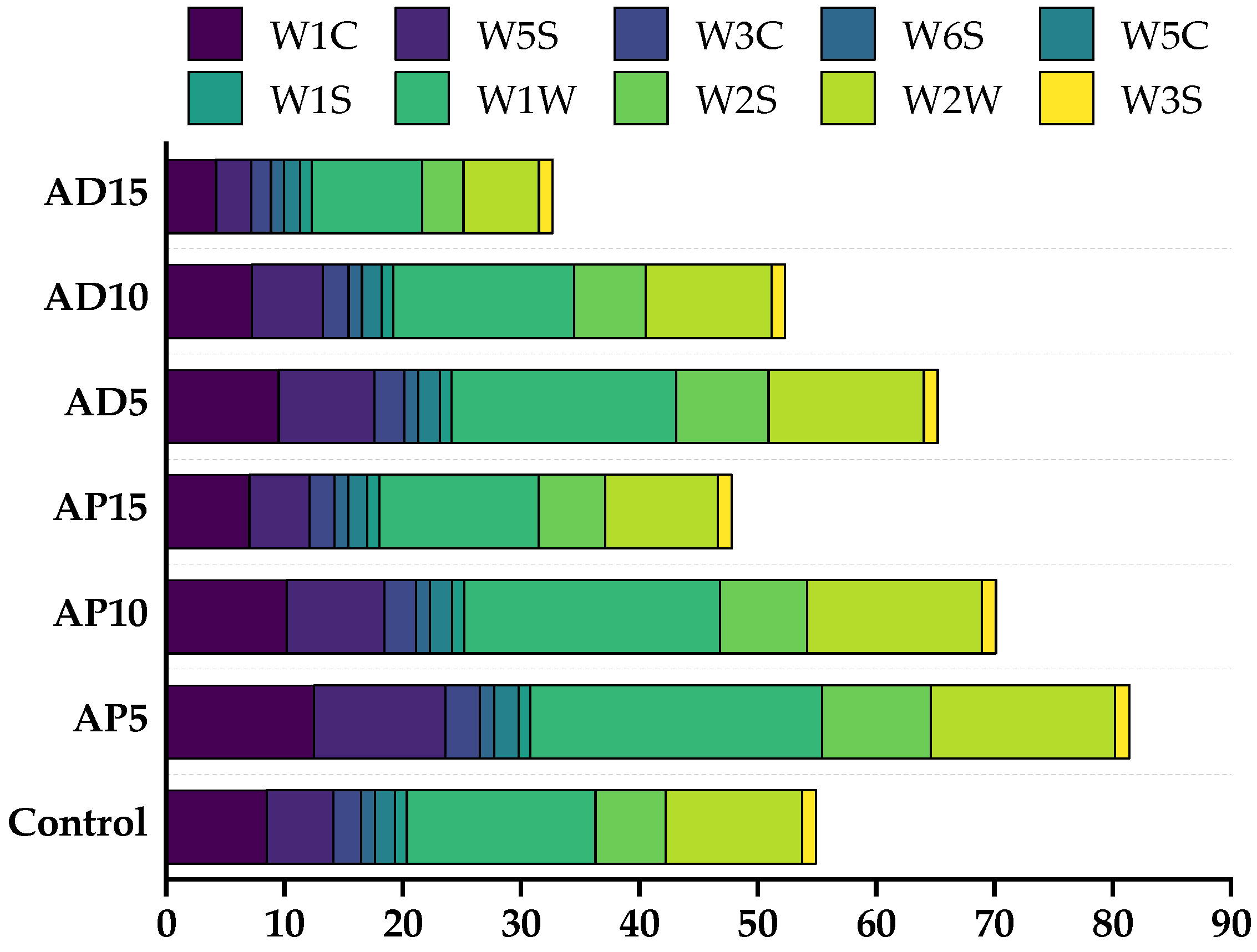3.1. Nutritional Composition
Table 2 compares the effects of incorporating two kinds of edible insect (AP and AD) at varying substitution levels on the proximate composition of CSB. The proximate composition of AP and AD was described in our earlier publications [
12,
34], as shown in
Table S1. For both types of edible insect, the protein content of CSB significantly elevated with higher substitution levels in comparison to the control (
p < 0.05). Specifically, the incorporation of 15% AP and 15% AD caused a considerable rise in protein content by 61.50% and 76.16%, respectively. The increase is likely due to the relatively lower protein content in wheat flour than that in both AP and AD [
35]. This result agrees with the observations from a prior study, which revealed that the incorporation of 10%
Alphitobius diaperinus powder in cookies resulted in a 26.91% increase in protein content [
36]. Another study by Biró et al. [
37], which observed a 55.17% rise in the protein content of oat biscuits incorporated with 15%
A. domesticus powder, further supports our findings.
Moreover, substitution with 15% AP significantly increased the fat and ash contents by 4.86-fold and 2.68-fold, respectively, compared to the control. Similarly, 15% AD substitution led to increases 3.35-fold in fat and 4.13-fold in ash content. This increase in fat content was due to the higher fat content in insect powder than in wheat flour. Based on the fat content analysis, the utilization of defatted insect powder is recommended to develop healthier products, as advocated by Roncolini et al. [
38]. The ash level corresponds to the mineral makeup of a food. These results suggest that fortification with insect powder effectively enhanced the mineral content of CSB compared to conventional wheat flour-based products. Kowalski et al. [
39] also found that high inclusion levels (i.e., 15% and 30%) of insect powder could increase the fat and ash contents of nut bars. With increasing substitution of AP or AD, the carbohydrate content in CSB decreased, attributable to the lower carbohydrate content in insect powder compared to wheat flour.
3.2. Color
Table 3 presents the color parameters of freshly prepared CSB. Color plays a key role in determining consumer acceptance of foods, since visual assessment often serves as the primary criterion for product recognition [
40]. Generally, the L* values of CSB fortified with AP and AD substantially reduced compared to the control. At the same inclusion levels, CSB fortified with AD exhibited lower L* values than those fortified with AP, which could be explained by the inherently darker color of AD compared to AP (see
Figure 1). In contrast, the a* and b* value increased after incorporating insect powder into CSB, indicating a shift in color chromaticity toward red and yellow. However, no significant differences were observed in a* value among control, AP5, AD5, and AP10. Although AD supplementation caused a slight increase in b* values, no significant difference (
p < 0.05) was found compared to the control. The phenolic compounds of AP or AD may undergo oxidation during the steaming process, resulting in color changes. Moreover, the high protein concentration in insect powder promotes Maillard reactions during steaming, leading to the formation of melanoidins and other browning pigments, which contribute to the increased yellowness of CSB [
28,
41]. These trends were in line with the findings of Wang et al. [
42], who reported that increasing the content of rose powder resulted in darker-colored CSB, along with elevated a* and b* values. The change in color may enhance consumers’ preference by suggesting freshness.
3.3. Textural Property
The textural attributes of CSB are closely relevant to the mouthfeel [
26], which is a critical factor affecting consumer acceptance. According to
Table 4, the hardness and chewiness of CSB increased progressively with higher incorporation levels of insect powder. Conversely, a general decreasing trend was noted in springiness, cohesiveness, and resilience. The increase in protein content promoted stronger interactions between the incorporated insect protein and wheat gluten, resulting in the formation of a more dense and rigid structure that ultimately enhanced the hardness of the CSB [
43]. Similarly, Bhatnagar et al. [
44] observed that protein supplementation increased the hardness of buns, which could be ascribed to the reduced gluten content in fortified products relative to the control. With the level of AD at 15%, the hardness and chewiness of CSB increased markedly, resulting in a less desirable mouthfeel. Due to the high energy and time demands of oral processing prior to swallowing, excessive chewiness and hardness can be detrimental to the palatability of CSB [
42]. However, changes in the texture of CSB induced by edible insect powder substitution do not necessarily reduce consumer acceptance. Consumer preferences for the texture of CSB exhibit regional variations [
2], highlighting the potential of edible insects to improve CSB quality.
3.4. Antioxidant Activity
Indeed, numerous bioactive compounds such as carotenoids, tocopherols, lignans, flavonoids, phytosterols, and phenolic acids are naturally present in wheat [
2]. The TPC and TFC of CSB fortified with AP or AD increased dose-dependently with insect powder inclusion. As displayed in
Table 5, TPC ranged from 5.63 to 32.66 mg GAE/100 g, with the highest increase (5.80 fold) observed in CSB containing 15% AP. Similarly, TFC increased significantly in fortified samples, ranging from 3.85 to 12.59 mg RE/100 g, compared to 0.45 mg RE/100 g in the control CSB. These results are in line with those found by Ogidi et al. [
45], who demonstrated that the fortification of 10%
Macroterms nigeriensis into wheat cookies significantly improved both TPC and TFC.
The AP15 group showed the highest improvement in antioxidant activity, with DPPH and ABTS values reaching 4.9 and 2.8 times higher than the control, respectively. The AD15 group also exhibited strong enhancement, achieving 3.2-fold and 2.2-fold increases in DPPH and ABTS values. The most pronounced improvements were observed at 10–15% fortification, consistent with the elevated polyphenol content—a phenomenon potentially attributable to insects’ abundance in bioactive constituents like chitins, tocopherols and ferulic acid [
12]. Additionally, the formation of Maillard reaction products during the CSB manufacturing process, which are known for their antioxidant activity [
46], further contributed to the enhanced antioxidant capacity. The radical scavenging activities significantly elevated in the AP- and AD-fortified CSB, compared to the control, which is in line with the findings of our previous study on the influence of edible insect incorporation in rice noodles [
47]. Nissen et al. [
46] obtained similar trends after adding cricket flour in gluten free sourdough bread. These results suggested that insect powder fortification not only improved the nutritional value but also enhanced the antioxidant profile of CSB.
3.5. In Vitro Starch Digestibility
Table 6 displays the eGI and starch composition of CSB fortified with AP or AD. The control exhibited the highest rapidly digestible starch (RDS) content (58.87%), which significantly decreased with higher levels of AP or AD incorporation (
p < 0.05). In contrast, slowly digestible starch (SDS) and resistant starch (RS) contents increased with the addition of AP or AD. Compared to the control, the AP15 group exhibited increases of 12.61% in SDS and 103.32% in RS, while the AD15 group showed increases of 13.48% and 122.92%, respectively. The incorporation of insect powder considerably lowered the eGI of CSB in a dose-dependent fashion. In comparison with the control, the incorporation of 15% AP and 15% AD resulted in a reduction in eGI by 20.55% and 23.71%, respectively. These results indicated that the addition of insect powder effectively inhibited starch digestion. This phenomenon could be explained by the elevated levels of protein, lipid, and polyphenols in insect-fortified CSB. Proteins and polyphenols retard starch digestibility through two primary mechanisms: firstly, by suppressing digestive enzyme activity, and secondly, by physically interacting with starch molecules to form a barrier that blocks enzyme access [
26,
48,
49]. Furthermore, the addition of insect powder reduced the gas retention capacity of the gluten network during fermentation and steaming, resulting in a denser crumb structure. This compact structure, which is more resistant to breakdown during digestion, consequently lowered starch digestibility [
50]. Zhao et al. [
51] reported similar changes when producing CSB with kiwi starch. They attributed these changes to the dense microstructure formed after kiwi starch substitution, which impeded physical contact between starch and digestive enzymes, thereby causing a decreased starch hydrolysis rate of CSB.
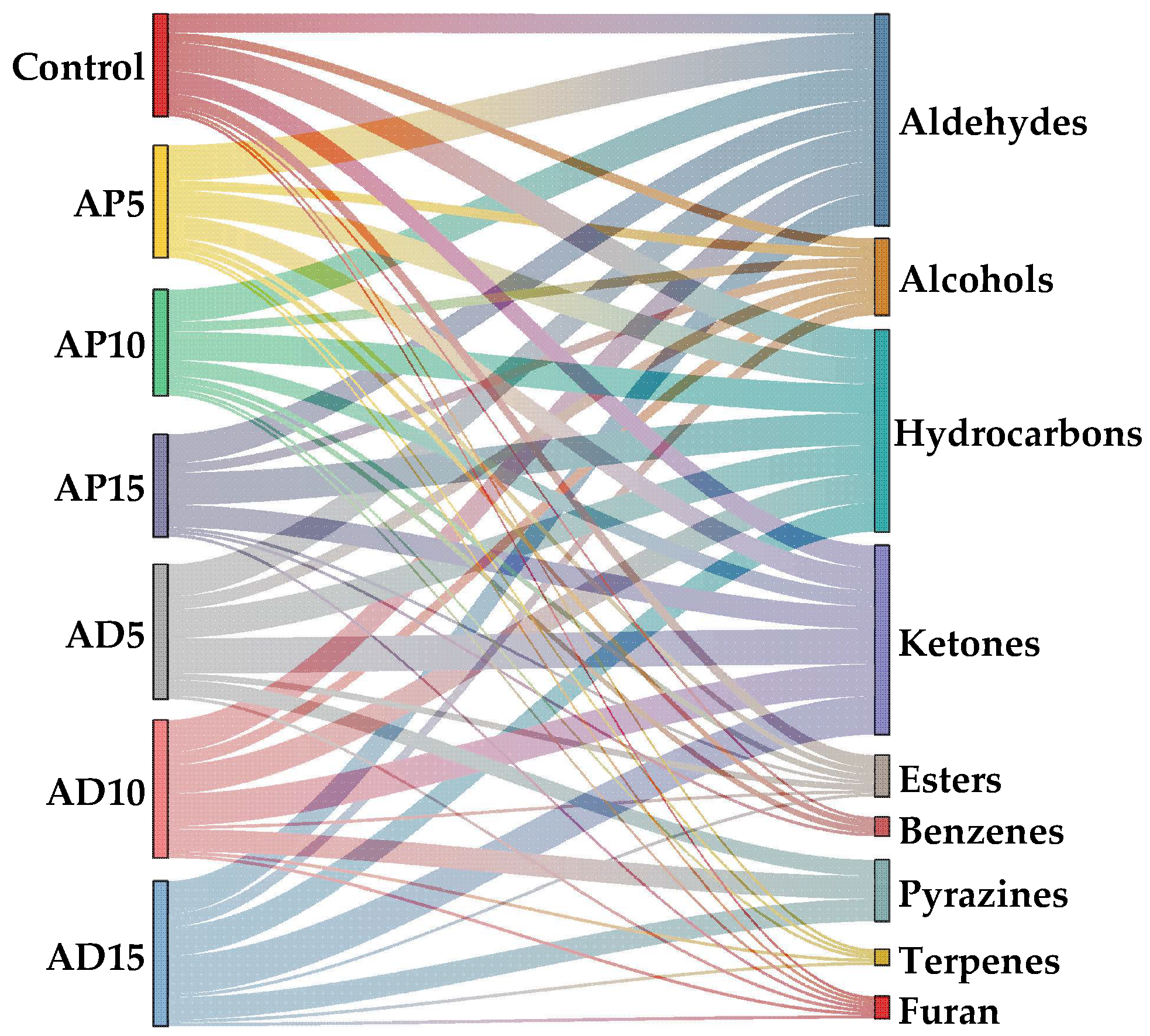
3.8. Volatile Flavor Compounds
Flavor is a critical determinant of consumer acceptance and overall quality evaluation of CSB [
55]. Therefore, this study used GC-MS for the analysis of the volatile flavor compounds in CSB with varying levels of edible insect powder. Fifty-seven VOCs were detected in the samples, comprising aldehydes, alcohols, hydrocarbons, ketones, esters, benzenes, pyrazines, terpenes, and furans. As shown in
Table 7, the incorporation of the two edible insects induced a greater diversity and complexity of VOCs compared to the control CSB. Seven new VOCs were identified in the AP-fortified CSB samples, including (E,Z)-2,6-nonadienal, heptanal, octanal, 2,6-dimethyl-undecane, (E,E)-3,5-Octadien-2-one, acetophenone, and 2-methoxy-4-vinylphenol, whereas in the AD-fortified samples, thirteen new VOCs were detected, including (E)-2-heptenal, 2-butyl-2-octenal, heptanal, hexanal, 1-undecanol, 2,3-nonanedione, 2-decanone, (E)-3-octen-2-one, 2,3-dimethyl-5-ethylpyrazine, 2,5-dimethyl-pyrazine, 2,5-dimethyl-3-(3-methylbutyl)-pyrazine, 3,5-diethyl-2-methyl-pyrazine, and 3-ethyl-2,5-dimethyl-pyrazine. These new VOCs, primarily aldehydes, ketones, and pyrazines, were likely generated by promoted Maillard reaction and lipid oxidation, thereby enriching the overall flavor profile of CSB.
As illustrated in
Figure 4, aldehydes were the dominant volatile compounds in both abundance and concentration across all sample groups, establishing them as the principal contributors to the CSB’s aroma. Aldehydes are predominantly generated through the oxidation of unsaturated fatty acids. Specifically, hexanal (grass) [
56] and (E)-2-octenal (fatty, nutty, and roasted) [
57] are primarily derived from linoleic acid oxidation [
58]. Nonanal (rose and citrus) [
59], heptanal (fatty, greasy, and fruity) [
60] and octanal (fruity and fatty) [
60] are secondary oxidation products of oleic acid, contributing pleasant fruity and fatty aromas [
60,
61]. The AP-fortified CSB contained high levels of nonanal, decanal (citric) [
62], (E,Z)-2,6-nonadienal (fatty and green) [
63], heptanal, and octanal, enhancing fruity flavor. Conversely, the AD-fortified CSB had richer concentrations of benzeneacetaldehyde (hawthorne and honey) [
64,
65], (E)-2-octenal, (E)-2-heptenal, 2-butyl-2-octenal (sweetish, metallic, and citrus) [
66], and hexanal. The elevated nonanal content in AP-fortified CSB may be associated with the high oleic acid concentration in AP [
67]. Ketones are primarily produced from lipid oxidation and Maillard reaction [
60]. The elevated relative content in AD-fortified CSB may be due to the greater amount of protein in AD, which promoted Maillard-derived ketone formation. Specifically, 2-decanone, 2-nonanone (rose and tea) [
68], 2-octanone, and (E)-3-octen-2-one were present at higher levels in AD-fortified CSB, likely resulting from the oxidation of free fatty acids [
61]. Hydrocarbons, formed through the cleavage of fatty acid alkoxy radicals [
60]. However, as a result of their generally high odor thresholds, hydrocarbons contributed little to the direct flavor of CSB but may still act as a complement to modify the overall flavor background and perception [
69].
The overall concentration of alcohols decreased after adding insect powder, a trend consistent with their oxidation into corresponding aldehydes, thereby explaining simultaneous aldehyde increase [
70]. Among key alcohols detected across all samples, ethanol was more abundant in the control than in the AP- and AD-fortified CSB, whereas 1-octen-3-ol (mushroom) [
60] was detected at higher levels in the AD- fortified CSB. Additionally, the ester level in the control was higher than that in the AP- and AD-fortified CSB [
71]. Esters play a critical role in flavor modulation attributed to their low odor thresholds. Esters derived from short-chain acids mainly impart fruity notes, whereas those formed from long-chain acids contribute mild fatty undertones [
72]. Meanwhile, The Maillard reaction products, such as pyrazines [
73], 2-pentylfuran (green bean and floral odor) [
32,
56], were more abundant in AD-fortified CSB compared to both the control and AP-fortified samples.
3.9. E-Tongue Analysis
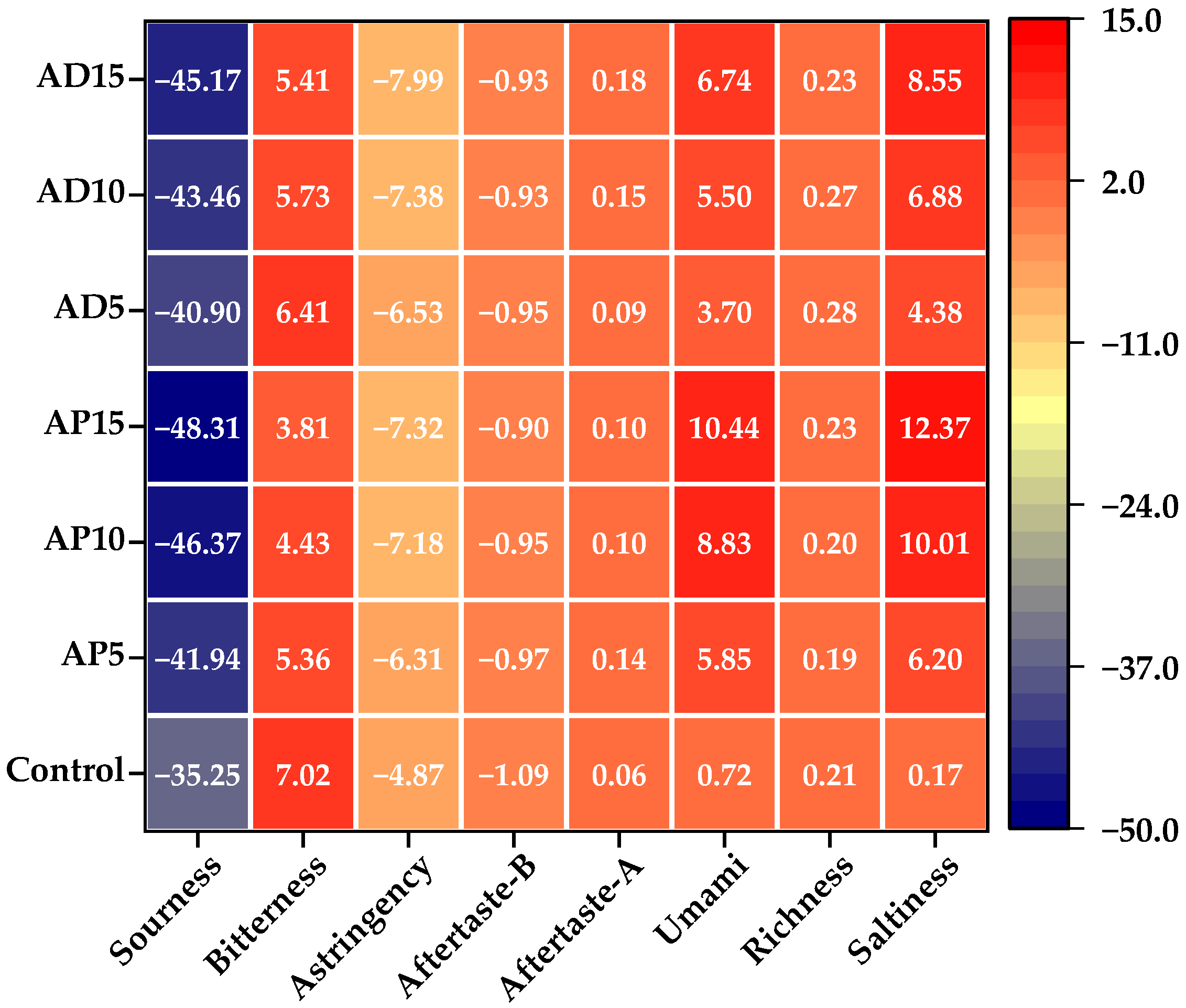
The E-tongue has been widely applied in food analysis owing to its extremely high sensitivity [
74]. E-tongue sensor responses toward flavor compounds in CSB with differential insect powder incorporation was visualized in the heat map presented in
Figure 5. In this representation, deeper red shades indicate higher response values, whereas deeper blue hues correspond to lower values. Among the eight taste attributes evaluated, the values for sourness, astringency, and aftertaste-B in all samples were below the detection threshold of the reference solution. Therefore, these tastes were considered negligible in the CSB samples. Notably, the incorporation of either AP or AD enhanced the umami and saltiness of CSB, which is likely resulting from the abundance of umami amino acids (e.g., glutamate) and minerals (e.g., sodium and potassium) in the insect powder [
14,
75].
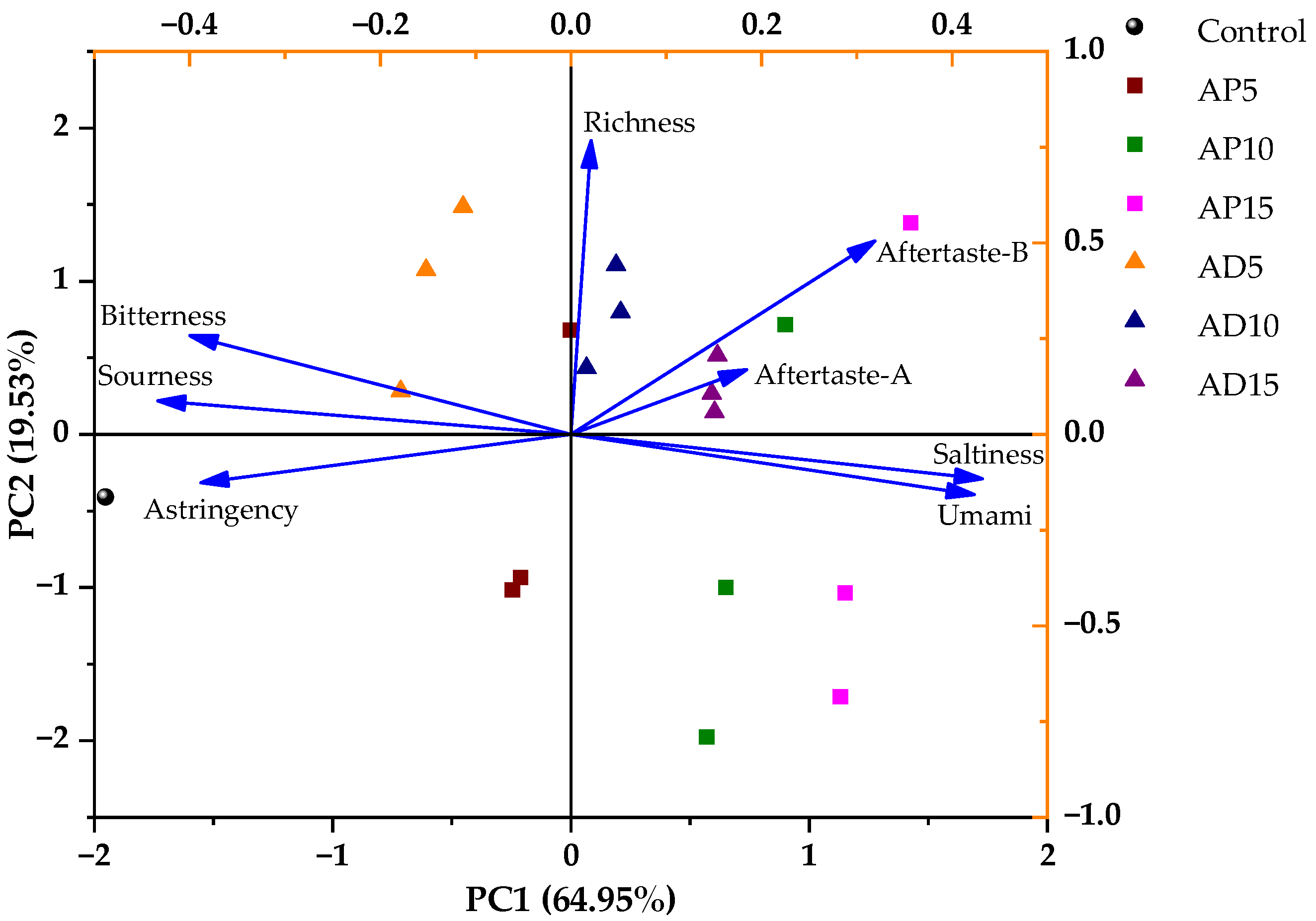
To further explore the differences in taste characteristics of CSB with varying insect powder content, PCA was applied to the E-tongue response values of each sample group. As illustrated in
Figure 6, PC1 and PC2 explained 64.95% and 19.53% of the total variance, respectively, cumulatively explaining 84.48% of the variance—sufficient to capture most of the sample information [
60]. PC1 was positively correlated with richness, aftertaste-A, aftertaste-B, saltiness, and umami, while it showed negative correlations with bitterness, sourness, and astringency. Among them, sourness and saltiness contributed more strongly to PC1, while richness was more influential on PC2. The shorter arrow length of aftertaste-A suggested its relatively lower contribution to the overall taste profile of CSB. The distribution of sample points correlated with distinct taste profiles. For example, AD15 displayed more pronounced aftertaste-A, while AP15 exhibited stronger umami and saltiness. It is worth noting that E-tongue analysis was used not for directly determining sensory acceptability, but for detecting subtle differences in taste characteristics among the CSB samples.