Influence of Thermal Treatment Conditions and Fruit Batches Variability on the Rheology and Physicochemical Profile of Golden Delicious Apple Purée
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Design and Statistical Analysis
2.3. Physical Analysis
2.3.1. Colour Analysis
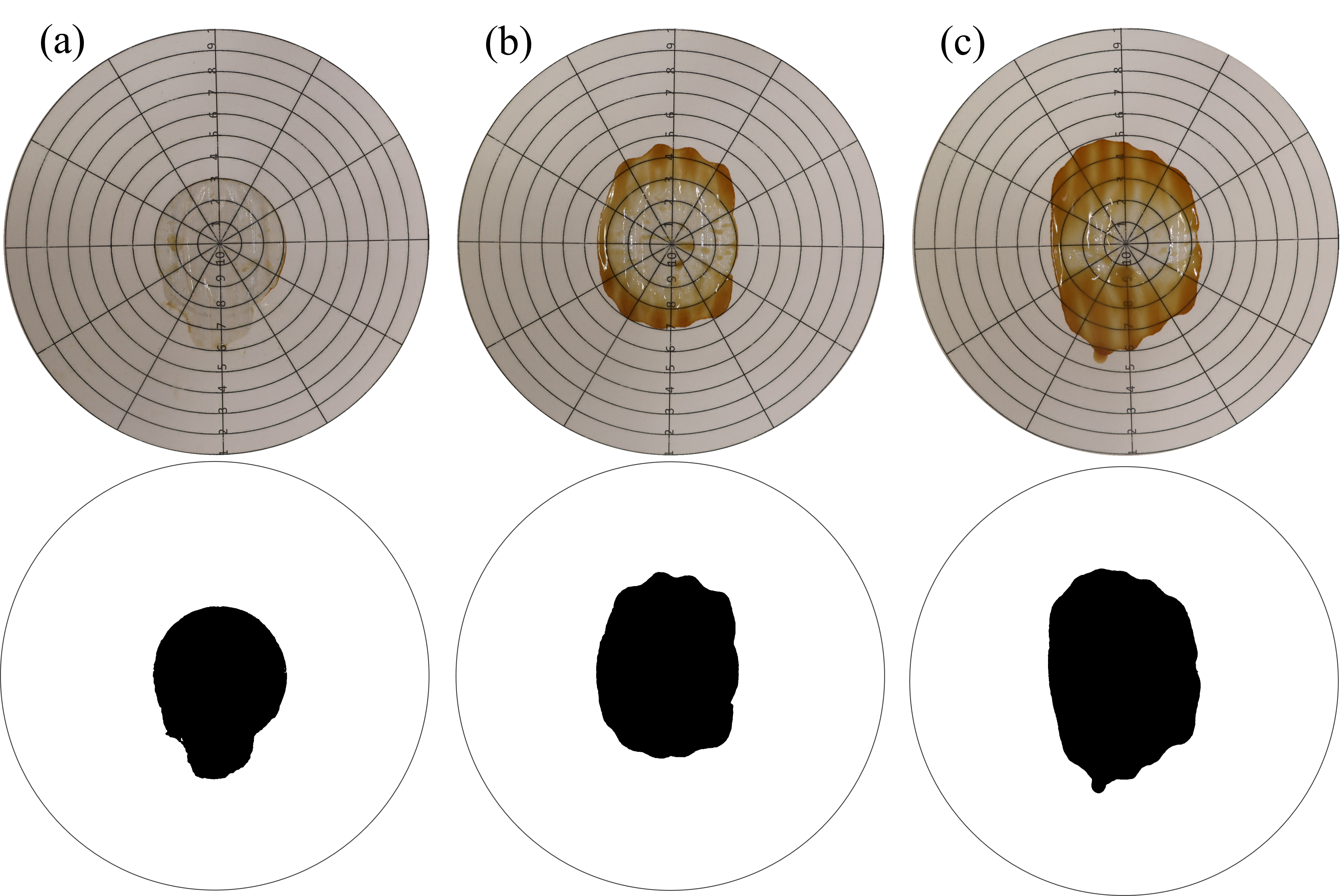
2.3.2. Consistency Determination
2.3.3. Syneresis Determination
2.3.4. Water Content, pH and Total Soluble Solids
2.4. Enzymatic Assays
2.4.1. Pectin Methylesterase Assay
2.4.2. Peroxidases Assay
2.4.3. Polyphenol Oxidase Assay
2.5. Chemical Analysis
2.5.1. Total Phenol Content
2.5.2. Antioxidant Capacity
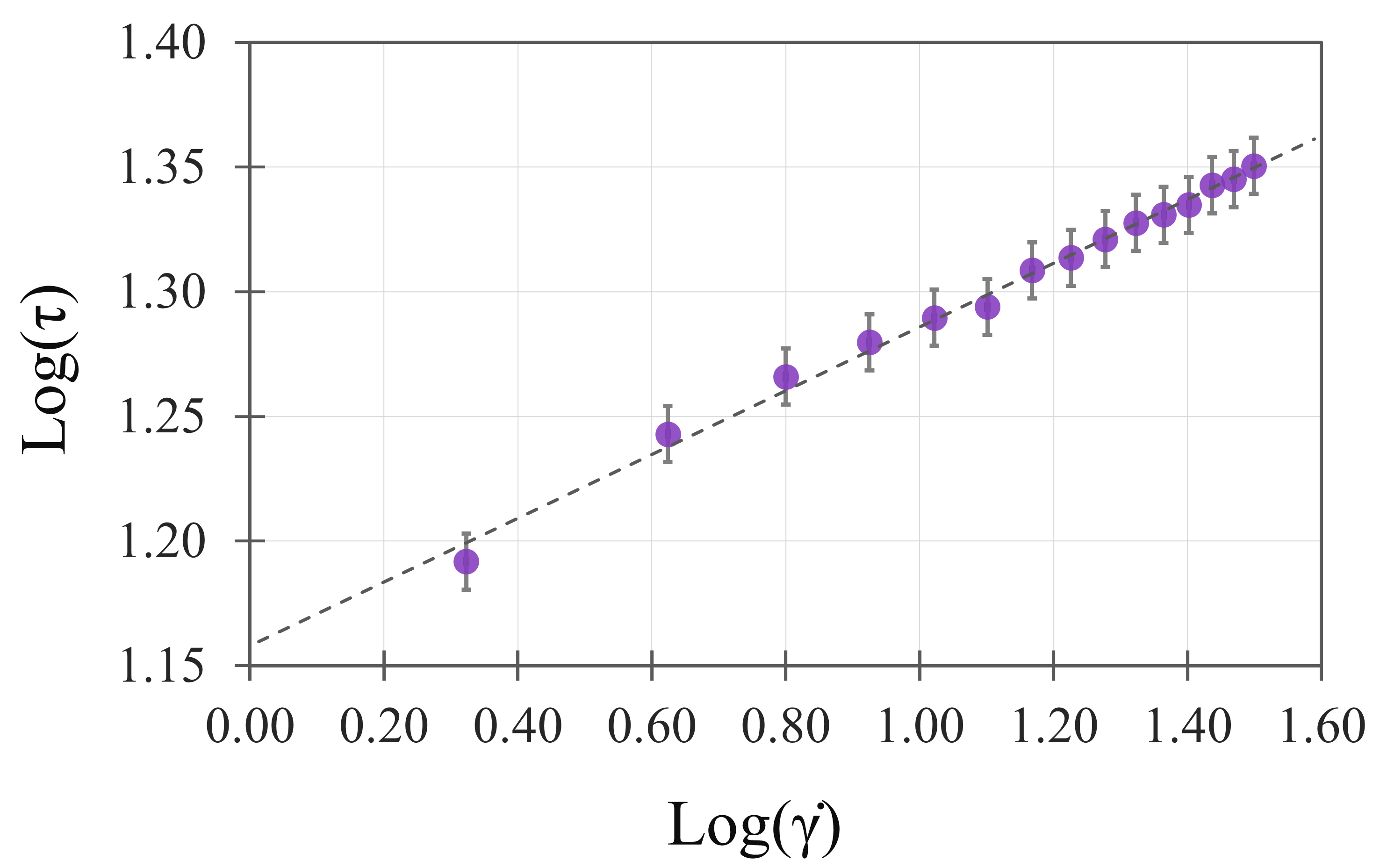
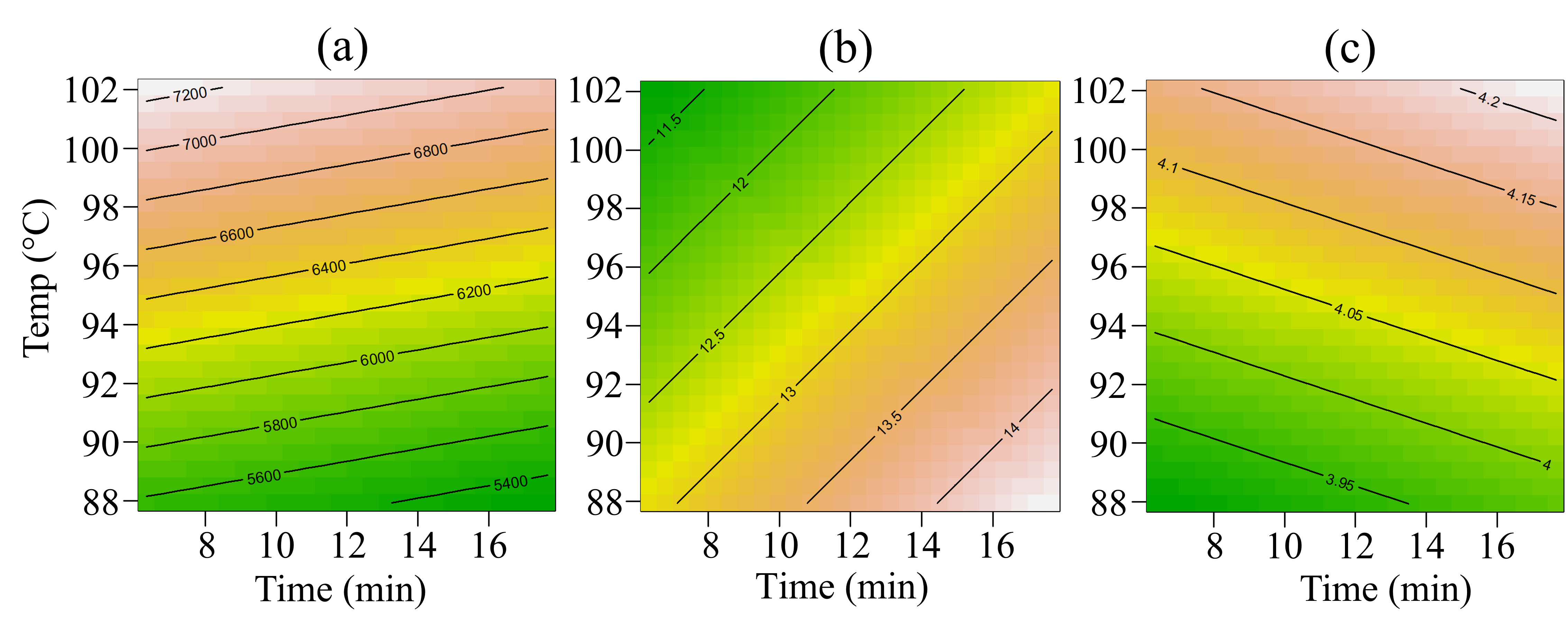
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Batch | Time | Temp | Viscosity | Syneresis | TSSs | pH | WC |
|---|---|---|---|---|---|---|---|
| (min:s) | (°C) | (mPa s) | (°Brix) | (%) | |||
| B17 | Control | 3312 | 15.9 | 11.100 | 4.1 | 88.1 | |
| B17 | 08:00 | 90.0 | 5764 | 13.6 | 13.500 | 3.8 | 87.2 |
| B17 | 16:00 | 90.0 | 6088 | 16.9 | 13.400 | 4.1 | 86.2 |
| B17 | 08:00 | 100.0 | 7420 | 10.0 | 13.200 | 4.3 | 85.9 |
| B17 | 16:00 | 100.0 | 7272 | 15.4 | 10.700 | 4.2 | 87.4 |
| B17 | 12:00 | 95.0 | 7296 | 12.2 | 13.700 | 4.3 | 86.2 |
| B17 | 12:00 | 95.0 | 6052 | 13.3 | 13.400 | 4.0 | 86.5 |
| B17 | 06:34 | 95.0 | 6128 | 11.4 | 13.900 | 4.2 | 85.4 |
| B17 | 17:42 | 95.0 | 5420 | 12.9 | 12.500 | 4.2 | 87.3 |
| B17 | 12:00 | 87.9 | 5190 | 15.6 | 14.400 | 3.8 | 86.4 |
| B17 | 12:00 | 102.1 | 9266 | 10.7 | 9.200 | 4.3 | 83.2 |
| B17 | 12:00 | 95.0 | 7220 | 12.5 | 13.100 | 4.1 | 86.2 |
| B17 | 12:00 | 95.0 | 6763 | 12.4 | 14.200 | 4.1 | 85.5 |
| B18 | Control | 4040 | 15.1 | 11.5 | 3.9 | 87.5 | |
| B18 | 08:00 | 90.0 | 6220 | 13.5 | 12.600 | 3.9 | 85.9 |
| B18 | 16:00 | 90.0 | 6024 | 13.6 | 12.200 | 4.0 | 86.7 |
| B18 | 8:00 | 100.0 | 5432 | 11.1 | 12.700 | 4.4 | 86.4 |
| B18 | 16:00 | 100.0 | 5408 | 13.1 | 13.100 | 4.0 | 85.6 |
| B18 | 12:00 | 95.0 | 6100 | 12.2 | 13.200 | 4.0 | 86.4 |
| B18 | 12:00 | 95.0 | 5043 | 11.9 | 12.300 | 4.0 | 87.0 |
| B18 | 06:34 | 95.0 | 7452 | 12.3 | 12.300 | 4.0 | 86.7 |
| B18 | 17:42 | 95.0 | 4264 | 12.0 | 10.200 | 4.0 | 85.6 |
| B18 | 12:00 | 87.9 | 5680 | 12.7 | 12.500 | 4.0 | 85.6 |
| B18 | 12:00 | 102.1 | 5748 | 11.1 | 11.300 | 4.0 | 86.3 |
| B18 | 12:00 | 95.0 | 6961 | 12.3 | 11.500 | 4.3 | 85.8 |
| B18 | 12:00 | 95.0 | 6085 | 12.4 | 11.600 | 4.2 | 84.9 |
| B19 | Control | 3384 | 17.5 | 10.1 | 3.8 | 88.7 | |
| B19 | 08:00 | 90.0 | 5204 | 12.2 | 10.600 | 3.9 | 86.7 |
| B19 | 16:00 | 90.0 | 5876 | 12.4 | 10.600 | 4.0 | 87.4 |
| B19 | 8:00 | 100.0 | 6616 | 14.9 | 10.200 | 4.0 | 86.8 |
| B19 | 16:00 | 100.0 | 5952 | 14.6 | 10.100 | 4.1 | 88.3 |
| B19 | 12:00 | 95.0 | 7116 | 12.4 | 10.300 | 4.1 | 87.8 |
| B19 | 12:00 | 95.0 | 5800 | 14.6 | 10.500 | 4.1 | 87.6 |
| B19 | 06:34 | 95.0 | 4504 | 12.2 | 10.200 | 3.9 | 87.3 |
| B19 | 17:42 | 95.0 | 6712 | 12.8 | 10.000 | 4.1 | 88.0 |
| B19 | 12:00 | 87.9 | 5472 | 13.0 | 11.200 | 4.0 | 87.3 |
| B19 | 12:00 | 102.1 | 9340 | 12.1 | 10.400 | 4.2 | 87.1 |
| B19 | 12:00 | 95.0 | 6252 | 13.0 | 10.800 | 4.1 | 87.9 |
| B19 | 12:00 | 95.0 | 6633 | 13.9 | 10.400 | 3.9 | 86.2 |
| Batch | Time | Temp | L | a | b | Chroma | Hue | ΔE | BI |
|---|---|---|---|---|---|---|---|---|---|
| B17 | Control | 46.0 | 18.0 | 27.3 | 34.8 | 1.0 | 0.0 | 12.3 | |
| B17 | 08:00 | 90.0 | 57.3 | 18.3 | 41.7 | 45.5 | 1.2 | 20.7 | 13.8 |
| B17 | 16:00 | 90.0 | 59.5 | 18.0 | 42.5 | 46.2 | 1.2 | 22.7 | 13.4 |
| B17 | 8:00 | 100.0 | 54.5 | 20.5 | 36.5 | 41.9 | 1.1 | 14.9 | 12.2 |
| B17 | 16:00 | 100.0 | 58.0 | 16.0 | 40.0 | 43.1 | 1.2 | 20.9 | 12.6 |
| B17 | 12:00 | 95.0 | 54.0 | 20.0 | 35.5 | 40.7 | 1.1 | 13.7 | 12.3 |
| B17 | 12:00 | 95.0 | 59.0 | 19.0 | 38.5 | 42.9 | 1.1 | 21.5 | 12.1 |
| B17 | 06:34 | 95.0 | 55.0 | 21.5 | 35.5 | 41.5 | 1.0 | 14.5 | 12.4 |
| B17 | 17:42 | 95.0 | 57.0 | 18.0 | 39.5 | 43.4 | 1.1 | 18.8 | 13.0 |
| B17 | 12:00 | 87.9 | 58.5 | 22.0 | 44.0 | 49.2 | 1.1 | 23.1 | 12.9 |
| B17 | 12:00 | 102.1 | 55.5 | 17.5 | 40.5 | 44.1 | 1.2 | 20.0 | 12.9 |
| B17 | 12:00 | 95.0 | 56.0 | 19.0 | 35.0 | 39.8 | 1.1 | 13.9 | 11.6 |
| B17 | 12:00 | 95.0 | 56.5 | 16.0 | 35.0 | 38.5 | 1.1 | 15.4 | 12.1 |
| B18 | Control | 45.0 | 19.0 | 29.0 | 36.4 | 1.0 | 0.0 | 13.6 | |
| B18 | 08:00 | 90.0 | 53.5 | 19.0 | 36.0 | 40.7 | 1.1 | 13.2 | 12.8 |
| B18 | 16:00 | 90.0 | 57.5 | 13.0 | 38.0 | 40.2 | 1.2 | 17.7 | 11.6 |
| B18 | 8:00 | 100.0 | 56.0 | 15.0 | 37.0 | 39.9 | 1.2 | 16.2 | 11.9 |
| B18 | 16:00 | 100.0 | 61.0 | 15.0 | 39.0 | 41.8 | 1.2 | 20.4 | 12.2 |
| B18 | 12:00 | 95.0 | 56.0 | 15.0 | 38.0 | 40.9 | 1.2 | 16.1 | 12.1 |
| B18 | 12:00 | 95.0 | 59.5 | 20.0 | 43.0 | 44.4 | 1.1 | 20.9 | 12.8 |
| B18 | 06:34 | 95.0 | 56.0 | 14.0 | 36.5 | 39.1 | 1.2 | 16.0 | 11.5 |
| B18 | 17:42 | 95.0 | 56.0 | 18.0 | 40.0 | 43.9 | 1.1 | 17.5 | 13.5 |
| B18 | 12:00 | 87.9 | 58.0 | 19.5 | 40.5 | 44.9 | 1.1 | 19.5 | 13.2 |
| B18 | 12:00 | 102.1 | 56.0 | 17.0 | 37.5 | 41.2 | 1.1 | 14.7 | 12.9 |
| B18 | 12:00 | 95.0 | 53.5 | 17.5 | 32.5 | 38.9 | 1.1 | 10.5 | 11.1 |
| B18 | 12:00 | 95.0 | 53.5 | 17.0 | 34.0 | 40.0 | 1.1 | 11.6 | 11.7 |
| B19 | Control | 44.3 | 17.0 | 28.0 | 35.4 | 1.1 | 0.0 | 13.6 | |
| B19 | 08:00 | 90.0 | 56.0 | 16.0 | 38.0 | 41.2 | 1.2 | 17.1 | 12.4 |
| B19 | 16:00 | 90.0 | 53.0 | 17.0 | 34.5 | 38.5 | 1.1 | 14.1 | 12.0 |
| B19 | 8:00 | 100.0 | 57.0 | 14.0 | 37.0 | 39.6 | 1.2 | 16.4 | 11.4 |
| B19 | 16:00 | 100.0 | 55.5 | 17.0 | 36.0 | 39.8 | 1.1 | 17.0 | 11.9 |
| B19 | 12:00 | 95.0 | 52.0 | 16.0 | 33.5 | 38.1 | 1.1 | 10.8 | 11.8 |
| B19 | 12:00 | 95.0 | 55.0 | 16.0 | 39.0 | 42.2 | 1.2 | 16.1 | 12.2 |
| B19 | 06:34 | 95.0 | 54.5 | 15.0 | 36.0 | 39.0 | 1.2 | 13.4 | 11.9 |
| B19 | 17:42 | 95.0 | 55.0 | 17.0 | 35.5 | 39.4 | 1.1 | 14.0 | 11.8 |
| B19 | 12:00 | 87.9 | 57.0 | 13.0 | 41.0 | 43.0 | 1.3 | 20.6 | 13.4 |
| B19 | 12:00 | 102.1 | 55.5 | 19.5 | 39.0 | 43.6 | 1.1 | 16.9 | 13.5 |
| B19 | 12:00 | 95.0 | 53.0 | 17.0 | 34.0 | 38.0 | 1.1 | 11.1 | 11.8 |
| B19 | 12:00 | 95.0 | 57.5 | 19.5 | 39.0 | 43.6 | 1.1 | 16.9 | 12.8 |
References
- Ismea Repot Mercati Agricoli. Available online: https://www.ismea.it/istituto-di-servizi-per-il-mercato-agricolo-alimentare (accessed on 7 June 2024).
- Buergy, A.; Rolland-Sabaté, A.; Leca, A.; Falourd, X.; Foucat, L.; Renard, C.M.G.C. Pectin Degradation Accounts for Apple Tissue Fragmentation during Thermomechanical-Mediated Puree Production. Food Hydrocoll. 2021, 120, 106885. [Google Scholar] [CrossRef]
- Awuah, G.B.; Ramaswamy, H.S.; Economides, A. Thermal Processing and Quality: Principles and Overview. Chem. Eng. Process. Process Intensif. 2007, 46, 584–602. [Google Scholar] [CrossRef]
- Castaldo, D.; Laratta, B.; Loiudice, R.; Giovane, A.; Quagliuolo, L.; Servillo, L. Presence of Residual Pectin Methylesterase Activity in Thermally Stabilized Industrial Fruit Preparations. LWT Food Sci. Technol. 1997, 30, 479–484. [Google Scholar] [CrossRef]
- Amiot, M.J.; Aubert, S.; Nicolas, J. Phenolic Composition and Browning Susceptibility of Various Apple and Pear Cultivars at Maturity. Acta Hortic. 1993, 343, 67–69. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.; Aubert, S.Y. Enzymatic Browning Reactions in Apple and Apple Products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef]
- Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Contreras-Esquivel, J.C.; Escobedo-García, S.; Morlett-Chávez, J.A.; Rodríguez-Herrera, R. Analytical Methods for Pectin Methylesterase Activity Determination: A Review. Food Anal. Methods 2017, 10, 3634–3646. [Google Scholar] [CrossRef]
- Espinosa-Muñoz, L.; Renard, C.M.G.C.; Symoneaux, R.; Biau, N.; Cuvelier, G. Structural Parameters That Determine the Rheological Properties of Apple Puree. J. Food Eng. 2013, 119, 619–626. [Google Scholar] [CrossRef]
- Mathew, A.G.; Parpia, H.A.B. Food Browning as a Polyphenol Reaction. In Advances in Food Research; Chichester, C.O., Mrak, E.M., Stewart, G.F.B.T.-A., Eds.; Academic Press: Cambridge, MA, USA, 1971; Volume 19, pp. 75–145. ISBN 0065-2628. [Google Scholar]
- Loew, O. Catalase, a New Enzym of General Occurrence with Special Reference to the Tobacco Plant; United States Department of Agriculture, Ed.; Government Printing Office: Washington, DC, USA, 1901. [Google Scholar]
- Arnold, M.; Gramza-Michałowska, A. Enzymatic Browning in Apple Products and Its Inhibition Treatments: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5038–5076. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of Polyphenol Oxidase and Peroxidase and Influence on Browning of Cold Stored Strawberry Fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef]
- Kim, A.N.; Lee, K.Y.; Rahman, M.S.; Kim, H.J.; Kerr, W.L.; Choi, S.G. Thermal Treatment of Apple Puree under Oxygen-Free Condition: Effect on Phenolic Compounds, Ascorbic Acid, Antioxidant Activities, Color, and Enzyme Activities. Food Biosci. 2021, 39, 100802. [Google Scholar] [CrossRef]
- Horie, F.; Kamei, M.; Nishibe, M.; Ogawa, Y.; Tanibuchi, M.; Gotow, N.; Oyama-Okubo, N.; Kohyama, K.; Kobayakawa, T.; Kusakabe, Y. Flavor Intensity Is Reduced in Pureed Food: A Study Using Instrumental and Sensory Analyses. Food Qual. Prefer. 2024, 115, 105121. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Pangborn, R.; Szczesniak, A. Effects of Hydrocolloids on Flavour and Odour Intensities of Aromatic Compounds. J. Texture Stud. 2007, 4, 467–482. [Google Scholar] [CrossRef]
- Sepehr, A.; Zaborowicz, M.; Gabardi, C.; Gabardi, N.; Biada, E.; Luzzini, M.; Zanchin, A.; Guerrini, L. Machine Learning Approach to Inline Monitoring of Apple Puree Consistency through Process Data and Fruit Characteristics. J. Food Eng. 2026, 403, 112712. [Google Scholar] [CrossRef]
- Cánovas, J.A.; Gea-Botella, S.; Borrás, F.; Martí, N.; Valero, M.; Saura, D.; Martínez-Madrid, M.C.; Laencina, J. Vitamin C Loss Kinetics and Shelf Life Study in Fruit-Based Baby Foods during Post Packaging Storage. Food Packag. Shelf Life 2020, 23, 100453. [Google Scholar] [CrossRef]
- Pitotti, A.; Elizalde, B.E.; Anese, M. Effect of Caramelization and Maillard Reaction Products on Peroxidase. J. Food Biochem. 1994, 18, 445–457. [Google Scholar] [CrossRef]
- de Castro, B.R.; Tribst, A.A.L.; Cristianini, M. Effect of High-Pressure Technologies on Enzymes Applied in Food Processing. In Enzyme Inhibitors and Activators; Şentürk, M., Ed.; IntechOpen: London, UK, 2017; pp. 49–72. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Lin, Y.; Nasiru, M.M.; Zhang, J.; Abid, M.; Murtaza, M.A.; Zhao, L. Comparative Study: Thermal and Non-Thermal Treatment on Enzyme Deactivation and Selected Quality Attributes of Fresh Carrot Juice. Int. J. Food Sci. Technol. 2022, 57, 827–841. [Google Scholar] [CrossRef]
- Martinez, M.V.; Whitaker, J.R. The Biochemistry and Control of Enzymatic Browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Guerra, L.; Romagnoli, G.; Vignali, G. Extraction of Golden Delicious Apple Puree: Experimental Comparison of Three Different Methods. J. Food Eng. 2012, 110, 169–174. [Google Scholar] [CrossRef]
- Buergy, A.; Rolland-Sabaté, A.; Leca, A.; Renard, C.M.G.C. Apple Puree’s Texture Is Independent from Fruit Firmness. LWT 2021, 145, 11324. [Google Scholar] [CrossRef]
- Maskan, M. Effect of Thermal Processing on Tristimulus Colour Changes of Fruits. Stewart Postharvest Rev. 2008, 2, 10. [Google Scholar] [CrossRef]
- Hirschler, R. Whiteness, yellowness, and Browning in Food Colorimetry. Color Food Technol. Psychophys. Asp. 2012, 93–104. [Google Scholar]
- Silva, F.M.; Sims, C.; Balaban, M.O.; Silva, C.L.M.; O’Keefe, S. Kinetics of Flavour and Aroma Changes in Thermally Processed Cupuaçu (Theobroma grandiflorum) Pulp. J. Sci. Food Agric. 2000, 80, 783–787. [Google Scholar] [CrossRef]
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; Van Loey, A.; Smout, C.; Hendrickx, M. Effect of Thermal Blanching and of High Pressure Treatments on Sweet Green and Red Bell Pepper Fruits (Capsicum annuum L.). Food Chem. 2008, 107, 1436–1449. [Google Scholar] [CrossRef]
- Terefe, N.S.; Matthies, K.; Simons, L.; Versteeg, C. Combined High Pressure-Mild Temperature Processing for Optimal Retention of Physical and Nutritional Quality of Strawberries (Fragaria × Ananassa). Innov. Food Sci. Emerg. Technol. 2009, 10, 297–307. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Effect of Combined High Pressure–Temperature Treatments on Color and Nutritional Quality Attributes of Pineapple (Ananas comosus L.) Puree. Innov. Food Sci. Emerg. Technol. 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Badin, E.E.; Rossi, Y.E.; Montenegro, M.A.; Ibarz, A.; Ribotta, P.D.; Lespinard, A.R. Thermal Processing of Raspberry Pulp: Effect on the Color and Bioactive Compounds. Food Bioprod. Process. 2020, 124, 469–477. [Google Scholar] [CrossRef]
- Liaotrakoon, W.; de Clercq, N.; van Hoed, V.; van de Walle, D.; Lewille, B.; Dewettinck, K. Impact of Thermal Treatment on Physicochemical, Antioxidative and Rheological Properties of White-Flesh and Red-Flesh Dragon Fruit (Hylocereus spp.) Purees. Food Bioprocess Technol. 2013, 6, 416–430. [Google Scholar] [CrossRef]
- Salazar-Orbea, G.L.; García-Villalba, R.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. High–Pressure Processing vs. Thermal Treatment: Effect on the Stability of Polyphenols in Strawberry and Apple Products. Foods 2021, 10, 2919. [Google Scholar] [CrossRef]
- Wang, Z.; Bureau, S.; Jaillais, B.; Renard, C.M.G.C.; Chen, X.; Sun, Y.; Lv, D.; Pan, L.; Lan, W. Infrared Guided Smart Food Formulation: An Innovative Spectral Reconstruction Strategy to Develop Anticipated and Constant Apple Puree Products. Food Innov. Adv. 2024, 3, 20–30. [Google Scholar] [CrossRef]
- Espinosa, L.; To, N.; Symoneaux, R.; Renard, C.M.G.C.; Biau, N.; Cuvelier, G. Effect of Processing on Rheological, Structural and Sensory Properties of Apple Puree. Procedia Food Sci. 2011, 1, 513–520. [Google Scholar] [CrossRef]
- Szczepańska, J.; Pinto, C.A.; Skąpska, S.; Saraiva, J.A.; Marszałek, K. Effect of Static and Multi-Pulsed High Pressure Processing on the Rheological Properties, Microbial and Physicochemical Quality, and Antioxidant Potential of Apple Juice during Refrigerated Storage. LWT 2021, 150, 112038. [Google Scholar] [CrossRef]
- El Bouchikhi, S.; Pagès, P.; El Alaoui, Y.; Ibrahimi, A.; Bensouda, Y. Syneresis Investigations of Lacto-Fermented Sodium Caseinate in a Mixed Model System. BMC Biotechnol. 2019, 19, 57. [Google Scholar] [CrossRef]
- Heydari, S.; Amiri-Rigi, A.; Ehsani, M.R.; Mohammadifar, M.A.; Khorshidian, N.; Koushki, M.R.; Mortazavian, A.M. Rheological Behaviour, Sensory Properties and Syneresis of Probiotic Yoghurt Supplemented with Various Prebiotics. Int. J. Dairy Technol. 2018, 71, 175–184. [Google Scholar] [CrossRef]
- Kertesz, Z.I. Pectic Enzymes: I. The Determination of Pectin-Methoxylase Activity. J. Biol. Chem. 1937, 121, 589–598. [Google Scholar] [CrossRef]
- Lee, M.; Macmillan, J.D. Mode of Action of Pectic Enzymes. I. Purification and Certain Properties of Tomato Pectinesterase. Biochemistry 1968, 7, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Ünal, M.Ü.; Şener, A. Extraction and Characterization of Pectin Methylesterase from Alyanak Apricot (Prunus armeniaca L). J. Food Sci. Technol. 2015, 52, 1194–1199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Kersten, E.; Barry, A.; Klein, S. Physicochemical Characterisation of Fluids and Soft Foods Frequently Mixed with Oral Drug Formulations Prior to Administration to Children. Pharmazie 2016, 71, 122–127. [Google Scholar] [PubMed]
- Maceiras, R.; Álvarez, E.; Cancela, M.A. Rheological Properties of Fruit Purees: Effect of Cooking. J. Food Eng. 2007, 80, 763–769. [Google Scholar] [CrossRef]
- ASTM E3070; Standard Test Method for Shear Thinning Index of Non-Newtonian Liquids Using a Rotational Viscometer. ASTM International: West Conshohocken, PA, USA, 2022; p. 4.
- Saravacos, G.D. Effect of Temperature on Viscosity of Fruit Juices and Purees. J. Food Sci. 1970, 35, 122–125. [Google Scholar] [CrossRef]
- Sila, D.N.; Van Buggenhout, S.; Duvetter, T.; Fraeye, I.; De Roeck, A.; Van Loey, A.; Hendrickx, M. Pectins in Processed Fruits and Vegetables: Part II—Structure–Function Relationships. Compr. Rev. Food Sci. Food Saf. 2009, 8, 86–104. [Google Scholar] [CrossRef]
- da Silva, J.A.L.; Gonçalves, M.P.; Rao, M.A. Kinetics and Thermal Behaviour of the Structure Formation Process in HMP/Sucrose Gelation. Int. J. Biol. Macromol. 1995, 17, 25–32. [Google Scholar] [CrossRef]
- Guillon, F.; Barry, J.L.; Thibault, J.-F. Effect of Autoclaving Sugar-Beet Fibre on Its Physico-Chemical Properties and Its in-Vitro Degradation by Human Faecal Bacteria. J. Sci. Food Agric. 1992, 60, 69–79. [Google Scholar] [CrossRef]
- Müller, S.; Kunzek, H. Material Properties of Processed Fruit and Vegetables I. Effect of Extraction and Thermal Treatment on Apple Parenchyma. Eur. Food Res. Technol. 1998, 206, 264–272. [Google Scholar]
- Vikram, V.B.; Ramesh, M.N.; Prapulla, S.G. Thermal Degradation Kinetics of Nutrients in Orange Juice Heated by Electromagnetic and Conventional Methods. J. Food Eng. 2005, 69, 31–40. [Google Scholar] [CrossRef]
- Ibarz, A.; PagaÂn, J.; Garza, S. Kinetic Models of Non-Enzymatic Browning in Apple Puree. J. Sci. Food Agric. 2000, 80, 1162–1168. [Google Scholar] [CrossRef]
- Ciou, J.-Y.; Lin, H.-H.; Chiang, P.-Y.; Wang, C.-C.; Charles, A.L. The Role of Polyphenol Oxidase and Peroxidase in the Browning of Water Caltrop Pericarp during Heat Treatment. Food Chem. 2011, 127, 523–527. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Antioxidant Activity of Water-Soluble Maillard Reaction Products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Barreiro, J.A.; Milano, M.; Sandoval, A.J. Kinetics of Colour Change of Double Concentrated Tomato Paste during Thermal Treatment. J. Food Eng. 1997, 33, 359–371. [Google Scholar] [CrossRef]
- Ávila, I.M.L.B.; Silva, C.L.M. Modelling Kinetics of Thermal Degradation of Colour in Peach Puree. J. Food Eng. 1999, 39, 161–166. [Google Scholar] [CrossRef]
- Rhim, J.W.; Nunes, R.V.; Jones, V.A.; Swartzel, K.R. Kinetics of Color Change of Grape Juice Generated Using Linearly Increasing Temperature. J. Food Sci. Technol. 1989, 54, 776–777. [Google Scholar] [CrossRef]
- Arora, B.; Sethi, S.; Joshi, A.; Sagar, V.R.; Sharma, R.R. Antioxidant Degradation Kinetics in Apples. J. Food Sci. Technol. 2018, 55, 1306–1313. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Nogales-Delgado, S. Polyphenoloxidase (PPO): Effect, Current Determination and Inhibition Treatments in Fresh-Cut Produce. Appl. Sci. 2021, 11, 7813. [Google Scholar] [CrossRef]
- Coseteng, M.Y.; Lee, C.Y. Changes in Apple Polyphenoloxidase and Polyphenol Concentrations in Relation to Degree of Browning. J. Food Sci. 1987, 52, 985–989. [Google Scholar] [CrossRef]
- Denès, J.-M.; Baron, A.; Drilleau, J.-F. Purification, Properties and Heat Inactivation of Pectin Methylesterase from Apple (Cv Golden Delicious). J. Sci. Food Agric. 2000, 80, 1503–1509. [Google Scholar] [CrossRef]
- Bodelón, O.G.; Avizcuri, J.-M.; Fernández-Zurbano, P.; Dizy, M.; Préstamo, G. Pressurization and Cold Storage of Strawberry Purée: Colour, Anthocyanins, Ascorbic Acid and Pectin Methylesterase. LWT—Food Sci. Technol. 2013, 52, 123–130. [Google Scholar] [CrossRef]


| Time (min:s) | Temp (°C) | Repetition |
|---|---|---|
| 12:00 | 87.9 | 1 |
| 08:00 | 90.0 | 1 |
| 16:00 | 90.0 | 1 |
| 12:00 | 95.0 | 4 |
| 06:20 | 95.0 | 1 |
| 17:42 | 95.0 | 1 |
| 08:00 | 100.0 | 1 |
| 16:00 | 100.0 | 1 |
| 12:00 | 102.1 | 1 |
| Batch | Variable | Linear | Quadratic | Lack | ||||
|---|---|---|---|---|---|---|---|---|
| Temp | Time | Interaction | Temp | Time | p-Value | of Fit | ||
| All | Consistency | ** | NS | NS | NS | NS | * | NS |
| All | Syneresis | * | * | NS | NS | NS | * | NS |
| All | pH | *** | NS | NS | NS | NS | ** | NS |
| All | ΔE | * | NS | NS | NS | NS | * | NS |
| All | Chroma | ** | NS | NS | ** | NS | * | NS |
| All | BI | ** | NS | NS | ** | NS | * | NS |
| Variable | Linear | Quadratic | Batch | Interaction with Batch | ||||
|---|---|---|---|---|---|---|---|---|
| Temp | Time | Interaction | Temp | Time | Temp | Time | ||
| TSSs | ** | * | NS | NS | NS | *** | * | NS |
| WC | NS | NS | NS | NS | NS | * | NS | NS |
| PME | * | NS | NS | NS | NS | NS | ** | * |
| PPOs | NS | NS | NS | NS | NS | * | NS | NS |
| POs | NS | NS | * | NS | NS | * | NS | NS |
| Variable | Unit | B17 | B18 | B19 |
| PME | units mL−1 | 2.592 a ± 0.331 | 2.824 a ± 0.500 | 3.519 a ± 0.248 |
| POs | ABS min−1 g−1 | 0.668 a ± 0.016 | 1.188 ab ± 0.025 | 2.079 b ± 0.049 |
| PPOs | ABS min−1 g−1 | 0.099 b ± 0.002 | 0.121 c ± 0.002 | 0.063 a ± 0.001 |
| TPC | mg kg−1 | 301.6 ± 144.1 | 323.7± 79.1 | 272.5 ± 18.6 |
| TEAC | mg kg−1 | 839.7 b ± 149.6 | 725.2 b ± 137.9 | 1227.7 a ± 250.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zanchin, A.; Perbellini, A.; Meggio, S.; Gabardi, N.; Luzzini, M.; Guerrini, L. Influence of Thermal Treatment Conditions and Fruit Batches Variability on the Rheology and Physicochemical Profile of Golden Delicious Apple Purée. Foods 2025, 14, 3912. https://doi.org/10.3390/foods14223912
Li S, Zanchin A, Perbellini A, Meggio S, Gabardi N, Luzzini M, Guerrini L. Influence of Thermal Treatment Conditions and Fruit Batches Variability on the Rheology and Physicochemical Profile of Golden Delicious Apple Purée. Foods. 2025; 14(22):3912. https://doi.org/10.3390/foods14223912
Chicago/Turabian StyleLi, Shichao, Alessandro Zanchin, Anna Perbellini, Sebastiano Meggio, Nicola Gabardi, Marco Luzzini, and Lorenzo Guerrini. 2025. "Influence of Thermal Treatment Conditions and Fruit Batches Variability on the Rheology and Physicochemical Profile of Golden Delicious Apple Purée" Foods 14, no. 22: 3912. https://doi.org/10.3390/foods14223912
APA StyleLi, S., Zanchin, A., Perbellini, A., Meggio, S., Gabardi, N., Luzzini, M., & Guerrini, L. (2025). Influence of Thermal Treatment Conditions and Fruit Batches Variability on the Rheology and Physicochemical Profile of Golden Delicious Apple Purée. Foods, 14(22), 3912. https://doi.org/10.3390/foods14223912








