Silicon Is Linked to Tea Quality Through Alteration Aluminum Uptake and Translocation in Camellia sinensis L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Analytical Parameters and Methods
2.3. Uptake Kinetics Parameters
2.4. Health Risk Assessment
2.5. Data Processing and Statistical Analysis
3. Results
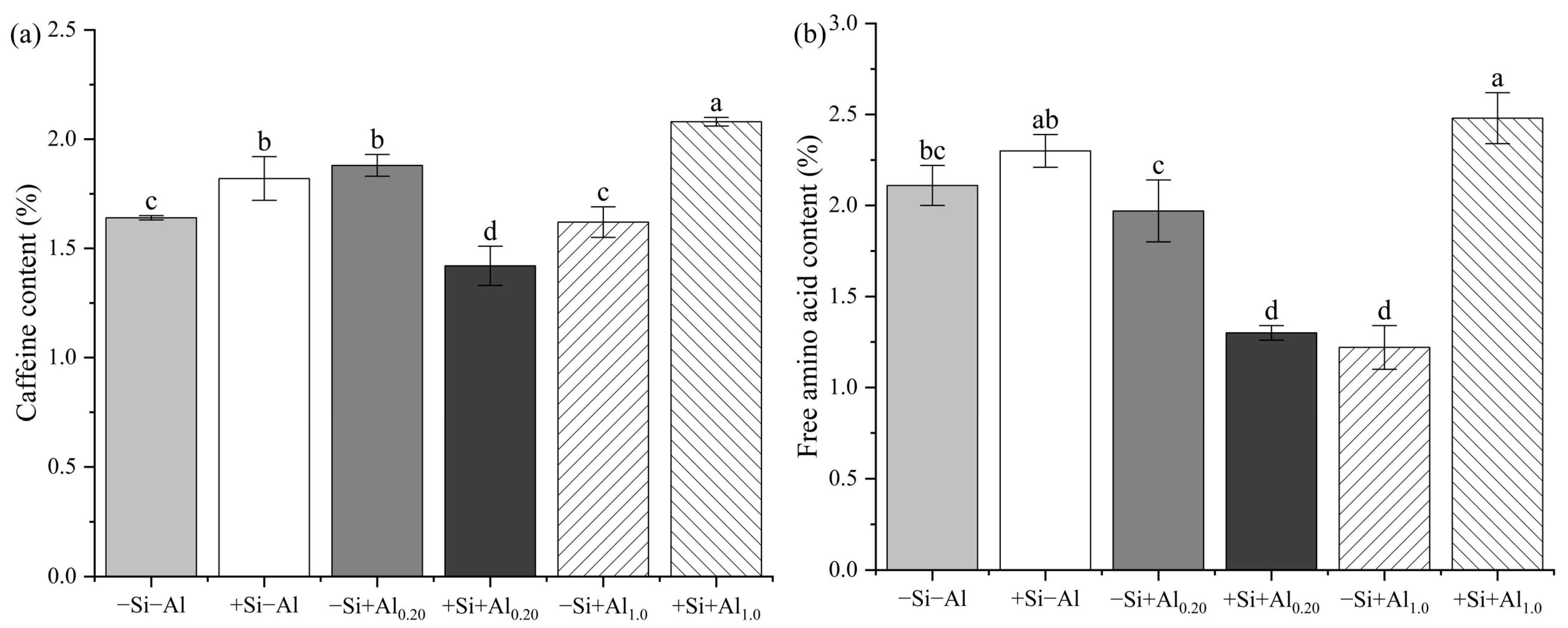
3.1. Caffeine and Free Amino Acid Contents in Young Tea Leaves
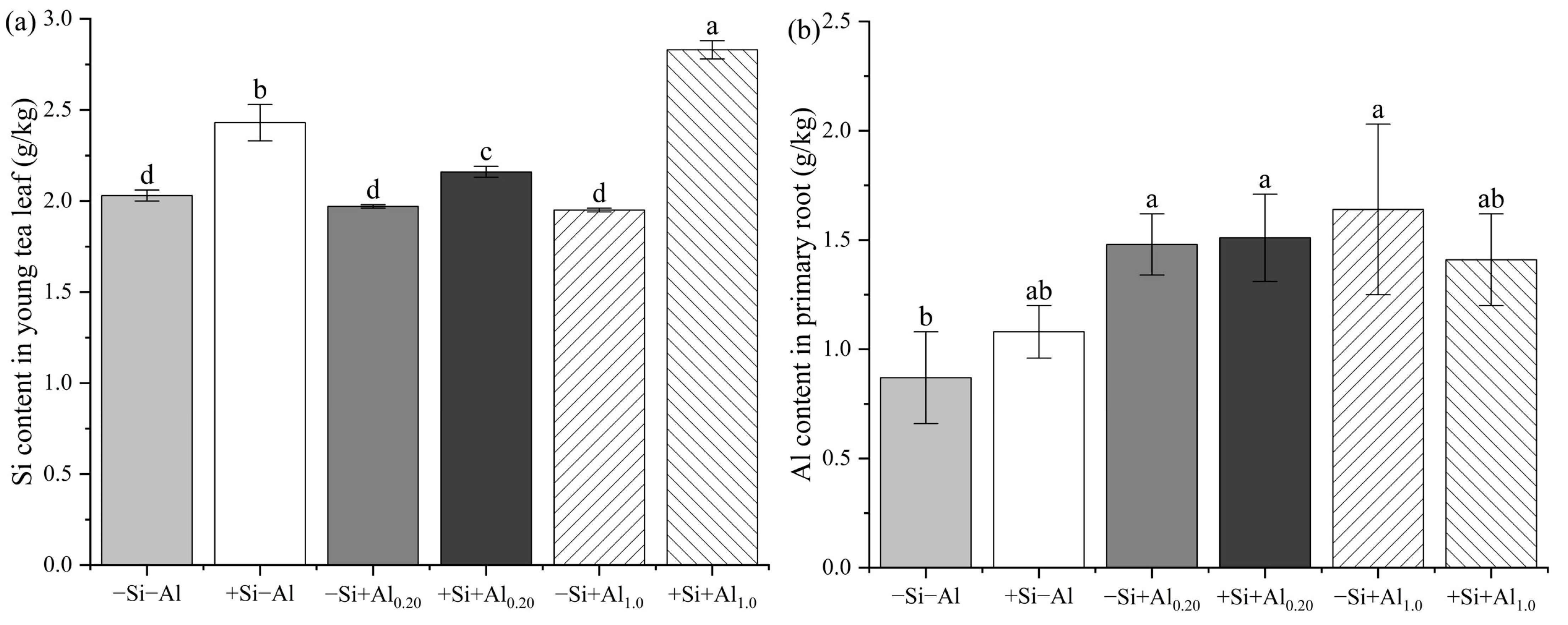
3.2. Al and Si Content in Young Tea Leaves
3.3. Al Content in Primary and Secondary Roots of Tea Seedlings
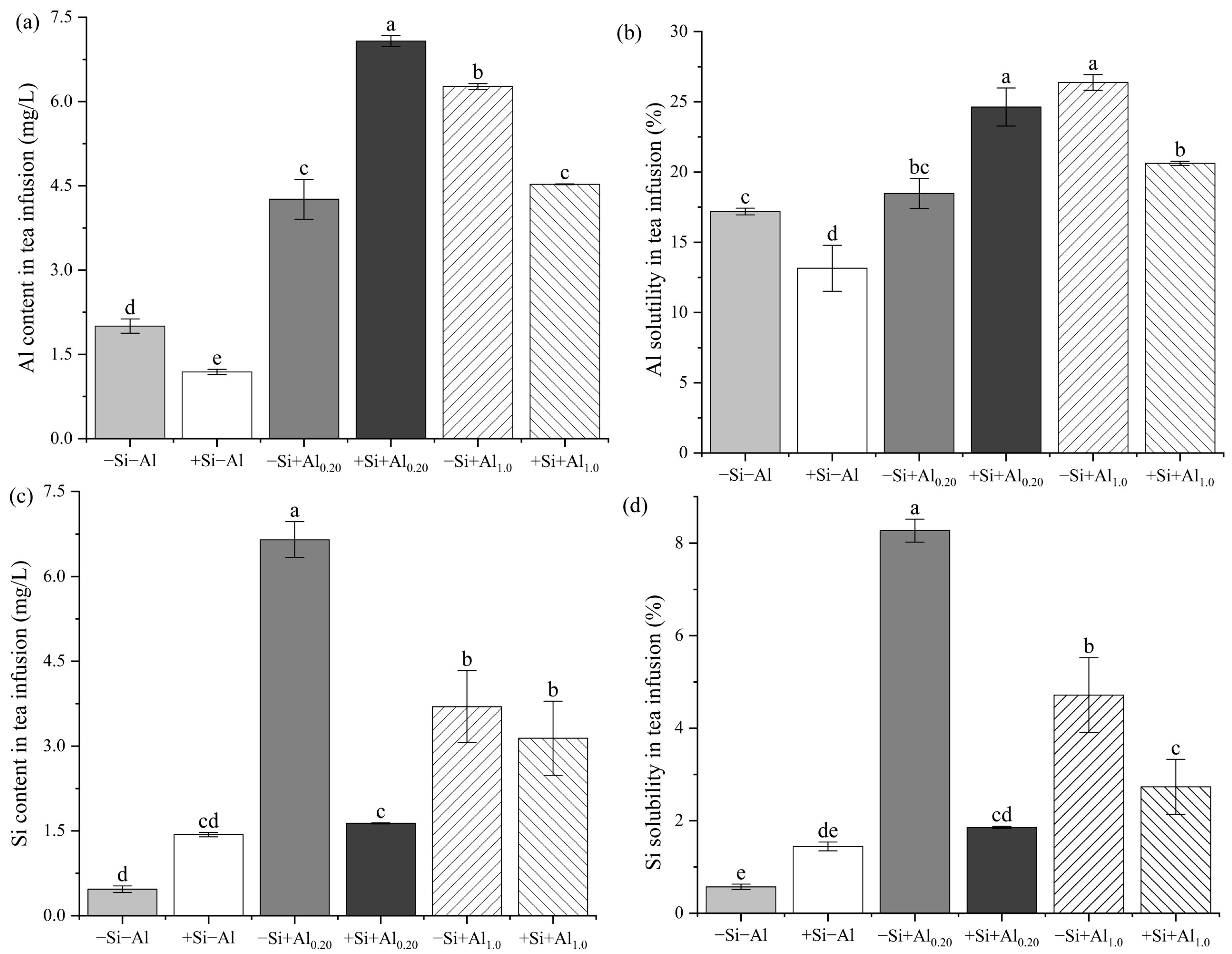
3.4. Al and Si Contents and Solubility in the Tea Infusion
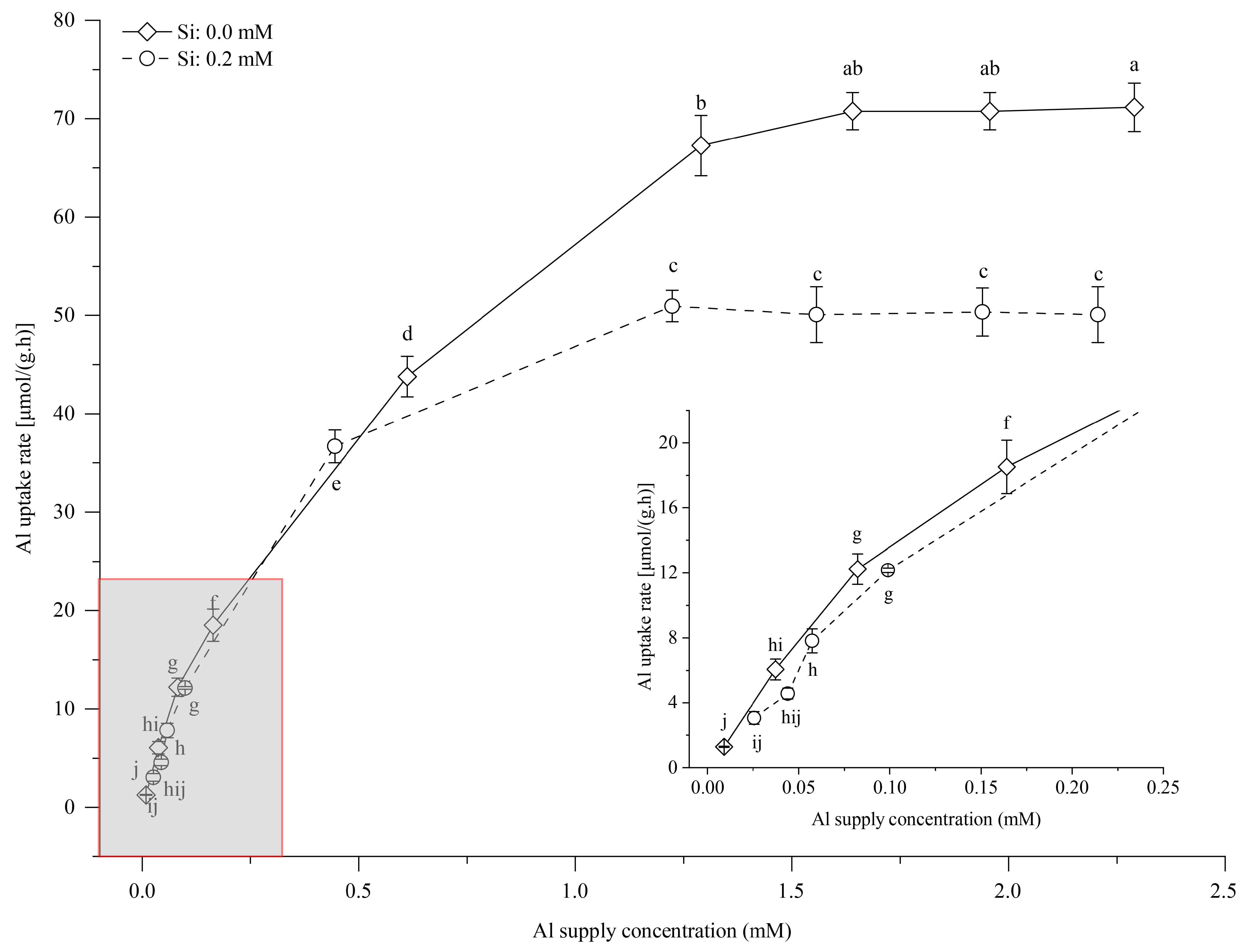
3.5. Effects of Si Supply on Al Uptake in Tea Seedlings
4. Discussion
4.1. Free Amino Acid and Caffeine Contents in Young Tea Leaves
4.2. Al Content in Young Tea Leaves and Roots
4.3. Si Content in Young Tea Leaves and Al Uptake Characteristics in Tea Seedlings
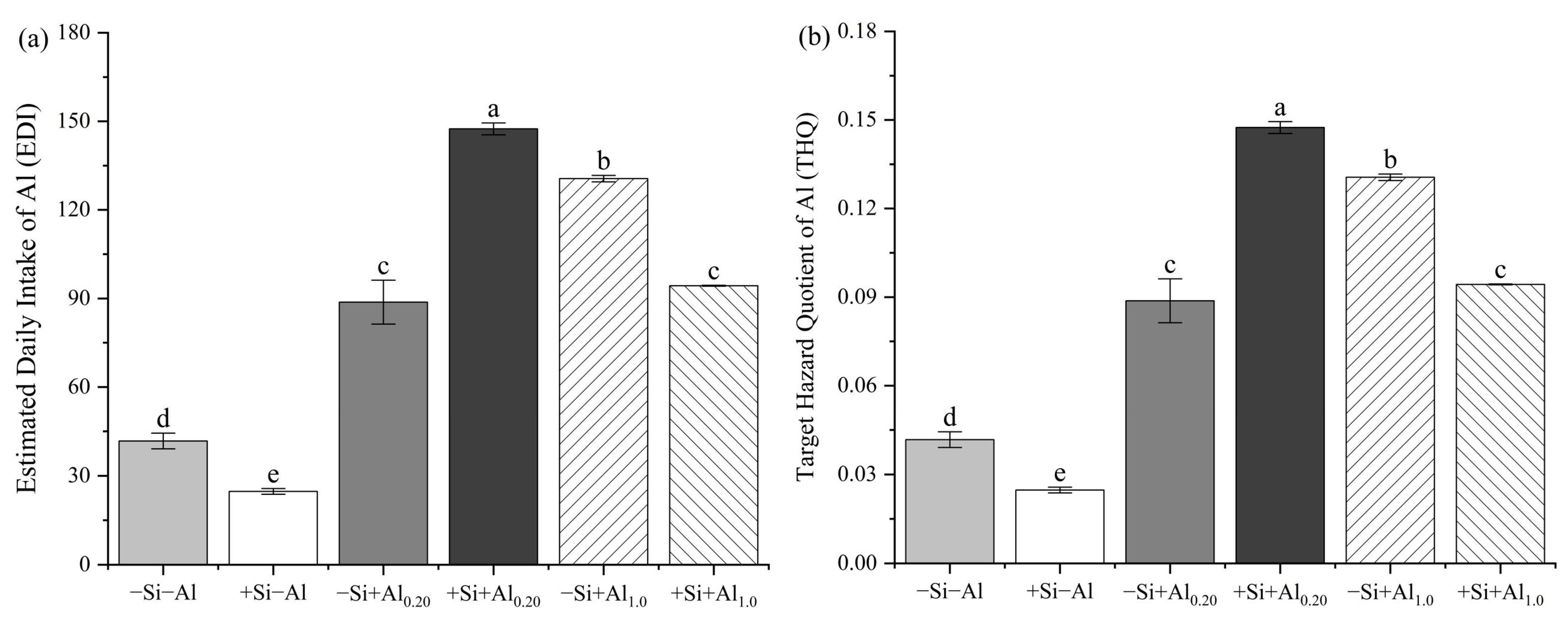
4.4. Health Risk Assessment of Al in the Tea Infusion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al | Aluminum |
| ANOVA | Analysis of variance |
| EDI | Estimated daily intake |
| EPA | Environmental Protection Agency |
| LSD | Least significant difference |
| Si | Silicon |
| THQ | Target hazard quotient |
| RfD | oral reference dose |
| Vmax | Maximum uptake rate |
| Km | Michaelis constant |
| α | rate of net influx |
References
- Exley, C. Aluminum should now be considered a primary etiological factor in Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2017, 1, 23–25. [Google Scholar] [CrossRef]
- Exley, C.; Korchazhkina, O.V. Promotion of formation of amyloid fibrils by aluminium adenosine triphosphate (AlATP). J. Inorg. Biochem. 2001, 84, 215–224. [Google Scholar] [CrossRef]
- Street, R.; Drábek, O.; Száková, J.; Mládková, L. Total content and speciation of aluminium in tea leaves and tea infusions. Food Chem. 2007, 104, 1662–1669. [Google Scholar] [CrossRef]
- US EPA. Regional Screening Level (RSL) Summary Table (TR=1E-6, HQ=1)-November 2024. Available online: https://semspub.epa.gov/work/HQ/405269.pdf (accessed on 12 September 2025).
- Wu, X.; Zhang, D.; Wang, F.; Luo, L.; Chen, Y.; Lu, S. Risk assessment of metal(loid)s in tea from seven producing provinces in China. Sci. Total Environ. 2023, 856, 159140. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Peng, A.; Yu, K.; Yu, S.; Li, Y.; Zuo, H.; Li, P.; Li, J.; Huang, J.; Liu, Z.; Zhao, J. Aluminum and fluoride stresses altered organic acid and secondary metabolism in tea (Camellia sinensis) plants: Influences on plant tolerance, tea quality and safety. Int. J. Mol. Sci. 2023, 24, 4640. [Google Scholar] [CrossRef]
- Beardmore, J.; Lopez, X.; Mujika, J.I.; Exley, C. What is the mechanism of formation of hydroxyaluminosilicates? Sci. Rep. 2016, 6, 30913. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wang, M.; Gao, Y.; Ning, Q.; Shi, Y. Transcriptomic and ionomic analysis provides new insight into the beneficial effect of Al on tea roots’ growth and nutrient uptake. Plant Cell Rep. 2019, 38, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium is essential for root growth and development of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef]
- Xie, X.; Yang, S.; Zhao, X.; Shang, T.; Han, X. Mechanisms of silicon-mediated amelioration for Camellia sinensis using physiology, transcriptomics, and metabolomics under cold stress. Beverage Plant Res. 2025, 5, e003. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Ding, Z.; Song, L.; Li, Y.; Ma, D.; Wang, Y.; Shen, J.; Jia, S.; Sun, H.; et al. Aluminum induced metabolic responses in two tea cultivars. Plant Physiol. Biochem. 2016, 101, 162–172. [Google Scholar] [CrossRef]
- Lima, M.D.R.; Barros, U.O.; Barbosa, M.A.M.; Segura, F.R.; Silva, F.F.; Batista, B.L.; Lobato, A.K.S. Silicon mitigates oxidative stress and has positive effects in Eucalyptus platyphylla under aluminium toxicity. Plant Soil Environ. 2016, 62, 164–170. [Google Scholar] [CrossRef]
- Singh, V.P.; Tripathi, D.K.; Kumar, D.; Chauhan, D.K. Influence of exogenous silicon addition on aluminium tolerance in rice seedlings. Biol. Trace Elem. Res. 2011, 144, 1260–1274. [Google Scholar] [CrossRef]
- Shen, X.; Xiao, X.; Dong, Z.; Chen, Y. Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol. Plant. 2014, 36, 3063–3069. [Google Scholar] [CrossRef]
- Barcelo, J.; Guevara, P.; Poschenrieder, C. Silicon amelioration of aluminium toxicity in teosinte (Zea mays L. ssp. mexicana). Plant Soil 1993, 154, 249–255. [Google Scholar] [CrossRef]
- Rahman, M.T.; Kawamura, K.; Koyama, H.; Hara, T. Varietal differences in the growth of rice plants in response to aluminum and silicon. Soil Sci. Plant Nutr. 1998, 44, 423–431. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, G.; Ye, M.; Liang, Y. Silicon relieves aluminum-induced inhibition of cell elongation in rice root apex by reducing the deposition of aluminum in the cell wall. Plant Soil 2021, 462, 189–205. [Google Scholar] [CrossRef]
- Cocker, K.M.; Evans, D.E.; Hodson, M.J. The amelioration of aluminium toxicity by silicon in wheat (Triticum aestivum L.): Malate exudation as evidence for an in planta mechanism. Planta 1998, 204, 318–323. [Google Scholar] [CrossRef]
- Unno, K.; Iguchi, K.; Tanida, N.; Fujitani, K.; Takamori, N.; Yamamoto, H.; Ishii, N.; Nagano, H.; Nagashima, T.; Hara, A.; et al. Ingestion of theanine, an amino acid in tea, suppresses psychosocial stress in mice. Exp. Physiol. 2013, 98, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, R.; Hu, B.; Li, W.; Sun, Y.; Tu, Y.; Zeng, X. Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chem. 2010, 123, 1259–1266. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A. Caffeine in tea Camellia sinensis—Content, absorption, benefits and risks of consumption. J. Nutr. Health Aging 2014, 18, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Weil, J.; De Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Q.; Zhang, L.-X.; Cui, L.-H.; Hou, J.; Xie, X.-Y.; Han, X.-Y. Effects of silicon fertilizer on the growth and silicon partitioning in tea plant parts. Plant Nutr. Fertil. Sci. 2023, 29, 712–721. [Google Scholar] [CrossRef]
- Xia, J.-G.; Yang, L.-Y.; Li, H.-X.; Wu, D.-Y. Effect of silicon applying on the quality of mengshan tea in Western Sichuan Basin. J. Tea Sci. 2007, 27, 83–87. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Huang, D. Effects of foliar application of different concentrations of organic-based biostimulant formulas on yield and quality of tea (Camellia sinensis L.) in red soil regions. J. Tea Sci. 2024, 44, 53–61. [Google Scholar]
- Hajiboland, R.; Bastani, S.; Bahrami-Rad, S.; Poschenrieder, C. Interactions between aluminum and boron in tea (Camellia sinensis) plants. Acta Physiol. Plant. 2015, 37, 54. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of nitrogen form and root-zone ph on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Xie, J.; Ye, J.; Pang, X.; Jia, X.L. Differences in the analysis of the quality indexes and characteristic amino acids of the different grades of Wuyi Shuixian (Camellia sinensis) tea. Food Sci. Technol. 2022, 42, e66122. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, P.; Zhong, N.; Huang, H.; Zheng, H. Impact of storage temperature on green tea quality: Insights from sensory analysis and chemical composition. Beverages 2024, 10, 35. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.; Lan, J.; Zhang, X.; Zhu, K.; Yang, S.; Lv, S. Detection of sodium formaldehyde sulfoxylate, aluminum, and borate compounds in bread and pasta products consumed by residents in Jilin Province, China. J. Food Prot. 2022, 85, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Huang, L.; Bai, J.; Zhang, G.; Wang, W.; Wang, D.; Wang, C.; Wang, Y.; Chen, G.; Liu, Z. Seasonal response of Suaeda salsa to hydrological connectivity in intertidal salt marshes through changing trait networks. CATENA 2023, 222, 106857. [Google Scholar] [CrossRef]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef]
- Dai, W.-m.; Zhang, K.-q.; Duan, B.-W.; Sun, C.-x.; Zheng, K.-l.; Cai, R.; Zhuang, J.-y. Rapid determination of silicon content in rice (Oryza sativa). Chin. J. Rice Sci. 2005, 19, 460–462. [Google Scholar] [CrossRef]
- Bora, K.; Sarkar, D.; Konwar, K.; Payeng, B.; Sood, K.; Paul, R.K.; Datta, R.; Das, S.; Khare, P.; Karak, T. Disentanglement of the secrets of aluminium in acidophilic tea plant (Camellia sinensis L.) influenced by organic and inorganic amendments. Food Res. Int. 2019, 120, 851–864. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmad, M.; Khan, M.A.; Sohail, A.; Sanaullah, M.; Ahmad, W.; Iqbal, D.N.; Khalid, K.; Wani, T.A.; Zargar, S. Assessment of carcinogenic and non-carcinogenic risk of exposure to potentially toxic elements in tea infusions: Determination by ICP-OES and multivariate statistical data analysis. J. Trace Elem. Med. Biol. 2024, 84, 127454. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- de Oliveira, L.M.; Das, S.; da Silva, E.B.; Gao, P.; Gress, J.; Liu, Y.; Ma, L.Q. Metal concentrations in traditional and herbal teas and their potential risks to human health. Sci. Total Environ. 2018, 633, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; York, L.M. Targeting root ion uptake kinetics to increase plant productivity and nutrient use efficiency1 [OPEN]. Plant Physiol. 2020, 182, 1854–1868. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Huang, C.-H.; Chang, C.-R. Characteristic of silicon uptake kinetic with different ratio of ammonium and nitrate in different silicon-efficiency rice. Soil Fertil. Sci. China 2017, 6, 122–126. [Google Scholar] [CrossRef]
- Li, L.; Fu, Q.-L.; Achal, V.; Liu, Y. A comparison of the potential health risk of aluminum and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi, China. Environ. Monit. Assess. 2015, 187, 228. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-l.; Liu, Y.; Li, L.; Achal, V. A survey on the heavy metal contents in Chinese traditional egg products and their potential health risk assessment. Food Addit. Contam. Part B 2014, 7, 99–105. [Google Scholar] [CrossRef]
- Shen, F.-M.; Chen, H.-W. Element composition of tea leaves and tea infusions and its impact on health. Bull. Environ. Contam. Toxicol. 2008, 80, 300–304. [Google Scholar] [CrossRef]
- Wang, J.; Qi, K. Preliminary report on spraying aluminum fertilizer on tea leaves. Guangdong Tea Ind. 1984, 8–11, 22. [Google Scholar]
- Huang, D.; Mao, Y.; Chen, X.; Tan, R.; Wang, H.; Wang, Y.; Gong, Z. Advances in aluminum accumulation and tolerance mechanisms in tea plant (Camellia sinensis). J. Tea Sci. 2018, 38, 125–132. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, X.-H.; Hu, X.-F.; Deng, Z.-Y.; Chen, F.-S. Effects of simulated acid rain and Al regulation on tea quality and its Al accumulation. J. Trop. Subtrop. Bot. 2011, 19, 254–259. [Google Scholar] [CrossRef]
- Wang, M.; Ning, Q.; Shi, Y. Study on physiological response of tea plant (Camellia sinensis) seedlings to different aluminum concentrations. J. Tea Sci. 2017, 37, 356–362. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, X.; Li, W.-W.; Shi, Y.; Lai, S.; Zhuang, J.; Yao, S.; Liu, Y.; Hu, J.; Gao, L.; et al. Proanthocyanidin–aluminum complexes improve aluminum resistance and detoxification of Camellia sinensis. J. Agric. Food Chem. 2020, 68, 7861–7869. [Google Scholar] [CrossRef]
- Morita, A.; Yanagisawa, O.; Takatsu, S.; Maeda, S.; Hiradate, S. Mechanism for the detoxification of aluminum in roots of tea plant (Camellia sinensis (L.) Kuntze). Phytochemistry 2008, 69, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xu, H.; Zhang, Y.; Chen, G. Silicon mediated redox homeostasis in the root-apex transition zone of rice plays a key role in aluminum tolerance. Plant Physiol. Biochem. 2023, 201, 107871. [Google Scholar] [CrossRef]
- Morita, A.; Yanagisawa, O.; Maeda, S.; Takatsu, S.; Ikka, T. Tea plant (Camellia sinensis L.) roots secrete oxalic acid and caffeine into medium containing aluminum. Soil Sci. Plant Nutr. 2011, 57, 796–802. [Google Scholar] [CrossRef]
- Haruyama, Y.; Fujiwara, T.; Yasuda, K.; Saito, M.; Suzuki, K. Localization of aluminum in epidermal cells of mature tea leaves. Quantum Beam Sci. 2019, 3, 9. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Gianoncelli, A.; Kourousias, G.; Green, K.; McKenna, B.A. Alleviation of Al toxicity by Si is associated with the formation of Al–Si complexes in root tissues of sorghum. Front. Plant Sci. 2017, 8, 109785. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, H.; Sheng, Y.; Li, Y.; Li, Y.; Ou, Y.; Zhang, Z.; Han, H.; Liu, S.; Chen, G. Silicon alleviates aluminum-induced inhibition of photosynthetic and growth attributes in rice by modulating competitive pathways between ethylene and polyamines and activating antioxidant defense. Plant Physiol. Biochem. 2025, 222, 109785. [Google Scholar] [CrossRef] [PubMed]
- Pontigo, S.; Godoy, K.; Jiménez, H.; Gutiérrez-Moraga, A.; Mora, M.d.l.L.; Cartes, P. Silicon-mediated alleviation of aluminum toxicity by modulation of Al/Si uptake and antioxidant performance in ryegrass plants. Front. Plant Sci. 2017, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liang, Y. Silicon prevents aluminum from entering root tip by promoting formation of root border cells in rice. Plant Physiol. Biochem. 2022, 175, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Xu, R.-k.; Li, X.-h. Proton release from tea plant (Camellia sinensis L.) roots induced by Al(III) under hydroponic conditions. Soil Res. 2012, 50, 482–488. [Google Scholar] [CrossRef]
- Fung, K.F.; Carr, H.P.; Poon, B.H.T.; Wong, M.H. A comparison of aluminum levels in tea products from Hong Kong markets and in varieties of tea plants from Hong Kong and India. Chemosphere 2009, 75, 955–962. [Google Scholar] [CrossRef]
| Treatment | Al Content in Young Tea Leaf (g/kg) | Al Content in Secondary Root (g/kg) | The Ratio of the Al Content in Young Tea Leaf to that in Secondary Root |
|---|---|---|---|
| −Si−Al | 0.29 ± 0.01 c | 0.76 ± 0.10 d | 0.39 ± 0.03 ab |
| +Si−Al | 0.23 ± 0.02 d | 0.51 ± 0.09 d | 0.46 ± 0.12 a |
| −Si+Al0.2 | 0.58 ± 0.01 b | 1.50 ± 0.22 c | 0.39 ± 0.05 ab |
| +Si+Al0.2 | 0.72 ± 0.05 a | 1.65 ± 0.01 bc | 0.44 ± 0.03 ab |
| −Si+Al1.0 | 0.59 ± 0.02 b | 1.92 ± 0.09 b | 0.31 ± 0.01 bc |
| +Si+Al1.0 | 0.55 ± 0.00 b | 2.32 ± 0.08 a | 0.24 ± 0.01 c |
| Si Supply Level (mM) | Kinetic Equation | R2 | Vmax [μmol/(g·h)] | Km (mM) | α [L/(g·h)] |
|---|---|---|---|---|---|
| 0.00 | y = −20.23x2 + 74.63x + 4.294 | 0.9950 ** | 73.12 ± 1.59 a | 0.500 ± 0.001 a | 0.146 ± 0.003 a |
| 0.20 | y = −19.79x2 + 62.78x + 4.590 | 0.9697 ** | 54.42 ± 0.21 b | 0.415 ± 0.016 b | 0.131 ± 0.006 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, D.; Bian, Y.; Bao, W.; Hu, Y.; Chang, C. Silicon Is Linked to Tea Quality Through Alteration Aluminum Uptake and Translocation in Camellia sinensis L. Foods 2025, 14, 3966. https://doi.org/10.3390/foods14223966
Liu H, Wang D, Bian Y, Bao W, Hu Y, Chang C. Silicon Is Linked to Tea Quality Through Alteration Aluminum Uptake and Translocation in Camellia sinensis L. Foods. 2025; 14(22):3966. https://doi.org/10.3390/foods14223966
Chicago/Turabian StyleLiu, Hui, Dongyang Wang, Yunting Bian, Wenzhe Bao, Yuanhui Hu, and Chunrong Chang. 2025. "Silicon Is Linked to Tea Quality Through Alteration Aluminum Uptake and Translocation in Camellia sinensis L." Foods 14, no. 22: 3966. https://doi.org/10.3390/foods14223966
APA StyleLiu, H., Wang, D., Bian, Y., Bao, W., Hu, Y., & Chang, C. (2025). Silicon Is Linked to Tea Quality Through Alteration Aluminum Uptake and Translocation in Camellia sinensis L. Foods, 14(22), 3966. https://doi.org/10.3390/foods14223966







