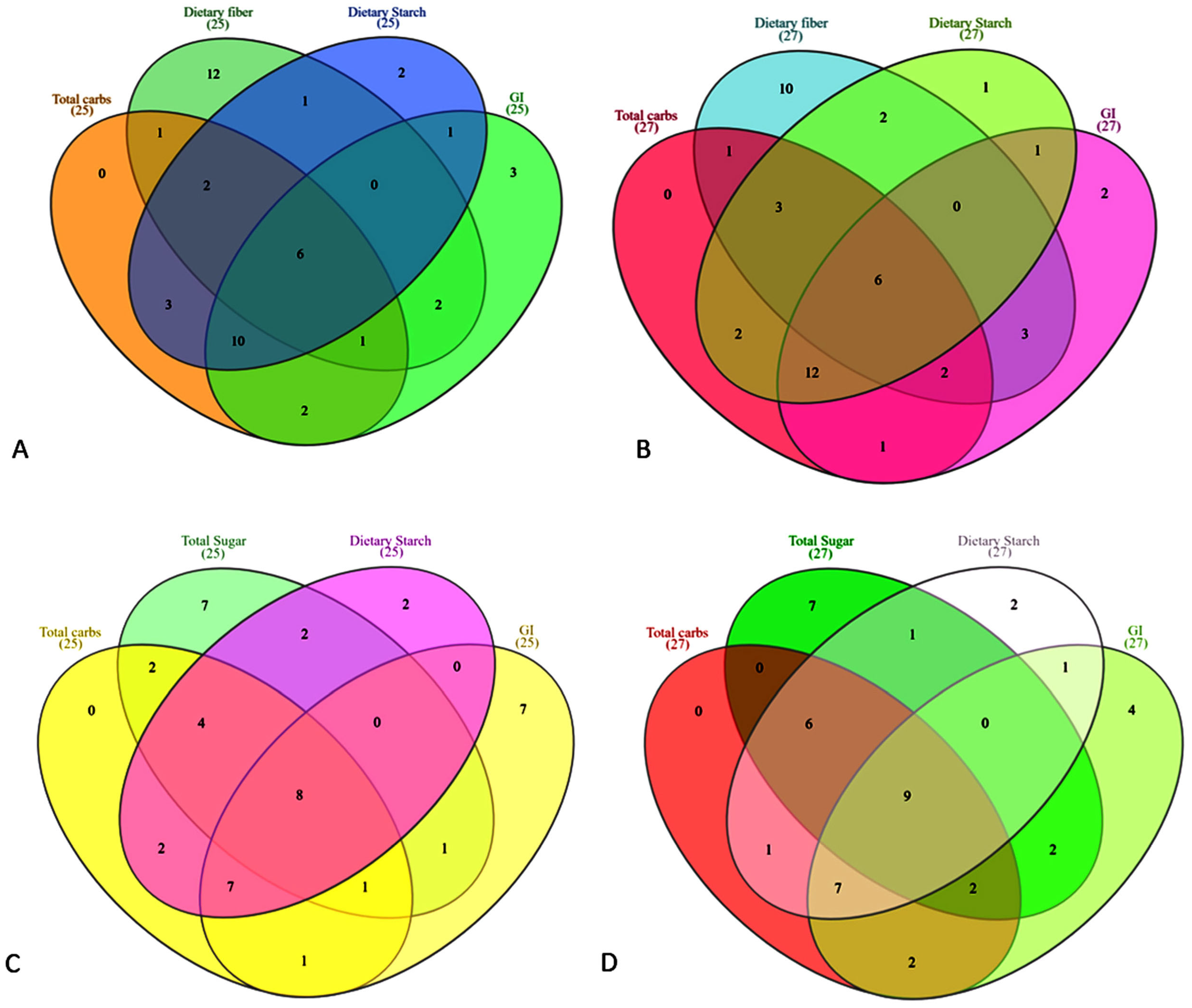
2.4. Venn Diagram
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Limitations and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| IBS | Irritable bowel syndrome |
| CGM | Continuous glucose monitoring |
| CRP | C-reactive Protein |
| CVD | Cardiovascular disease |
| CKD | Chronic Kidney disease |
| DS | Dietary Starch |
| DF | Dietary fiber |
| DGTAC | Dietary guidelines technical advisory committee |
| DGA | Dietary guidelines for Americans |
| FDA | Food and drug administration |
| FGM/isCGM | Flash/Intermittently scanned continuous glucose monitoring |
| GI | Glycemic index |
| GL | Glycemic load |
| GR | Glycemic response |
| GLP-1 | Glucagon-like peptide |
| HbA1c | Hemoglobin A1c |
| HHS | U.S. Department of Health and Human Services |
| iAUC | Incremental area under the curve |
| IDF | Insoluble dietary fiber |
| KFCD | Korean Food Composition Database |
| LDL | Low density lipoprotein |
| LE | Life expectancy |
| MLRA | Multiple linear regression analysis |
| NAFLD | Non-alcoholic fatty liver disease |
| NSP | Non-starch Polysaccharides |
| OS | Oligosaccharides |
| PCA | Principle component analysis |
| RG | Refined Grain |
| RS | Resistant starch |
| ROS | Reactive oxygen species |
| RT-CGM | Real-time Continuous glucose monitoring |
| SCFAs | Short-chain fatty acids |
| SDF | Soluble dietary fiber |
| T2DM | Type 2 Diabetes mellitus |
| TC | Total Carbohydrates |
| TS | Total Starch |
| WHO | World Health Organization |
| WG | Whole Grain |
| WGC | Whole grain Council |
References
- O’Neil, C.E.; Nicklas, T.A.; Zanovec, M.; Cho, S. Whole-grain consumption is associated with diet quality and nutrient intake in adults: The National Health and Nutrition Examination Survey, 1999–2004. J. Am. Diet. Assoc. 2010, 110, 1461–1468. [Google Scholar] [CrossRef]
- Hur, I.Y.; Reicks, M. Relationship between whole-grain intake, chronic disease risk indicators, and weight status among adolescents in the National Health and Nutrition Examination Survey, 1999–2004. J. Acad. Nutr. Diet. 2012, 112, 46–55. [Google Scholar] [CrossRef]
- Manhire, A.; Henry, C.; Hartog, M.; Heaton, K. Unrefined carbohydrate and dietary fibre in treatment of diabetes mellitus. J. Hum. Nutr. 1981, 35, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, C.; Coulston, A.; Reaven, G. To what extent does increased dietary fiber improve glucose and lipid metabolism in patients with noninsulin-dependent diabetes mellitus (NIDDM)? Am. J. Clin. Nutr. 1986, 43, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Wolever, T.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef] [PubMed]
- Chutkan, R.; Fahey, G.; Wright, W.L.; McRorie, J. Viscous versus nonviscous soluble fiber supplements: Mechanisms and evidence for fiber-specific health benefits. J. Am. Assoc. Nurse Pract. 2012, 24, 476–487. [Google Scholar] [CrossRef]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; Jenkins, A.L.; Smircic-Duvnjak, L.; Sievenpiper, J.L. Effect of viscous fiber supplementation on obesity indicators in individuals consuming calorie-restricted diets: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021, 60, 101–112. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Gallaher, D.D. Whole grain intake and cardiovascular disease: A review. Curr. Atheroscler. Rep. 2004, 6, 415–423. [Google Scholar] [CrossRef]
- Cheng, Z.; Qiao, D.; Zhao, S.; Zhang, B.; Lin, Q.; Xie, F. Whole grain rice: Updated understanding of starch digestibility and the regulation of glucose and lipid metabolism. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3244–3273. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Chaichoompu, E.; Ruengphayak, S.; Wattanavanitchakorn, S.; Wansuksri, R.; Yonkoksung, U.; Suklaew, P.O.; Chotineeranat, S.; Raungrusmee, S.; Vanavichit, A.; Toojinda, T. Development of Whole-Grain Rice Lines Exhibiting Low and Intermediate Glycemic Index with Decreased Amylose Content. Foods 2024, 13, 3627. [Google Scholar] [CrossRef] [PubMed]
- Topping, D. Cereal complex carbohydrates and their contribution to human health. J. Cereal Sci. 2007, 46, 220–229. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Raggio, A.M.; Shen, L.; McCutcheon, K.; Keenan, M.J. The importance of GLP-1 and PYY in resistant starch’s effect on body fat in mice. Mol. Nutr. Food Res. 2015, 59, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef]
- May, K.S.; den Hartigh, L.J. Gut microbial-derived short chain fatty acids: Impact on adipose tissue physiology. Nutrients 2023, 15, 272. [Google Scholar] [CrossRef]
- Byrne, C.; Chambers, E.; Morrison, D.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Jovanovski, E.; Khayyat, R.; Zurbau, A.; Komishon, A.; Mazhar, N.; Sievenpiper, J.L.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Jenkins, A.L. Should viscous fiber supplements be considered in diabetes control? Results from a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2019, 42, 755–766. [Google Scholar] [CrossRef]
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef]
- Jayasinghe, T.; Jenkins, J.; Medara, N.; Choowong, P.; Dharmarathne, G.; Kong, F.; Cho, H.; Kim, S.H.; Zhang, Y.; Franco-Duarte, R. Dietary Fibre Modulates Body Composition, Blood Glucose, Inflammation, Microbiome, and Metabolome in a Murine Model of Periodontitis. Nutrients 2025, 17, 1146. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzmanr, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Miller, J.B. International tables of glycemic index. Am. J. Clin. Nutr. 1995, 62, 871S–890S. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture, Agricultural Research Service. USDA Nutrient Database for Standard Reference, Release 14: Nutrient Data Laboratory Home Page; US Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2001. [Google Scholar]
- English, R.; Lewis, J. Food for Health: A Guide to Good Nutrition with Nutrient Values for 650 Australian Foods; Australia Government Pubishing Service: Canberra, Australia, 1991. [Google Scholar]
- AlEssa, H.B.; Bhupathiraju, S.N.; Malik, V.S.; Wedick, N.M.; Campos, H.; Rosner, B.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Cândido, F.G.; Pereira, E.V.; Alfenas, R.d.C.G. Use of the glycemic index in nutrition education. Rev. Nutr. 2013, 26, 89–96. [Google Scholar] [CrossRef]
- Esfahani, A.; Wong, J.M.; Mirrahimi, A.; Srichaikul, K.; Jenkins, D.J.; Kendall, C.W. The glycemic index: Physiological significance. J. Am. Coll. Nutr. 2009, 28 (Suppl. 4), 439S–445S. [Google Scholar] [CrossRef]
- Singh, M.K.; Han, S.; Ju, S.; Ranbhise, J.S.; Akter, S.; Kim, S.S.; Kang, I. Fruit Carbohydrates and Their Impact on the Glycemic Index: A Study of Key Determinants. Foods 2025, 14, 646. [Google Scholar] [CrossRef]
- Englyst, K.N.; Englyst, H.N. Carbohydrate bioavailability. Br. J. Nutr. 2005, 94, 1–11. [Google Scholar] [CrossRef]
- Petkova, N.; Popova, V.; Ivanova, T.; Mazova, N.; Panayotov, N.; Stoyanova, A. Nutritional composition of different cape gooseberry genotypes (Physalis peruviana L.)—A comparative study. Food Res. 2021, 5, 191–202. [Google Scholar] [CrossRef]
- Englyst, K.; Liu, S.; Englyst, H. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 2007, 61, S19–S39. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Teymoori, F.; Farhadnejad, H.; Moslehi, N.; Mirmiran, P.; Mokhtari, E.; Azizi, F. The association of dietary insulin and glycemic indices with the risk of type 2 diabetes. Clin. Nutr. 2021, 40, 2138–2144. [Google Scholar] [CrossRef]
- O’Dea, K.; Nestel, P.J.; Antonoff, L. Physical factors influencing postprandial glucose and insulin responses to starch. Am. J. Clin. Nutr. 1980, 33, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Musa-Veloso, K.; Poon, T.; Harkness, L.S.; O’Shea, M.; Chu, Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018, 108, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Truswell, A. Cereal grains and coronary heart disease. Eur. J. Clin. Nutr. 2002, 56, 1–14. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Chen, C.; Zhang, J. Physicochemical property of starch-soluble dietary fiber conjugates and their resistance to enzymatic hydrolysis. Int. J. Food Prop. 2015, 18, 2457–2471. [Google Scholar] [CrossRef]
- Nimptsch, K.; Brand-Miller, J.C.; Franz, M.; Sampson, L.; Willett, W.C.; Giovannucci, E. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am. J. Clin. Nutr. 2011, 94, 182–190. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Coulston, A.; Hollenbeck, C.; Reaven, G. Utility of studies measuring glucose and insulin responses to various carbohydrate-containing foods. Am. J. Clin. Nutr. 1984, 39, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, F.X. Glycemic index and disease. Am. J. Clin. Nutr. 2002, 76, 290S–298S. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Møller, B.K.; Raben, A.; Pedersen, D.; Tetens, I.; Holst, J.J.; Astrup, A. The use of glycaemic index tables to predict glycaemic index of composite breakfast meals. Br. J. Nutr. 2004, 91, 979–989. [Google Scholar] [CrossRef]
- Venn, B.; Green, T. Glycemic index and glycemic load: Measurement issues and their effect on diet–disease relationships. Eur. J. Clin. Nutr. 2007, 61, S122–S131. [Google Scholar] [CrossRef]
- Armendáriz-Anguiano, A.L.; Jimenez-Cruz, A.; Bacardi-Gascon, M.; Hurtado-Ayala, L. Effect of a low glycemic load on body composition and Homeostasis Model Assessment (HOMA) in overweight and obese subjects. Nutr. Hosp. 2011, 26, 170–175. [Google Scholar]
- Li, M.; Li, L.; Sun, B.; Ma, S. Interaction of wheat bran dietary fiber-gluten protein affects dough product: A critical review. Int. J. Biol. Macromol. 2024, 255, 128199. [Google Scholar] [CrossRef]
- Kaline, K.; Bornstein, S.; Bergmann, A.; Hauner, H.; Schwarz, P. The importance and effect of dietary fiber in diabetes prevention with particular consideration of whole grain products. Horm. Metab. Res. 2007, 39, 687–693. [Google Scholar] [CrossRef]
- Hou, Q.; Li, Y.; Li, L.; Cheng, G.; Sun, X.; Li, S.; Tian, H. The metabolic effects of oats intake in patients with type 2 diabetes: A systematic review and meta-analysis. Nutrients 2015, 7, 10369–10387. [Google Scholar] [CrossRef]
- Li, X.; Cai, X.; Ma, X.; Jing, L.; Gu, J.; Bao, L.; Li, J.; Xu, M.; Zhang, Z.; Li, Y. Short-and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: A randomized control trial. Nutrients 2016, 8, 549. [Google Scholar] [CrossRef]
- Li, M.; Ma, S. A review of healthy role of dietary fiber in modulating chronic diseases. Food Res. Int. 2024, 191, 114682. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Flint, A.; Gutzwiller, J.-P.; Naslund, E.; Beglinger, C.; Hellstrom, P.; Long, S.; Morgan, L.; Holst, J.; Astrup, A. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4382–4389. [Google Scholar] [PubMed]
- Drucker, D.J. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003, 26, 2929–2940. [Google Scholar] [CrossRef]
- Schirra, J.; Göke, B. The physiological role of GLP-1 in human: Incretin, ileal brake or more? Regul. Pept. 2005, 128, 109–115. [Google Scholar] [CrossRef]
- Ma, W.; Nguyen, L.H.; Song, M.; Wang, D.D.; Franzosa, E.A.; Cao, Y.; Joshi, A.; Drew, D.A.; Mehta, R.; Ivey, K.L. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Recent advances in understanding the structure and function of the human microbiome. Front. Microbiol. 2022, 13, 825338. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Roumia, H.; Kókai, Z.; Mihály-Langó, B.; Csobod, É.C.; Benedek, C. Ancient Wheats—A Nutritional and Sensory Analysis Review. Foods 2023, 12, 2411. [Google Scholar] [CrossRef]
- Magi, C.E.; Rasero, L.; Mannucci, E.; Bonaccorsi, G.; Ranaldi, F.; Pazzagli, L.; Faraoni, P.; Mulinacci, N.; Bambi, S.; Longobucco, Y. USE of ancient grains for the management of diabetes mellitus: A systematic review with meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1110–1128. [Google Scholar] [CrossRef]
- Djurle, S.; Andersson, A.A.; Andersson, R. Milling and extrusion of six barley varieties, effects on dietary fibre and starch content and composition. J. Cereal Sci. 2016, 72, 146–152. [Google Scholar] [CrossRef]
- Messia, M.C.; Candigliota, T.; De Arcangelis, E.; Marconi, E. Arabinoxylans and [beta]-glucans assessment in cereals. Ital. J. Food Sci. 2017, 29, 112–123. [Google Scholar]
- Sterna, V.; Zute, S.; Jansone, I.; Kantane, I. Chemical composition of covered and naked spring barley varieties and their potential for food production. Pol. J. Food Nutr. Sci. 2017, 67, 151–158. [Google Scholar] [CrossRef]
- Andersson, R.; Fransson, G.; Tietjen, M.; Åman, P. Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. J. Agric. Food Chem. 2009, 57, 2004–2008. [Google Scholar] [CrossRef]
- Mathews, R.; Shete, V.; Chu, Y. The effect of cereal Β-glucan on body weight and adiposity: A review of efficacy and mechanism of action. Crit. Rev. Food Sci. Nutr. 2023, 63, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Haros, C.M.; Schoenlechner, R. Pseudocereals: Chemistry and Technology; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.-P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Kendall, C.; Nguyen, T.; Teitel, J.; Marchie, A.; Chiu, M.; Taha, A.; Faulkner, D.; Kemp, T.; Wong, J. Effect on hematologic risk factors for coronary heart disease of a cholesterol reducing diet. Eur. J. Clin. Nutr. 2007, 61, 483–492. [Google Scholar] [CrossRef]
- Jung, S.-J.; Oh, M.-R.; Park, S.-H.; Chae, S.-W. Effects of rice-based and wheat-based diets on bowel movements in young Korean women with functional constipation. Eur. J. Clin. Nutr. 2020, 74, 1565–1575. [Google Scholar] [CrossRef]
- Törrönen, R.; Kolehmainen, M.; Sarkkinen, E.; Poutanen, K.; Mykkänen, H.; Niskanen, L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J. Nutr. 2013, 143, 430–436. [Google Scholar] [CrossRef]
- Elbalshy, M.; Haszard, J.; Smith, H.; Kuroko, S.; Galland, B.; Oliver, N.; Shah, V.; de Bock, M.I.; Wheeler, B.J. Effect of divergent continuous glucose monitoring technologies on glycaemic control in type 1 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabet. Med. 2022, 39, e14854. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, P.A.; Montoya, L.; Ruelas, V.; Xing, D.; Chen, V.; Beck, R.; Peters, A.L. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol. Ther. 2013, 15, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Singh, R.; Zhang, B.; Kumar, S.; Li, G. WaveFlex biosensor based on S-tapered and waist-expanded technique for detection of glycosylated hemoglobin. Biomed. Opt. Express 2023, 14, 6100–6113. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-Y.; Cho, M.-K. Effects of Continuous Glucose Monitoring on Glycemic Control in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 571. [Google Scholar] [CrossRef]
- Chellamani, N.; Albelwi, S.A.; Shanmuganathan, M.; Amirthalingam, P.; Paul, A. Diabetes: Non-invasive blood glucose monitoring using federated learning with biosensor signals. Biosensors 2025, 15, 255. [Google Scholar] [CrossRef]
- Nie, Z.; Rong, M.; Li, K. Blood glucose prediction based on imagingphotoplethysmography in combination with Machine learning. Biomed. Signal Process. Control 2023, 79, 104179. [Google Scholar] [CrossRef]
- Gupta, S.S.; Kwon, T.-H.; Hossain, S.; Kim, K.-D. Towards non-invasive blood glucose measurement using machine learning: An all-purpose PPG system design. Biomed. Signal Process. Control 2021, 68, 102706. [Google Scholar]
- Elsheakh, D.N.; Mohamed, E.-H.; Eldamak, A.R. Blood glucose monitoring biosensor based on multiband split-ring resonator monopole antenna. Biosensors 2025, 15, 250. [Google Scholar] [CrossRef]
- Guoqiang, G.; Liang, Q.; Yani, Z.; Pengyun, W.; Fanzhuo, K.; Yuyang, Z.; Zhiyuan, L.; Xing, N.; Xue, Z.; Qiongya, L. Recent advances in glucose monitoring utilizing oxidase electrochemical biosensors integrating carbon-based nanomaterials and smart enzyme design. Front. Chem. 2025, 13, 1591302. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. bmj 2016, 353, i2716. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Chen, X.; Yu, M.; Pan, Q.; Guo, L. Whole grain food diet slightly reduces cardiovascular risks in obese/overweight adults: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 82. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Zhu, L.; Fang, Z.; He, L.; Ai, D.; Jin, Y. Whole grain and cereal fiber intake and the risk of type 2 diabetes: A meta-analysis. Int. J. Mol. Epidemiol. Genet. 2019, 10, 38. [Google Scholar]
- Wu, W.; Qiu, J.; Wang, A.; Li, Z. Impact of whole cereals and processing on type 2 diabetes mellitus: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1447–1474. [Google Scholar] [CrossRef] [PubMed]
- Hullings, A.G.; Sinha, R.; Liao, L.M.; Freedman, N.D.; Graubard, B.I.; Loftfield, E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr. 2020, 112, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Gasperi, V.; Catani, M.V.; Savini, I. The impact of whole grain intake on gastrointestinal tumors: A focus on colorectal, gastric, and esophageal cancers. Nutrients 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Evan, Y.Y.; Wesselius, A.; Mehrkanoon, S.; Brinkman, M.; van Den Brandt, P.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; Gunter, M.; Huybrechts, I. Grain and dietary fiber intake and bladder cancer risk: A pooled analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 112, 1252–1266. [Google Scholar] [CrossRef]
- Mann, K.D.; Hopkins, S.; Foster, J.; Seal, C.J. Investigating the impact of replacing refined grain foods with whole-grain foods on fibre intake in the UK. Proc. Nutr. Soc. 2018, 77, E134. [Google Scholar] [CrossRef]
- Seal, C. The importance of whole grains in improving diet quality: Is it a valid public health policy goal? Cereal Foods World 2019, 64, 1–6. [Google Scholar] [CrossRef]
- Thorne, M.J.; Thompson, L.; Jenkins, D. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am. J. Clin. Nutr. 1983, 38, 481–488. [Google Scholar] [CrossRef]
- Fadnes, L.T.; Økland, J.-M.; Haaland, Ø.A.; Johansson, K.A. Estimating impact of food choices on life expectancy: A modeling study. PLoS Med. 2022, 19, e1003889. [Google Scholar]
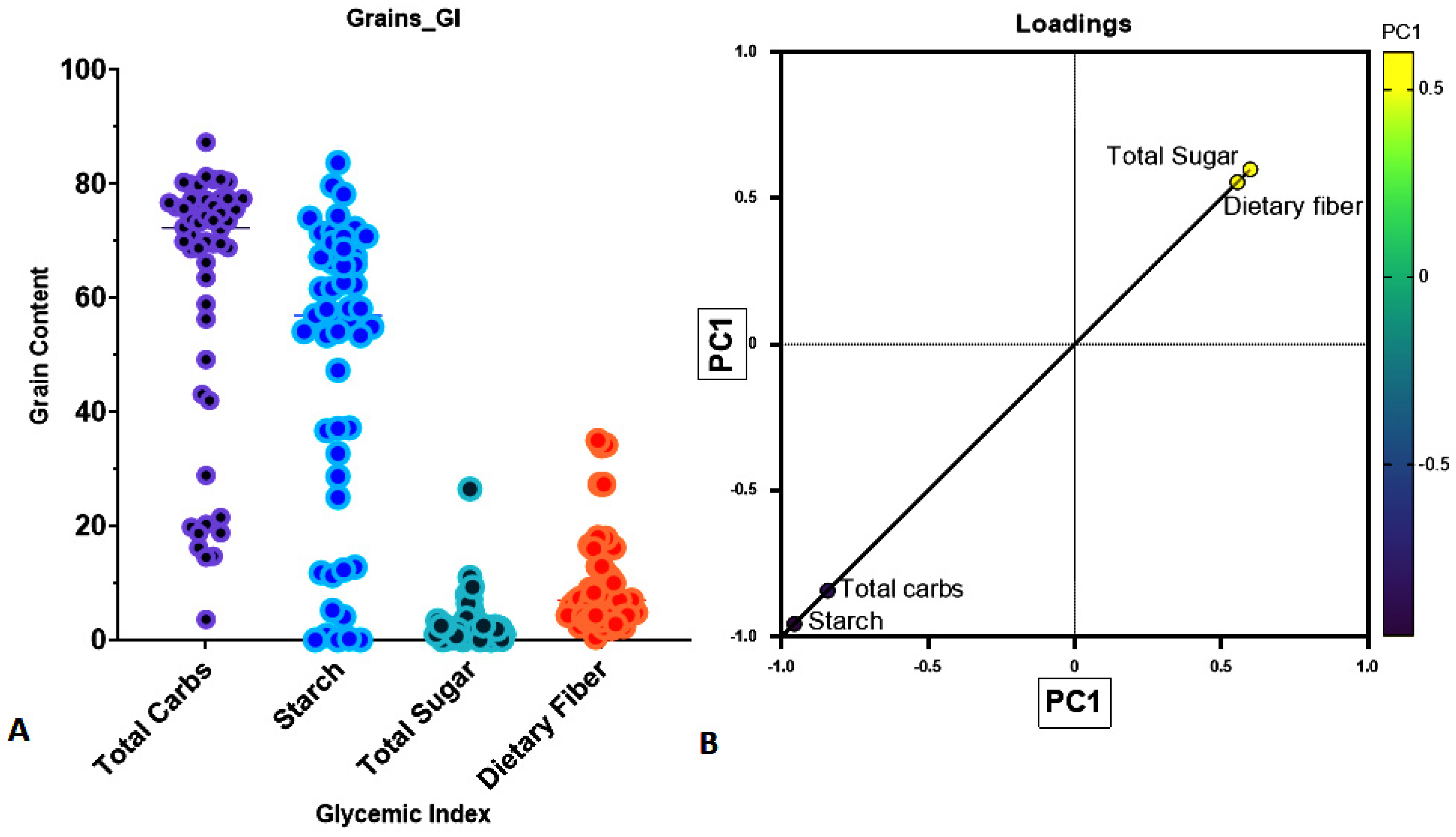
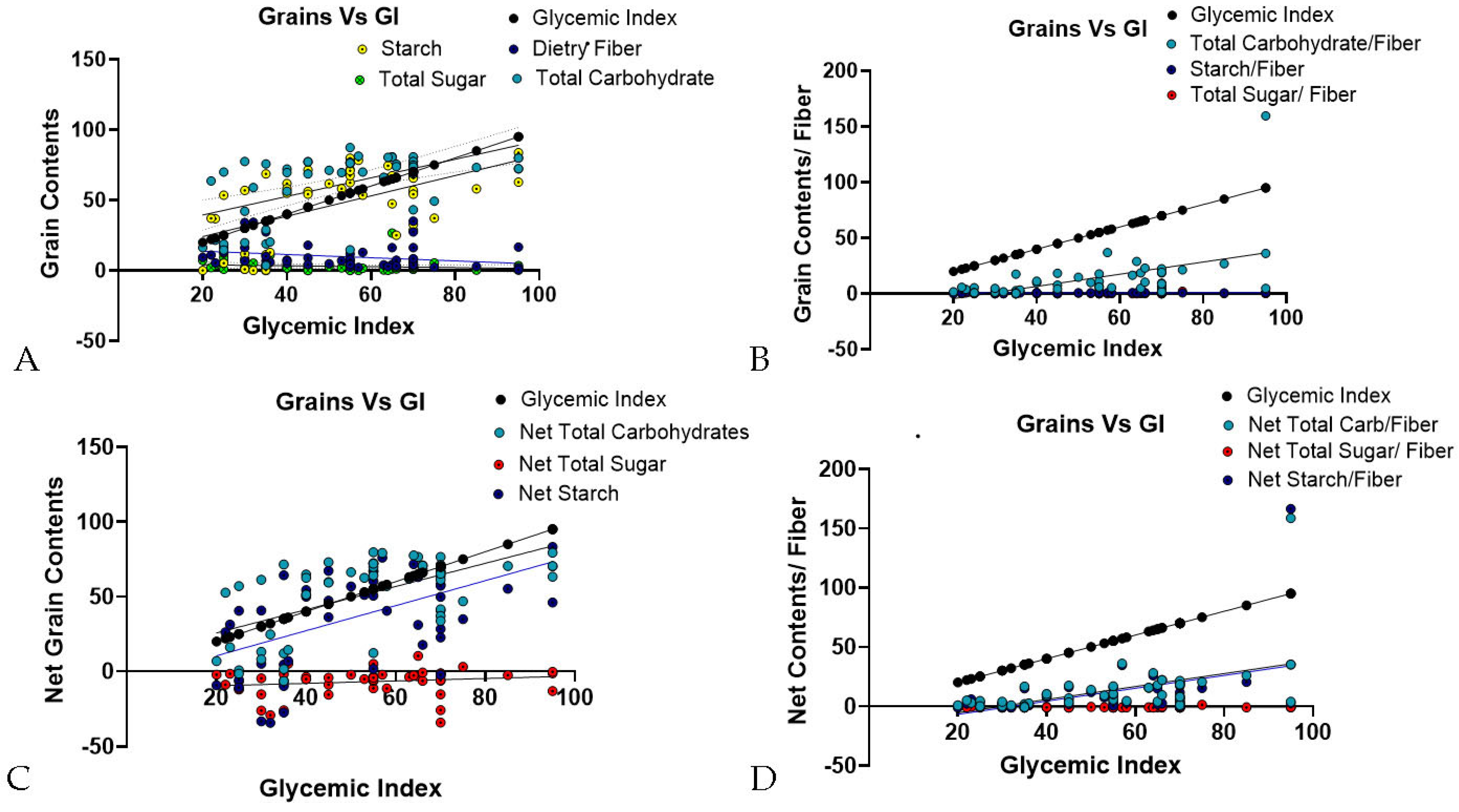
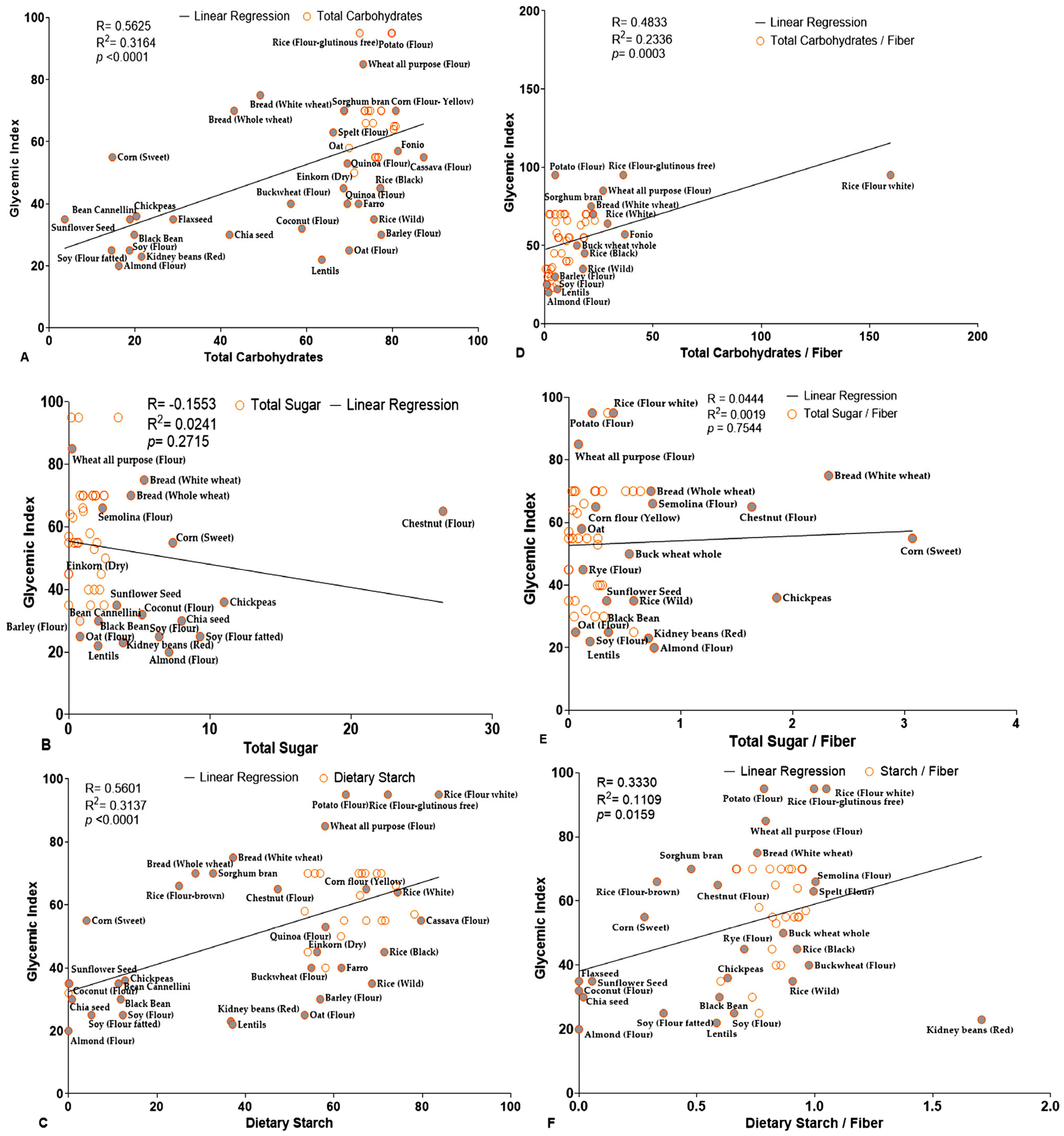
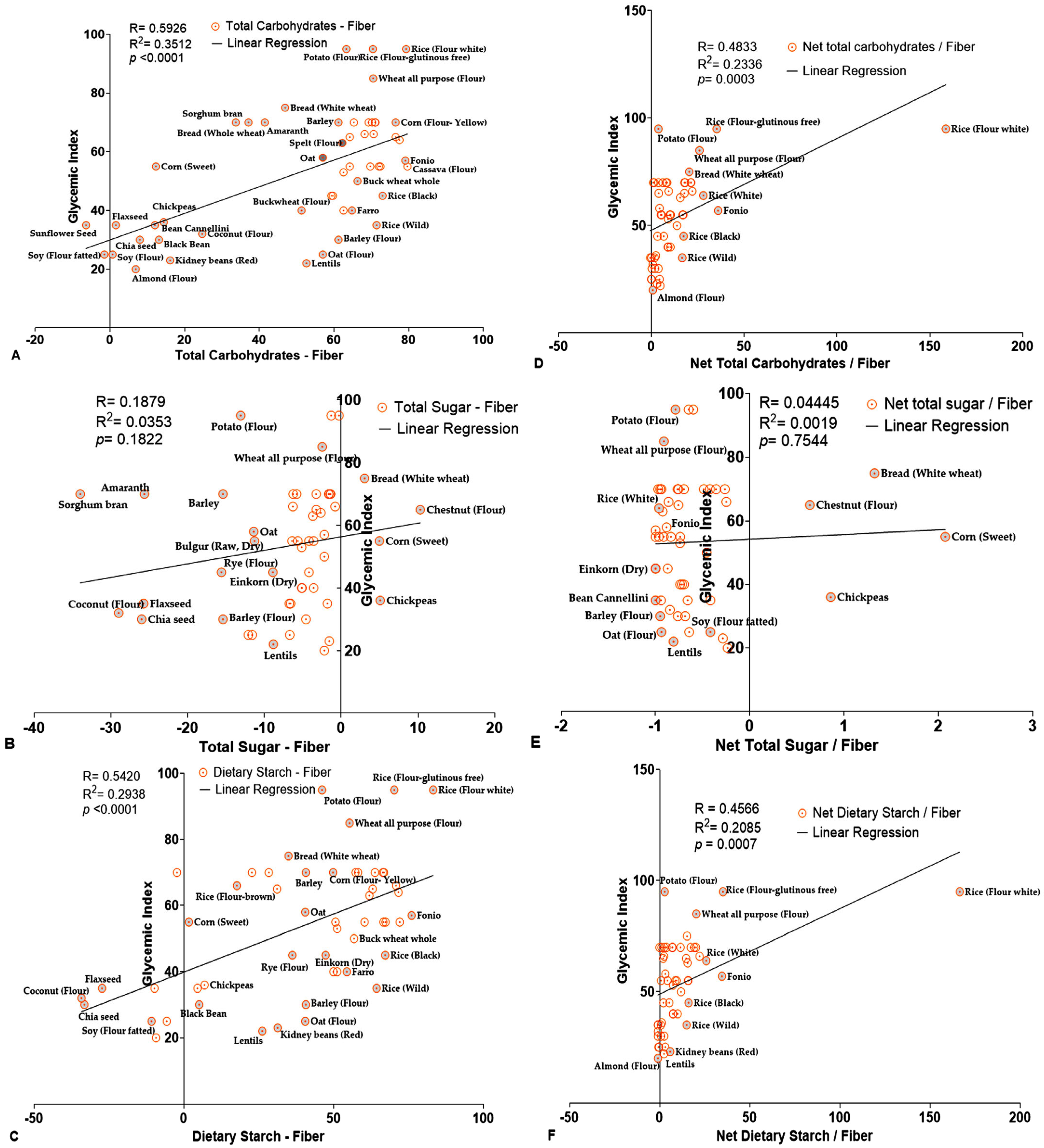
| S.no. | Grains | Total Carbohydrate (TC) | Dietary Fiber (DF) | Dietary Starch (DS) | Total Sugar (TS) | Glycemic Index (GI) | Glycemic Load (GL) |
|---|---|---|---|---|---|---|---|
| 1 | Amaranth | 68.8 | 27.34 | 55.7 | 1.7 | 70 | 43.4 |
| 2 | Barley | 77.4 | 16.2 | 56.9 | 0.8 | 70 | 67 |
| 3 | Buck wheat (Whole) | 71.1 | 4.8 | 61.6 | 2.6 | 50 | 22 |
| 4 | Bulgur (Raw, Dry) | 75.9 | 11.7 | 62.3 | 0.4 | 55 | 10.4 |
| 5 | Black Bean | 19.8 | 6.69 | 11.8 | 2.1 | 30 | 2 |
| 6 | Bean Cannellini | 18.8 | 6.76 | 11.3 | 0 | 35 | 21.4 |
| 7 | Bread (White) | 49.2 | 2.3 | 37.2 | 5.34 | 75 | 11 |
| 8 | Bread wheat (Whole) | 43.1 | 6 | 28.7 | 4.41 | 70 | 19 |
| 9 | Chia seed | 42 | 34 | 0.8 | 8 | 30 | 12.6 |
| 10 | Chickpeas | 20.3 | 5.92 | 12.8 | 11 | 36 | 9 |
| 11 | Corn flour (Yellow) | 80.8 | 4.3 | 67.3 | 1.04 | 65 | 10.1 |
| 12 | Corn flour (White) | 76.7 | 7 | 67.3 | 0.6 | 55 | 10.4 |
| 13 | Corn (Sweet) | 14.7 | 2.4 | 4.08 | 7.37 | 55 | 10.4 |
| 14 | Einkorn (Dry, Raw) | 68.6 | 8.9 | 56.2 | 0 | 45 | 8.1 |
| 15 | Farro | 72.1 | 7.3 | 61.7 | 2.2 | 40 | 11.3 |
| 16 | Flaxseed | 34.4 | 23.1 | 1.3 | 1.55 | 35 | 0.6 |
| 17 | Almond flour | 16.2 | 9.3 | 0 | 7.1 | 20 | 15.1 |
| 18 | Barley flour | 77.4 | 16.2 | 56.9 | 0.8 | 30 | 16.8 |
| 19 | Buckwheat flour | 56.3 | 5 | 54.9 | 1.4 | 40 | 28.2 |
| 20 | Cassava flour | 87.3 | 7.66 | 79.7 | 2 | 55 | 20.9 |
| 21 | Chestnut flour | 80.4 | 16.2 | 47.32 | 26.5 | 65 | 46.1 |
| 22 | Coconut flour | 58.9 | 34.2 | 0 | 5.2 | 32 | 18.1 |
| 23 | Corn flour (Yellow) | 80.8 | 4.3 | 54.1 | 1.04 | 70 | 53.8 |
| 24 | Oat flour | 69.9 | 12.9 | 53.4 | 0.8 | 25 | 3 |
| 25 | Potato flour | 79.9 | 16.6 | 62.7 | 3.5 | 95 | 78.9 |
| 26 | Quinoa flour | 69.5 | 6.95 | 58.1 | 1.8 | 40 | 22.9 |
| 27 | Rice flour (brown) | 75.5 | 7.3 | 25 | 1 | 66 | 32 |
| 28 | Rice flour (glutinous free) | 72.4 | 2 | 72.2 | 0.7 | 95 | 76.1 |
| 29 | Rice flour (white) | 79.8 | 0.5 | 83.7 | 0.2 | 95 | 76.1 |
| 30 | Rye flour | 77.2 | 17.9 | 54.1 | 2.3 | 45 | 28.9 |
| 31 | Semolina flour | 73.8 | 3.2 | 74 | 2.4 | 66 | 14.7 |
| 32 | Sorghum flour | 77.4 | 8.16 | 66.3 | 1.9 | 70 | 46.5 |
| 33 | Soy flour | 18.7 | 18 | 12.3 | 6.4 | 25 | 4.5 |
| 34 | Soy flour (fatted) | 35.2 | 16 | 5.3 | 9.3 | 25 | 4.5 |
| 35 | Spelt flour | 66.2 | 4 | 65.9 | 0.3 | 63 | 28 |
| 36 | Wheat flour (all purpose) | 73.2 | 2.72 | 58 | 0.24 | 85 | 62.6 |
| 37 | Fonio | 81.3 | 2.2 | 78.2 | 0 | 57 | 17.5 |
| 38 | Kidney beans (Red) | 21.5 | 5.4 | 36.7 | 3.85 | 23 | 6 |
| 39 | Lentils | 63.54 | 10.93 | 37.1 | 2.083 | 22 | 3 |
| 40 | Millet | 74.4 | 3.33 | 67.1 | 1.7 | 70 | 51.1 |
| 41 | Oat | 69.9 | 12.9 | 53.4 | 1.5 | 58 | 16 |
| 42 | Quinoa flour | 69.5 | 6.95 | 58.1 | 1.8 | 53 | 9 |
| 43 | Rice (Black) | 77.2 | 4.2 | 71.4 | 0 | 45 | 33.8 |
| 44 | Rice (Brown) | 76.7 | 4.3 | 71.6 | 0.7 | 55 | 18 |
| 45 | Rice (Red) | 76.2 | 4.2 | 70.8 | 0 | 55 | 38.8 |
| 46 | Rice (White) | 80.3 | 2.77 | 74.4 | 0.1 | 64 | 26 |
| 47 | Sorghum bran | 68.7 | 35 | 32.7 | 1 | 70 | 46.5 |
| 48 | Sorghum flour (white) | 73.5 | 3.3 | 69.7 | 1.9 | 70 | 46.5 |
| 49 | Sorghum (White) | 74.9 | 3.9 | 70.7 | 2.5 | 70 | 46.5 |
| 50 | Sorghum (Whole grain) | 73.6 | 8.3 | 65.6 | 2.5 | 70 | 46.5 |
| 51 | Sunflower Seed | 24.5 | 7.2 | 1 | 3.4 | 35 | 7 |
| 52 | Rice (Wild) | 75.7 | 4.3 | 68.6 | 2.5 | 35 | 7.3 |
| S.no. | Grains | Carbohydrate-to-Fiber Ratio | Available Carbohydrate | ||||
|---|---|---|---|---|---|---|---|
| Total Carbohydrate (TC/DF) | Total Sugar (TS/DF) | Dietary Starch (DS/DF) | Total Carbohydrate (TC-DF) | Total Sugar (TS-DF) | Dietary Starch (DS-DF) | ||
| 1 | Amaranth | 2.516 | 0.0622 | 0.810 | 41.460 | −25.64 | 28.360 |
| 2 | Barley (Flour) | 4.778 | 0.0494 | 0.735 | 61.200 | −15.40 | 40.700 |
| 3 | Buck wheat whole grain | 14.813 | 0.5417 | 0.866 | 66.300 | −2.20 | 56.800 |
| 4 | Bulgur (Raw, Dry) | 6.487 | 0.0342 | 0.821 | 64.200 | −11.30 | 50.600 |
| 5 | Black Bean | 2.960 | 0.3139 | 0.596 | 13.110 | −4.59 | 5.110 |
| 6 | Bean Cannellini | 2.781 | 0.0000 | 0.601 | 12.040 | −6.76 | 4.540 |
| 7 | Bread (White) | 21.391 | 2.3217 | 0.756 | 46.900 | 3.04 | 34.900 |
| 8 | Bread (Whole wheat) | 7.183 | 0.7350 | 0.666 | 37.100 | −1.59 | 22.700 |
| 9 | Chia seed | 1.235 | 0.2353 | 0.019 | 8.000 | −26.00 | −33.200 |
| 10 | Chickpeas | 3.429 | 1.8581 | 0.631 | 14.380 | 5.08 | 6.880 |
| 11 | Corn flour (Yellow) | 18.791 | 0.2419 | 0.833 | 76.500 | −3.26 | 63.000 |
| 12 | Corn flour (White) | 10.957 | 0.0857 | 0.877 | 69.700 | −6.40 | 60.300 |
| 13 | Corn (Sweet) | 6.125 | 3.0708 | 0.278 | 12.300 | 4.97 | 1.680 |
| 14 | Einkorn (Dry, Raw) | 7.708 | 0.0000 | 0.819 | 59.700 | −8.90 | 47.300 |
| 15 | Farro | 9.877 | 0.3014 | 0.856 | 64.800 | −5.10 | 54.400 |
| 16 | Flaxseed | 1.489 | 0.0671 | 0.380 | 11.30 | −21.55 | −21.80 |
| 17 | Almond (Flour) | 1.742 | 0.7634 | 0.000 | 6.900 | −2.20 | −9.300 |
| 18 | Barley (Flour) | 4.778 | 0.0494 | 0.735 | 61.200 | −15.40 | 40.700 |
| 19 | Buckwheat (Flour) | 11.260 | 0.2800 | 0.975 | 51.300 | −3.60 | 49.900 |
| 20 | Cassava (Flour) | 11.397 | 0.2611 | 0.913 | 79.640 | −5.66 | 72.040 |
| 21 | Chestnut (Flour) | 4.963 | 1.6358 | 0.589 | 64.200 | 10.30 | 31.120 |
| 22 | Coconut (Flour) | 1.722 | 0.1520 | 0.000 | 24.700 | −29.00 | −34.200 |
| 23 | Corn (Flour-Yellow) | 18.791 | 0.2419 | 0.670 | 76.500 | −3.26 | 49.800 |
| 24 | Oat (Flour) | 5.419 | 0.0620 | 0.764 | 57.000 | −12.10 | 40.500 |
| 25 | Potato (Flour) | 4.813 | 0.2108 | 0.785 | 63.300 | −13.10 | 46.100 |
| 26 | Quinoa (Flour) | 10.000 | 0.2590 | 0.836 | 62.550 | −5.15 | 51.150 |
| 27 | Rice (Flour-brown) | 10.342 | 0.1370 | 0.331 | 68.200 | −6.30 | 17.700 |
| 28 | Rice (Flour-glutinous free) | 36.200 | 0.3500 | 0.997 | 70.400 | −1.30 | 70.200 |
| 29 | Rice (Flour white) | 159.600 | 0.4000 | 1.049 | 79.300 | −0.30 | 83.200 |
| 30 | Rye (Flour) | 4.313 | 0.1285 | 0.701 | 59.300 | −15.60 | 36.200 |
| 31 | Semolina (Flour) | 23.063 | 0.7500 | 1.003 | 70.600 | −0.80 | 70.800 |
| 32 | Sorghum (Flour) | 9.485 | 0.2328 | 0.857 | 69.240 | −6.26 | 58.140 |
| 33 | Soy (Flour) | 1.039 | 0.3556 | 0.658 | 0.700 | −11.60 | −5.700 |
| 34 | Soy (Flour fatted) | 2.20 | 0.5813 | 0.151 | 19.20 | −6.70 | −10.700 |
| 35 | Spelt (Flour) | 16.550 | 0.0750 | 0.995 | 62.200 | −3.70 | 61.900 |
| 36 | Wheat all purpose (Flour) | 26.912 | 0.0882 | 0.792 | 70.480 | −2.48 | 55.280 |
| 37 | Fonio | 36.955 | 0.0000 | 0.962 | 79.100 | −2.20 | 76.000 |
| 38 | Kidney beans (Red) | 3.981 | 0.7130 | 1.707 | 16.100 | −1.55 | 31.300 |
| 39 | Lentils | 5.813 | 0.1906 | 0.584 | 52.610 | −8.85 | 26.170 |
| 40 | Millet | 22.342 | 0.5105 | 0.902 | 71.070 | −1.63 | 63.770 |
| 41 | Oat | 5.419 | 0.1163 | 0.764 | 57.000 | −11.40 | 40.500 |
| 42 | Quinoa (Flour) | 10.000 | 0.2590 | 0.836 | 62.550 | −5.15 | 51.150 |
| 43 | Rice (Black) | 18.381 | 0.0000 | 0.925 | 73.000 | −4.20 | 67.200 |
| 44 | Rice (Brown) | 17.837 | 0.1628 | 0.934 | 72.400 | −3.60 | 67.300 |
| 45 | Rice (Red) | 18.143 | 0.0000 | 0.929 | 72.000 | −4.20 | 66.600 |
| 46 | Rice (White) | 28.989 | 0.0361 | 0.927 | 77.530 | −2.67 | 71.630 |
| 47 | Sorghum bran | 1.963 | 0.0286 | 0.476 | 33.700 | −34.00 | −2.300 |
| 48 | Sorghum (Flour white) | 22.273 | 0.5758 | 0.948 | 70.200 | −1.40 | 66.400 |
| 49 | Sorghum (White) | 19.205 | 0.6410 | 0.944 | 71.000 | −1.40 | 66.800 |
| 50 | Sorghum (Whole grain) | 8.867 | 0.3012 | 0.891 | 65.300 | −5.80 | 57.300 |
| 51 | Sunflower Seed | 3.403 | 0.4722 | 0.041 | 17.3 | −3.80 | −6.20 |
| 52 | Rice (Wild) | 17.605 | 0.5814 | 0.906 | 71.400 | −1.80 | 64.300 |
| PC Summary | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Eigenvalue | 2.291 | 0.8830 | 0.7591 | 0.06726 |
| Proportion of variance | 57.27% | 22.08% | 18.98% | 1.68% |
| Cumulative proportion of variance | 57.27% | 79.34% | 98.32% | 100.00% |
| Grains Carbohydrate | Carbohydrate Content to Fiber Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Values | Total Carbohydrates | Total Sugar | Dietary Starch | Dietary Fiber | Total Carbohydrates | Total Sugar | Dietary Starch |
| R | 0.5625 | 0.5601 | −0.1553 | −0.2650 | 0.4833 | 0.0444 | 0.3330 |
| R2 | 0.3164 | 0.3137 | 0.0241 | 0.0702 | 0.2336 | 0.00197 | 0.1109 |
| p-values | <0.0001 | <0.0001 | 0.2715 | 0.0577 | 0.0003 | 0.7544 | 0.0159 |
| Significance | **** | **** | ns | ns | *** | ns | * |
| Available Carbohydrate | Available Carbohydrate-to-Dietary Fiber | |||||
|---|---|---|---|---|---|---|
| Values | Total Carbohydrates | Total Sugar | Dietary Starch | Total Carbohydrates | Total Sugar | Dietary Starch |
| R | 0.5926 | 0.1879 | 0.5420 | 0.4833 | 0.04445 | 0.4566 |
| R2 | 0.3512 | 0.03531 | 0.2938 | 0.2336 | 0.001975 | 0.2085 |
| p-values | <0.0001 | 0.1822 | <0.0001 | 0.0003 | 0.7544 | 0.0007 |
| Significance | **** | ns | **** | *** | ns | *** |
| Grains Carbohydrates | Carbohydrates Content-to-Fiber Ratio | |||||
|---|---|---|---|---|---|---|
| Values | TC | TS | DS | TC | TS | DS |
| R | 0.5625 | −0.1553 | 0.5601 | 0.4833 | 0.04444 | 0.3330 |
| R2 | 0.3164 | 0.02413 | 0.3137 | 0.2336 | 0.001975 | 0.1109 |
| p-values | <0.0001 | 0.2715 | <0.0001 | 0.0003 | 0.7544 | 0.0159 |
| Net Grains Carbohydrates | Net Carbohydrates Content-to-Fiber Ratio | |||||
|---|---|---|---|---|---|---|
| Values | TC | TS | DS | TC | TS | DS |
| R | 0.5926 | 0.1879 | 0.5420 | 0.4833 | 0.04445 | 0.4566 |
| R2 | 0.3512 | 0.03531 | 0.2938 | 0.2336 | 0.001975 | 0.2085 |
| p-values | <0.0001 | 0.1822 | <0.0001 | 0.0003 | 0.7544 | 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.K.; Yun, H.R.; Ranbhise, J.S.; Han, S.; Ju, S.; Akter, S.; Yeo, S.G.; Kim, S.S.; Kang, I. Grains, Cereals, and Legumes: Implications in Glycemic Index and Perspectives. Foods 2025, 14, 4038. https://doi.org/10.3390/foods14234038
Singh MK, Yun HR, Ranbhise JS, Han S, Ju S, Akter S, Yeo SG, Kim SS, Kang I. Grains, Cereals, and Legumes: Implications in Glycemic Index and Perspectives. Foods. 2025; 14(23):4038. https://doi.org/10.3390/foods14234038
Chicago/Turabian StyleSingh, Manish Kumar, Hyeong Rok Yun, Jyotsna S. Ranbhise, Sunhee Han, Songhyun Ju, Salima Akter, Seung Geun Yeo, Sung Soo Kim, and Insug Kang. 2025. "Grains, Cereals, and Legumes: Implications in Glycemic Index and Perspectives" Foods 14, no. 23: 4038. https://doi.org/10.3390/foods14234038
APA StyleSingh, M. K., Yun, H. R., Ranbhise, J. S., Han, S., Ju, S., Akter, S., Yeo, S. G., Kim, S. S., & Kang, I. (2025). Grains, Cereals, and Legumes: Implications in Glycemic Index and Perspectives. Foods, 14(23), 4038. https://doi.org/10.3390/foods14234038








