Effects of the Brazilian Native Fruit Jaboticaba (Plinia cauliflora) Peel on Inflammatory and Oxidative Stress Pathways: Insights from a Pilot Study in Hemodialysis Patients and Renal Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion and Non-Inclusion Criteria
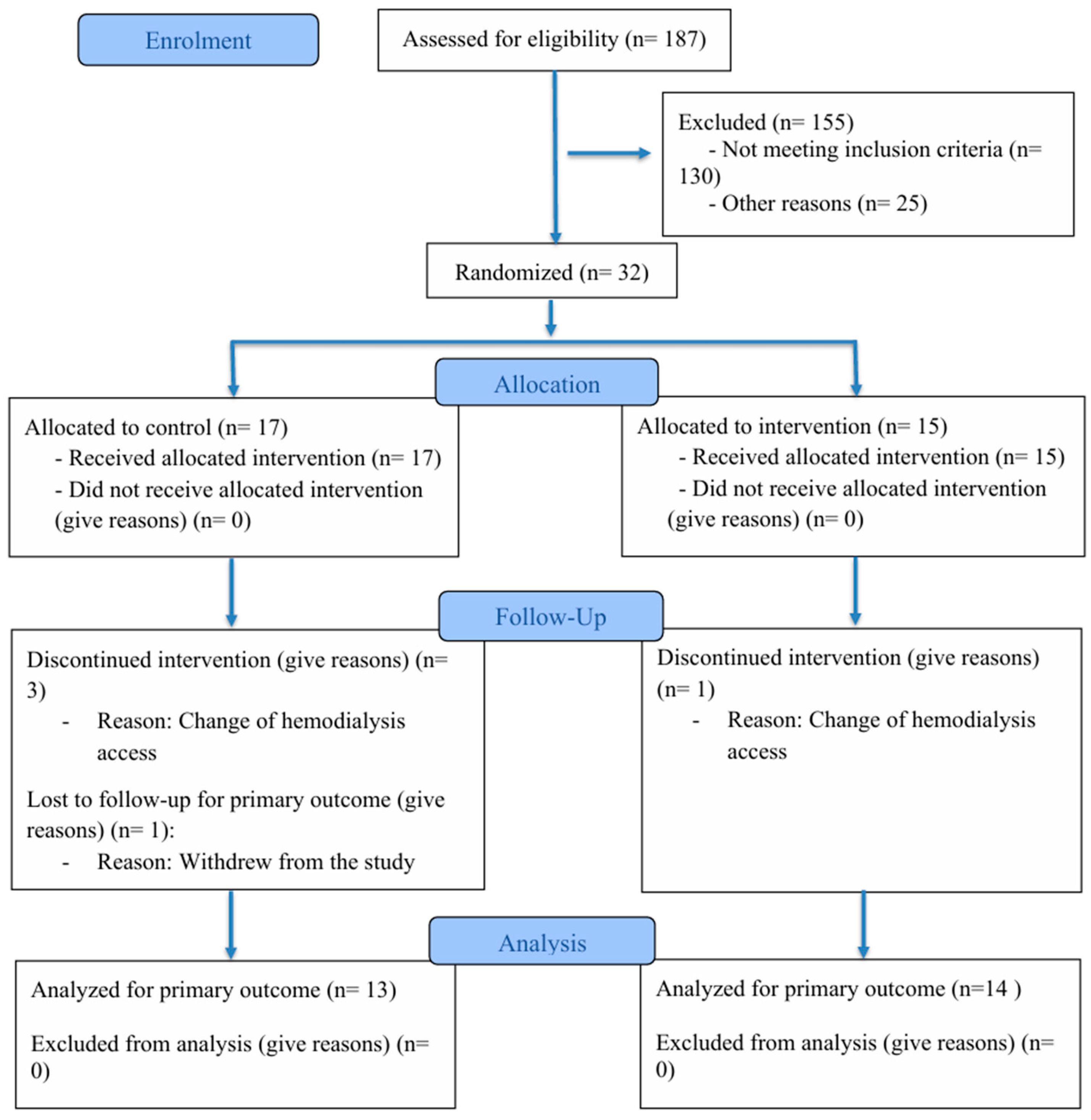
2.3. Randomization Implementation and Blinding
2.4. Composition of Jaboticaba Formulation
2.4.1. Chemical Characterization
Determination of Total Phenolic Content
Preparation of the Gallic Acid Calibration Curve
Sample Preparation
2.4.2. Determination of Monomeric Anthocyanin Content
Preparation of Buffers for the Total pH Differential Method
Spectrometry (pH Differential Method)
2.5. Experimental Design
2.6. Primary and Secondary Outcomes
2.7. Anthropometric Measurement
2.8. Blood Collection and Biochemical Analyses
2.9. Assessment of Oxidative Stress Biomarkers
2.10. Assessment of Inflammatory Cytokines
2.11. Uremic Toxins Analysis
2.12. Cell Culture
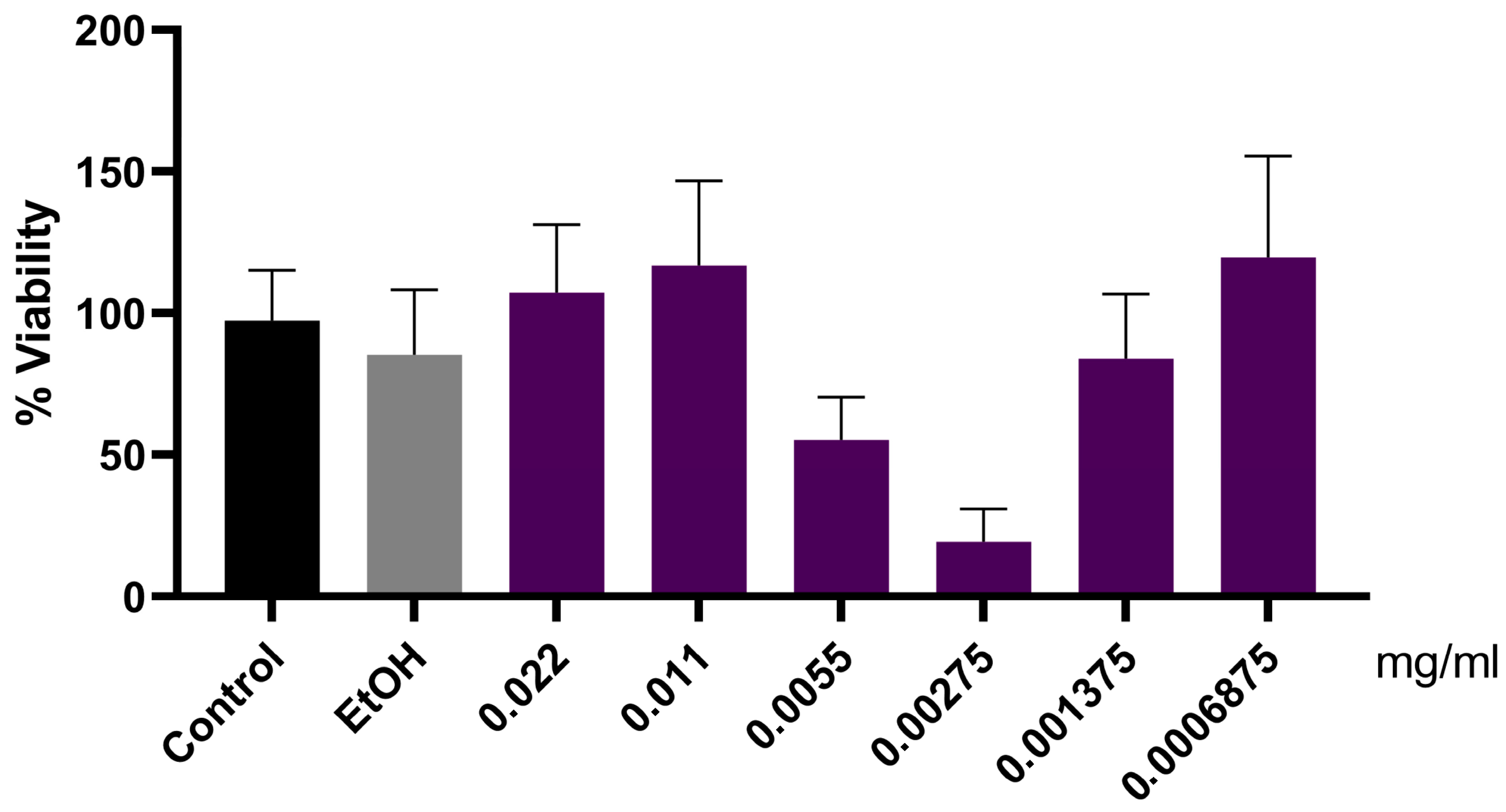
2.13. MTT Cellular Viability Assay
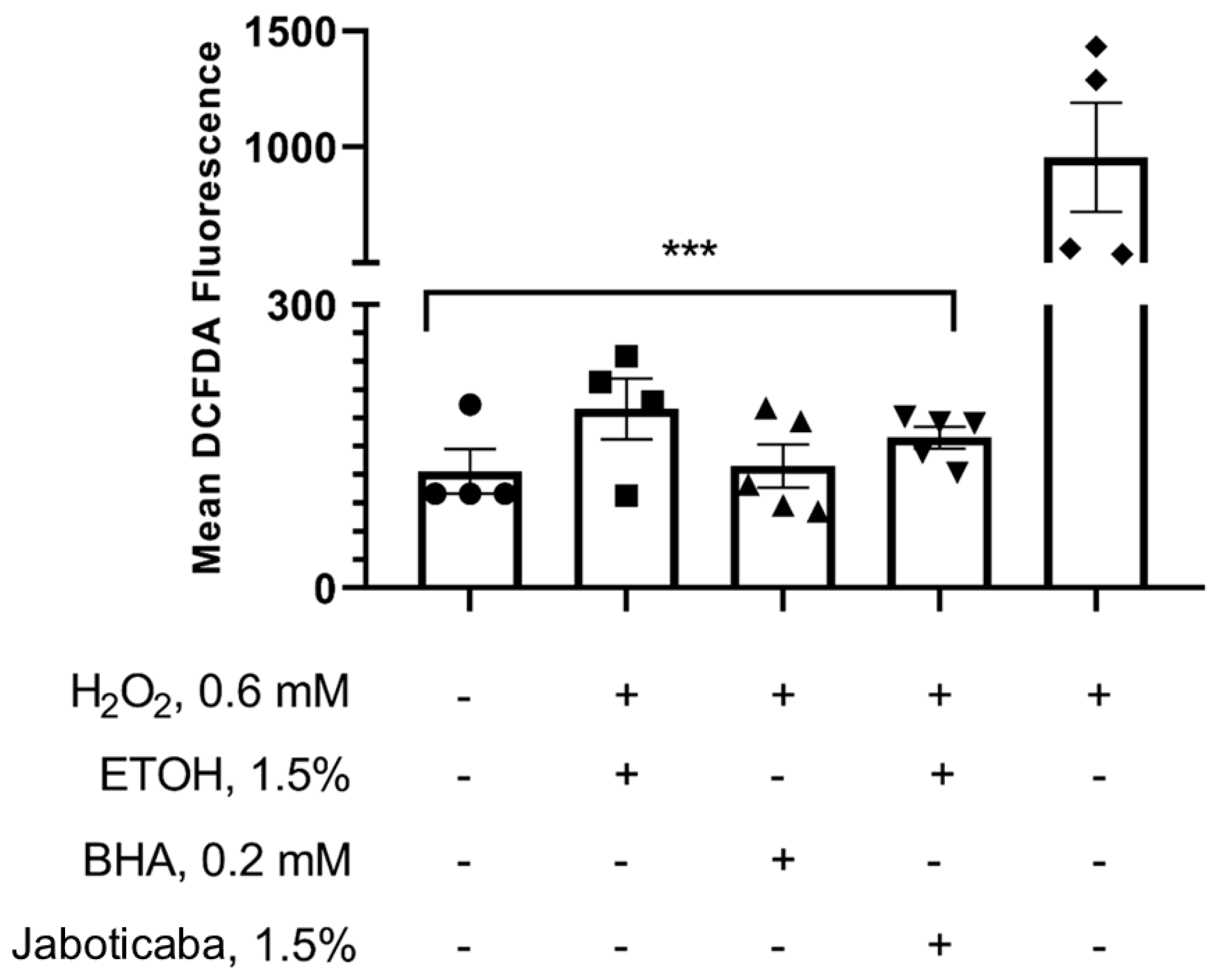
2.14. Determination of Reactive Oxygen Species (ROS) Levels
2.15. Transcriptomic Data Reanalysis of Public RNA-Seq Datasets
2.16. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| HD | Hemodialysis |
| ROS | Reactive oxygen species |
| IAA | Indole-3-acetic acid |
| p-CS | p-Cresyl sulfate |
| IS | Indoxyl sulfate |
| IL | Interleukin |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-17E | Interleukin-17E |
| TLR-4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-alpha |
| NF-κB | Nuclear factor-κB |
| NLRs | NOD-like receptors |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| AVF | Arteriovenous fistula |
| BMI | Body mass index |
| FDA | Food and Drug Administration |
| MDA | Malondialdehyde |
| WHO | World Health Organization |
| CRP | C-reactive protein |
| TBARS | Thiobarbituric acid-reactive substances |
| TCA | Trichloroacetic acid |
| DNPH | Dinitrophenylhydrazine |
| ELISA | Enzyme-linked immunosorbent assay |
| TMB | Tetramethylbenzidine |
| HPLC | High-performance liquid chromatography |
| ATCC | American type culture collection |
| DMEM | Dulbecco modified eagle medium |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| H2DCFDA | 2′,7′-Dichlorofluorescein diacetate |
| sICAM-1 | Soluble intercellular adhesion molecule-1 |
| sVCAM-1 | Soluble vascular cell adhesion molecule-1 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| SIRT-1 | Sirtuin 1 |
| mtROS | Mitochondrial ROS |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme oxygenase-1 |
| NAD(P)H | Nicotinamide adenine dinucleotide phosphate |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| TSLP | Thymic stromal lymphopoietin |
References
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as Medicine: Targeting the Uraemic Phenotype in Chronic Kidney Disease. Nat. Rev. Nephrol. 2020, 17, 153–171. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Borges, N.A.; Ribeiro, M.; Yee-Moon Wang, A.; Mafra, D. Protect the Kidneys and Save the Heart Using the Concept of Food as Medicine. J. Ren. Nutr. 2023, 33, S110–S117. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects–A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Inada, K.O.P.; Leite, I.B.; Martins, A.B.N.; Fialho, E.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Jaboticaba Berry: A Comprehensive Review on Its Polyphenol Composition, Health Effects, Metabolism, and the Development of Food Products. Food Res. Int. 2021, 147, 110518. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.M.; Franca, A.S. Jabuticaba (Plinia sp.) Peel as a Source of Pectin: Characterization and Effect of Different Extraction Methods. Foods 2022, 12, 117. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Jaboticaba (Myrtaceae cauliflora) Fruit and Its by-Products: Alternative Sources for New Foods and Functional Components. Trends Food Sci. Technol. 2021, 112, 118–136. [Google Scholar] [CrossRef]
- da Silva Brito, T.G.; da Silva, A.P.S.A.; da Cunha, R.X.; da Fonseca, C.S.M.; da Silva Araujo, T.F.; de Lima Campos, J.K.; Nascimento, W.M.; de Araújo, H.D.A.; Silva, J.P.R.; Tavares, J.F.; et al. Anti-Inflammatory, Hypoglycemic, Hypolipidemic, and Analgesic Activities of Plinia cauliflora (Mart.) Kausel (Brazilian Grape) Epicarp. J. Ethnopharmacol. 2021, 268, 113611. [Google Scholar] [CrossRef]
- Rodrigues, L.; Donado-Pestana, C.M.; Moura, M.H.C.; Rossi e Silva, R.; Pessoa, É.V.M.; Genovese, M.I. Phenolic Compounds from Jaboticaba (Plinia jaboticaba (Vell.) Berg) Ameliorate Intestinal Inflammation and Associated Endotoxemia in Obesity. Food Res. Int. 2021, 141, 110139. [Google Scholar] [CrossRef]
- Soares, E.; Soares, A.C.; Trindade, P.L.; Monteiro, E.B.; Martins, F.F.; Forgie, A.J.; Inada, K.O.P.; de Bem, G.F.; Resende, A.; Perrone, D.; et al. Jaboticaba (Myrciaria jaboticaba) Powder Consumption Improves the Metabolic Profile and Regulates Gut Microbiome Composition in High-Fat Diet-Fed Mice. Biomed. Pharmacother. 2021, 144, 112314. [Google Scholar] [CrossRef]
- Dragano, N.R.V.; Marques, A.Y.C.; Cintra, D.E.C.; Solon, C.; Morari, J.; Leite-Legatti, A.V.; Velloso, L.A.; Maróstica-Júnior, M.R. Freeze-Dried Jaboticaba Peel Powder Improves Insulin Sensitivity in High-Fat-Fed Mice. Br. J. Nutr. 2013, 110, 447–455. [Google Scholar] [CrossRef]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The Systemic Nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef]
- Deng, L.; Guo, S.; Liu, Y.; Zhou, Y.; Liu, Y.; Zheng, X.; Yu, X.; Shuai, P. Global, Regional, and National Burden of Chronic Kidney Disease and Its Underlying Etiologies from 1990 to 2021: A Systematic Analysis for the Global Burden of Disease Study 2021. BMC Public Health 2025, 25, 636. [Google Scholar] [CrossRef]
- Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; ElHafeez, S.A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global Incidence, Prevalence, Years Lived with Disability (YLDs), Disability-Adjusted Life-Years (DALYs), and Healthy Life Expectancy (HALE) for 371 Diseases and Injuries in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Zoccali, C.; Mark, P.B.; Sarafidis, P.; Agarwal, R.; Adamczak, M.; Bueno de Oliveira, R.; Massy, Z.A.; Kotanko, P.; Ferro, C.J.; Wanner, C.; et al. Diagnosis of Cardiovascular Disease in Patients with Chronic Kidney Disease. Nat. Rev. Nephrol. 2023, 19, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Li, X.J.; Shan, Q.Y.; Wu, X.; Miao, H.; Zhao, Y.Y. Gut Microbiota Regulates Oxidative Stress and Inflammation: A Double-Edged Sword in Renal Fibrosis. Cell. Mol. Life Sci. 2024, 81, 480. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L. Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease. Front. Pharmacol. 2022, 13, 800950. [Google Scholar] [CrossRef]
- Chan, K.; Moe, S.M.; Saran, R.; Libby, P. The Cardiovascular–Dialysis Nexus: The Transition to Dialysis Is a Treacherous Time for the Heart. Eur. Heart J. 2021, 42, 1244. [Google Scholar] [CrossRef]
- Granata, S.; Masola, V.; Zoratti, E.; Scupoli, M.T.; Baruzzi, A.; Messa, M.; Sallustio, F.; Gesualdo, L.; Lupo, A.; Zaza, G. NLRP3 Inflammasome Activation in Dialyzed Chronic Kidney Disease Patients. PLoS ONE 2015, 10, e0122272. [Google Scholar] [CrossRef]
- Kooman, J.P.; Dekker, M.J.; Usvyat, L.A.; Kotanko, P.; van der Sande, F.M.; Schalkwijk, C.G.; Shiels, P.G.; Stenvinkel, P. Inflammation and Premature Aging in Advanced Chronic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2017, 313, F938–F950. [Google Scholar] [CrossRef]
- Sun, Y.; Johnson, C.; Zhou, J.; Wang, L.; Li, Y.F.; Lu, Y.; Nanayakkara, G.; Fu, H.; Shao, Y.; Sanchez, C.; et al. Uremic Toxins Are Conditional Danger- or Homeostasis-Associated Molecular Patterns. Front. Biosci.-Landmark 2018, 23, 348–387. [Google Scholar] [CrossRef]
- Sepe, V.; Libetta, C.; Gregorini, M.; Rampino, T. The Innate Immune System in Human Kidney Inflammaging. J. Nephrol. 2021, 35, 381. [Google Scholar] [CrossRef] [PubMed]
- Plonsky-Toder, M.; Magen, D.; Pollack, S. Innate Immunity and CKD: Is There a Significant Association? Cells 2023, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Cestari, A.P.; Gasparotto, F.M.; Kassuya, C.A.L.; Lacerda, T.M.R.; Donadel, G.; Moura, C.S.; Ceranto, D.B.; Jacomassi, E.; Alberton, O.; Tramontini, S.B.; et al. Ateroprotective Effects of Plinia cauliflora in. New Zealand Rabbits: Beyond the Lipid-Lowering Effect. Front. Pharmacol. 2024, 15, 1244632. [Google Scholar] [CrossRef]
- Aslan, A.; Gok, O.; Beyaz, S.; Ağca, C.A.; Erman, O.; Zerek, A. Ellagic Acid Prevents Kidney Injury and Oxidative Damage via Regulation of Nrf-2/NF-ΚB Signaling in Carbon Tetrachloride Induced Rats. Mol. Biol. Rep. 2020, 47, 7959–7970. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.M.F.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of Curcumin Supplementation on Expression of Inflammatory Transcription Factors in Hemodialysis Patients: A Pilot Randomized, Double-Blind, Controlled Study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Duarte, P.A.; Lapa, J.; Miguel, M.A.L.; Monteiro, M. Jabuticaba (Myrciaria jaboticaba) Juice Obtained by Steam-Extraction: Phenolic Compound Profile, Antioxidant Capacity, Microbiological Stability, and Sensory Acceptability. J. Food Sci. Technol. 2018, 55, 52–61. [Google Scholar] [CrossRef]
- Plaza, M.; Batista, Â.G.; Cazarin, C.B.B.; Sandahl, M.; Turner, C.; Östman, E.; Maróstica Júnior, M.R. Characterization of Antioxidant Polyphenols from Myrciaria jaboticaba Peel and Their Effects on Glucose Metabolism and Antioxidant Status: A Pilot Clinical Study. Food Chem. 2016, 211, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Balisteiro, D.M.; de Araujo, R.L.; Giacaglia, L.R.; Genovese, M.I. Effect of Clarified Brazilian Native Fruit Juices on Postprandial Glycemia in Healthy Subjects. Food Res. Int. 2017, 100, 196–203. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available online: https://www.fda.gov/media/72309/download (accessed on 20 November 2025).
- Association of Official Analytical Chemists Official Methods of Analysis. AOAC-Official Methods of Analysis of AOAC INTERNATIONAL; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. In Handbook of Food Analytical Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2005; Volume 2, pp. 7–17. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 00, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis. 18th Edition, Association of Officiating Analytical Chemists, Washington DC, Method 935.14 and 992.24. 2005. Available online: https://www.scirp.org/reference/ReferencesPapers?ReferenceID=2033299 (accessed on 12 November 2025).
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Volume 894, ISBN 9241208945. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. [49] Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Meert, N.; Schepers, E.; Glorieux, G.; Van Landschoot, M.; Goeman, J.L.; Waterloos, M.A.; Dhondt, A.; Van Der Eycken, J.; Vanholder, R. Novel Method for Simultaneous Determination of p-Cresylsulphate and p-Cresylglucuronide: Clinical Data and Pathophysiological Implications. Nephrol. Dial. Transplant. 2012, 27, 2388–2396. [Google Scholar] [CrossRef]
- Borges, N.A.; Barros, A.F.; Nakao, L.S.; Dolenga, C.J.; Fouque, D.; Mafra, D. Protein-Bound Uremic Toxins from Gut Microbiota and Inflammatory Markers in Chronic Kidney Disease. J. Ren. Nutr. 2016, 26, 396–400. [Google Scholar] [CrossRef]
- Melo, M.N.D.O.; Ochioni, A.C.; Zancan, P.; Oliveira, A.P.; Grazi, M.; Garrett, R.; Holandino, C.; Baumgartner, S. Viscum album Mother Tinctures: Harvest Conditions and Host Trees Influence the Plant Metabolome and the Glycolytic Pathway of Breast Cancer Cells. Front. Pharmacol. 2022, 13, 1027931. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Schmitt, J.; Schneider, K.; Schwarzbach, H.; Aziz-Kalbhenn, H.; Kelber, O.; Müller, J.; Kinscherf, R. Phytohustil® and Root Extract of Althaea Officinalis L. Exert Anti-Inflammatory and Anti-Oxidative Properties and Improve the Migratory Capacity of Endothelial Cells in Vitro. Front. Pharmacol. 2022, 13, 948248. [Google Scholar] [CrossRef]
- Geraldi, M.V.; de Souza, Á.C.; Norde, M.M.; Berni, P.R.; Reguengo, L.M.; Geloneze, B.; Marostica, M.R. Jaboticaba Peel Improves Postprandial Glucose and Inflammation: A Randomized Controlled Trial in Adults with Metabolic Syndrome. Nutr. Res. 2024, 125, 36–49. [Google Scholar] [CrossRef]
- Ramos Junior, O.J.F.; Tavares, I.R.G.; Lima, R.C.; Conte-Junior, C.A.; Alvares, T.S. Jaboticaba Berry (Myrciaria jaboticaba) Supplementation Protects against Micro- and Macrovascular Dysfunction Induced by Eccentric Exercise: A Randomized Clinical Trial. Food Funct. 2024, 15, 7148–7160. [Google Scholar] [CrossRef] [PubMed]
- Junior, O.J.F.R.; Veiga, N.S.; Alvares, T.S. Jaboticaba Berry (Myrciaria jaboticaba) Consumption Improves Plasma GSH Levels and Accelerates Muscle Recovery Following Exercise-Induced Muscle Damage: A Randomized, Placebo-Controlled Trial. Eur. J. Nutr. 2025, 64, 251. [Google Scholar] [CrossRef] [PubMed]
- Frountzas, M.; Karanikki, E.; Toutouza, O.; Sotirakis, D.; Schizas, D.; Theofilis, P.; Tousoulis, D.; Toutouzas, K.G. Exploring the Impact of Cyanidin-3-Glucoside on Inflammatory Bowel Diseases: Investigating New Mechanisms for Emerging Interventions. Int. J. Mol. Sci. 2023, 24, 9399. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, M.; Ying, X.; Zhou, C. Ellagic Acid Protects Rats from Chronic Renal Failure via MiR-182/FOXO3a Axis. Mol. Immunol. 2021, 138, 150–160. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, X.; Liang, M.; Qin, R.; Qin, F.; Wang, X. Ellagic Acid Ameliorates Renal Ischemic-Reperfusion Injury Through NOX4/JAK/STAT Signaling Pathway. Inflammation 2020, 43, 298–309. [Google Scholar] [CrossRef]
- He, X.M.; Zhou, Y.Z.; Sheng, S.; Li, J.J.; Wang, G.Q.; Zhang, F. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxidative Med. Cell. Longev. 2020, 2020, 2963540. [Google Scholar] [CrossRef]
- Sun, Z.R.; Liu, H.R.; Hu, D.; Fan, M.S.; Wang, M.Y.; An, M.F.; Zhao, Y.L.; Xiang, Z.M.; Sheng, J. Ellagic Acid Exerts Beneficial Effects on Hyperuricemia by Inhibiting Xanthine Oxidase and NLRP3 Inflammasome Activation. J. Agric. Food Chem. 2021, 69, 12741–12752. [Google Scholar] [CrossRef]
- Xiong, Y.; Cheng, Z.; Zhang, Y.; Liu, T.; Wan, Z.; Xia, C.; Zhou, B.; Shan, C.; Song, D.; Miao, F. Ellagic Acid Alleviates DSS–Induced Ulcerative Colitis by Inhibiting ROS/NLRP3 Pathway Activation and Modulating Gut Microbiota in Mice. Eur. J. Nutr. 2025, 64, 64. [Google Scholar] [CrossRef]
- Mohammed, E.T.; Hashem, K.S.; Abdelazem, A.Z.; Foda, F.A.M.A. Prospective Protective Effect of Ellagic Acid as a SIRT1 Activator in Iron Oxide Nanoparticle-Induced Renal Damage in Rats. Biol. Trace Elem. Res. 2020, 198, 177–188. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, Y.; Sasaki, M.; Sato, A.; Kamiya, T.; Kondo, K.; Iida, K. Terminalia bellirica (Gaertn.) Roxb. Extract and Gallic Acid Attenuate LPS-Induced Inflammation and Oxidative Stress via MAPK/NF-ΚB and Akt/AMPK/Nrf2 Pathways. Oxidative Med. Cell. Longev. 2018, 2018, 9364364. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.Q.; Shen, H. Gallic Acid Improved Inflammation via NF-ΚB Pathway in TNBS-Induced Ulcerative Colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, T.; Weng, A.; Huang, X.; Yao, Y.; Fu, Z.; Li, Y.; Liu, A.; Li, X.; Chen, D.; et al. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Through Enhancing Nrf2 Signaling. Front. Immunol. 2020, 11, 580593. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, N.; Sadeghi, A.; Movahedinia, S.; Rahimi Naiini, M.; Rajizadeh, M.A.; Bahri, F.; Nazari-Robati, M. Ellagic Acid Ameliorates Aging-Induced Renal Oxidative Damage through Upregulating SIRT1 and NRF2. BMC Complement. Med. Ther. 2023, 23, 77. [Google Scholar] [CrossRef]
- Gu, L.; Deng, W.S.; Liu, Y.; Jiang, C.H.; Sun, L.C.; Sun, X.F.; Xu, Q.; Zhou, H. Ellagic Acid Protects Lipopolysaccharide/d-Galactosamine-Induced Acute Hepatic Injury in Mice. Int. Immunopharmacol. 2014, 22, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, S.D.; Zhao, X.L.; Ni, H.Y.; Song, X.R.; Wang, W.; Yao, L.P.; Zhao, X.H.; Fu, Y.J. Cyanidin-3-Glucoside Protects Liver from Oxidative Damage through AMPK/Nrf2 Mediated Signaling Pathway in Vivo and in Vitro. J. Funct. Foods 2020, 73, 104148. [Google Scholar] [CrossRef]
- Zhu, W.; Tang, H.; Li, J.; Guedes, R.M.; Cao, L.; Guo, C. Ellagic Acid Attenuates Interleukin-1β-Induced Oxidative Stress and Exerts Protective Effects on Chondrocytes through the Kelch-like ECH-Associated Protein 1 (Keap1)/Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Pathway. Bioengineered 2022, 13, 9233–9247. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Barbarossa, A.; Carocci, A.; Meleleo, D. Evaluation of the Potential Protective Effect of Ellagic Acid against Heavy Metal (Cadmium, Mercury, and Lead) Toxicity in SH-SY5Y Neuroblastoma Cells. Foods 2024, 13, 419. [Google Scholar] [CrossRef]
- Al-Zharani, M.; Mubarak, M.; Rudayni, H.A.; Al-Doaiss, A.A.; Abd-Elwahab, M.M.; Al-Eissa, M.S. Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats. Nutrients 2023, 15, 1888. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research Progress of Quercetin in Cardiovascular Disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine Growth Factor Rev. 2011, 22, 189. [Google Scholar] [CrossRef]
- Anders, H.J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2564. [Google Scholar] [CrossRef] [PubMed]
- Islamuddin, M.; Qin, X. Renal Macrophages and NLRP3 Inflammasomes in Kidney Diseases and Therapeutics. Cell Death Discov. 2024, 10, 229. [Google Scholar] [CrossRef]
- Yuan, Q.; Tang, B.; Zhang, C. Signaling Pathways of Chronic Kidney Diseases, Implications for Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, C.; Zheng, D.; Wang, Y.; Lee, V.W.S.; Wang, Y.M.; Zheng, G.; Tan, T.K.; Yu, D.; Alexander, S.I.; et al. IL-25 Induces M2 Macrophages and Reduces Renal Injury in Proteinuric Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 1229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Niu, Z.; Tan, J.; Yang, J.; Liu, Y.; Ma, H.; Lee, V.W.S.; Sun, S.; Song, X.; Guo, M.; et al. IL-25 Elicits Innate Lymphoid Cells and Multipotent Progenitor Type 2 Cells That Reduce Renal Ischemic/Reperfusion Injury. J. Am. Soc. Nephrol. 2015, 26, 2199–2211. [Google Scholar] [CrossRef]
- Poudel, B.; Ekperikpe, U.S.; Mandal, S.; Wilson, G.E.; Shields, C.A.; Cornelius, D.C.; Williams, J.M. Chronic Treatment with IL-25 Increases Renal M2 Macrophages and Reduces Renal Injury in Obese Dahl Salt-Sensitive Rats during the Prepubescent Stage. Am. J. Physiol.-Ren. Physiol. 2023, 325, F87. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, J.; Wang, M.; Liu, J.; Wang, Z.; Jiang, H.; Ye, D.; Zhang, J.; Wan, J. The Expression of Interleukin-25 Increases in Human Coronary Artery Disease and Is Associated with the Severity of Coronary Stenosis. Anatol. J. Cardiol. 2020, 23, 151. [Google Scholar] [CrossRef]
- Yuan, Q.; Peng, N.; Xiao, F.; Shi, X.; Zhu, B.; Rui, K.; Tian, J.; Lu, L. New Insights into the Function of Interleukin-25 in Disease Pathogenesis. Biomark. Res. 2023, 11, 36. [Google Scholar] [CrossRef]
- Polak-Szczybyło, E.; Tabarkiewicz, J. IL-17A, IL-17E and IL-17F as Potential Biomarkers for the Intensity of Low-Grade Inflammation and the Risk of Cardiovascular Diseases in Obese People. Nutrients 2022, 14, 643. [Google Scholar] [CrossRef]
- Borowczyk, J.; Shutova, M.; Brembilla, N.C.; Boehncke, W.H. IL-25 (IL-17E) in Epithelial Immunology and Pathophysiology. J. Allergy Clin. Immunol. 2021, 148, 40–52. [Google Scholar] [CrossRef]
- Feng, D.; Gao, J.; Liu, R.; Liu, W.; Gao, T.; Yang, Y.; Zhang, D.; Yang, T.; Yin, X.; Yu, H.; et al. CARM1 Drives Triple-Negative Breast Cancer Progression by Coordinating with HIF1A. Protein Cell 2024, 15, 744–765. [Google Scholar] [CrossRef]
- Qiu, S.; Zhong, C.; Zhao, B.; Li, G.; Wang, J.; Jehan, S.; Li, J.; Zhao, X.; Li, D.; Sui, G. Transcriptome Analysis of Signaling Pathways Targeted by Ellagic Acid in Hepatocellular Carcinoma Cells. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129911. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.F.M.F.; Baptista, B.G.; Nascimento, D.; Esgalhado, M.; Mafra, D. Urolithin as a Metabolite of Ellagitannins and Ellagic Acid from Fruits and Nuts Produced by the Gut Microbiota: Its Role on Non-Communicable Diseases. Curr. Nutr. Rep. 2025, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Qu, J.; Shen, X. NF-ΚB/P65 Antagonizes Nrf2-ARE Pathway by Depriving CBP from Nrf2 and Facilitating Recruitment of HDAC3 to MafK. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499. [Google Scholar] [CrossRef]
- Griesser, E.; Vemula, V.; Raulien, N.; Wagner, U.; Reeg, S.; Grune, T.; Fedorova, M. Cross-Talk between Lipid and Protein Carbonylation in a Dynamic Cardiomyocyte Model of Mild Nitroxidative Stress. Redox Biol. 2017, 11, 438–455. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in Cardiovascular Diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Mróz, K.; Paszek, E.; Baran, M.; Ząbczyk, M.; Butenas, S.; Undas, A. Elevated Carbonylated Proteins Are Associated with Major Cardiovascular Events in Patients with Chronic Coronary Syndrome: A Cohort Study. Kardiol. Pol. 2024, 82, 708–715. [Google Scholar] [CrossRef]
- Quatrin, A.; Conte, L.; Silva, D.T.D.; Figueiredo, C.G.; Somacal, S.; Roehrs, M.; Teixeira, C.F.; Barbisan, F.; Augusti, P.R.; Maróstica Júnior, M.R.; et al. The Hepatoprotective Effect of Jaboticaba Peel Powder in a Rat Model of Type 2 Diabetes Mellitus Involves the Modulation of Thiol/Disulfide Redox State through the Upregulation of Glutathione Synthesis. J. Nutr. Metab. 2018, 2018, 9794629. [Google Scholar] [CrossRef]
- Calloni, C.; Martínez, L.S.; Gil, D.F.; da Silva, D.M.; Rosales, P.F.; Agostini, F.; Moura e Silva, S.; Parmegiani Jahn, M.; Salvador, M. Jabuticaba (Plinia trunciflora (O. Berg) Kausel) Improved the Lipid Profile and Immune System and Reduced Oxidative Stress in Streptozotocin-Induced Diabetic Rats. J. Food Biochem. 2020, 44, e13383. [Google Scholar] [CrossRef]
- Andreucci, M.; Faga, T.; Pisani, A.; Serra, R.; Russo, D.; De Sarro, G.; Michael, A. Quercetin Protects against Radiocontrast Medium Toxicity in Human Renal Proximal Tubular Cells. J. Cell. Physiol. 2018, 233, 4116–4125. [Google Scholar] [CrossRef]
- Erjavec, L.C.; Parra, L.G.; Sendyk, D.E.; Recabarren, M.; Herbón, C.; Favale, N.O.; Tulino, S.; Carballo, M.; López Nigro, M.; Magnani, N.; et al. Non-Beneficial Effects of Resveratrol on Renal Epithelial Cell Cultures: Implications in Cell Differentiation. BioFactors 2025, 51, e70018. [Google Scholar] [CrossRef]
| Variables | All (n = 27) | Control (n = 13) | Jaboticaba (n = 14) | p-Value * |
|---|---|---|---|---|
| Age (years) | 55 (19.5) | 56 (15) | 51 (20) | 0.64 |
| Sex% (Male/Female) | 16/11 | 8/5 | 8/6 | 1.00 |
| Time on HD (months) | 26 (48.5) | 22 (27) | 30.5 (73.2) | 0.75 |
| BMI (kg/m2) | 24.3 (3.7) | 24.3 (4.0) | 24.3 (3.3) | 0.98 |
| Kt/V | 1.17 (0.45) | 1.14 (0.34) | 1.17 (0.72) | 0.36 |
| Parameters | Control | Jaboticaba | ||||
|---|---|---|---|---|---|---|
| Before | After | p-Value * | Before | After | p-Value * | |
| Albumin (g/dL) | 4.11 (3.98–4.23) | 4.12 (3.99–4.25) | 0.99 | 4.08 (3.96–4.20) | 4.19 (4.07–4.32) | 0.33 |
| Phosphorus (mg/dL) | 4.36 (3.51–5.22) | 4.55 (3.66–5.44) | 0.97 | 4.85 (4.03–5.66) | 4.70 (3.85–5.54) | 0.98 |
| Potassium (mmol/L) | 5.58 (4.98–6.17) | 5.10 (4.48–5.72) | 0.37 | 5.65 (5.12–6.19) | 5.18 (4.60–5.75) | 0.28 |
| Magnesium (mg/dL) | 2.57 (2.32–2.81) | 2.43 (2.18–2.67) | 0.22 | 2.45 (2.22–2.68) | 2.36 (2.13–2.60) | 0.57 |
| Glucose (mg/dL) | 93.75 (76.34–111.16) | 95.29 (76.78–113.80) | 0.99 | 97.75 (81.52–113.98) | 120.06 (103.37–136.75) | 0.15 |
| Parameters | Control | Jaboticaba | ||||
|---|---|---|---|---|---|---|
| Before | After | p-Value * | Before | After | p-Value * | |
| C-reactive protein (mg/dL) | 3.13 (1.6–4.6) | 4.16 (2.4–5.8) | 0.72 | 3.59 (2.2–4.9) | 3.29 (1.8–4.7) | 0.98 |
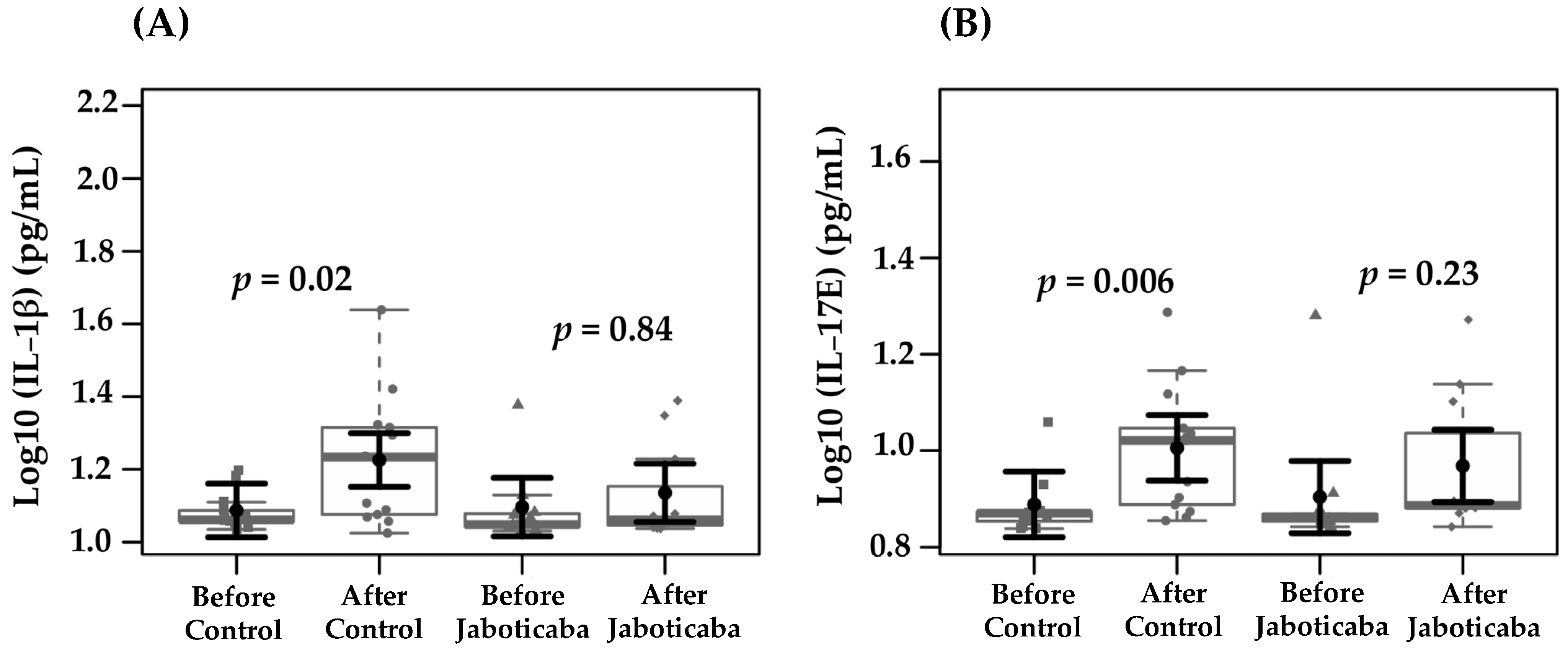
| Interleukin-1β (pg/mL) | 1.08 (1.0–1.1) | 1.22 (1.1–1.2) | 0.02 | 1.09 (1.0–1.1) | 1.13 (1.0–1.2) | 0.84 |
| Interleukin-17E (pg/mL) | 0.88 (0.8–0.9) | 1.00 (0.9–1.0) | 0.006 | 0.90 (0.8–0.9) | 0.96 (0.89–1.04) | 0.23 |
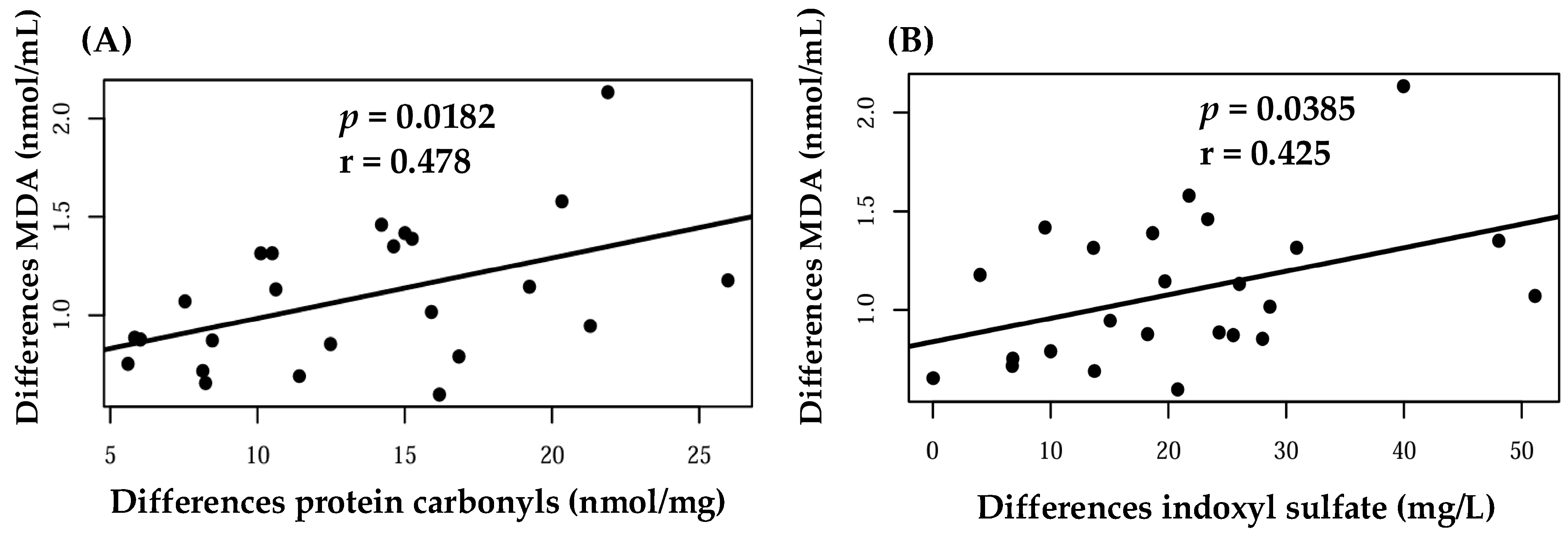
| Malondialdehyde (nmol/mL) | 0.95 (0.7–1.1) | 1.09 (0,8–1,3) | 0.81 | 0.96 (0.7–1.1) | 0.99 (0.7–1.2) | 0.99 |
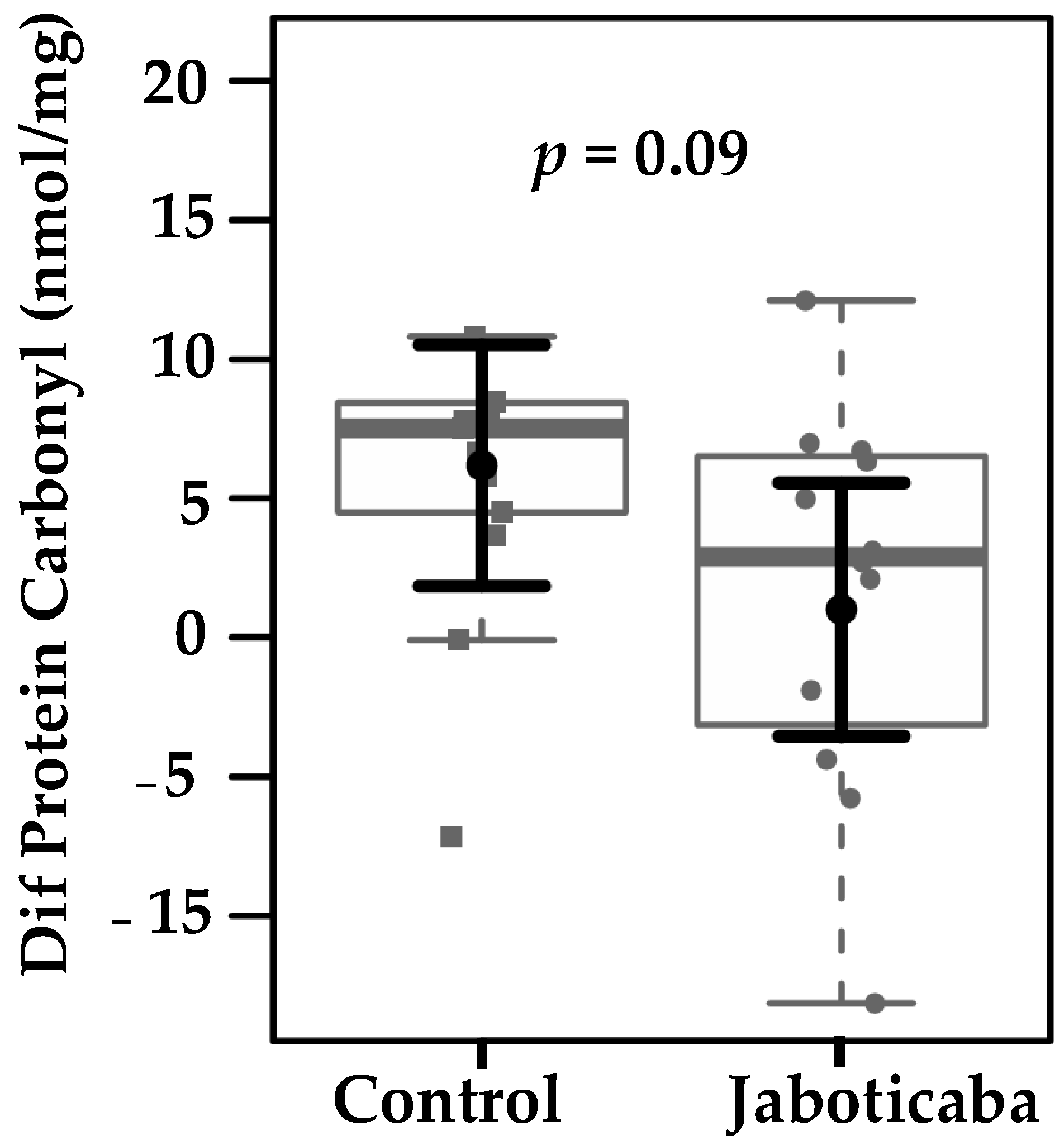
| Protein carbonyl (nmol/mg) | 9.54 (6.4–12.6) | 10.7 (7.7–13.8) | 0.91 | 14.0 (10.9–17.2) | 10.74 (7.5–13.9) | 0.31 |
| Indoxyl sulfate (mg/L) | 28.23 (18.2–38.2) | 24.4 (14.5–34.4) | 0.20 | 22.4 (13.0–31.8) | 24.48 (15.0–33.9) | 0.63 |
| p-cresyl sulfate (mg/L) | 12.45 (3.3–21.5) | 10.7 (1.6–19.8) | 0.94 | 21.1 (11.1–31.6) | 14.2 (3.9–24.4) | 0.14 |
| Indole-3-acetic acid (ug/L) | 897.4 (418.1–1376.7) | 944.1 (464.8–1423.4) | 0.91 | 1389.1 (888.3–1889.8) | 1383.8 (883.1–1884.6) | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.S.; Brito, J.S.d.; Ribeiro-Alves, M.; Coutinho-Wolino, K.S.; Duarte, R.d.S.P.; Valverde, R.H.F.; Einicker-Lamas, M.; Berretta, A.A.; Sanz, C.L.; Nakao, L.S.; et al. Effects of the Brazilian Native Fruit Jaboticaba (Plinia cauliflora) Peel on Inflammatory and Oxidative Stress Pathways: Insights from a Pilot Study in Hemodialysis Patients and Renal Cell Models. Foods 2025, 14, 4030. https://doi.org/10.3390/foods14234030
Lima LS, Brito JSd, Ribeiro-Alves M, Coutinho-Wolino KS, Duarte RdSP, Valverde RHF, Einicker-Lamas M, Berretta AA, Sanz CL, Nakao LS, et al. Effects of the Brazilian Native Fruit Jaboticaba (Plinia cauliflora) Peel on Inflammatory and Oxidative Stress Pathways: Insights from a Pilot Study in Hemodialysis Patients and Renal Cell Models. Foods. 2025; 14(23):4030. https://doi.org/10.3390/foods14234030
Chicago/Turabian StyleLima, Ligia Soares, Jessyca Sousa de Brito, Marcelo Ribeiro-Alves, Karen Salve Coutinho-Wolino, Rodrigo dos Santos P. Duarte, Rafael Hospodar Felippe Valverde, Marcelo Einicker-Lamas, Andresa A. Berretta, Carmen Lucía Sanz, Lia S. Nakao, and et al. 2025. "Effects of the Brazilian Native Fruit Jaboticaba (Plinia cauliflora) Peel on Inflammatory and Oxidative Stress Pathways: Insights from a Pilot Study in Hemodialysis Patients and Renal Cell Models" Foods 14, no. 23: 4030. https://doi.org/10.3390/foods14234030
APA StyleLima, L. S., Brito, J. S. d., Ribeiro-Alves, M., Coutinho-Wolino, K. S., Duarte, R. d. S. P., Valverde, R. H. F., Einicker-Lamas, M., Berretta, A. A., Sanz, C. L., Nakao, L. S., Stenvinkel, P., & Mafra, D. (2025). Effects of the Brazilian Native Fruit Jaboticaba (Plinia cauliflora) Peel on Inflammatory and Oxidative Stress Pathways: Insights from a Pilot Study in Hemodialysis Patients and Renal Cell Models. Foods, 14(23), 4030. https://doi.org/10.3390/foods14234030











