Sustainable Approach to Prolong Cold Storage Shelf Life of Plant-Based Meat Using Lactic Acid Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. PBM Product Preparation
2.3. Lactic Acid Bacteria Preparation
2.4. PBM Sample Preparation and Storage
2.5. Microbial Analysis of PBM Samples
2.6. pH Value and Titratable Acidity
2.7. Determination of Volatile Organic Compounds (VOCs) by HS-SPME/GC-MS
2.8. Physicochemical Characteristics of the PBM Products
2.8.1. Color Analysis of the PBM Products
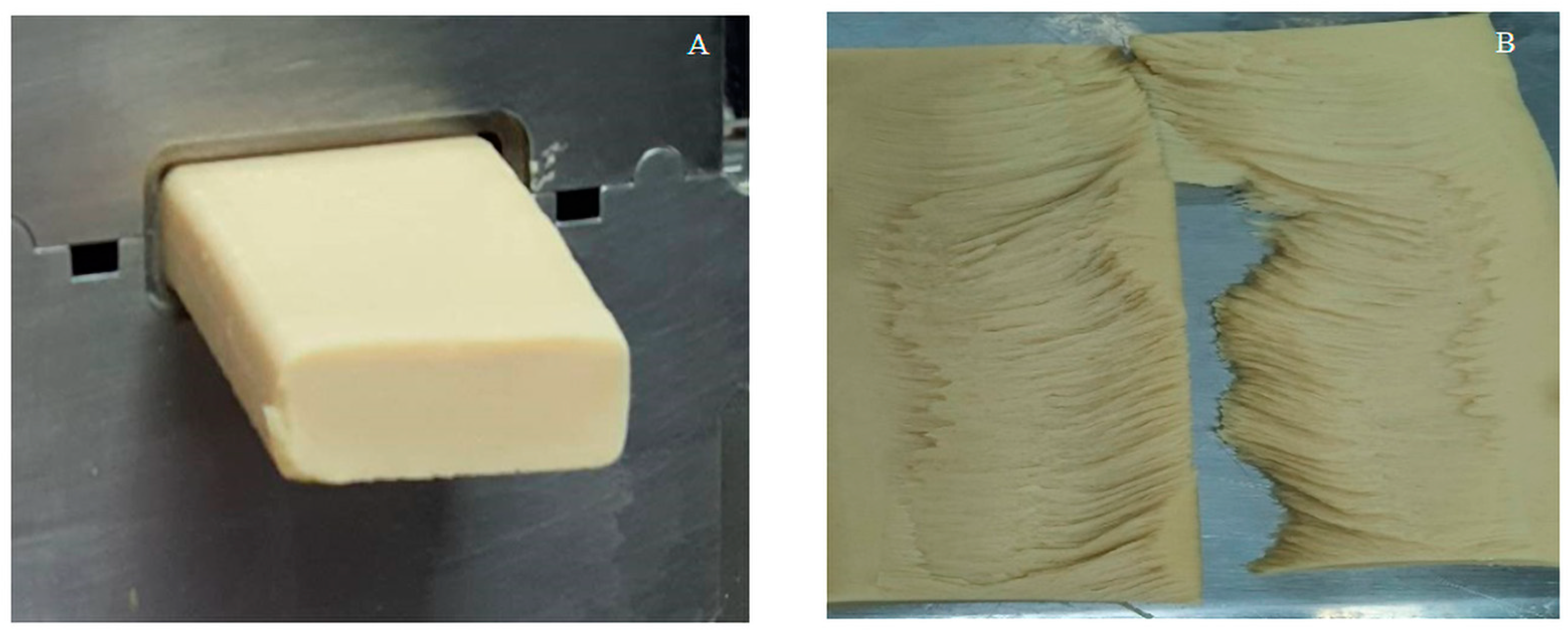
2.8.2. Textural Analysis
2.8.3. Environmental Scanning Electron Microscopy
2.9. Amino Acid Analysis
2.10. Statistical Analysis
3. Results
3.1. Number of Microorganisms in the PBM Samples
3.2. pH and TA of PBM
3.3. Volatile Organic Compounds
3.4. Color Characteristics
3.5. Textural Properties
3.6. Microstructures of PBM
3.7. Amino Acid Profiles
4. Discussion
4.1. Number of Microorganisms in the PBM Samples
4.2. pH and TA Values of PBM
4.3. Volatile Organic Compounds
4.4. Color Characteristics
4.5. Textural Properties
4.6. Microstructures of PBM
4.7. Amino Acid Profiles
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutierrez, I.B.; Ortega, C.V.; Manners, R. Evaluating animal-based foods and plant-based alternatives using multi-criteria and SWOT Analyses. Int. J. Environ. Res. Public Health 2020, 17, 7969. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Sanctorum, H. Alternative proteins, evolving attitudes: Comparing consumer attitudes to plant-based and cultured meat in Belgium in two consecutive years. Appetite 2021, 161, 105161. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects. 2017. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2017_world_population_prospects-2017_revision_databooklet.pdf (accessed on 2 May 2024).
- Hadi, J.; Brighwell, G. Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Graça, J.S.; Esmerino, E.A.; Silva, M.C.; Sant’Ana, A.S.; Duarte, M.C.K.; Freitas, M.Q. Impact of probiotics and prebiotics on food texture. Curr. Opin. Food Sci. 2020, 33, 38–44. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Güngören, A.; Koluman, A.; İlhak, O.İ.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of lactic acid bacteria postbiotics, evaluation in-vitro antibacterial effect, microbial and chemical quality on chicken drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzym. Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, B.; Zhou, C.; Ren, W.; Wu, M. Effect of high-moisture extrusion process on quality attribute and fibrous formation mechanism of pea protein: Insight into dynamic changes of textural protein. Innov. Food Sci. Emerg. Technol. 2023, 89, 103486. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, Z.; Cheng, Q.I.; Li, Q.Z.; Zhang, Y.J.; Liu, L.; Shi, A.M.; Wang, Q. Plant-based meat substitutes by high-moisture extrusion: Visualizing the whole process in data systematically from raw material to the products. J. Integr. Agric. 2022, 21, 8–2435. [Google Scholar] [CrossRef]
- Guyony, V.; Fayolle, F.; Jury, V. Die dimensions impact on fibrous plant protein formation during high moisture extrusion. Appl. Food Res. 2022, 2, 100228. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Kaplan, D.L.; Wang, Q. High-moisture extruded protein fiber formation toward plant-based meat substitutes applications: Science, technology, and prospect. Trends Food Sci. Technol. 2022, 128, 202–216. [Google Scholar] [CrossRef]
- Prasert, W.; Pantoa, T.; Chitisankul, W.T.; Pengpinij, W. Effects of Sacha inchi (Plukenetia volubilis L.) oil and extrusion process conditions on physicochemical properties of fortified omega-3 fibrous high moisture meat analogs. J. Food Process. Preserv. 2022, 46, e17227. [Google Scholar] [CrossRef]
- Kumari, S.; Alam, A.N.; Hossain, M.J.; Lee, E.Y.; Hwang, Y.H.; Joo, S.T. Sensory Evaluation of Plant-Based Meat: Bridging the Gap with Animal Meat, Challenges and Future Prospects. Foods 2024, 13, 108. [Google Scholar] [CrossRef]
- Wang, Y.; Tuccillo, F.; Lampi, A.; Knaapila, A.; Pulkkinen, M.; Kariluoto, S.; Coda, R.; Edelmann, M.; Jouppila, K.; Sandell, M.; et al. Flavor challenges in extruded plant-based meat alternatives: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2898–2929. [Google Scholar] [CrossRef]
- Barmettler, K.; Waser, S.; Stephan, R. Microbiological Quality of Plant-based Meat-alternative Products Collected at Retail Level in Switzerland. J. Food Prot. 2025, 88, 100402. [Google Scholar] [CrossRef]
- Hai, D.; Guo, B.; Qiao, M.; Jiang, H.; Song, L.; Meng, Z.; Huang, X. Evaluating the potential safety risk of plant-based meat analogues by analyzing microbial community composition. Foods 2023, 13, 117. [Google Scholar] [CrossRef]
- Kyrylenko, A.; Eijlander, R.T.; Alliney, G.; de Bos, E.L.-V.; Wells-Bennik, M.H. Levels and types of microbial contaminants in different plant-based ingredients used in dairy alternatives. Int. J. Food Microbiol. 2023, 407, 110392. [Google Scholar] [CrossRef]
- Roch, F.-F.; Dzieciol, M.; Quijada, N.M.; Alteio, L.V.; Mester, P.-J.; Selberherr, E. Microbial community structure of plant-based meat alternatives. npj Sci. Food 2024, 8, 27. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.; Wang, Q.; Suo, H.; Hamdy, A.M.; Song, J. Antifungal effect of metabolites from a new strain Lactiplantibacillus plantarum LPP703 isolated from naturally fermented yak yogurt. Foods 2023, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Gai, Y.; Zhou, H.; Fu, S.; Zhang, D. Assessment of spoilage microbiota of rainbow trout (Oncorhynchus mykiss) during storage by 16S rDNA sequencing. J. Food Qual. 2022, 2022, 5367984. [Google Scholar] [CrossRef]
- Ou, M.; Lou, J.; Lao, L.; Guo, Y.; Pan, D.; Yang, H.; Wu, Z. Plant-based meat analogue of soy proteins by the multi-strain solid-state mixing fermentation. Food Chem. 2023, 414, 135671. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Hussain, R.; Khan, Q.F.; Zhang, H. Functional microbiota in Chinese traditional Baijiu and Mijiu Qu(starters): A review. Food Res. Int. 2020, 138, 109830. [Google Scholar] [CrossRef] [PubMed]
- Chugh, B.; Kamal-Eldin, A. Bioactive compounds produced by probiotics in food products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Hugo, C.J.; Hugo, A. Current trends in natural preservatives for fresh sausage products. Trends Food Sci. Technol. 2015, 45, 12–23. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Schweiggert-Weisz, U.; Eisner, P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016, 205, 229–238. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Saint-Eve, A.; Panouille, M.; Souchon, I.; Bonnarme, P.; Dugar-Bony, E.; Irlinger, F. Design of microbial consortia for the fermentation of pea-protein-enriched T emulsions. Food Microbiol. 2019, 293, 124–136. [Google Scholar] [CrossRef]
- Tamang, J.P.; Tamang, B.; Schillinger, U.; Guigas, C.; Holzapfel, W.H. Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. Food Microbiol. 2009, 135, 28–33. [Google Scholar] [CrossRef]
- Ruggirello, M.; Nucera, D.; Cannoni, M.; Peraino, A.; Rosso, F.; Fontana, M.; Cocolin, L.; Dolci, P. Antifungal activity of yeasts and lactic acid bacteria isolated from cocoa bean fermentations. Food Res. Int. 2019, 115, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, H.; Yu, H.; Qin, G.; Tan, Z.; Wang, Y.; Pang, H. Screening of Lactobacillus plantarum subsp. plantarum with Potential Probiotic Activities for Inhibiting ETEC K88 in Weaned Piglets. Molecules 2020, 25, 4481. [Google Scholar]
- Abriouel, H.; Gómez, N.C.; Manetsberger, J.; Benomar, N. Dual effects of a bacteriocin-producing Lactiplantibacillus pentosus CF-6HA, isolated from fermented aloreña table olives, as potential probiotic and antimicrobial agent. Heliyon 2024, 10, E28408. [Google Scholar] [CrossRef]
- Dai, M.; Li, Y.; Xu, L.; Wu, D.; Zhou, Q.; Li, P.; Gu, Q. A Novel Bacteriocin From Lactobacillus pentosus ZFM94 and Its Antibacterial Mode of Action. Nutr. Immunol. 2021, 8, 710862. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicki, R.; Sójka, M.; Bonikowski, R.; Żyżelewicz, D.; Kołodziejczyk, K.; Klewicka, E. Antifungal Activity of Lactobacillus pentosus ŁOCK 0979 in the Presence of Polyols and Galactosyl-Polyols. Probiotics Antimicrob. Proteins 2018, 10, 186–200. [Google Scholar] [CrossRef]
- Ruiz, Y.P.; Reyes, D.M.; Quijano, R.R.; Lopez, D.G.; Figuroa, M.S.; Ovando, A.V. Antifungal Capacity of Microcapsules Containing Lactiplantibacillus plantarum TEP15 or Lactiplantibacillus pentosus TEJ4. Processes 2024, 12, 763. [Google Scholar] [CrossRef]
- Diguta, C.F.; Nitoi, G.D.; Matei, F.; Luta, G.; Cornea, C.P. The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods 2022, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Seetapan, N.; Raksa, P.; Limparyoon, N.; Srirajan, S.; Makmoon, T.; Israkarn, K.; Gamonpilas, C.; Methacanon, P.; Fuongfuchat, A. High moisture extrusion of meat analogues using mung bean (Vigna radiata L.) protein and flour blends: Investigations on morphology, texture and rheology. Int. J. Food Sci. Technol. 2023, 58, 1922–1930. [Google Scholar] [CrossRef]
- Treesuwan, K.; Jirapakkul, W.; Tongchitpakdee, S.; Chonhenchob, V.; Mahakarnchanakul, W.; Tongkhao, K. Antimicrobial mechanism of salt/acid solution on microorganisms isolated from trimmed young coconut. Microorganisms 2023, 11, 873. [Google Scholar] [CrossRef]
- Pöri, P.; Lille, M.; Edelmann, M.; Aisala, H.; Santangelo, D.; Coda, R.; Sozer, N. Technological and sensory properties of plant-based meat analogues containing fermented sunflower protein concentrate. J. Future Foods 2023, 8, 100244. [Google Scholar] [CrossRef]
- Valtonen, A.; Aisala, H.; Nisov, A.; Nikinmaa, M.; Honkapää, K.; Sozer, N. Synergistic use of fermentation and extrusion processing to design plant protein-based sausages. LWT—Food Sci. Technol. 2023, 184, 115067. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Pantoa, T.; Prasert, W.; Sangkhiaw, J.; Rojviriya, C.; Phoovasawat, C.; Kantrong, H. Microalgae-enriched high-moisture meat analogues: Improved physicochemical, functional, and digestibility properties. Foods 2025, 14, 2838. [Google Scholar] [CrossRef]
- Chen, C.; Rui, X.; Lu, Z.; Li, W.; Dong, M. Enhanced shelf-life of tofu by using bacteriocinogenic Weissella hellenica D1501 as bioprotective cultures. Food Control 2014, 46, 203–209. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis; Association of the Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- da Silva Oliveira, W.; Neves, D.A.; Ballus, C.A. Mature chemical analysis methods for food chemical properties evaluation. In Evaluation Technologies for Food Quality; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–90. [Google Scholar]
- Limsangouan, N.; Rodkwan, N.; Pengpinit, W.; Tumpanuvatr, T.; Pengpinit, P.; Paopun, Y.; Kantrong, H. Physical property changes promoting shelf-life extension of soy protein-based high moisture meat analog under high pressure treatment. J. Food Sci. Technol. 2024, 61, 918–927. [Google Scholar] [CrossRef]
- Bassani, J.C.; Martins, V.F.R.; Barbosa, J.; Coelho, M.; Sousa, C.; Steffens, J.; Backes, G.T.; Pereira, H.; Pintado, M.E.; Teixeira, P.C.; et al. Exploring the development of a clean-label vegan burger enriched with fermented microalgae. Foods 2025, 14, 2884. [Google Scholar] [CrossRef]
- Supjaroen, P.; Niamsi, W.; Thummarati, P.; Laiwattanapaisal, W. An in vitro cell model of intestinal barrier function using a low-cost 3D-printed transwell device and paper-based cell membrane. Int. J. Mol. Sci. 2025, 26, 2524. [Google Scholar] [CrossRef]
- Windham, W. AOAC Official Method 994.12, Amino Acids in Feeds, Alternative III, Acid Hydrolysis Method; AOAC International: Rockville, MD, USA, 1995; Available online: https://ci.nii.ac.jp/naid/10018325012/ (accessed on 26 May 2024).
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J. Chromatogr. B 2014, 964, 116–127. [Google Scholar] [CrossRef]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2007; p. 1. [Google Scholar]
- Ministry of Agriculture and Cooperatives. Food Consumption Data of Thailand; The Agricultural Co-operative Federation of Thailand: Bangkok, Thailand, 2016. [Google Scholar]
- Piranavatharsan, U.; Jinadasa, B.; Jayasinghe, C. Validation of thiobarbituric acid reactive substances (TBARS) method for measuring secondary lipid oxidation products in fresh Indian mackerel (Astrologer kanagurta). Food Humanit. 2023, 1, 1194–1199. [Google Scholar] [CrossRef]
- Gorny, J. Summary of CA and MA requirements and recommendations for fresh-cut (minimally processed) fruits and vegetables. Acta Hortic. 2003, 600, 609–616. [Google Scholar] [CrossRef]
- Sandhya. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT-Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Treesuwan, K.; Jirapakkul, W.; Tongchitpakdee, S.; Chonhenchob, V.; Mahakarnchanakul, W.; Tongkhao, K. Sulfite-free treatment combined with modified atmosphere packaging to extend trimmed young coconut shelf life during cold storage. Food Control 2022, 139, 109099. [Google Scholar] [CrossRef]
- Bouchibane, M.; Cheriguene, A.; Chougrani, F.; Bououdina, M.; Kaced, A.; Dahou, A.E.; Benbouziane, B.; Saada, D.A. Technological and genotypic characteristics of lactic acid bacteria isolated from Algerian artisanal dairy products. Int. Dairy J. 2023, 146, 105747. [Google Scholar] [CrossRef]
- Chadli, A.; Benbouziane, B.; Bouderoua, K.; Bentahar, M.C.; Benabdelmoumene, D. Assessment of potential probiotic properties and biotechnological activities of lactobacillus strains isolated from traditional algerian fermented wheat ELHAMOUM. Asian J. Dairy Food Res. 2024, 352, 1–7. [Google Scholar] [CrossRef]
- Thammavongs, B.; Corroler, D.; Panoff, J.-M.; Auffray, Y.; Boutibonnes, P. Physiological response of Enterococcus faecalis JH2-2 to cold shock: Growth at low temperatures and freezing/thawing challenge. Lett. Appl. Microbiol. 1996, 23, 398–402. [Google Scholar] [CrossRef]
- Han, K.; Park, S.; Sathiyaseelan, A.; Wang, M.-H. Isolation and characterization of Enterococcus faecium from fermented Korean soybean paste with antibacterial effects. Fermentation 2023, 9, 760. [Google Scholar] [CrossRef]
- Betancourt, M.M.; García, J.G.; Luna, L.; Maldonado, L.; Cardona, J.; Torrico, D.D. Effects of fermentation conditions on the physicochemical and sensory properties of plant-based yogurts. LWT—Food Sci. Technol. 2025, 230, 118242. [Google Scholar] [CrossRef]
- Başak, B.; Alagha, O. Trace metals solubility in rainwater: Evaluation of rainwater quality at a watershed area, Istanbul. Environ. Monit. Assess. 2010, 167, 493–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, X.; Li, M. Preparation, characterization and in vitro stability of iron-chelating peptides from mung beans. Food Chem. 2021, 349, 129101. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Q.; Hu, J.-N.; Han, X.-Y.; Li, W.; Zhao, L.-C. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef]
- Lorjaroenphon, Y.; Cadwallader, K.R. Identification of character-impact odorants in a cola-flavored carbonated beverage by quantitative analysis and omission studies of aroma reconstitution models. J. Agric. Food Chem. 2015, 63, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L. Odour Thresholds. Compilations of Odour Threshold Values in Air, Water and Other Media. 2011. Available online: https://www.academia.edu/117947016/ODOUR_THRESHOLDS_EDITION (accessed on 18 September 2025).
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, J.; Gong, S.; Li, M.; Yang, T.; Zhang, J. Physicochemical properties of potato starch fermented by amylolytic Lactobacillus plantarum. Int. J. Biol. Macromol. 2020, 158, 656–661. [Google Scholar] [CrossRef]
- Mittermaier, S.; Weghuber, J.; Miesenberger, J.; Kratzer, R.; Brunner, T. Technological and sensory properties of plant-based meat analogues containing fermented sunflower protein concentrate. Curr. Res. Food Sci. 2023, 6, 100420. [Google Scholar]
- Zhang, T.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Effect of lactic acid bacteria fermentation on the structural and rheological properties of soy protein isolate gels. Food Hydrocoll. 2019, 97, 105222. [Google Scholar]
- Zhao, X.; Li, L.; Luo, D. Textural and sensory improvements of pea protein-based meat analogues via bacterial fermentation. J. Food Sci. Technol. 2020, 57, 3949–3959. [Google Scholar]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. Biomed. Res. Int. 2018, 1, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Pederson, C.S. A Study of the Species Lactobacillus plantarum (Orla-Jensen). J. Bacteriol. 1936, 31, 217–224. [Google Scholar] [CrossRef]
- Li, W.; Guan, Y.; Chen, S.; Du, X.; Yan, B.; Wang, Z.; Ma, R.; Zhang, Y.; Huang, H.; Li, D.; et al. Effect of Lactobacillus pentosus fermentation on molecular structure and gel quality of peanut protein. J. Future Foods 2023, 5, 257–265. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Kashyap, S.; Devi, S.; Pasanna, R.M.; Preston, T.; Kurpad, A.V. True Digestibility of tryptophan in plant and animal protein. J. Nutr. 2024, 154, 3203–3209. [Google Scholar] [CrossRef] [PubMed]

| Condition | Time (Days) | Number of Microorganisms (log CFU/g) | ||||
|---|---|---|---|---|---|---|
| Total Plate Count | Yeast and Mold | Lactic Acid Bacteria | Total MYP Count | |||
| Pinkish Cloudy Colonies | Yellowish Non-Cloudy Colonies | |||||
| Control | 0 7 14 21 | 6.96 ± 1.13 Ca 4.93 ± 0.16 Db 4.76 ± 1.25 Bb 5.63 ± 0.23 Cb | ND ND ND ND | 2.55 ± 0.20 Ca 2.38 ± 0.52 Ca 2.95 ± 0.70 Ba 1.60 ± 1.39 Ba | 5.40 ± 0.88 ND ND ND | 4.44 ± 1.18 Aa No data No data 5.06 ± 0.31 Aa |
| LM-treated | 0 7 14 21 | 9.07 ± 0.11 Aa 8.85 ± 0.10 Ab 8.69 ± 0.22 Abc 8.66 ± 0.08 Ac | ND ND ND ND | 9.10 ± 0.14 Aa 8.90 ± 0.12 Aab 8.70 ± 0.23 Ab 8.68 ± 0.05 Ab | ND ND ND ND | 3.59± 0.15 Aa 3.64 ± 0.14 Aa 3.03 ± 0.88 Aa 2.90 ± 0.34 BCa |
| LS-treated | 0 7 14 21 | 8.62 ± 0.26 Ba 8.39 ± 0.08 Ca 8.41 ± 0.20 Aa 8.22 ± 0.14 Ba | ND ND ND ND | 8.62 ± 0.08 Ba 8.35 ± 0.06 Bb 8.52 ± 0.15 Aa 8.24 ± 0.06 Ab | ND ND ND ND | 3.79 ± 0.25 Aa 3.69 ± 0.15 Aa 3.61 ± 0.15 Aa 3.40 ± 0.35 Ba |
| PA-treated | 0 7 14 21 | 8.96 ± 0.15 ABa 8.62 ± 0.04 Ba 8.60 ± 0.30 Aa 8.64 ± 0.03 Aa | ND ND ND ND | 9.06 ± 0.20 Aa 8.67 ± 0.04 ABa 8.61 ± 0.28 Aa 8.71 ± 0.07 Aa | ND ND ND ND | 3.96 ± 0.40 Aa 3.74 ± 0.33 Aab 310 ± 0.14 Ab 2.37 ± 0.34 Cc |
| Condition | Time (Days) | pH | TA (%) |
|---|---|---|---|
| Control | 0 7 14 21 | 7.00 ± 0.02 Aa 6.90 ± 0.15 Aa 6.77 ± 0.13 Aa 7.01 ± 0.05 Aa | 0.22 ± 0.03 Ba 0.21 ± 0.03 Ca 0.19 ± 0.02 Ca 0.09 ± 0.01 Ca |
| LM-treated | 0 7 14 21 | 4.91 ± 0.11 Ca 4.90 ± 0.04 BCa 4.86 ± 0.07 Ca 4.88 ± 0.05 Ca | 0.53 ± 0.05 Aa 0.43 ± 0.03 Bb 0.54 ± 0.02 Aa 0.37 ± 0.03 Bb |
| LS-treated | 0 7 14 21 | 4.87 ± 0.08 Ca 4.84 ± 0.04 Ca 4.75 ± 0.04 Ca 4.85 ± 0.04 Ca | 0.51 ± 0.03 Aab 0.55 ± 0.04 Aa 0.43 ± 0.02 Bc 0.45 ± 0.04 Abc |
| PA-treated | 0 7 14 21 | 5.13 ± 0.08 Ba 5.12 ± 0.16 Ba 5.16 ± 0.07 Ba 5.05 ± 0.14 Ba | 0.56 ± 0.05 Aa 0.47 ± 0.02 Ba 0.55 ± 0.09 Aa 0.40 ± 0.05 ABa |
| Condition | Time (Days) | Relative Concentration (µg/g) | ||||
|---|---|---|---|---|---|---|
| Acetoin (RI = 1282) | 1-Hexanol (RI = 1361) | Acetic Acid (RI = 1454) | 1-Nonadecene (RI > 1800) | Maltol (RI > 1800) | ||
| Control | 0 21 | ND ND | 0.07 0.02 | ND ND | 3.23 3.60 | 0.06 0.07 |
| LM-treated | 0 21 | 0.02 0.01 | 0.02 0.01 | 0.14 0.07 | 0.28 0.08 | 0.03 0.01 |
| LS-treated | 0 21 | ND 0.02 | 0.04 0.02 | 0.30 0.30 | 0.26 0.27 | 0.05 0.02 |
| PA-treated | 0 21 | ND ND | 0.06 0.11 | 0.06 0.17 | 1.89 ND | 0.06 ND |
| Condition | Time (Days) | L* | a* | b* | ∆E |
|---|---|---|---|---|---|
| Control | 0 7 14 21 | 75.01 ± 0.25 Ca 75.32 ± 0.47 Ca 74.98 ± 0.27 Ca 74.56 ± 0.16 Ca | 0.90 ± 0.09 Aa 0.87 ± 0.07 Ca 0.65 ± 0.06 Cb 0.75 ± 0.13 Cab | 20.49 ± 0.43 Aa 20.34 ± 0.62 Aa 20.85 ± 0.23 Aa 20.81 ± 0.10 Aa | - ** - - - |
| LM-treated | 0 7 14 21 | 79.49 ± 0.53 ABa 77.79 ± 0.12 ABa 78.58 ± 0.56 Aa 78.42 ± 0.67 Aa | 0.93 ± 0.11 Aa 1.32 ± 0.17 Ba 1.00 ± 0.28 Ba 1.12 ± 0.28 Ba | 20.58 ± 0.25 Aa 19.80 ± 0.47 Aba 19.91 ± 0.48 Aa 19.87 ± 0.14 Aa | 4.48 2.57 3.74 3.99 |
| LS-treated | 0 7 14 21 | 79.83 ± 0.08 Aa 78.05 ± 0.08 Ac 78.58 ± 0.30 Ab 78.27 ± 0.18 Abc | 0.61 ± 0.08 Ad 1.33 ± 0.07 Ba 1.04 ± 0.05 Bc 1.18 ± 0.03 ABb | 20.90 ± 0.47 Aa 19.72 ± 0.10 Aba 19.86 ± 0.24 Aa 19.36 ± 0.83 Aa | 4.85 2.84 3.75 4.01 |
| PA-treated | 0 7 14 21 | 78.94 ± 0.28 Ba 77.23 ± 0.25 Bb 77.31 ± 0.23 Bb 77.29 ± 0.67 Bb | 1.05 ± 0.24 Ab 1.56 ± 0.12 Aa 1.29 ± 0.11 Aab 1.43 ± 0.15 Aa | 20.13 ± 0.33 Aa 19.13 ± 0.49 Ba 19.41 ± 0.78 Aa 19.95 ± 0.44 Aa | 3.95 2.36 2.81 2.94 |
| Condition | Time (Days) | Hardness (N) | Resilience (%) | Cohesiveness | Springiness | Gumminess (N) | Chewiness (N) |
|---|---|---|---|---|---|---|---|
| Control | 0 7 14 21 | 67.75 ± 6.72 Ba 75.53 ± 7.51 Ba 67.34 ± 2.51 Ba 69.71 ± 2.61 Ba | 15.78 ± 0.06 ABb 15.02 ± 0.55 Ac 14.76 ± 0.11 Ac 16.90 ± 0.54 Aa | 0.40 ± 0.01 BCab 0.39 ± 0.01 Cb 0.38 ± 0.01 Bb 0.42 ± 0.01 Aa | 0.90 ± 0.01 Ca 0.89 ± 0.01 Aa 0.89 ± 0.01 Aa 0.88 ± 0.01 Ba | 27.04 ± 2.94 Ba 29.47 ± 3.77 Ba 25.46 ± 0.86 Ba 29.13 ± 0.68 Ba | 24.26 ± 2.57 Ba 26.34 ± 3.27 Ba 22.55 ± 0.96 Ba 25.63 ± 0.86 Ca |
| LM-treated | 0 7 14 21 | 82.89 ± 13.36 ABa 74.59 ± 4.50 Ba 99.57 ± 19.75 Aa 105.07 ± 24.35 Aa | 16.63 ± 0.90 Aa 15.08 ± 0.55 Ab 14.87 ± 0.44 Ab 14.84 ± 0.63 Bb | 0.46 ± 0.01 Aa 0.42 ± 0.01 ABb 0.43 ± 0.01 Ab 0.42 ± 0.02 Ab | 0.94 ± 0.02 Aa 0.91 ± 0.02 Ab 0.93 ± 0.01 Aab 0.92 ± 0.01 Aab | 38.05 ± 6.87 Aa 31.22 ± 1.77 Ba 42.77 ± 9.23 Aa 44.15 ± 11.75 Aa | 35.88 ± 5.78 Aa 28.32 ± 1.61 Ba 39.65 ± 8.52 Aa 40.46 ± 10.58 ABa |
| LS-treated | 0 7 14 21 | 96.27 ± 1.51 Aa 99.08 ± 7.97 Aa 108.77 ± 5.49 Aa 111.83 ± 10.99 ABa | 15.32 ± 0.25 Ba 15.74 ± 0.25 Aa 15.36 ± 0.08 Aa 15.77 ± 0.36 ABa | 0.43 ± 0.01 Ba 0.44 ± 0.01 Aa 0.44 ± 0.01 Aa 0.44 ± 0.01 Aa | 0.92 ± 0.01 Ba 0.91 ± 0.01 Aa 0.92 ± 0.01 Aa 0.92 ± 0.01 Aa | 41.06 ± 1.20 Ac 43.34 ± 3.06 Abc 47.70 ± 2.49 Aab 49.47 ± 4.62 Aa | 37.85 ± 1.10 Ac 39.53 ± 2.55 Abc 44.06 ± 2.54 Aab 45.33 ± 3.76 Aa |
| PA-treated | 0 7 14 21 | 82.16 ± 10.80 ABa 77.51 ± 12.45 Ba 97.35 ± 3.95 Aa 86.52 ± 12.46 ABa | 13.88 ± 0.80 Ca 15.14 ± 0.37 Aa 15.77 ± 0.80 Aa 15.15 ± 0.82 Ba | 0.39 ± 0.03 Ca 0.41 ± 0.01 BCa 0.43 ± 0.01 Aa 0.43 ± 0.01 Aa | 0.91 ± 0.01 BCa 0.89 ± 0.04 Aa 0.91 ± 0.03 Aa 0.91 ± 0.01 Aa | 32.21 ± 6.91 ABa 33.36 ± 7.34 Ba 42.09 ± 1.43 Aa 36.69 ± 5.53 ABa | 29.47 ± 6.34 ABa 30.67 ± 7.13 Ba 38.52 ± 2.58 Aa 33.13 ± 4.85 BCa |
| Amino Acid Profile (mg/100 g) | Control | LM-Treated | LS-Treated |
|---|---|---|---|
| Histidine | 512.26 ± 53.47 | 551.82 ± 7.52 | 533.89 ± 75.01 |
| Threonine | 688.00 ± 5.76 | 728.52 ± 28.84 | 673.88 ± 28.23 |
| Valine | 854.74 ± 21.14 | 880.38 ± 1.76 | 857.05 ± 17.27 |
| Methionine | 321.74 ± 46.89 | 334.49 ± 39.38 | 340.50 ± 39.80 |
| Phenylalanine | 1078.31 ± 99.93 | 1127.67 ± 71.62 | 1092.59 ± 71.80 |
| Isoleucine | 767.58 ± 41.19 | 787.29 ± 24.45 | 761.62 ± 24.42 |
| Leucine | 1459.67 ± 63.80 | 1521.33 ± 27.15 | 1467.33 ± 27.61 |
| Lysine | 997.34 ± 34.93 | 1030.08 ± 33.66 | 989.17 ± 33.09 |
| Tryptophan | 37.96 ± 26.95 | 106.03 ± 86.18 | 98.02 ± 86.38 |
| Aspartic acid | 1858.00 ± 137.72 | 1906.33 ± 151.27 | 1819.67 ± 15.89 |
| Glutamic acid | 5260.67 ± 66.67 | 5610.33 ± 271.72 | 5265.33 ± 27.23 |
| Serine | 1055.07 ± 62.25 | 1153.67 ± 23.69 | 995.59 ± 23.69 |
| Glycine | 790.68 ± 26.05 | 823.80 ± 18.76 | 790.59 ± 18.20 |
| Arginine | 1168.00 ± 39.36 | 1203.33 ± 4.73 | 1161.67 ± 4.01 |
| Alanine | 752.18 ± 19.85 | 776.24 ± 12.26 | 765.19 ± 12.26 |
| Tyrosine | 623.71 ± 26.20 | 670.14 ± 11.50 | 655.25 ± 11.01 |
| Cystine | 434.25 ± 61.19 | 418.82 ± 68.11 | 502.76 ± 68.30 |
| Proline | 1480.67 ± 74.33 | 1566.67 ± 13.58 | 1524.00 ± 13.96 |
| Essential Amino Acid | Requirement Pattern 1 (mg/kg BW per Day) | Amino Acid Requirement 2 (mg/Day) | Amino Acid Score LM-Treated PBM 3 (%) |
|---|---|---|---|
| Histidine | 10 | 554.50 | 99.52 |
| Valine | 26 | 1441.70 | 61.07 |
| Methionine | 10 | 554.50 | 60.32 |
| Phenylalanine | 25 | 1386.25 | 81.35 |
| Isoleucine | 20 | 1109.00 | 70.99 |
| Leucine | 39 | 2162.55 | 70.35 |
| Lysine | 30 | 1663.50 | 61.92 |
| Tryptophan | 4 | 221.80 | 47.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treesuwan, K.; Tongkhao, K.; Kantrong, H.; Yodin, K.; Klinsoda, J.; Pengpinit, P. Sustainable Approach to Prolong Cold Storage Shelf Life of Plant-Based Meat Using Lactic Acid Bacteria. Foods 2025, 14, 3923. https://doi.org/10.3390/foods14223923
Treesuwan K, Tongkhao K, Kantrong H, Yodin K, Klinsoda J, Pengpinit P. Sustainable Approach to Prolong Cold Storage Shelf Life of Plant-Based Meat Using Lactic Acid Bacteria. Foods. 2025; 14(22):3923. https://doi.org/10.3390/foods14223923
Chicago/Turabian StyleTreesuwan, Khemmapas, Kullanart Tongkhao, Hataichanok Kantrong, Kanokwan Yodin, Jutamat Klinsoda, and Pathika Pengpinit. 2025. "Sustainable Approach to Prolong Cold Storage Shelf Life of Plant-Based Meat Using Lactic Acid Bacteria" Foods 14, no. 22: 3923. https://doi.org/10.3390/foods14223923
APA StyleTreesuwan, K., Tongkhao, K., Kantrong, H., Yodin, K., Klinsoda, J., & Pengpinit, P. (2025). Sustainable Approach to Prolong Cold Storage Shelf Life of Plant-Based Meat Using Lactic Acid Bacteria. Foods, 14(22), 3923. https://doi.org/10.3390/foods14223923








