Abstract
In this study, one of the main problems related to the development of new foods and the improvement of existing staple foods is examined. The effect of stalk pigweed flour in relation to the main raw material, wheat flour, at levels of 0%, 5%, 10%, and 15% was evaluated with respect to key characteristics of flour mixtures, dough, and biscuits with this additive. A selection of informative features was made, revealing that out of the 39 studied parameters, covering sensory, physicochemical, geometric, colorimetric, and spectral characteristics, only 19 proved to be informative. Principal component analysis showed that the relationship between amaranth green powder (AGP) concentration and the first two principal components explained up to 99% of the variance. The optimal addition level of 7.17% AGP was identified based on the convergence of biscuit characteristics. pH decreased from 6.55 to 6.28, electrical conductivity increased from 1075 to 3759 µS/cm, and sensory scores for aroma and taste peaked near 7% before declining at higher concentrations. It was demonstrated that the relationship between the amount of amaranth in biscuits and the first two principal components can be described with up to 99% accuracy. It was determined that the optimal amount of amaranth flour in biscuits is +7.17%. The results obtained provide a basis for further research into the rapid automated analysis of biscuits with added pigweed flour, which will contribute to the development of new foods with improved characteristics. It is suggested to carry out more research to study the effect of flours from other amaranth types, enhancing different varieties and cultivated in diverse ecological regions. This work also explores the viability of pigweed as a nutritious and sustainable flour alternative while providing a multivariate approach in view of newly developed bakery formulations.
1. Introduction
The quest for new sources of nutritional and bioactive substances has increased in recent decades [1]. Pseudocereals, like amaranths, are a good alternative to traditional cereal crops for their potential in the development of gluten-free foods and/or those with a low glycemic index [1,2]. They are a good complement to other grain crops in arid and semi-arid areas that do not thrive well [3,4]. Amaranths are highly adaptable to a wide range of ecological conditions due to a set of versatile physiological traits, genetic diversity, and phenotypic plasticity. Still, in many cases they are considered underutilized [5]. In countries where amaranths are grown for their grain, the stems are usually considered as agricultural waste; however, they are rich in fibers, microelements, and biologically active substances [6]. In the face of global climate changes, finding new and diverse ways to tap these resources would contribute to green transition efforts [7].
The genus Amaranthus L. (Amaranthaceae) comprises 70 widely distributed annual and perennial species [8]. Amaranth seeds and green biomass are a part of the traditional cuisines of Northern America, the Indian subcontinent, Africa, and some European countries [9,10,11]. Amaranthus hypochondriacus (L.) B.L.Rob., A. cruentus L., and A. caudatus L. are cultivated for their seeds (known as grain amaranths) [10,12,13,14,15], while A. hybridus L., A. tricolor L., A. viridis L., A. blitum L., A. dubius Mart., A. graecizans L., and A. retroflexus L. are known as leafy vegetables [11,16,17]. Amaranthus caudatus L., A. hybridus L., and A. tricolor L. are also decorative and A. retroflexus L. and A. albus L. are applied in some traditional medicine systems [18,19,20].
The inclusion of fruit and vegetables in bakery goods, especially raw ingredients that are potentially destined as agricultural waste, is recognized as a valuable source of functional dietary fibers and phenolic compounds [21]. Blended fresh and dry leafy vegetables were successfully used as fortification agents in various bakery products, improving mineral content and fatty acid and amino acid profiles, as well as contributing as antioxidant agents [22]. Amaranths were reported to contain valuable nutritional and mineral compounds, some essential amino acids, carotenoids, antioxidants (e.g., hydroxycinnamic acid), etc. The works of [22,23,24] presented a wide survey of nutritional and therapeutic features of Amaranthus spp. and their potential as ingredients in various food products, among which are baked foods and biscuits. Amaranth seeds and related products (flours, coarse blends, etc.) are well-studied and common ingredients of various gluten-free foods [6,25]. On the other hand, the potential of the remaining parts of the plant—roots, leaves, and herbage—is less studied as a functional additive in the food industry. Amaranths are known as traditional leafy vegetables worldwide, but they are more seldom used as ingredients for bakery goods [26,27]. As seeds were the primary use of these crops in their area of origin, the Americas, some authors even consider the secondary use of amaranths as vegetables to be related to their accidental distribution as weeds and the ignorance of local communities towards usage of the seeds [28].
The whole above-ground part of the amaranth plant could be used as an additive to basic foods like bread, cakes, biscuits, porridge, and pasta as a way for efficient utilization of green biomass from agriculture [29,30]. Amaranth greens, usually young leaves, are cooked like spinach in stews or soups and added to salads or traditional pastries [11,31,32]. Pastries with amaranth greens in Bulgaria were part of Lenten fare, using young plants as filling or directly as part of the filo dough [11,31]. Still, consumption of wild amaranth greens in Europe was mostly in remote areas with poor soils and as emergency/wartime food when organoleptic qualities were least prioritized [31].
As species availability and local preferences towards the developmental stage of the plants vary, raw amaranth collected from the wild and/or available on the market could have very diverse nutritional and gustatory properties. Biscuits made with red morphs of A. tricolor leaves were reported to have significantly more protein and fibers than the control [30]. Compared to spinach pasta with added A. caudatus, leaf powder had insignificantly higher content of fats and protein [33]. Same authors report on similar sensory ratings for both spinach and amaranth, with only half of the panelists recognizing amaranth as an ingredient. Green leaf powder of A. cruentus added to corn snacks resulted in elevated protein and fiber content, and hence harder products, which was negatively perceived by sensory panelists, especially in cases of higher percentages of amaranth leaf powder (3%) [34]. Hiscock et al. [35] reported that even boiled leaves of genotypes in the same Amaranthus species were ranked with both similar and contradictory sensory properties [35]. Same authors outline that panelists most frequently describe the samples as “leafy”, followed by “spinach”-like, “soft”, and “tasteless”, which could be improved with the addition of other vegetables. When incorporated in noodles, amaranth leaf powder (from botanical unspecified market origin) was found to significantly increase the fat content whilst reducing the amounts of total glycemic carbohydrates [36], with the taste described as “leafy” also [37]. Salty muffins with up to 6% amaranth leaf powder had no statistically significant difference regarding the sensory parameters to the control group [38]. Conversely, the addition of amaranth leaf powder (7% of the dough) was shown to significantly increase total phenolic content but received the lowest sensory rates compared to the control and other vegetable breads [39]. The aim of the current study is to assess the qualities and organoleptic characteristics of salty butter biscuits with different amounts of amaranth green powder (AGP) prepared from different wild Amaranthus species growing in Bulgaria and to establish the optimal quantity to be included in biscuits in order to improve their nutritional value whilst preserving acceptable sensorial and physical characteristics.
Unlike most studies that focus on amaranth seeds or cultivated varieties, this research utilizes the whole above-ground biomass of wild Amaranthus species, typically considered agricultural waste, and applies a multifactorial evaluation approach combining physico-chemical, geometric, colorimetric, spectral, and sensory analyses to determine optimal flour substitution levels in biscuits.
2. Materials and Methods
2.1. Plant Material
The AGP contains equal parts from five species: Amaranthus albus, A. blitum, A. deflexus, A. hybridus, and A. retroflexus. These are widely distributed in Bulgaria and plants were collected from 29 wild populations from six floristic regions: Central Stara Planina Mt., Thracian lowlands, West Frontier Mts., Strandzha Mt., Tundzha Hilly plain, and West Rhodopi Mt. The collected plant material (whole herbage) was sorted, inspected for damages, washed under running water, and then dried at room temperature with natural ventilation under shade conditions. After drying, the herbage was turned into powder with a high-speed multifunctional blender model BN1200AL (Gorenje PLC, Velenje, Slovenia). The powder samples were stored in paper bags in dark and aerated place at 16–22 °C.
2.2. Biscuit Formulation and Methodology of Preparation
The following ingredients were used for the preparation of the biscuits: type 500 all-purpose white flour (Topaz Mel Ltd., Sofia, Bulgaria), iodized salt, 82% cow butter (Milky group Ltd., Haskovo, Bulgaria), and hen egg yolks obtained according to Ordinance 1/2008 on the requirements for the marketing of table eggs. For the experiment, only the quantities of the flour and the AGP were changed; all other ingredients were constant (Table 1).

Table 1.
Ingredients of the salty butter biscuits with amaranth green powder.
The stages of preparation for the salty butter biscuits with added AGP are given in Figure 1. Some of them are conducted at room temperature; refrigeration was used so as to avoid melting the butter. The total technological time to prepare the butter biscuits is 2.5–3 h.
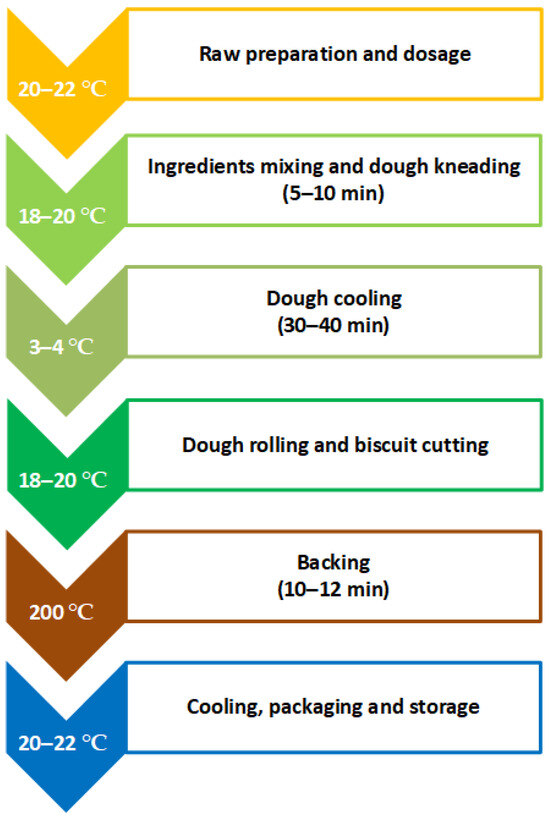
Figure 1.
Technology for preparing salty butter biscuits with added AGP.
2.3. Testing of Physico-Chemical Parameters
2.3.1. Measurement of pH, Electrical Conductivity (EC), Total Dissolved Solids (TDS), and Oxidation-Reduction Potential (ORP)
The protocol described in the standard method, AACC 02-52.01 [40], was applied. Briefly, a 5 g sample was added to 50 mL distilled water at 70 °C. The mixture was homogenized by stirring and left to cool down to room temperature. Three independent measurements of every parameter were conducted and the mean and standard deviation were calculated.
The quantities of the samples were measured with Pocket Scale MH-200 (Zhezhong Weighing Apparatus Factory, Yongkang City, China). pH; EC, µS/cm; TDS, ppm was measured with a multifunctional device PH-TDS-EC-TEMP Meter (Nanjing Tsung Water Technology Company Ltd., Nanjing, China).
The protocol described in AACC Method 02-52.01 [40] was used for sample preparation. The oxidation–reduction potential (ORP, mV) of the aqueous extract was then measured potentiometrically using a platinum electrode with an Ag/AgCl reference electrode Model ORP-2069 (Shanghai Longway Optical Instruments Co., Ltd., Shanghai, China).
2.3.2. Temperature Measurement (T)
T, °C was measured with a digital thermometer V&A VA6502 (Shanghai Yihua V&A Instrument Co., Shanghai, China).
2.3.3. Determination of Loss After Baking
After baking, the biscuits were cooled down to room temperature and after that they were weighed on a technical scale Boeco BBL-64 (Boeckel GmbH, Hamburg, Germany), with a maximum capacity of 300 g and a resolution of 0.1 g. The losses were calculated with the following formula:
where TL, % are thermal losses; a is the weight before baking, g; b—the weight after baking, g.
2.3.4. Determination of the Spread Factor After Baking
To determine the spread factor (SF), the diameter (D, mm) and height (h, mm) were measured after baking. The diameter and height were measured in three different positions and the mean values were calculated. A digital caliper model SEB-DC-023 (Shanghai Shangerbo import & export Co., Ltd., Shanghai, China) was used, with a maximal length measurement of 150 mm and a resolution of 0.05 mm (Shanghai Shangerbo import & export Co., Ltd., Shanghai, China). Spread factor was determined with the following formula:
where D is the diameter of the biscuit after baking, mm; h—height of the biscuit after baking, mm. The obtained value has no dimension.
2.4. Organoleptic Analysis
The organoleptic analysis of the biscuits was performed according to the Bulgarian State Standard EN ISO 13299:2016 (Sensorial analysis. Methodology. General manual for establishing sensorial profile) [41].
The sensory panel consisted of nine evaluators (five academic staff and four graduate students) from the Food Technologies Department, selected based on their prior training in sensory evaluation and familiarity with biscuit formulations. The tasting was conducted in a controlled environment using individual booths under standardized daylight-balanced lighting (D65 equivalent) to minimize visual bias. Samples were coded with randomized three-digit numbers and presented in a randomized order to avoid positional effects. Panelists were instructed to cleanse their palates with water between samples to ensure consistent evaluation across all biscuit variants.
The requirements for the code of ethics of the university community were met (http://uni-sz.bg/truni5, accessed on 25 June 2022, in Bulgarian).
The butter biscuits examined in this study are confectionery products compliant with the Bulgarian national standard BNS 441:1987 (Biscuits. General requirements) [42], which defines biscuits as products suitable for human consumption. The pigweed (Amaranthus spp.) used as an ingredient is also recognized as food-grade according to Bulgarian Regulation No. 5 of 3 September 2018 on organic production, labeling, and control of plant products. Sampling and organoleptic analyses were conducted in accordance with Bulgarian Regulation No. 2 of 27 March 2024 and Regulation No. 12 of 10 November 2021, which govern food sampling and laboratory testing and do not require ethical approval when no human or psychological factors are involved. Organoleptic evaluation followed the international standard BDS EN ISO 13299:2016, which specifies sensory analysis methods for food and does not involve the collection of personal data or medical information from participants.
A 5-point Likert scale was used to evaluate the biscuits (1—completely does not correspond to the indicator; 5—completely corresponds to the indicator). Finally, an overall average score of the organoleptic characteristics of the biscuits was calculated.
2.5. Color Analysis
The color digital images were acquired with the video sensor of a mobile phone LG L70 (LG Electronics, Inc., Seoul, Republic of Korea). The sensor is VB6955CM (STMicroelectronics International N.V., Geneva, Switzerland), resolution 2600 × 1952 pix and 1.4 × 1.4 μm pixel.
Color digital images were obtained in RGB color model, converted to Lab color model according to CIE Lab 1976. Color component conversion functions at observer 2o and illumination D65 were used.
The color difference ΔE of the biscuits with added amaranth green powder was determined. It varied in the range 0–100; biscuits with color close to 0 were closer to those of the control sample, while those that were closer to 100 differed from the control sample. Color difference below 20 was difficult to distinguish with the naked eye.
Lc, ac, bc were color components of the control sample; La, aa, ba—components of the sample with the additive.
The following color indices were calculated: C1 indicates the brightness of the brown color. Brown nuances normally have moderate-to-low L value, slightly positive a value and moderately high b value; C2 indicates the darkness of the brown color with special reference to lower brightness; C3 indicates the yellow-brown nuances with specific reference on the yellow component but preserving the brown nuance as well; C4 indicates the density of the brown color and refers to very dark brown nuances.
The color indices have the following formulae:
Spectral characteristics were obtained. The conversion of the values from XYZ and LMS models to reflectance spectra in the VIS region, in the 390–730 nm range, was performed using mathematical formulas presented by Vilaseca et al. [43]. These formulas were for observer 2o and illumination D65.
The following spectral indices were calculated: S1 emphasizes the balance between red and green, which can be useful in distinguishing between reddish-brown and greenish-yellow colors; S2 measures the steepness of the spectral curve between two wavelengths. It shows the dominance of certain color transitions useful for analyzing color changes in the spectrum; S3 measures the depth of a reflection feature useful for identifying colors with strong absorption in specific spectral regions.
The spectral indices were determined at two wavelengths, 530 and 630 nm, applying the following formulas:
2.6. Statistical Analysis
The data were statistically processed with Matlab 2017b (The Mathworks Inc., Natick, MA, USA). Preparation and visualization of data were performed with MS Office 2016 (Microsoft Corp., Washington, DC, USA).
All measurements were made in three independent repeats and the means were used in the tables and graphs. The statistical functions of standard deviation and maximum allowable error were used. All data were processed at an accepted significance level of α < 0.05.
One-way ANOVA was used to analyze the data. A post hoc LSD test was also performed to determine the degree of significance of differences between average values after statistically significant differences (p < 0.05) were found. The non-parametric Kruskal–Wallis test was used when there was no normal distribution.
2.6.1. Formation of Feature Vectors
Feature vectors that describe the flour mixtures (F1–F4), the dough (F5–F16), and the biscuits (F17–F39) were established. The features and their meaning are given in Table 2.

Table 2.
Features and their meanings.
2.6.2. Selection of the Informative Features
For the selection of the informative features, the RReliefF method was used. The ReliefF method registers relations between the features and is noise resilient. The features with a weight coefficient above 0.6 are considered as informative [44]. A vector was defined from the selected features. In this context, informative variables are those features that provide the most relevant and discriminative information for distinguishing among the analyzed samples, as identified by their high ReliefF weights.
2.6.3. Principal Component Analysis (PCA)
The data from the feature vector were reduced using the PCA method [45]. It is a statistical method to reduce the dimensionality of data. A large set of data can be processed and transformed into a smaller set of variables, the so-called principal components, each of which is a linear combination of the original variables arranged according to the depression size derived from the data.
Possible combinations between % additive and different informative features are given in Table 3. Such analysis was performed for all cases of added green amaranth powder (from 0 to 15%), and only the selected informative features were used.

Table 3.
Feature/%AWF combinations table.
A regression model more frequently used for analysis of food products was applied [46]. It describes the relation between independent and dependent variables according to the following formula:
z is the dependent variable, while x and y are the independent variables; b refers to the model coefficients.
For the assessment of the model, the determination coefficients (R2), model coefficients, their standard error (SE), p-values, and Fisher criterion (F) were used. Analysis of the residues was performed.
To define the correct quantity of the amaranth green powder additive a linear programming algorithm was applied using the function “linprog” in Matlab 2017b (The Mathworks Inc., Natick, MA, USA) Software System. Linear programming involved finding the solution for a vector x so that the linear function fTx, with linear constraints
fulfills one of the following conditions:
where f, x, b, beq, l, and u are vectors, and A and Aeq are matrices.
An “Interior-point-legacy” function was used. The function arrives at an appropriate solution by traversing the inside of the data area.
3. Results
3.1. Composition of the Amaranth Green Powder
The main characteristics of the green powder of the used amaranth species are presented in Table 4.

Table 4.
Main characteristics of the green powder from the used amaranth species.
3.2. Analysis of the Amaranth Green Powder and the Mixtures of Wheat Flour and AGP
The results showed that the AGP mixture had a slightly acidic pH, high TDS and EC values, and low ORP values (Table 5). The data suggest a high mineral content of AGP, which may have an impact on both oxidative stability and nutritional value when incorporated into butter biscuits.

Table 5.
Main physico-chemical characteristics of the amaranth green powder and flour mixtures.
Statistically significant differences were registered between the different flour–AGP mixtures (from 0% to 15%). (Table 5) The increase in the AGP content caused a slight decrease in the pH value (from 6.55 to 6.28); hence, the acidity of the mixture slightly raised.
With an increase in the amount of the additive, the concentration of dissolved ions and minerals in the flour also rises, as evidenced by significant increases in both TDS and EC. TDS increases from 627 ppm at 0% additive to 2523 ppm at 15%, and EC rises from 1075 µS/cm to 3759 µS/cm over the same range, reflecting an improvement in ionic strength and mineral content. Furthermore, ORP also increases from 65 mV to 78 mV, indicating that higher additive concentrations lead to a shift toward a more oxidative environment.
The results indicate that the additive strongly alters the physicochemical properties of the flour, making it more acidic, more mineral-rich, and more oxidatively active, which could, in turn, affect its behavior during baking as well as its nutritional value.
3.3. Analysis of Dough
The physicochemical properties of biscuit dough containing different additive levels (0%, 5%, 10%, and 15%) are summarized in Table 6. The pH remained largely consistent across all formulations (6.24–6.35), indicating near-neutral conditions with slight acidity. From Table 6 it is evident that both TDS and EC increased with rising additive concentrations, reflecting higher levels of dissolved minerals and enhanced ionic strength. Specifically, TDS increased from 1834.5 ppm at 0% to 2439 ppm at 15%, while EC rose from 2962.5 µS/cm to 3932.5 µS/cm, reflecting enhanced ionic strength. ORP values remained relatively stable around 65 mV, suggesting that the additive has minimal effect on the dough’s redox balance. Overall, these results indicate that the additive enhances mineral content and ionic activity without significantly altering pH or redox stability, preserving the chemical integrity of the dough.

Table 6.
Main physico-chemical characteristics of biscuit dough. All data have statistically significant difference at p < 0.05.
Figure 2 illustrates the overall changes in the surface characteristics of butter biscuit dough with increasing levels of amaranth flour. As the amount of plant flour increases, both the color characteristics and the surface structure of the product are noticeably altered.

Figure 2.
Color images of biscuit doughs with additive. (a) 0% additive; (b) 5% additive; (c) 10% additive; (d) 15% additive.
Figure 3 presents the Lab and spectral characteristics of biscuit dough containing amaranth. Lab values and spectral data generally change according to the color properties of the additive as its proportion in the dough increases. With higher additive levels, the “L” value decreases, indicating that the dough becomes darker. The “a” and “b” values adjust according to the hue of the additive: “a” decreases because the additive lacks a reddish tint, while “b” increases due to the additive’s greenish-yellow hue. The resulting spectral data reflect the loss of lightness, accompanied by wavelength shifts corresponding to the reflectance characteristics.
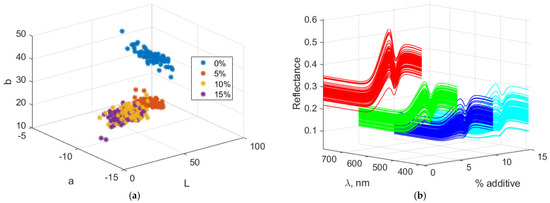
Figure 3.
Color and spectral characteristics of biscuit dough. (a) Lab color characteristics; (b) spectral characteristics.
Figure 4 illustrates the color difference (ΔE) of biscuit dough relative to the control (0% additive) across varying additive levels. Increasing the additive concentration resulted in progressively larger ΔE values, indicating color changes that are readily perceptible to the naked eye.
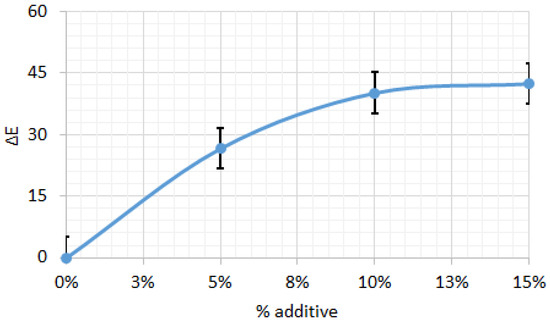
Figure 4.
Color difference for biscuit dough between control sample and those with different percentages of additive. All data have statistically significant difference at p < 0.05.
The color index values of biscuit dough with varying percentages of additive are presented in Table 7. Increasing the additive content resulted in significant changes, highlighting its strong impact on the dough’s appearance. With higher additive concentrations, the C1 value decreased, indicating a reduction in the intensity of this color index, while C3 and C4 values increased, reflecting enhanced color characteristics associated with these indices. C2 initially increased, reaching a maximum at 10% additive, before slightly declining, suggesting a more complex effect on this particular index. Overall, the additive substantially altered the color profile of the dough, enhancing or, in some cases, diminishing specific colors at different concentrations.

Table 7.
Values of color indices of biscuits dough with additive. All data have statistically significant difference at p < 0.05.
The values of the spectral indices of biscuit dough with different percentages of additive are presented in Table 8. The data show significant variations in the indices with changes in additive levels. Index S1 remains constant across all additive concentrations, indicating that this spectral characteristic is not significantly affected. Index S2 decreases sharply from 0.87 at 0% additive to 0.24 at 15% additive, indicating a substantial change in the spectral properties of the dough due to the additive. Index S3 remains practically unchanged across different additive levels. In conclusion, the additive has a strong impact on the spectral index S2, while the indices S1 and S3 remain stable and are not significantly affected.

Table 8.
Values of spectral indices of biscuits dough with additive. All data have statistically significant difference at p < 0.05.
3.4. Biscuit Analysis
The main physicochemical characteristics of the biscuits show that increasing the amount of amaranth flour results in a slight decrease in pH. TDS and EC are strongly increased, while ORP remains approximately constant across all levels of the additive (Table 9).

Table 9.
Main physico-chemical characteristics of biscuits with Amaranth green powder. All data have statistically significant difference at p < 0.05.
Table 10 presents data on the changes in the geometric characteristics of the biscuits and their heat losses during baking. Biscuit diameter remains relatively constant across all additive levels, while height increases slightly, peaking at 10% additive before slightly decreasing at 15%. Variations in the spread factor are minor, with no clear trend observed. Heat losses rise with increasing additive content, reaching a maximum at 10%, and stabilize at 17% for 5% and 15% additive levels.

Table 10.
The geometric properties and thermal losses of butter biscuits with amaranth powder. All data have statistically significant difference at p < 0.05.
The results of the sensory evaluation of biscuits with varying levels of amaranth flour are presented in Table 11.

Table 11.
Average sensory scores of salty butter biscuits with different amounts of amaranth green powder (n = 9). All data have statistically significant difference at p < 0.05.
Increasing the additive by 5% generally leads to a decline in sensory attributes, including appearance, texture, aroma, taste, and chewiness. At 10% additive, however, most of these attributes improve, reaching values comparable to the control (0% additive). Further increasing the additive to 15% causes a slight decrease in sensory scores. These findings suggest that incorporating up to 10% amaranth flour achieves an optimal balance between enhanced sensory qualities and overall acceptability, while higher levels may negatively affect the sensory profile of the biscuits (Figure 5).
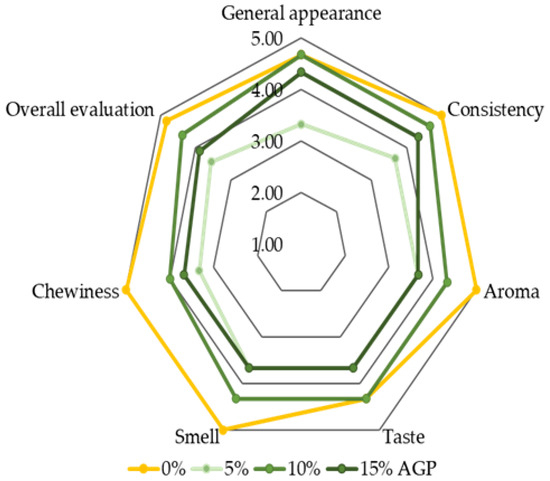
Figure 5.
A 5-point Likert scale: 1—completely does not correspond to the indicator; 5—completely corresponds to the indicator; AGP—amaranth green powder.
Color digital images of the resulting biscuits are shown in Figure 6. Increasing the amount of amaranth flour in the biscuits visibly alters both the color characteristics and the surface structure of the product.

Figure 6.
Color images of biscuits with pigweed powder. (a) 0% additive; (b) 5% additive; (c) 10% additive; (d) 15% additive.
Figure 7 illustrates the Lab and spectral characteristics of biscuits containing amaranth. Increasing the amount of amaranth flour leads to a decrease in the “L” value, indicating that the biscuits darken as the additive level rises. This color component decreases from 56.81 at 0% additive to 36.90 at 15%. The “a” component (red/green) has negative values, indicating that the color lies within the green spectrum. With increasing additive levels, “a” becomes more negative, changing from −4.74 at 0% to −6.33 at 15%, showing a slight intensification of the green hue. The “b” component (yellow/blue) increases with the amount of additive, reaching a maximum of 39.15 at 10%, and then slightly decreases to 35.37 at 15%. This suggests that the yellowing of the biscuits increases up to a certain point and then slightly decreases at the highest additive level.
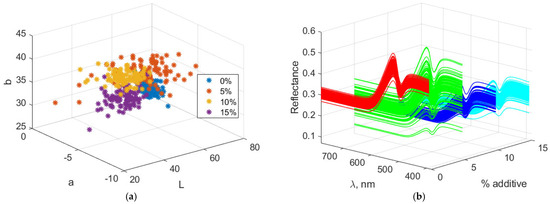
Figure 7.
Color and spectral characteristics of biscuits with amaranth powder. (a) Lab color characteristics; (b) Spectral characteristics.
The VIS reflection spectra reveal a general decrease in reflectance with increasing levels of the additive, indicating progressive darkening of the biscuits due to higher light absorption. A slight increase in reflectance is observed in the green wavelength range, which becomes more pronounced at higher additive levels. Reflectance in the yellow wavelength range rises up to 10% additive, then decreases slightly at 15%, suggesting a reduction in yellow hue intensity. Overall, the spectra indicate a trend toward darker and more muted colors with increasing additive content.
Figure 8 shows the color difference (ΔE) relative to the control biscuit sample, depending on the amount of amaranth flour. The ΔE results indicate significant color changes with increasing additive levels, reaching a maximum of ΔE = 63.27 at 5% additive. As the additive percentage increases to 10% and 15%, the color difference slightly decreases to ΔE = 55.88 and 51.51, respectively, suggesting that the initial addition of the additive causes a more pronounced color change, while higher concentrations produce a less marked effect. This trend indicates that the most significant variation in color occurs at lower additive levels, with the rate of change decreasing thereafter, likely due to saturation effects or interactions among the ingredients in the biscuit formulation.
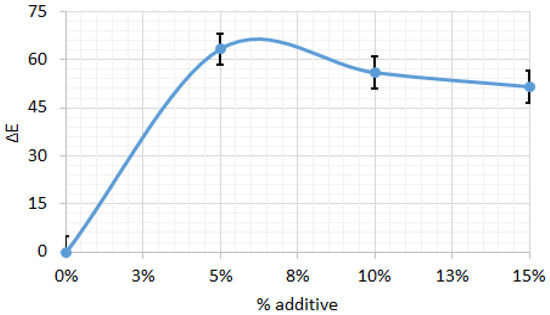
Figure 8.
Color difference for biscuits between control sample and those with different percentage of amaranth powder. All data have statistically significant difference at p < 0.05.
Table 12 shows the color index values of biscuits containing varying amounts of amaranth flour. The C1 index increases at 5% additive but decreases with higher concentrations, indicating a gradual loss of brightness. The C2 and C3 indices rise progressively at 10% additive, reflecting enhanced color intensity and saturation, and continue to increase up to 15%, indicating further color deepening. These results suggest that moderate additive levels, around 10%, provide optimal color characteristics, while higher concentrations lead to more complex or less desirable color changes.

Table 12.
Values of color indices of biscuits with amaranth powder. All data have statistically significant difference at p < 0.05.
Table 13 presents the spectral index values of biscuits containing varying percentages of amaranth flour. The indices S1 and S2 exhibit clearly differentiated trends with increasing additive concentration. S1 shows a slight increase up to 10% additive, followed by a decrease at 15%, indicating a positive effect that levels off at higher concentrations. In contrast, S2 decreases markedly as the additive concentration rises, suggesting a reduction in the measured property (e.g., color or texture) with higher amaranth flour content. S3 shows minimal variation, with a slight overall decrease and a small increase at 15%, reflecting a negligible effect of the additive.

Table 13.
Values of spectral indices of biscuits with amaranth powder. All data have statistically significant difference at p < 0.05.
3.5. Determination of the Optimal Amount of Pigweed Flour in Biscuits
Figure 9 presents the results of informative feature selection using the RReliefF method. Features with weight coefficients above 0.6 indicate that TDS, EC, and ORP are the most influential variables in this model, showing significant changes across different additive levels, with weights of 0.97 and 1.00. Other important features include C1, C3, C4, S2, and ΔE, all exhibiting high coefficients ranging from 0.85 to 0.92. In contrast, features such as pH and ORP have relatively low coefficients in some cases, suggesting that their changes may be context-dependent. Furthermore, features with high weight coefficients but relatively low mean values, such as C2 and C4, are variably affected under different conditions.
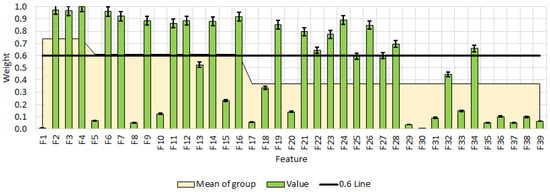
Figure 9.
Feature selection by RReliefF method. All data have statistically significant difference at p < 0.05. F1—pH; F2—TDS; F3—EC; F4—ORP; F5—pH; F6—TDS; F7—EC; F8—ORP; F9—C1; F10—C2; F11—C3; F12—C4; F13—S1; F14—S2; F15—S3; F16—dE; F17—pH; F18—TDS; F19—EC; F20—ORP; F21—C1; F22—C2; F23—C3; F24—C4; F25—S1; F26—S2; F27—S3; F28—dE; F29—D; F30—h; F31—SF; F32—TL; F33—GA; F34—Color; F35—Aroma; F36—Taste; F37—Smell; F38—Chewiness; F39—OA.
The following vector of informative features is formed:
FV = [F2 F3 F4 F6 F7 F9 F11 F12 F14 F16 F19 F21 F22 F23 F24 F26 F27 F28 F34]
An analysis of principal components was conducted to investigate the relationship between % additive and the selected biscuit features. Prior to analysis, all data were normalized to the [0, 1] range. Table 14 presents the normalized values of % additive relative to the selected features.

Table 14.
Normed values of % additive/feature combinations.
TDS shows the highest normalized values for F2 and F3, reaching 0.75 and 0.71 at 15% additive, respectively, indicating that both features gain importance as the additive level increases. In contrast, ORP exhibits minimal variation, remaining low across all additive levels. Other features, such as C1 (F12) and C2 (F14), display more variable behavior: C1 peaks at 0% additive with a value of 0.71 and declines slightly at higher levels, while C2 shows low variability. Changes in ΔE (F16) and C (F34) are particularly pronounced: ΔE rises sharply to 0.98 at 15%, whereas C (F34) jumps from 0.00 to 0.73 at 10% additive before decreasing. The observed variability among features highlights that each characteristic differs considerably in its relevance and effectiveness relative to additive level, underscoring the need for targeted analysis to optimize additive use.
The results of the PCA are presented in Figure 10. Increasing the proportion of amaranth flour in the biscuits was found to significantly influence several key characteristics, including the color difference of both the dough and biscuits (ΔE—F16, F28) and biscuit consistency (C—F34). Furthermore, as the level of supplementation increased from 0% to 15%, a positive shift in PC1 was observed, indicating a pronounced effect of the additive on the overall product characteristics. Among these, the TDS of the dough (F6) exhibited a moderately positive response. Similarly, the EC values of the flour blends, dough, and biscuits (F3, F7, and F19) were moderately positively affected by the additive. Conversely, negative effects were observed in the ORP of the flour blends (F4) as well as in certain color attributes of the dough and biscuits, particularly lightness and browning (C1—F9 and F21). Overall, PC1 accounted for the largest proportion of the additive’s influence on biscuit properties, explaining 69.96% of the total variance. This demonstrates the relevance of these parameters in describing the impact of the additive on biscuit quality.
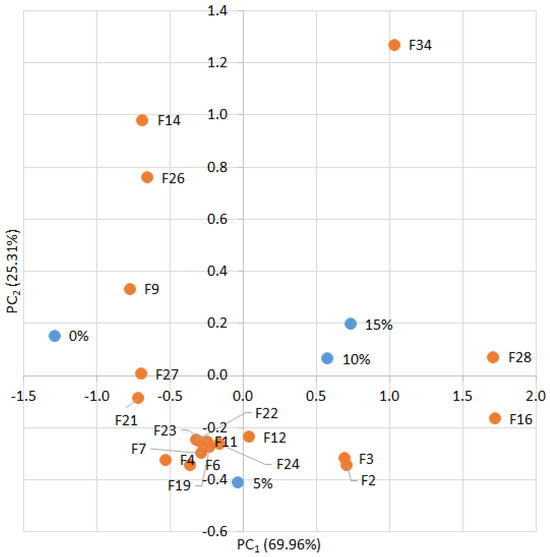
Figure 10.
PCA of connections % additive/feature. F1—pH; F2—TDS; F3—EC; F4—ORP; F5—pH; F6—TDS; F7—EC; F8—ORP; F9—C1; F10—C2; F11—C3; F12—C4; F13—S1; F14—S2; F15—S3; F16—dE; F17—pH; F18—TDS; F19—EC; F20—ORP; F21—C1; F22—C2; F23—C3; F24—C4; F25—S1; F26—S2; F27—S3; F28—dE; F29—D; F30—h; F31—SF; F32—TL; F33—GA; F34—Color; F35—Aroma; F36—Taste; F37—Smell; F38—Chewiness; F39—OA.
These findings provide valuable insights for formulation strategies aimed at optimizing biscuit quality in accordance with the desired outcomes associated with the incorporation of amaranth flour.
A regression model of the form %A = f(PC1, PC2) was obtained. Non-informative coefficients with p > α were removed. The model equation is as follows:
Table 15 presents the evaluation of the obtained model. The coefficient of determination was R2 = 0.99. According to the Fisher criterion, F(5, 6) = 611.2 >> F_critical = 4.39, with a significance level of p << 0.05. The standard error was SE = 0.35.

Table 15.
Assessment of regression model %A = f(PC1, PC2).
The regression model predicting %A from the two principal components, PC1 and PC2, and their interactions exhibits excellent predictive strength, with R2 = 0.99. This indicates that the model explains 99% of the variance in %A. All predictors and their interactions were significant at p < 0.001. PC1 has a positive effect on %A, whereas PC2 has a negative effect. The interaction terms PC12 and PC22 indicate that the effects of PC1 and PC2 increase or decrease with their respective magnitudes.
Residual analysis showed that the residuals closely align with a straight line on the normal probability plot and are approximately normally distributed. These results provide strong evidence that the regression model describes the experimental data with sufficient accuracy.
The appropriate amount of amaranth flour in the biscuits was determined, and the result is visualized in Figure 11. The figure presents model form. The star is the point from the model with the appropriate amount of amaranth additive in wheat flour. The presented value is the appropriate amount of additive. The positive value of PC1 indicates that the optimal 7.17% additive preserves certain properties more closely to the lower percentages (0%, 5%, 10%). This renders the supplemented biscuits more similar to the characteristics of the control sample. In this context, these properties are more closely associated with weight, diameter, spread ratio, and certain sensory attributes such as taste and aroma of the control sample. The second principal component, PC2, is close to zero, indicating minimal or no deviation at the 7.17% additive level. This suggests that critical biscuit characteristics are maintained without extreme changes, resulting in a product that is neither over- nor under-modified while retaining the beneficial properties of the additive.
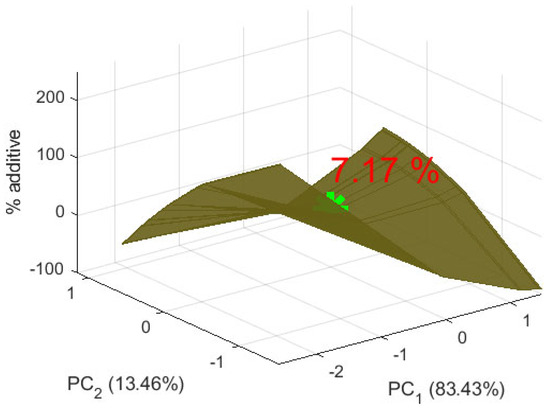
Figure 11.
Appropriate amount of additive in biscuits.
The results indicate that the addition of varying amounts of amaranth flour strongly influences the color and structure of the biscuits. As described in the analysis, increasing the level of supplementation leads to significant changes in certain biscuit characteristics, such as color difference and the organoleptic attribute of consistency. Positive changes in PC1 suggest that the additive exerts an influence on the properties represented by this principal component, with a moderate positive effect on TDS and EC, and an impact on ORP and color attributes. These findings are consistent with previous reports on the role of amaranth flour in modulating the physical and sensory properties of biscuits [47,48].
Polyunsaturated fatty acids (PUFAs) have demonstrable effects on the texture and aroma of baked goods, and comparative studies support these claims.
The incorporation of PUFA-rich ingredients into baked goods has been shown to influence both texture and sensory perception. For instance, Martínez et al. [49] demonstrated that reformulating puff pastry with oils derived from chia and poppy seeds, both rich in PUFAs, resulted in a softer crumb structure and enhanced aroma, attributed to the oxidative breakdown of unsaturated fatty acids during baking. Similarly, Dominguez et al. [50] reported that structuring PUFA-rich oils with monoglycerides improved lipid dispersion and contributed to a more cohesive, less brittle texture in semisolid bakery matrices. These findings align with the current study’s observation that amaranth green powder, rich in PUFAs, subtly modifies the biscuit texture and aroma, suggesting that the lipid profile of the additive plays a functional role in shaping sensory outcomes.
Comparative sensory analyses further support the impact of PUFA-rich additives on aroma and mouthfeel. Pimdit et al. [51] found that puff pastries formulated with salatrim, a fat replacer containing PUFAs, exhibited higher moisture retention and softer texture, though some sensory attributes were less pronounced compared to conventional fat formulations. Moreover, the American Society of Baking notes that PUFAs, despite their susceptibility to oxidation, are valued in commercial baking for their ability to enhance flavor complexity and contribute to a lighter mouthfeel [52]. These parallels reinforce the current study’s findings that moderate inclusion of amaranth green powder (around 7%) optimizes both nutritional and sensory characteristics, aligning with broader trends in functional bakery product development.
The optimal additive level, determined as 7.17% via PCA, represents the point at which the desired properties closely resemble those of the control sample, maintaining close alignment with characteristics such as weight, diameter, and sensory attributes related to taste and aroma. These results complement observations by Karki et al. [53] and Raihan et al. [54], who reported that lower levels of amaranth flour better preserve sensory qualities while providing nutritional benefits [53,54]. Overall, the findings of this study suggest that 7.17% is the optimal level for balancing the nutritional advantages of amaranth flour with the preservation of the sensory quality of the biscuits.
4. Conclusions
This study presents the evaluation of the effects of incorporating varying pigweed stalk (Amaranthus spp.) flour on the characteristics of butter biscuits. Several analyses were conducted, and it was found that the addition of 7.17% pigweed flour improved both the physicochemical and sensory properties of the biscuits. Determining the optimal level of pigweed flour provides guidance for product formulation and can enhance overall biscuit quality. The results of this study complement the existing literature on pigweed flour-enriched biscuits by providing data on color and spectral indices, allowing a more detailed and in-depth assessment of the product, as these indices are associated with changes in composition and biscuit properties.
As determined by the simultaneous consideration of physico-chemical parameters and sensory characteristics, 7.17% AGP could be considered the optimal addition. With the increase in AGP levels from 0% to 15%, the pH of the flour mixture reduced slowly (from 6.55 ± 0.06 to 6.28 ± 0.05), which demonstrated that acidification for the flour mixture was very mild. Meanwhile, TDS and EC increased from 627 ± 97 to 2523 ± 243 ppm and from 1075 ± 108 to 3759 ± 421 µS/cm, which indicates more mineral burden with an increase in the ions of the affected areas. The ORP also significantly increased (from 65 ± 19 mV to 78 ± 18 mV), reflecting a shift toward an oxidative environment. These changes may enhance nutritive values, but they might influence dough rheology and final product quality. Sensory also corroborated the 7.17% limitation, as biscuits with AGP over 10% started to diminish on panelists’ scores for aroma, taste, and chewiness, which may be due to increased vegetable notes and firmer texture. Thus 7.17% becomes an equilibrium where nutrients are enriched and without significant deterioration in consumers’ acceptability and essential technological characteristics.
Increasing amounts of pigweed flour could cause biscuits to maintain their diameter, rise in height, and show a higher spread ratio, which was also noted for heat losses. Also, 39 properties of the flour, dough, and biscuits (geometric, physicochemical, color, and sensory) were determined; 19 were selected as the most informative. Geometric parameters, electrical conductivity, firmness, taste, color, and spectral indices were the most pertinent descriptors for characterization of the effect of pigweed flour incorporated in this study. The associations of these 19 features with additive level were detailed by principal component analysis, accounting for up to 99% of the variance. Pigweed flour is appropriate to produce some types of butter biscuits, given its distinct chemical composition and high dietary fiber level.
These results can be used to correct the feeding level of stalk pigweed flour for better product nutrition and functional food development. Considering the diversity of species in the genus amaranthus, additional studies are necessary to evaluate the effect of amaranth-derived flour from other species.
These studies would contribute to further knowledge regarding the nutritional and technological characteristics of the species, cultivated or in natura, as well as its potential use as a functional ingredient by the food industry.
The results from this study demonstrate the industrial potential of the findings in relation to food application. The proved influence of the additive reiterates the value of amaranth as a health food and a rich agri-food with immunity power that enhances the nutritional and functional potentiality in bakery goods. This promotes the environmentally friendly use of agricultural resources and represents a practicable strategy for the development of new products in industrial food production.
Author Contributions
Conceptualization, Z.Z., N.G., T.K. and S.B.; methodology, N.G. and S.T.; software, Z.Z., S.T. and M.T.; validation, D.D., T.I. and Z.Z.; formal analysis, N.G.; investigation, S.B., Z.Z. and T.K.; resources, N.G., T.I., D.D. and M.T.; data curation, Z.Z. and S.B.; writing—original draft preparation, Z.Z., S.B. and N.G.; writing—review and editing, N.G. and M.T.; visualization, Z.Z.; supervision, Z.Z., S.B. and N.G.; project administration, S.B.; funding acquisition, S.B. and N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by the Bulgarian National Science Fund in the frame of the KΠ-06-H066/11 “Traditional food practices—a basis for diversification of the food market and social innovation in rural areas” project. Also, this study is funded by the EU and the Bulgarian Ministry of Education and Science through project BG-RRP-2.004-0006-C02 “Development of scientific research and innovation at Trakia University in the service of health and sustainable well-being”.
Institutional Review Board Statement
The butter biscuits presented in this work are a confectionery product regulated in BNS 441:1987 (Biscuits. General requirements). In the specified standard, biscuits are defined as a product appropriate for human consumption. According to Bulgarian Regulation No. 5 of 3.09.2018 on organic production, labeling, and control of plant products. This includes leafy vegetables, among which pigweed (Amaranthus spp.) can be classified as food for humans if cultivated for consumption. Organoleptic analysis, according to BNS EN ISO 13299:2016, Bulgarian Regulation No. 2 of 27 March 2024; Bulgarian Regulation No. 12 of 10 November 2021, is part of control studies that do NOT fall under medical or biomedical ethical supervision. This is because the ethical requirements are related to patients and people with mental problems, who are NOT the subject of this study. Also, the organoleptic analysis of food products does not require the use of personal data from those participating in it. The aforementioned standards and regulations do not provide for an obligation for approval by an ethics committee if human subjects are not affected in a medical or psychological aspect. Therefore, ethical approval was waived, and informed consent was obtained from all participants prior to participation.
Informed Consent Statement
Written consent was obtained from all participants before the sensory evaluation.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AACC | American Association of Cereal Chemists |
| AGP | Amaranth green powder |
| ANOVA | Analysis of variance |
| CIE | International Commission on Illumination |
| DW | Dry weight |
| EC | Electrical conductivity |
| GA | General appearance |
| ISO | International standard organization |
| LMS | Long, medium, short—Color model |
| LSD | Least Significant Difference |
| OA | Overall acceptance |
| ORP | Oxidation-reduction potential |
| pH | Active acidity |
| PR | People Republic |
| PUFA | Polyunsaturated fatty acids |
| RGB | Red, green, blue—color model |
| SE | Standard error |
| SF | Spread factor |
| TDS | Totally dissolved solids |
| TL | Thermal losses |
| USA | United states of America |
| ΔE | Color difference |
| PCA | Principal Component Analysis |
References
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1537–1544. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Sharma, N.; Singh, A.; Phimolsiripol, Y.; Brennan, C.S. Glycaemic response of pseudocereal-based gluten-free food products: A review. Int. J. Food Sci. Technol. 2022, 57, 4936–4944. [Google Scholar] [CrossRef]
- Amaya-Farfan, J.; Marcílio, R.; Spehar, C.R. Deveria o Brasil investir em novos grãos para a sua alimentação? A proposta do amaranto (Amaranthus sp.). Segur. Aliment. Nutr. 2005, 12, 47–56. [Google Scholar] [CrossRef]
- Trucco, F.; Tranel, P.J. Amaranthus. In Wild Crop Relatives: Genomic and Breeding Resources: Vegetables; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 11–21. [Google Scholar] [CrossRef]
- Aderibigbe, O.R.; Ezekiel, O.O.; Owolade, S.O.; Korese, J.K.; Sturm, B.; Hensel, O. Exploring the potentials of underutilized grain amaranth (Amaranthus spp.) along the value chain for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef]
- Barba de la Rosa, A.P.; Fomsgaard, I.S.; Laursen, B.; Mortensen, A.G.; Olvera-Martínez, L.; Silva-Sánchez, C.; Mendoza-Herrera, A.; González-Castañeda, J.; De León-Rodríguez, A. Amaranth (Amaranthus hypochondriacus) as an Alternative Crop for Sustainable Food Production: Phenolic Acids and Flavonoids with Potential Impact on Its Nutraceutical Quality. J. Cereal Sci. 2009, 49, 117–121. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, S.; Agarwal, A.; Sabharwal, M.; Tripathi, A.D. Amaranthus crop for food security and sustainable food systems. Planta 2024, 260, 59. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Amaranthus: A Promising Crop of Future; Springer: Singapore, 2016; pp. 13–48. [Google Scholar] [CrossRef]
- Mueller-Bieniek, A.; Walanus, A.; Zaitz, E. Cultivated plants in medieval Kraków (Poland), with special reference to amaranth (Amaranthus lividus L. cf. var lividus) and ruderal communities. Acta Palaeobot. 2015, 55, 97–114. [Google Scholar] [CrossRef]
- Srivastava, A.; Snehi, S.K.; Raj, S.K. Molecular detection and characterization of begomoviruses infecting Amaranthus, a protein-rich crop. In Geminivirus: Detection, Diagnosis and Management; Gaur, R.K., Sharma, P., Czosnek, H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 25–32. [Google Scholar] [CrossRef]
- Ivanova, T.; Marchev, A.; Chervenkov, M.; Bosseva, Y.; Georgiev, M.; Kozuharova, E.; Dimitrova, D. Catching the Green—Diversity of Ruderal Spring Plants Traditionally Consumed in Bulgaria and Their Potential Benefit for Human Health. Diversity 2023, 15, 435. [Google Scholar] [CrossRef]
- Akubugwo, I.E.; Obasi, N.A.; Chinyere, G.C.; Ugbogu, A.E. Nutritional and chemical value of Amaranthus hybridus L. leaves from Afikpo, Nigeria. Afr. J. Biotechnol. 2007, 6, 2833–2839. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Achigan-Dako, E.G.; Sogbohossou, O.E.; Maundu, P. Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Pospišil, A.; Pospišil, M.; Varga, B.; Svečnjak, Z. Grain yield and protein concentration of two amaranth species (Amaranthus spp.) as influenced by nitrogen fertilization. Eur. J. Agron. 2006, 25, 250–253. [Google Scholar] [CrossRef]
- Medoua, G.N.; Oldewage-Theron, W.H. Effect of drying and cooking on nutritional value and antioxidant capacity of morogo (Amaranthus hybridus), a traditional leafy vegetable grown in South Africa. J. Food Sci. Technol. 2014, 51, 736–742. [Google Scholar] [CrossRef]
- Managa, G.M.; Nemadodzi, L.E. Comparison of agronomic parameters and nutritional composition on red and green amaranth species grown in open field versus greenhouse environment. Agriculture 2023, 13, 685. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Palmisano, R.; Genovese, S.; Taddeo, V.A. A re-investigation of the phytochemical composition of the edible herb Amaranthus retroflexus L. J. Pharm. Biomed. Anal. 2017, 143, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.; Yang, Y.; Xia, T.; Zhong, R.; Peng, M.; Chen, Y.; Ding, Z.; Feng, F.; Li, S.; et al. Study on quality standard for Amaranthus retroflexus. China Pharm. 2021, 32, 1741–1746. [Google Scholar] [CrossRef]
- Costea, M.; Tardif, F.J. The biology of Canadian weeds. 126. Amaranthus albus L., A. blitoides S. Watson, and A. blitum L. Can. J. Plant Sci. 2003, 83, 1039–1066. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2017, 58, 2119–2135. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Roy, S.; Chakraborty, R.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M.; Rengasamy, K.R.R. Underutilized green leafy vegetables: Frontier in fortified food development and nutrition. Crit. Rev. Food Sci. Nutr. 2023, 63, 11679–11733. [Google Scholar] [CrossRef]
- Kachiguma, N.A.; Mwase, W.; Maliro, M.; Damaliphetsa, A. Chemical and mineral composition of amaranth (Amaranthus L.) species collected from central Malawi. J. Food Res. 2015, 4, 92–102. [Google Scholar] [CrossRef]
- Sattar, M.; Saeed, F.; Afzaal, M.; Rasheed, A.; Asif, A.; Sharif, S.; Hussain, M.; Asad Ur Rehman, H.; Raza, M.A.; Munir, H.; et al. An overview of the nutritional and therapeutic properties of amaranth. Int. J. Food Prop. 2024, 27, 263–272. [Google Scholar] [CrossRef]
- Petkova, Z.Y.; Antova, G.A.; Angelova-Romova, M.I.; Vaseva, I.C. A comparative study on chemical and lipid composition of amaranth seeds with different origin. Bulg. Chem. Commun. 2019, 51, 262–267. [Google Scholar]
- Odhav, B.; Beekrum, S.; Akula, U.; Baijnath, H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Compos. Anal. 2007, 20, 430–435. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci. Rep. 2019, 9, 20413. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Lin, Y.P.; Oba, S.; Yoshioka, Y.; Hoshikawa, K. Prospects and potentials of underutilized leafy Amaranths as vegetable use for health-promotion. Plant Physiol. Biochem. 2022, 182, 104–123. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Available online: http://www.fao.org/3/I8429EN/i8429en.pdf (accessed on 12 November 2025).
- Singh, M.; Liu, S.X. Evaluation of amaranth flour processing for noodle making. J. Food Process Preserv. 2021, 45, e15270. [Google Scholar] [CrossRef]
- Markova, M. Food and Nutrition: Between Nature and Culture; Prof. Marin Drinov Academic Publishing House: Sofia, Bulgaria, 2011; ISBN 978-954-322-462-3. [Google Scholar]
- Nedelcheva, A. An ethnobotanical study of wild edible plants in Bulgaria. Eurasia J. Biosci. 2013, 7, 77–94. [Google Scholar] [CrossRef]
- Borneo, R.; Aguirre, A. Chemical composition, cooking quality, and consumer acceptance of pasta made with dried amaranth leaves flour. LWT-Food Sci. Technol. 2008, 41, 1748–1751. [Google Scholar] [CrossRef]
- Beswa, D.; Dlamini, N.R.; Amonsou, E.O.; Siwela, M.; Derera, J. Effects of amaranth addition on the provitamin A content, and physical and antioxidant properties of extruded provitamin A-biofortified maize snacks. J. Sci. Food Agric. 2016, 96, 287–294. [Google Scholar] [CrossRef]
- Hiscock, L.; Bothma, C.; Hugo, A.; Van Biljon, A.; Van Rensburg, W.S.J. Overall liking and sensory profiling of boiled Amaranthus leaves using the Check-all-that-apply question. CYTA J. Food. 2018, 16, 822–830. [Google Scholar] [CrossRef]
- Qumbisa, N.D.; Ngobese, N.Z.; Kolanisi, U.; Siwela, M.; Gebashe, F.C. Effect of Amaranthus leaf powder addition on the nutritional composition, physical quality, and consumer acceptability of instant noodles. S Afr. J. Bot. 2022, 145, 258–264. [Google Scholar] [CrossRef]
- Qumbisa, N.D.; Ngobese, N.; Kolanisi, U. Potential of using Amaranthus leaves to fortify instant noodles in the South African context: A review. Afr. J. Food Agric. Nutr. Dev. 2020, 20, 16099–16111. [Google Scholar] [CrossRef]
- Choi, S. Quality characteristics of muffins added with amaranth leaf powder. Culin. Sci. Hosp. Res. 2016, 22, 51–64. [Google Scholar] [CrossRef]
- Oyinloye, P.O.; Ajala, A.S.; Asogwa, N.T.; Galani, Y.J.H. Fortification of dough with moringa, coriander, and amaranth improves the nutritional composition, health-benefiting properties, and sensory attributes of Nigerian wheat bread. Food Sci. Nutr. 2023, 12, 615–626. [Google Scholar] [CrossRef] [PubMed]
- AACC International. International. Approved Methods of Analysis. Method 02-52.01. Hydrogen-Ion Activity (pH); Method 44-15.02. Electrometric Method; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- International Organization for Standardization (ISO). EN ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- Bulgarian Institute for Standardization (BIS). BNS 441:1987; Biscuits—General Requirements. BIS: Sofia, Bulgaria, 1987.
- Vilaseca, M.; Pujol, J.; Arjona, M.; Martínez-Verdú, F. Color visualization system for near-infrared multispectral images. In Proceedings of the 2nd European Conference on Colour Graphics Imaging Vision (CGIV), Aachen, Germany, 5–8 April 2004; pp. 431–436. [Google Scholar]
- Farrell, A.; Wang, G.; Rush, S.A.; Martin, J.A.; Belant, J.L.; Butler, A.B.; Godwin, D. Machine learning of large-scale spatial distributions of wild turkeys with high-dimensional environmental data. Ecol. Evol. 2019, 9, 5938–5949. [Google Scholar] [CrossRef]
- Mladenov, M. Model-based approach for assessment of freshness and safety of meat and dairy products using a simple method for hyperspectral analysis. J. Food Nutr. Res. 2020, 59, 108–119. [Google Scholar]
- Georgieva Ts Mihaylova, A.; Daskalov, P. Research of the possibilities for determination of some basic soil properties using image processing. In Proceedings of the 7th International Conference on Energy Efficiency Agric Eng (EE&AE), Ruse, Bulgaria, 11–13 June 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Inglett, G.; Chen, G.; Liu, S. Physical properties of gluten-free sugar cookies made from amaranth-oat composites. LWT-Food Sci. Technol. 2015, 63, 214–220. [Google Scholar] [CrossRef]
- Liu, S.; Chen, D.; Xu, J. Evaluation of gluten-free amaranth and navy bean flour blends on quality of sugar cookies. J. Food Res. 2017, 6, 63–73. [Google Scholar] [CrossRef]
- Martínez, E.; Ferreira, D.M.; Nunes, M.A.; Oliveira, M.B.P.P.; Pardo, J.E.; Álvarez-Ortí, M. Reformulation of Puff Pastry Using Oils from Agri-Food Residues, Chia, and Poppy Seeds to Produce a Functional Spanish Cake: ‘Miguelitos de la Roda’. Agriculture 2025, 15, 399. [Google Scholar] [CrossRef]
- Domínguez, M.; Carrín, M.E.; Palla, C.A. Structuring PUFA-Rich Oils with Monoglycerides: Physicochemical and Nutritional Properties of Fat Substitutes with High-Lipid Quality. Food Biosci. 2024, 60, 104107. [Google Scholar] [CrossRef]
- Pimdit, S.; Charoen, R.; Wattanachai, S. Effects of Fat Replacers on the Physical, Chemical, and Sensory Characteristics of Puff Pastry. Int. Food Res. J. 2014, 21, 264–273. [Google Scholar]
- American Society of Baking. Polyunsaturated Fat (PUFA): Functional Roles in Baking Applications. ASB Technical Bulletin. Available online: https://asbe.org/article/polyunsaturated-fat-pufa/ (accessed on 2 October 2025).
- Karki, R.; Mishra, A.; Ojha, P.; Subedi, U. Comparative study on the sensory quality of prepared biscuit and cake from Amaranthus and Sorghum. J. Food Sci. Technol. Nepal 2016, 9, 79–84. [Google Scholar] [CrossRef]
- Raihan, M.; Saini, C.S. Evaluation of various properties of composite flour from oats, sorghum, amaranth, and wheat flour and production of cookies thereof. Int. Food Res. J. 2017, 24, 2278–2284. Available online: http://www.ifrj.upm.edu.my/24%20(06)%202017/(1).pdf (accessed on 12 November 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).