Profiling Neuroactive Compounds in Organic, Conventional, and Processed Tomatoes
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Standard Preparation
2.2. Study Design
2.3. UHPLC-MS-DAD Analysis of Carotenoids
2.3.1. Sample Preparation
2.3.2. Method Optimization
2.4. UHPLC-QTOF-MS Non-Target Analysis
2.4.1. Sample Preparation
2.4.2. Method Optimization
2.4.3. Data Acquisition Quality
2.4.4. Data Processing and Compound Annotation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Neuroactive Compound Profile in Four Types of Tomatoes
3.1.1. Carotenoids
3.1.2. Identification of Neuroactive Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- JPND. Linking Pre-Diagnosis Disturbances of Physiological Systems to Neurodegenerative Diseases. Available online: https://www.neurodegenerationresearch.eu/initiatives/annual-calls-for-proposals/linking-pre-diagnosis-disturbances-of-physiological-systems-to-neurodegenerative-diseases/ (accessed on 13 November 2025).
- Mao, X.Y.; Yin, X.X.; Guan, Q.W.; Xia, Q.X.; Yang, N.; Zhou, H.H.; Liu, Z.Q.; Jin, W.L. Dietary nutrition for neurological disease therapy: Current status and future directions. Pharmacol. Ther. 2021, 226, 107861. [Google Scholar] [CrossRef]
- Ceppa, F.A.; Izzo, L.; Sardelli, L.; Raimondi, I.; Tunesi, M.; Albani, D.; Giordano, C. Human Gut-Microbiota Interaction in Neurodegenerative Disorders and Current Engineered Tools for Its Modeling. Front. Cell. Infect. Microbiol. 2020, 10, 297. [Google Scholar] [CrossRef]
- Di Lecce, G.; Martínez-Huélamo, M.; Tulipani, S.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Setup of a UHPLC-QqQ-MS method for the analysis of phenolic compounds in cherry tomatoes, tomato sauce, and tomato juice. J. Agric. Food Chem. 2013, 61, 8373–8380. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Sina, A.A.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- Mun, H.I.; Kwon, M.C.; Lee, N.-R.; Son, S.Y.; Song, D.H.; Lee, C.H. Comparing Metabolites and Functional Properties of Various Tomatoes Using Mass Spectrometry-Based Metabolomics Approach. Front. Nutr. 2011, 8, 659646. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; García-Alonso, J.; Periago, M.J. Bioactive compounds of tomato: Cancer chemopreventive effects and influence on the transcriptome in hepatocytes. J. Funct. Foods 2018, 42, 271–280. [Google Scholar] [CrossRef]
- Ara, T.; Sakurai, N.; Takahashi, S.; Waki, N.; Suganuma, H.; Aizawa, K.; Matsumura, Y.; Kawada, T.; Shibata, D. TOMATOMET: A metabolome database consists of 7118 accurate mass values detected in mature fruits of 25 tomato cultivars. Plant Direct 2021, 29, e00318. [Google Scholar] [CrossRef]
- Gaouar, Z.L.; Chefirat, B.; Saadi, R.; Djelad, S.; Rezk-Kallah, H. Pesticide residues in tomato crops in Western Algeria. Food Addit. Contam. Part. B Surveill. 2021, 14, 281–286. [Google Scholar] [CrossRef]
- Margenat, A.; Matamoros, V.; Díez, S.; Cañameras, N.; Comas, J.; Bayona, J.M. Occurrence and human health implications of chemical contaminants in vegetables grown in peri-urban agriculture. Environ. Int. 2019, 124, 49–57. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Santos, L. Uptake and translocation of UV-filters and synthetic musk compounds into edible parts of tomato grown in amended soils. Sci. Total Environ. 2021, 792, 148482. [Google Scholar] [CrossRef]
- Araújo, D.F.d.S.; da Silva, A.M.R.B.; Lima, L.L.d.A.; Vasconcelos, M.A.d.S.; Andrade, S.A.C.; Sarubbo, L.A. The concentration of minerals and physicochemical contaminants in conventional and organic vegetables. Food Control 2014, 44, 242–248. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Baker, N.C.; Williams, A.J.; Singh, R.R.; Trezzi, J.-P.; Wilmes, P.; Kolber, P.L.; Kruger, R.; Paczia, N.; Linster, C.L.; et al. Connecting environmental exposure and neurodegeneration using cheminformatics and high resolution mass spectrometry: Potential and challenges. Env. Sci. Process Impacts 2019, 21, 1426–1445. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Caruso, M.; Martens, S. Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res. Int. 2020, 134, 109228. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Carlin, S.; Sicari, V.; Martens, S. Differences in the composition of phenolic compounds, carotenoids, and volatiles between juice and pomace of four citrus fruits from Southern Italy. Eur. Food Res. Technol. 2020, 246, 1991–2005. [Google Scholar] [CrossRef]
- Dumont, D.; Danielato, G.; Chastellier, A.; Oyant, L.H.S.; Fanciullino, A.-L.; Lugan, R. Multi-targeted metabolic profiling of carotenoids, phenolic compounds and primary metabolites in goji (Lycium spp.) berry and tomato (Solanum lycopersicum) reveals inter and intra genus biomarkers. Metabolites 2020, 10, 422. [Google Scholar] [CrossRef]
- Yurekten, O.; Payne, T.; Tejera, N.; Amaladoss, F.X.; Martin, C.; Williams, M.; O’donovan, C. MetaboLights: Open data repository for metabolomics. Nucleic Acids Res. 2023, 52, D640–D646. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R.; Ebbels, T.M.D.; Beger, R.D.; Ekman, D.R.; Epps, D.J.T.; Kamp, H.; Leonards, P.E.G.; Loizou, G.D.; MacRae, J.I.; van Ravenzwaay, B.; et al. Use cases, best practice and reporting standards for metabolomics in regulatory toxicology. Nat. Commun. 2019, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Marković, K.; Krbavčić, I.; Krpan, M.; Bicanic, D.; Vahčić, N. The lycopene content in pulp and peel of five fresh tomato cultivars. Acta Aliment. 2010, 39, 90–98. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Gas-Pascual, E.; Orchard, C.J.; Sari, E.N.; Riedl, K.M.; Schwartz, S.J.; Francis, D.M.; Cooperstone, J.L. Analysis of tomato carotenoids: Comparing extraction and chromatographic methods. J. AOAC Int. 2019, 102, 1069–1079. [Google Scholar] [CrossRef]
- Maldonado-Reina, A.J.; López-Ruiz, R.; Sáez, J.M.; Romero-González, R.; Frenich, A.G. Tracing the dissipation of difenoconazole, its metabolites and co-formulants in tomato: A comprehensive analysis by chromatography coupled to high resolution mass spectrometry in laboratory and greenhouse trials. Environ. Pollut. 2024, 349, 123924. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Lamuela-Raventós, R.M. Evaluation of a method to characterize the phenolic profile of organic and conventional tomatoes. J. Agric. Food Chem. 2012, 60, 3373–3380. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventos, R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012, 130, 222–227. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Teleky, B.-E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Evaluation of the Bioactive Compounds Found in Tomato Seed Oil and Tomato Peels Influenced by Industrial Heat Treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.P.P.; Gómez, H.A.G.; Junior, S.S.; Maraschin, M.; Tecchio, M.A.; Borges, C.V. Functional and Nutraceutical Compounds of Tomatoes as Affected by Agronomic Practices, Postharvest Management, and Processing Methods: A Mini Review. Front. Nutr. 2022, 9, 868492. [Google Scholar] [CrossRef] [PubMed]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of Vitamin C, Total Phenolics, and Antioxidant Capacity as Affected by Processing Tomatoes to Different Products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Sohel, M.; Haidary, M.M.H.; Mostaq, M.S.; Akter, S.; Nahar, A.; Labony, F.Z.; Ahmed, A.; Hasan, M.S.; Babu, M.H.; et al. Therapeutic potential of clinically proven natural products in the management of dementia. Heliyon 2024, 10, e27233. [Google Scholar] [CrossRef]
- Ośko, J.; Nasierowska, K.; Grembecka, M. Application of In Vitro Digestion Models in the Evaluation of Dietary Supplements. Foods 2024, 13, 2135. [Google Scholar] [CrossRef]
- de Bie, T.H.; Balvers, M.G.J.; de Vos, R.C.H.; Witkamp, R.F.; Jongsma, M.A. The influence of a tomato food matrix on the bioavailability and plasma kinetics of oral gamma-aminobutyric acid (GABA) and its precursor glutamate in healthy men. Food Funct. 2022, 13, 8399–8410. [Google Scholar] [CrossRef]
- Craggs, M.; Gibson, G.R.; Whalley, P.; Collins, C.D. Bioaccessibility of Difenoconazole in Rice following Industry Standard Processing and Preparation Procedures. J. Agric. Food Chem. 2020, 68, 10167–10173. [Google Scholar] [CrossRef]
- Shaher, S.A.A.; Mihailescu, D.F.; Amuzescu, B. Aspartame Safety as a Food Sweetener and Related Health Hazards. Nutrients 2023, 15, 3627. [Google Scholar] [CrossRef] [PubMed]
- Flores-Gutierrez, C.A.; Torres-Sanchez, E.D.; Reyes-Uribe, E.; Torres-Jasso, J.H.; Reyna-Villela, M.Z.; Rojas-Bravo, D.; Salazar-Flores, J. The Association between Pesticide Exposure and the Development of Fronto-Temporal Dementia-Cum-Dissociative Disorders: A Review. Brain Sci. 2023, 13, 1194. [Google Scholar] [CrossRef] [PubMed]
- Winter, B. Linear Models and Linear Mixed Effects Models in R with Linguistic Applications. arXiv 2013. [Google Scholar] [CrossRef]
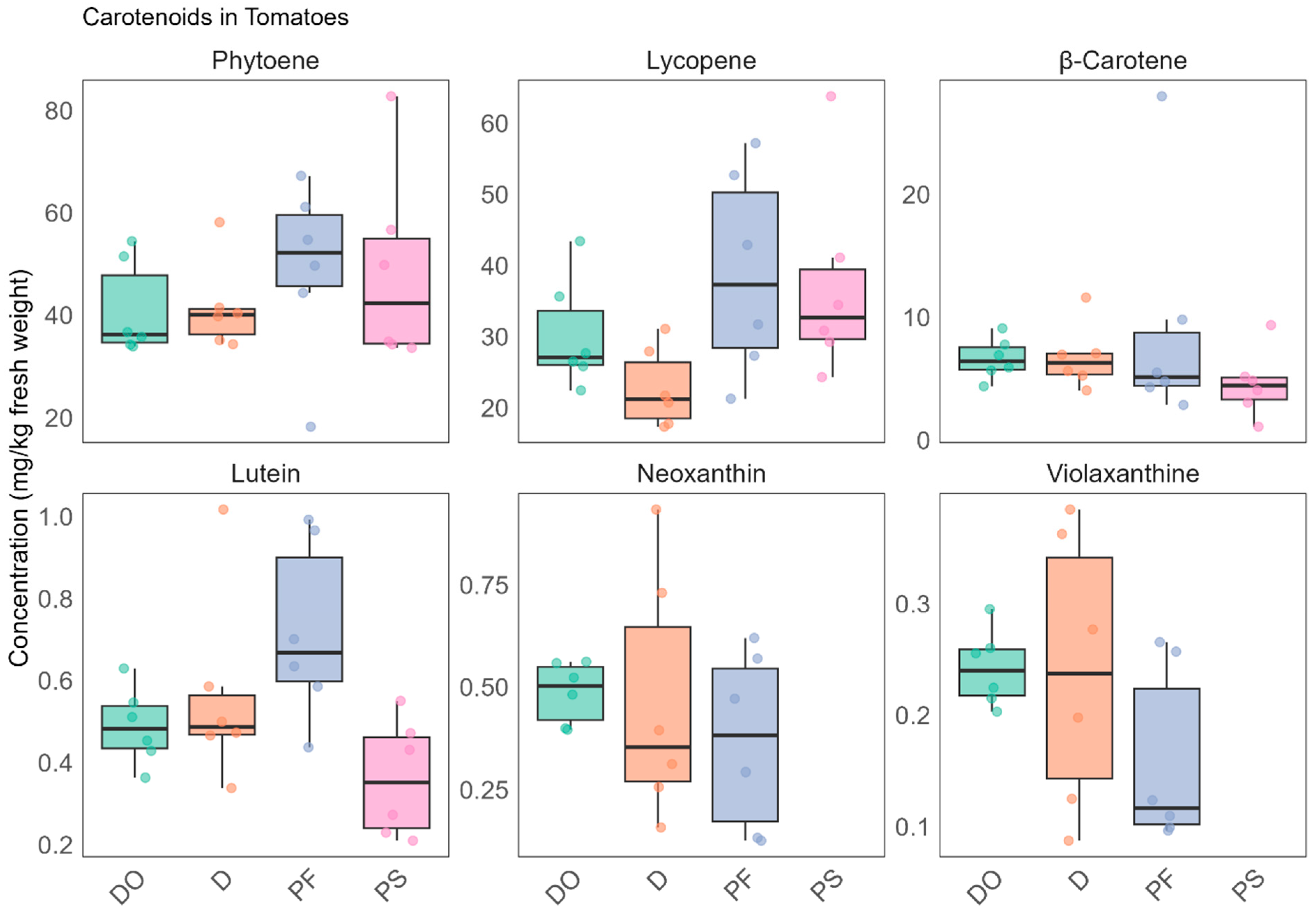
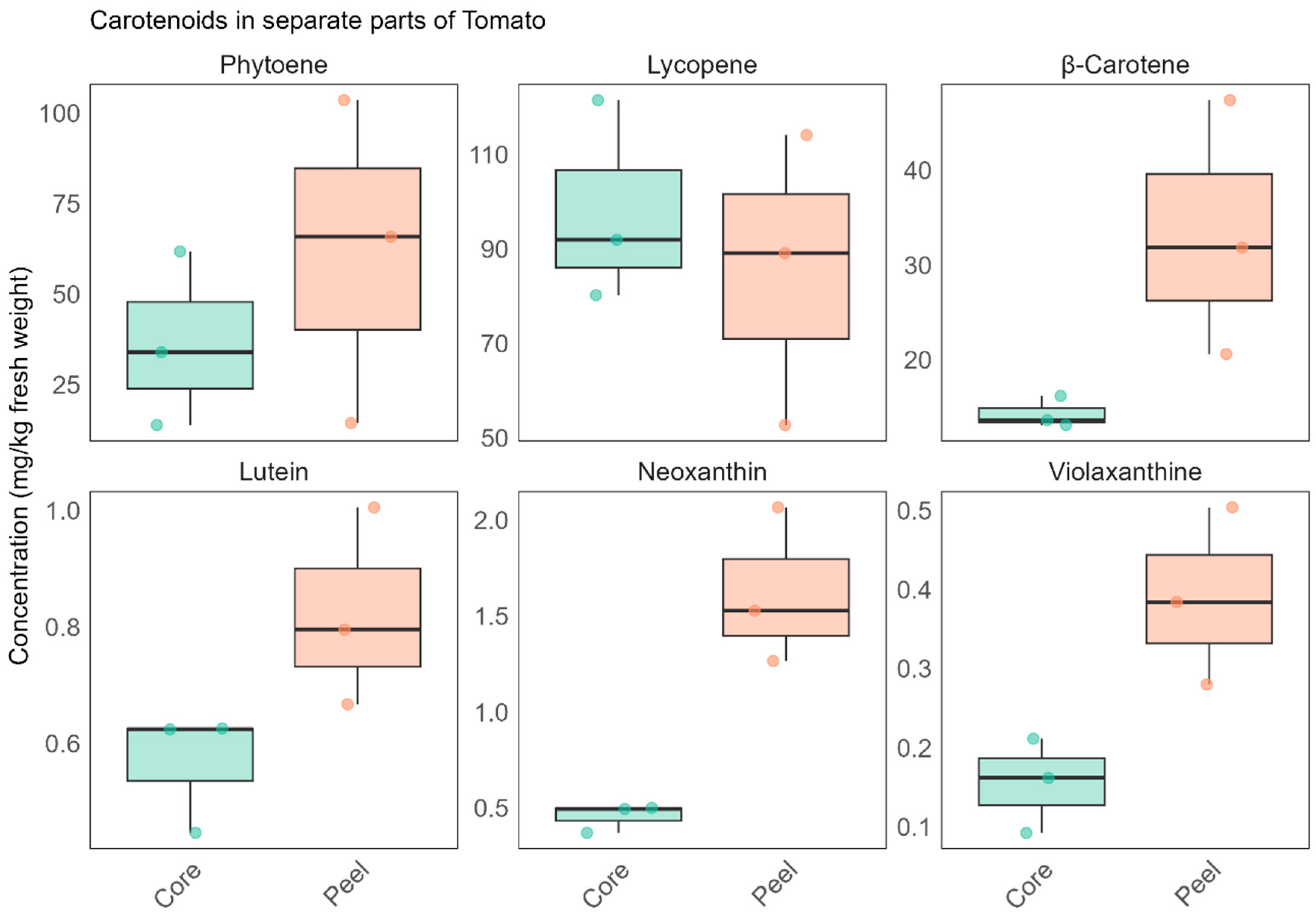
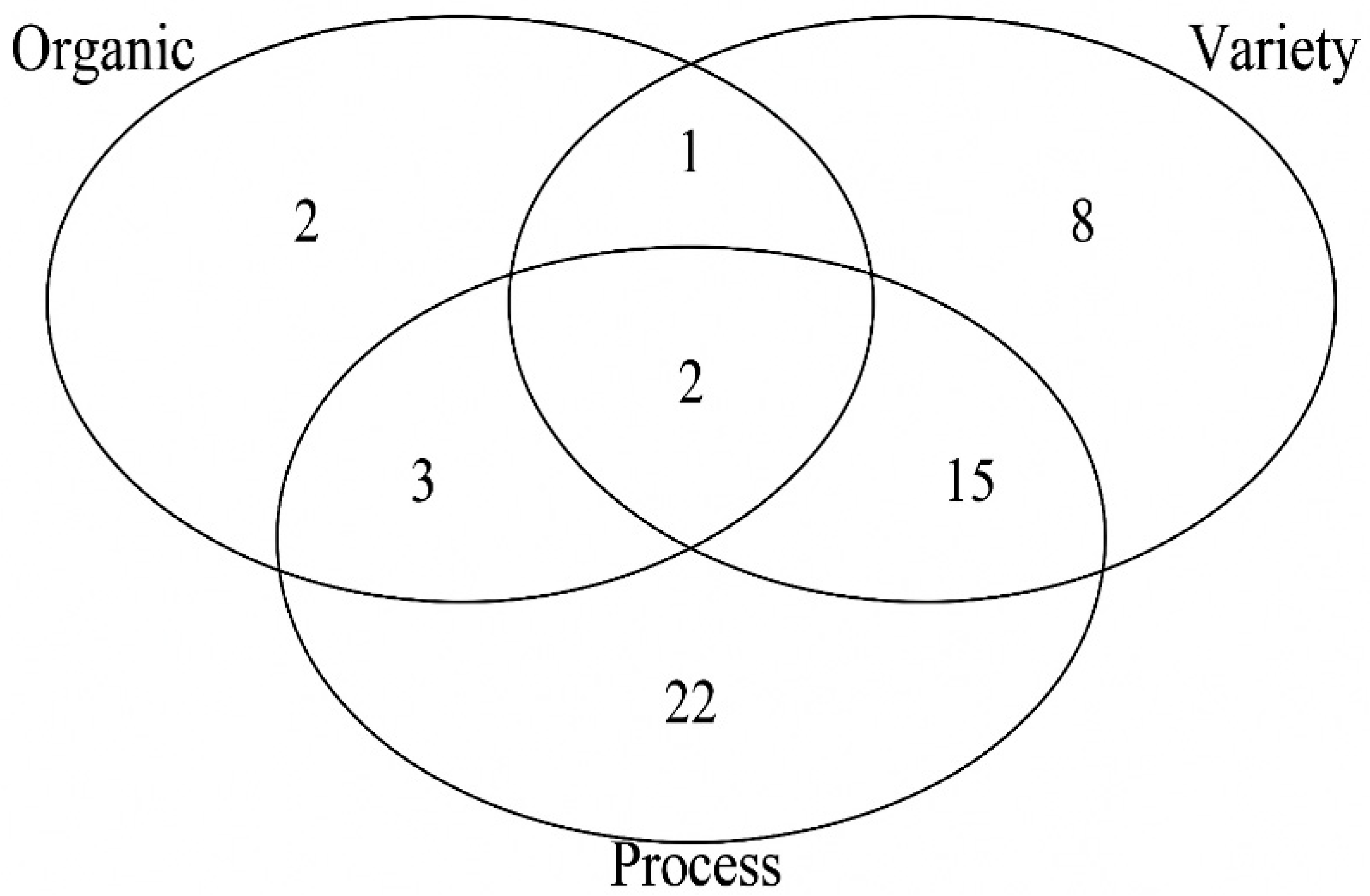

| Neuro-Activity | Class | Compound | m/z | RT [min] | Ion Adduct | Level | Organic | Variety | Process |
|---|---|---|---|---|---|---|---|---|---|
| disrupting | Food additive | Amino(nitrophenyl)methylsulfonyl-propanoic acid | 289.052 | 3.25 | [M + H]+ | L3 | = | + | + |
| Aspartame (I) | 295.1154 | 6.2 | [M + H]+ | L1 | + | − | − | ||
| Aspartame (II) | 295.1297 | 5.43 | [M + H]+ | L2 | = | = | = | ||
| Hydroquinone | 109.0296 | 3.97 | [M − H]− | L2 | = | − | + | ||
| Industrial chemicals | 1-Naphthylamine | 144.0812 | 4.56 | [M + H]+ | L2 | = | = | = | |
| Derivative of acetaminophen | 152.0709 | 1.58 | [M + H]+ | L3 | = | = | − | ||
| Difenoconazole | 406.0727 | 13.41 | [M + H]+ | L1 | − | = | = | ||
| Methylsulfonyl(pyridin-ylmethyl)piperidinyl-pyridin | 332.1436 | 12.07 | [M + H]+ | L3 | = | = | = | ||
| protective | Amino Acids and Derivatives | Glutamine | 147.0773 | 0.87 | [M + H]+ | L2 | = | − | − |
| Glutamylphenylalanine | 295.1299 | 3.70 | [M + H]+ | L1 | = | = | = | ||
| Guanine | 152.0709 | 1.58 | [M + H]+ | L1 | = | = | = | ||
| Leucine-leucine (I) | 245.1846 | 5.96 | [M + H]+ | L1 | − | = | + | ||
| Leucine-leucine (II) | 245.1867 | 5.91 | [M + H]+ | L1 | − | = | + | ||
| Leucylvaline | 231.1711 | 4.96 | [M + H]+ | L1 | = | = | + | ||
| N-acetyltryptophan | 245.0922 | 6.88 | [M − H]− | L2 | = | = | = | ||
| phenylalanine | 166.0861 | 3.4 | [M − H]− | L2 | = | = | = | ||
| Tryptophan | 205.0976 | 4.51 | [M − H]− | L2 | + | = | + | ||
| Tyramine | 138.0902 | 1.95 | [M − H]− | L2 | = | + | = | ||
| Fatty Acids and Lipids | 10,16-Dihydroxypalmitic acid | 287.2223 | 12.04 | [M − H]− | L2 | = | = | + | |
| 2-Isopropylmalic acid | 175.0609 | 5.42 | [M − H]− | L2 | = | = | = | ||
| Elaidic/Oleic acid | 281.2471 | 16.57 | [M − H]− | L3 | = | = | + | ||
| Hydroxyadipic acid | 161.0453 | 3.6 | [M − H]− | L1 | = | = | − | ||
| Linoleic acid | 279.2332 | 16.1 | [M − H]− | L2 | = | = | + | ||
| Neurotransmitters | Adenosine | 268.1041 | 2.59 | [M + H]+ | L1 | = | − | + | |
| Dopa | 198.0744 | 2.01 | [M + H]+ | L1 | = | = | = | ||
| Glutamic acid | 148.0614 | 0.87 | [M + H]+ | L1 | = | = | + | ||
| Serotonin | 177.1027 | 2.74 | [M + H]+ | L1 | = | + | − | ||
| Tryptamine | 161.1080 | 4.60 | [M + H]+ | L2 | = | + | = | ||
| Tyrosine | 182.0811 | 1.91 | [M + H]+ | L1 | = | = | = | ||
| Nucleotides and Nucleosides | Adenine | 136.0623 | 1.16 | [M + H]+ | L2 | = | + | + | |
| Adenosine monophosphate | 346.0540 | 1.32 | [M + H]+ | L2 | = | + | = | ||
| Guanosine 5′-monophosphate (I) | 362.0507 | 1.23 | [M − H]− | L2 | − | + | = | ||
| Guanosine 5′-monophosphate (II) | 362.0507 | 1.69 | [M − H]− | L2 | = | + | + | ||
| Uridine 5′-monophosphate | 323.0289 | 1.14 | [M − H]− | L2 | = | = | + | ||
| Phenolic Compound/Polyphenols and Derivatives | 5-caffeoylshikimic acid | 335.0783 | 6.36 | [M + H]+ | L2 | = | = | = | |
| Ascorbic Acid | 191.0191 | 1.30 | [M + H]+ | L2 | = | = | = | ||
| Caffeic acid | 179.0352 | 5.74 | [M − H]− | L1 | = | − | = | ||
| Chlorogenic Acid | 353.0878 | 5.27 | [M − H]− | L1 | = | + | = | ||
| Citric acid | 191.0189 | 1.31 | [M − H]− | L1 | = | + | + | ||
| Crepenynic acid | 277.2194 | 15.71 | [M − H]− | L2 | = | = | + | ||
| Cryptochlorogenic acid | 353.0886 | 5.34 | [M − H]− | L1 | = | + | = | ||
| Dihydrocoumarin | 147.0441 | 3.41 | [M − H]− | L2 | = | = | = | ||
| Eriodictyol (I) | 287.0562 | 8.68 | [M − H]− | L2 | = | = | + | ||
| Eriodictyol (II) | 287.0564 | 9.04 | [M − H]− | L2 | = | = | − | ||
| Eriodictyol (III) | 287.0550 | 6.99 | [M − H]− | L3 | = | + | + | ||
| Ferulic acid | 193.0500 | 5.14 | [M − H]− | L1 | = | − | − | ||
| Feruloyltyramine | 341.1390 | 8.72 | [M − H]− | L1 | = | = | + | ||
| Hesperetin | 301.0725 | 9.66 | [M − H]− | L1 | = | = | = | ||
| Homoeriodictyol | 301.0726 | 9.97 | [M − H]− | L1 | = | = | − | ||
| Hydroxycinnamic acid | 163.0399 | 2.02 | [M − H]− | L1 | = | − | − | ||
| Isoquercetin | 463.1240 | 8.06 | [M − H]− | L2 | = | + | + | ||
| Malonyltryptophan | 289.0828 | 6.90 | [M − H]− | L1 | = | = | = | ||
| Naringenin | 271.0607 | 9.58 | [M − H]− | L1 | = | = | − | ||
| Naringenin chalcone | 271.0639 | 9.51 | [M − H]− | L2 | = | = | + | ||
| Naringin | 581.1870 | 7.67 | [M − H]− | L2 | = | = | + | ||
| Neochlorogenic Acid | 353.0874 | 4.22 | [M − H]− | L1 | = | + | + | ||
| Phenyllactic acid | 165.0551 | 6.96 | [M − H]− | L1 | = | = | + | ||
| Quercetin | 301.0363 | 9.58 | [M − H]− | L2 | = | = | + | ||
| Quinic Acid | 191.0553 | 1.0 | [M − H]− | L2 | = | + | = | ||
| Resveratrol | 227.0714 | 7.0 | [M − H]− | L3 | = | = | − | ||
| Rutin | 609.1462 | 7.99 | [M − H]− | L1 | = | + | + | ||
| Plant Alkaloids and Secondary Metabolites | 5′-Deoxy-5′-(methylthio)adenosine | 298.0973 | 4.73 | [M + H]+ | L3 | + | − | + | |
| N-Caffeoyl putrescin | 251.1392 | 4.02 | [M + H]+ | L3 | = | + | + | ||
| N-Feruloylputrescine | 265.1548 | 5.10 | [M + H]+ | L2 | = | = | = | ||
| Sibricose A3 | 461.1302 | 3.73 | [M + H]+ | L2 | = | − | = | ||
| Tomatidinol (I) | 414.3376 | 8.69 | [M + H]+ | L1 | = | = | + | ||
| Tomatidinol (II) | 414.3376 | 8.86 | [M + H]+ | L2 | = | = | + | ||
| Tomatine | 1034.5546 | 10.14 | [M − H]− | L2 | = | = | + | ||
| Vitamins and Coenzymes | 4-Pyridoxic acid | 182.0455 | 2.72 | [M − H]− | L1 | = | = | = | |
| Pantothenic Acid | 220.1185 | 4.23 | [M + H]+ | L1 | + | = | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačič, A.; Garcia-Aloy, M.; Masuero, D.; Sáez, V.; Brus, A.; Franceschi, P.; Vrhovšek, U. Profiling Neuroactive Compounds in Organic, Conventional, and Processed Tomatoes. Foods 2025, 14, 3927. https://doi.org/10.3390/foods14223927
Kovačič A, Garcia-Aloy M, Masuero D, Sáez V, Brus A, Franceschi P, Vrhovšek U. Profiling Neuroactive Compounds in Organic, Conventional, and Processed Tomatoes. Foods. 2025; 14(22):3927. https://doi.org/10.3390/foods14223927
Chicago/Turabian StyleKovačič, Ana, Mar Garcia-Aloy, Domenico Masuero, Vania Sáez, Anže Brus, Pietro Franceschi, and Urška Vrhovšek. 2025. "Profiling Neuroactive Compounds in Organic, Conventional, and Processed Tomatoes" Foods 14, no. 22: 3927. https://doi.org/10.3390/foods14223927
APA StyleKovačič, A., Garcia-Aloy, M., Masuero, D., Sáez, V., Brus, A., Franceschi, P., & Vrhovšek, U. (2025). Profiling Neuroactive Compounds in Organic, Conventional, and Processed Tomatoes. Foods, 14(22), 3927. https://doi.org/10.3390/foods14223927







