Comparative Analysis of Volatile Organic Compounds in Freshwater-Cultured and Saline–Alkaline Selectively Bred Tilapia Using Electronic Nose, GC-IMS, and HS-SPME-GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Electronic Nose (E-Nose) Analysis
2.3. Volatile Organic Compounds (VOCs) Detection Based on Gas Chromatography-Ion Mobility Spectrometry (GC-IMS)
2.4. VOCs Determination Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS)
2.5. Odor Activity Value (OAV) Analysis
- OAV: Odor Activity Value
- C: Compound Concentration
- T: Odor Threshold
2.6. Data Analysis
3. Results and Discussion
3.1. E-Nose Analysis of FW and SAWG7
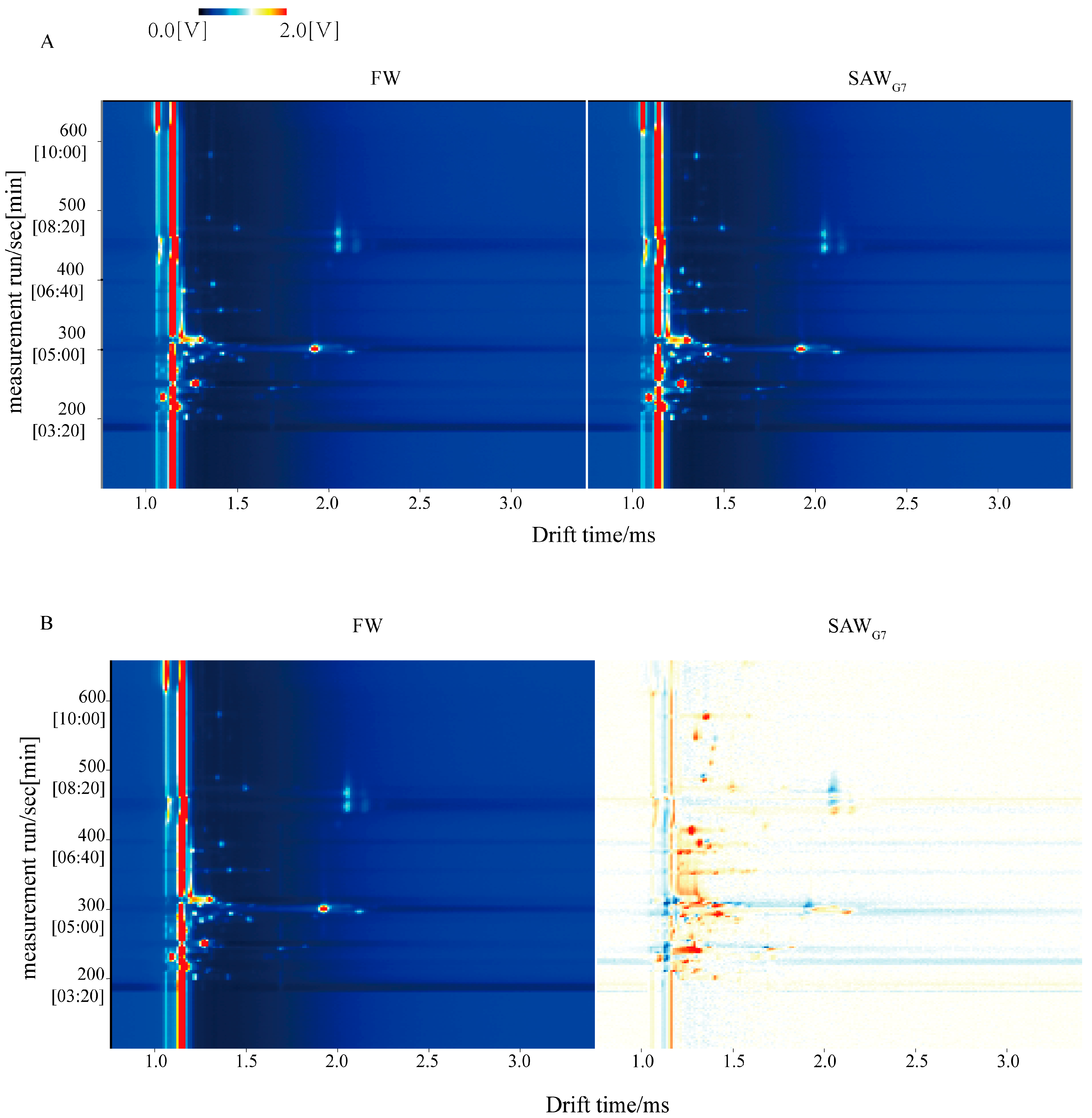
3.2. VOCs in FW and SAWG7 Identified by GC-IMS
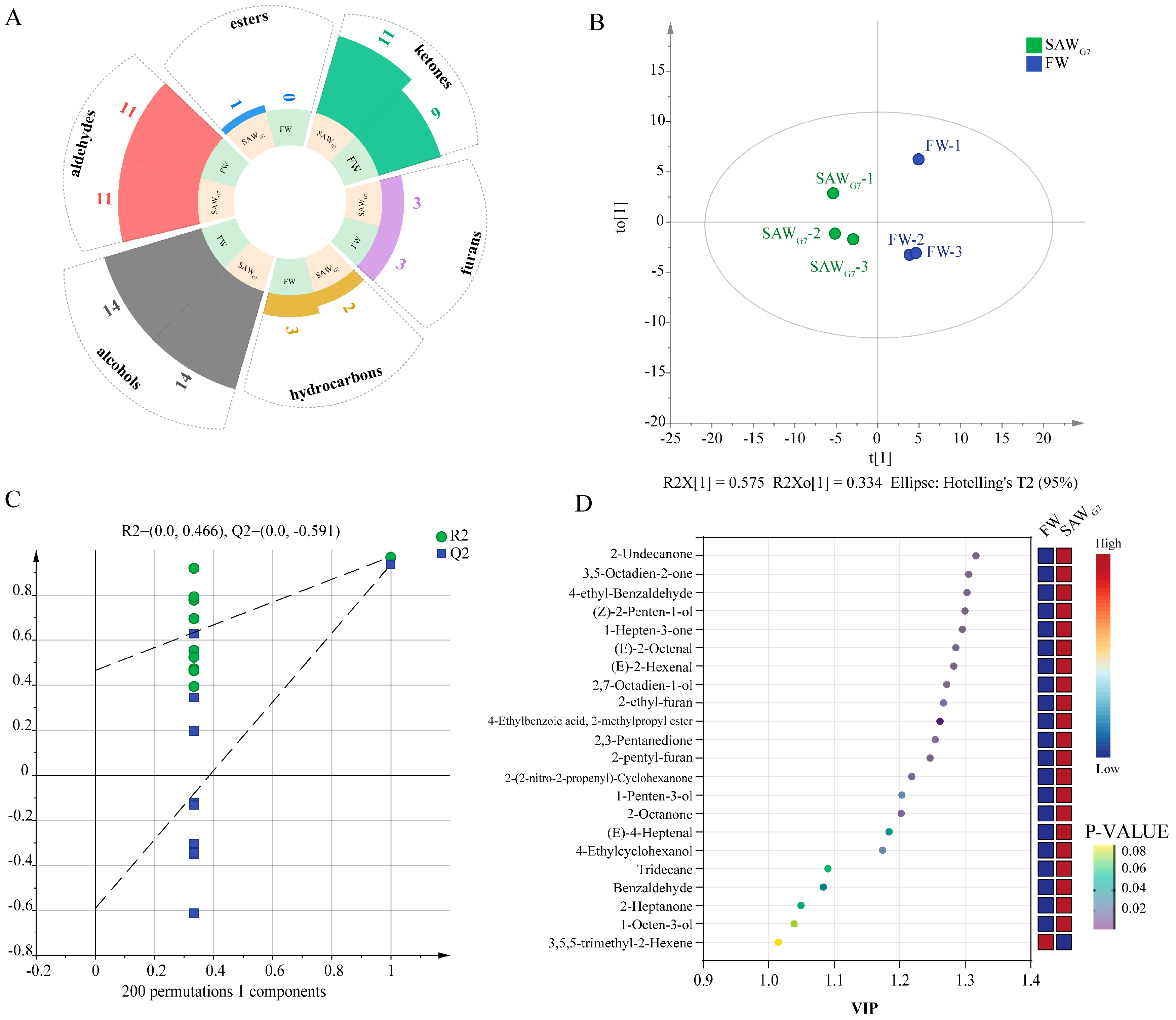
3.3. VOCs in FW and SAWG7 Identified by GC-MS
3.4. Identification of Key Aroma Compounds in Two Species of Tilapia by OAV
3.5. Comparative Analysis of E-Nose, GC-IMS, and GC-MS Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, Q.; Wang, J.; Li, J.; Li, J. Effect of high alkalinity on shrimp gills: Histopathological alternations and cell specific responses. Ecotoxicol. Environ. Saf. 2023, 256, 114902. [Google Scholar] [CrossRef]
- Yao, Z.L.; Lai, Q.F.; Zhou, K.; Rizalita, R.-E.; Wang, H. Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress: Alkalinity stress for Oryzias latipes. J. Appl. Ichthyol. 2010, 26, 397–402. [Google Scholar] [CrossRef]
- Li, Y.; Gao, P.; Zhou, K.; Yao, Z.; Sun, Z.; Qin, H.; Lai, Q. Effects of saline and alkaline stresses on the survival, growth, and physiological responses in juvenile mandarin fish (Siniperca chuatsi). Aquaculture 2024, 591, 741143. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, K.; Liang, G.; Li, X.; Niu, M.; Wang, H.; Wang, C.; Mu, C.; Zhu, R. Comparative study on non-volatile flavor substances of Scylla paramamosain cultured in inland low saline-alkaline water. J. Food Compos. Anal. 2023, 118, 105157. [Google Scholar] [CrossRef]
- Jiang, X.; Niu, M.; Qin, K.; Hu, Y.; Li, Y.; Che, C.; Wang, C.; Mu, C.; Wang, H. Enhancement of nutrient composition and non-volatile flavor substances in muscle tissue of red drum (Sciaenops ocellatus) through inland low salinity saline-alkaline water culture. J. Agric. Food Chem. 2024, 72, 7326–7335. [Google Scholar] [CrossRef]
- Wang, S.; Guo, K.; Luo, L.; Zhang, R.; Xu, W.; Song, Y.; Zhao, Z. Fattening in saline and alkaline water improves the color, nutritional and taste quality of adult Chinese Mitten Crab Eriocheir sinensis. Foods 2022, 11, 2573. [Google Scholar] [CrossRef]
- El Asely, A.; Amin, A.; Abd El-Naby, A.S.; Samir, F.; El-Ashram, A.; Dawood, M.A.O. Ziziphus mauritiana supplementation of Nile tilapia (Oreochromis niloticus) diet for improvement of immune response to Aeromonas hydrophila infection. Fish Physiol. Biochem. 2020, 46, 1561–1575. [Google Scholar] [CrossRef]
- El Asely, A.M.; Reda, R.M.; Salah, A.S.; Mahmoud, M.A.; Dawood, M.A. Overall performances of Nile tilapia (Oreochromis niloticus) associated with using vegetable oil sources under suboptimal temperature. Aquac. Nutr. 2020, 26, 1154–1163. [Google Scholar] [CrossRef]
- Song, L.; Zhao, Y.; Song, Y.; Zhao, L.; Ma, C.; Zhao, J. Effects of saline-alkaline water on growth performance, nutritional processing, and immunity in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 544, 737036. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Xu, X.; Zhao, Y. Influence of thermal processing on flavor and sensory profile of sturgeon meat. Food Chem. 2022, 374, 131689. [Google Scholar] [CrossRef]
- Menis-Henrique, M.E.C. Methodologies to advance the understanding of flavor chemistry. Curr. Opin. Food Sci. 2020, 33, 131–135. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Ma, D.; Fan, J.; Zhong, Z.; Zhu, H. Metabolism responses in the intestine of Oreochromis mossambicus exposed to salinity, alkalinity and salt-alkalinity stress using LC-MS/MS-based metabolomics. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 45, 101044. [Google Scholar] [CrossRef]
- Sun, Z.; Yao, Z.; Gao, P.; Zhou, K.; Li, Y.; Wei, Y.; Lai, Q. Effects of fishery utilization on the physicochemical index and microbial community composition in saline-alkaline water. ACS Omega 2024, 9, 18872–18881. [Google Scholar] [CrossRef]
- Choi, H.-Y.; Woo, H.-E.; Go, E.-S.; Kim, J.-S.; Choi, J.-H. Flavor characteristics of garlic fish cakes using electronic nose and tongue analyses. Sci. Rep. 2024, 14, 6048. [Google Scholar] [CrossRef]
- Teixeira, E.; Noronha, J.P.; Barbosa, V.; Anacleto, P.; Maulvault, A.; Nunes, M.L.; Marques, A.; Dinizb, M.S. Determination of target biogenic amines in fish by GC-MS: Investigating seafood quality. Ann. Med. 2019, 51 (Suppl. S1), 73. [Google Scholar] [CrossRef]
- Parastar, H.; Weller, P. Towards greener volatilomics: Is GC-IMS the new Swiss army knife of gas phase analysis? TrAC Trends Anal. Chem. 2024, 170, 117438. [Google Scholar] [CrossRef]
- Chen, P.; Dai, Y.; Weng, W.; Ren, Z.; Li, P.; Shi, L. Comparative analysis of volatile compounds of an oyster enzymatic hydrolysate adsorbed by V-type starches based on electronic nose, GC-IMS, and GC-MS. Food Res. Int. 2025, 209, 116194. [Google Scholar] [CrossRef]
- Jagannivasan, A.; Gopakumar, S.T.; Sharma, S.R.K.; Suresh, G.; Raveendranathan, D.N.; Peter, R.; Gop, A.P.; Achamveetil, G. Profiling the antioxidant biomarkers in marine fish larvae: A comparative assessment of different storage conditions to select the optimal strategy. Fish Physiol. Biochem. 2024, 50, 557–574. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, L.; Zi, Y.; Chen, J.; Xu, J.; Shi, W.; Wang, X.; Zhong, J. Development of tilapia muscle-based Shanghai smoked fish and the effect of salt amounts in the preparation process. LWT 2023, 186, 115240. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, N.; Zhang, Q.; Tian, Z.; Li, M.; Shi, W. Evaluation of aroma characteristics of Penaeus vannamei with different drying methods using HS-SPME-GC-MS, MMSE-GC-MS, and sensory evaluation. Food Chem. 2024, 449, 138957. [Google Scholar] [CrossRef]
- Oliveros, M.C.C.; Pavón, J.L.P.; Pinto, C.G.; Laespada, M.E.F.; Cordero, B.M.; Forina, M. Electronic nose based on metal oxide semiconductor sensors as a fast alternative for the detection of adulteration of virgin olive oils. Anal. Chim. Acta 2002, 459, 219–228. [Google Scholar] [CrossRef]
- Upadhyay, R.; Sehwag, S.; Mishra, H.N. Electronic nose guided determination of frying disposal time of sunflower oil using fuzzy logic analysis. Food Chem. 2017, 221, 379–385. [Google Scholar] [CrossRef]
- Pan, Y.; Qiao, L.; Liu, S.; He, Y.; Huang, D.; Wu, W.; Liu, Y.; Chen, L.; Huang, D. Explorative study on volatile organic compounds of Cinnamon based on GC-IMS. Metabolites 2024, 14, 274. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, G.; Sun, X.; Li, Y.; Huang, H.; Fu, Y. Comparing the volatile and soluble profiles of fermented and integrated Chinese bayberry wine with HS-SPME GC–MS and UHPLC Q-TOF. Foods 2023, 12, 1546. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the key aroma volatile compounds in nine different grape varieties wine by headspace gas chromatography–ion mobility spectrometry (HS-GC-IMS), odor activity values (OAV) and sensory analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mi, S.; Liu, R.; Sang, Y.; Wang, X. Evaluation of volatile compounds in milks fermented using traditional starter cultures and probiotics based on odor activity value and chemometric techniques. Molecules 2020, 25, 1129. [Google Scholar] [CrossRef]
- Chen, R.; Sun, L.; Zhang, S.; Li, Q.; Wen, S.; Lai, X.; Li, Q.; Cao, J.; Sun, S. Mechanisms and quality variations of non-volatile and volatile metabolites in black tea from various ages of tea trees: Insights from metabolomics analysis. Food Chem. X 2024, 22, 101470. [Google Scholar] [CrossRef]
- Lu, Q.; Ding, W.; Guo, X.; Xiao, T.; Wang, X. Drivers of consumer preference derived from active volatiles for cooked Eriocheir sinensis. Animals 2023, 13, 541. [Google Scholar] [CrossRef]
- van Houcke, J.; Medina, I.; Linssen, J.; Luten, J. Biochemical and volatile organic compound profile of European flat oyster (Ostrea edulis) and Pacific cupped oyster (Crassostrea gigas) cultivated in the Eastern Scheldt and Lake Grevelingen, The Netherlands. Food Control 2016, 68, 200–207. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Jia, F.; Wu, J.; Jin, W.; Zhao, W.; Cao, J.; Cheng, Y.; Shi, L.; Yun, S.; et al. Exploring the effect of pH-shifting on the gel properties and interaction of heat-induced Flammulina velutipes polysaccharide-porcine myofibrillar protein for improving the quality of Flammulina velutipes-pork patties. Food Chem. 2025, 465, 142187. [Google Scholar] [CrossRef]
- Qiu, D.; Gan, R.; Feng, Q.; Shang, W.; He, Y.; Li, C.; Shen, X.; Li, Y. Flavor formation of tilapia byproduct hydrolysates in Maillard reaction. J. Food Sci. 2024, 89, 1554–1566. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Xie, J. Progress on odor deterioration of aquatic products: Characteristic volatile compounds, analysis methods, and formation mechanisms. Food Biosci. 2023, 53, 102666. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; O’Keefe, S.F.; Jahncke, M.L.; Mallikarjunan, P.; Flick, G.J. Analysis of crab meat volatiles as possible spoilage indicators for blue crab (Callinectes sapidus) meat by gas chromatography–mass spectrometry. Food Chem. 2010, 122, 930–935. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Schnabel, K.O.; Belitz, H.D.; von Ranson, C. Untersuchungen zur Struktur-Aktivitäts-Beziehung bei Geruchsstoffen 1. Mitteilung: Wahrnehmungsschwellenwerte und Geruchsqualitäten von gesättigten aliphatischen und alicyclischen Verbindungen mit Sauerstoff-Funktion. Z Leb. Unters Forch 1988, 187, 215–223. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C. Volatile flavor components of corn tortillas and related products. J. Agric. Food Chem. 1995, 43, 1878–1882. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Büttner, A.; Schieberle, P. Evaluation of key aroma compounds in hand-squeezed grapefruit juice (Citrus paradisi Macfayden) by quantitation and flavor reconstitution experiments. J. Agric. Food Chem. 2001, 49, 1358–1363. [Google Scholar] [CrossRef]


| Fatty Acid | FW (%) | SAWG7 (%) |
|---|---|---|
| C4:0 | 15.80 ± 4.44 a | 16.81 ± 13.17 a |
| C14:0 | 0.96 ± 0.09 a | 0.95 ± 0.15 a |
| C15:0 | 0.25 ± 0.05 b | 0.35 ± 0.06 a |
| C16:0 | 22.90 ± 0.19 a | 19.70 ± 0.93 b |
| C16:1 | 2.00 ± 0.26 a | 1.90 ± 0.14 a |
| C17:0 | 0.30 ± 0.05 b | 0.50 ± 0.05 a |
| C18:0 | 7.60 ± 0.15 b | 8.5 ± 0.28 a |
| C18:1N9T | 0.20 ± 0.03 | / |
| C18:1N9C | 21.40 ± 0.36 a | 13.90 ± 2.62 b |
| C19:0 | 8.00 ± 0.16 b | 15.70 ± 0.15 a |
| C18:2N6C | 21.50 ± 0.20 a | 16.50 ± 0.63 b |
| C20:0 | 0.20 ± 0.04 b | 0.30 ± 0.03 a |
| C18:3N6 | 0.90 ± 0.01 a | 0.30 ± 0.08 b |
| C20:1 | 1.10 ± 0.22 a | 1.00 ± 0.05 a |
| C18:3N3 | 1.20 ± 0.10 a | 1.00 ± 0.06 b |
| C20:2 | 1.60 ± 0.05 a | 1.10 ± 0.02 b |
| C22:0 | 0.30 ± 0.01 a | 0.30 ± 0.05 a |
| C20:3N6 | 1.70 ± 0.04 a | 0.80 ± 0.04 b |
| C20:3N3 | 0.30 ± 0.03 a | 0.30 ± 0.04 a |
| C20:4N6 | 5.00 ± 0.25 a | 4.80 ± 0.03 a |
| C20:5 | 0.20 ± 0.02 b | 0.70 ± 0.17 a |
| C24:1 | / | 1.50 ± 0.13 |
| C22:6NS | 3.90 ± 0.11 b | 11.10 ± 0.30 a |
| SFA | 48.00 ± 0.58 | 53.48 ± 1.65 |
| MUFA | 21.06 ± 0.17 | 15.51 ± 0.59 |
| PUFA | 30.94 ± 0.09 | 31.01 ± 0.15 |
| EPA + DHA | 4.10 | 11.80 |
| n-3/n-6(%) | 0.19 | 0.58 |
| Volatile Compounds | Threshold | OAV | ||
|---|---|---|---|---|
| (μg/kg) | SAWG7 | FW | ||
| alcohols | 1-Octen-3-ol | 1.5 [34] | 57.88 | 27.2 |
| 1-Heptanol | 3 [35] | 2.86 | 1.57 | |
| aldehydes | Pentanal | 12 [36] | 1.72 | 1.03 |
| Hexanal | 5 [34] | 88.80 | 51.54 | |
| Heptanal | 3 [36] | 6.530 | 3.66 | |
| Octanal | 0.7 [37] | 20.45 | 14.41 | |
| Nonanal | 1 [36] | 22.10 | 22.77 | |
| ketones | 1-Hepten-3-one | 0.04 [38] | 19.25 | 4.50 |
| 2,5-Octanedione | 29 [35] | 1.12 | 0.72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, Y.; Zhang, D.; Li, J.; Zhang, L.; Zhao, Y.; Zhao, J.; Zhang, J.; Wu, J. Comparative Analysis of Volatile Organic Compounds in Freshwater-Cultured and Saline–Alkaline Selectively Bred Tilapia Using Electronic Nose, GC-IMS, and HS-SPME-GC-MS. Foods 2025, 14, 3946. https://doi.org/10.3390/foods14223946
Wang Z, Yang Y, Zhang D, Li J, Zhang L, Zhao Y, Zhao J, Zhang J, Wu J. Comparative Analysis of Volatile Organic Compounds in Freshwater-Cultured and Saline–Alkaline Selectively Bred Tilapia Using Electronic Nose, GC-IMS, and HS-SPME-GC-MS. Foods. 2025; 14(22):3946. https://doi.org/10.3390/foods14223946
Chicago/Turabian StyleWang, Zhi, Yi Yang, Dongxue Zhang, Jiashu Li, Longsheng Zhang, Yan Zhao, Jinliang Zhao, Junling Zhang, and Jikui Wu. 2025. "Comparative Analysis of Volatile Organic Compounds in Freshwater-Cultured and Saline–Alkaline Selectively Bred Tilapia Using Electronic Nose, GC-IMS, and HS-SPME-GC-MS" Foods 14, no. 22: 3946. https://doi.org/10.3390/foods14223946
APA StyleWang, Z., Yang, Y., Zhang, D., Li, J., Zhang, L., Zhao, Y., Zhao, J., Zhang, J., & Wu, J. (2025). Comparative Analysis of Volatile Organic Compounds in Freshwater-Cultured and Saline–Alkaline Selectively Bred Tilapia Using Electronic Nose, GC-IMS, and HS-SPME-GC-MS. Foods, 14(22), 3946. https://doi.org/10.3390/foods14223946





