Impacts of Roasting Intensity and Cultivar on Date Seed Beverage Quality Traits and Volatile Compounds Using Digital Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physicochemical Characterization of Date Seed Beverage Brew
2.2.1. pH Measurement
2.2.2. Color Measurement
2.3. Near-Infrared Spectroscopy (NIR) Analysis
2.4. E-Nose and Data Extraction
2.5. Identification and Quantification of Volatiles by HS-SPME-GC-MS
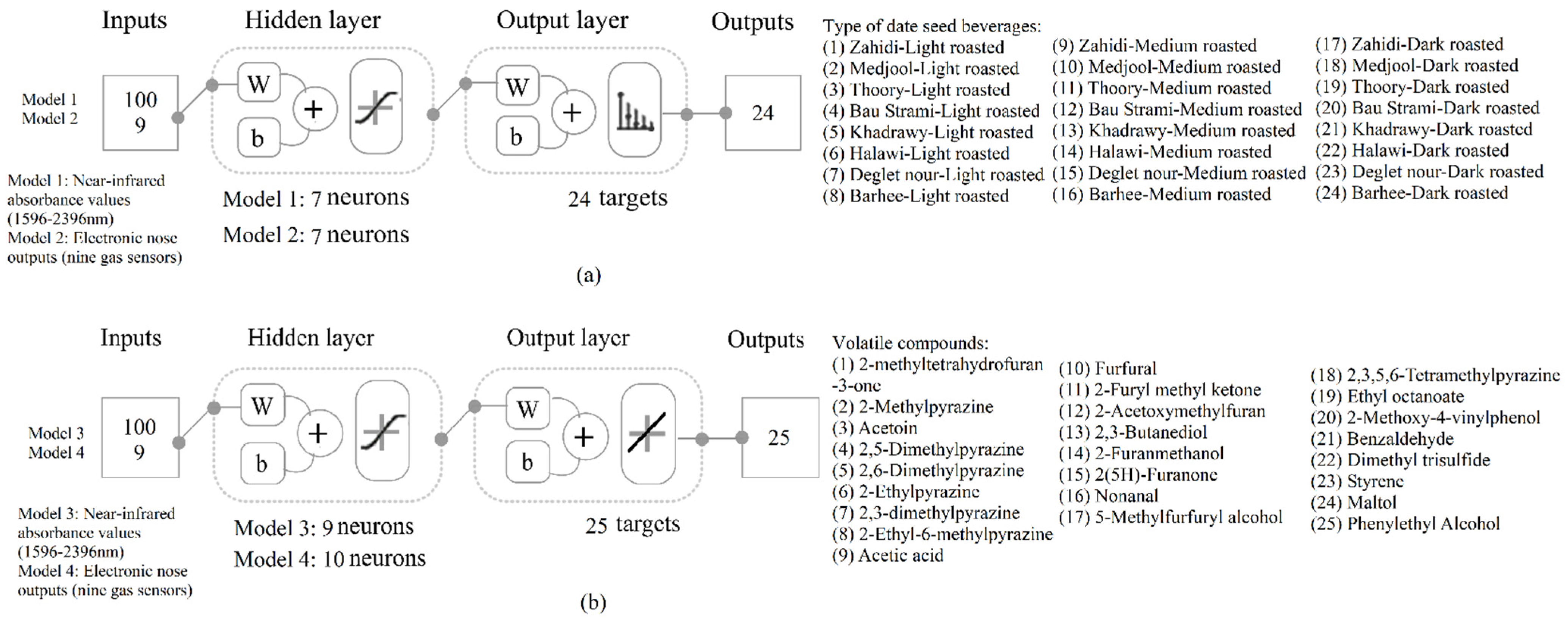
2.6. Statistical Analysis and Machine Learning (ML) Modeling
3. Results and Discussion
3.1. Physicochemical Properties
3.1.1. pH
3.1.2. Color Measurement
3.2. Near-Infrared Spectroscopy (NIR) Analysis
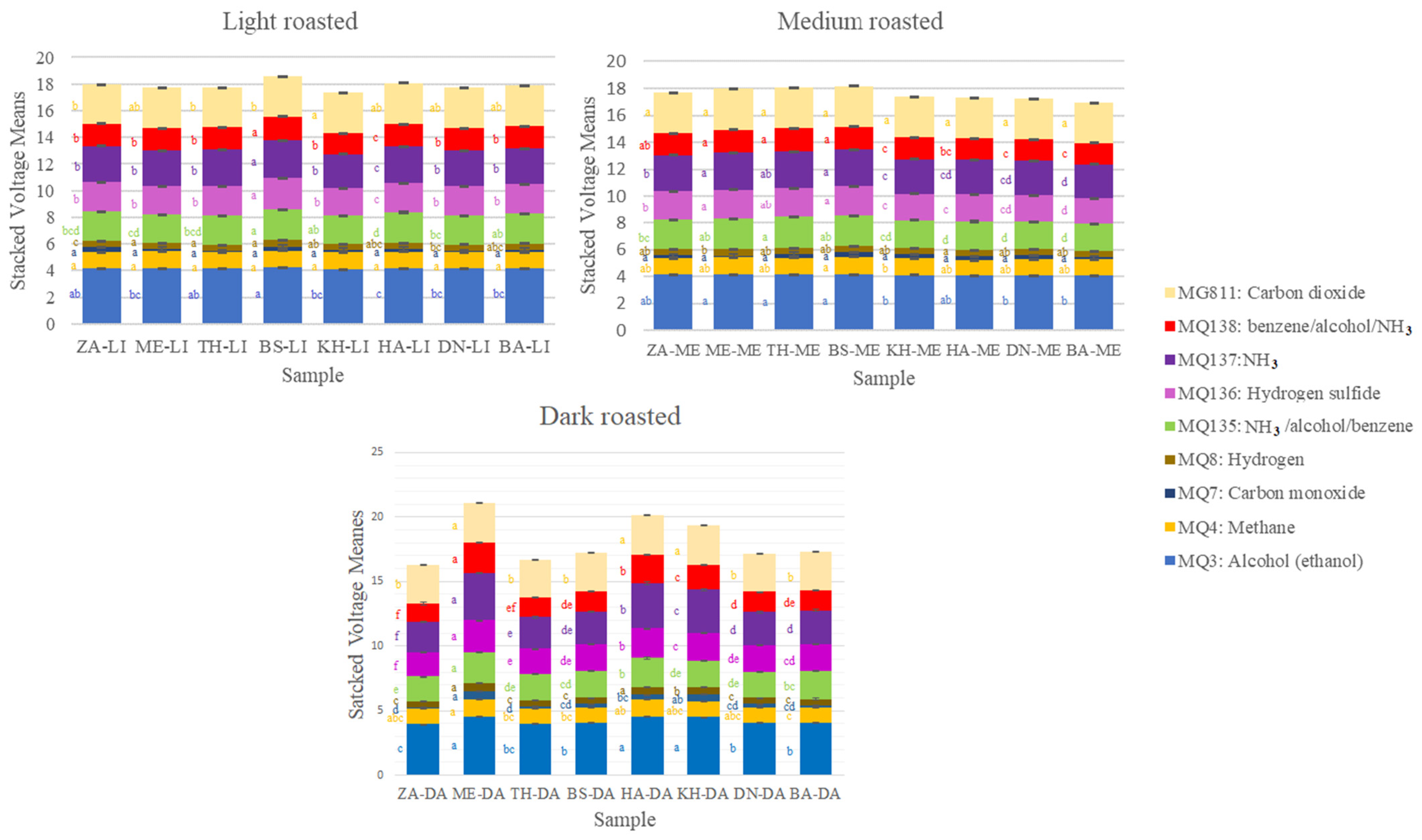
3.3. Electronic Nose Outputs
3.4. Identification and Quantification of Volatile Compounds in Date Seed Beverage
| No. | Compound Name | Molecular Formula | Aroma | RT * (min) | Concentration (μg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light Roasting | ||||||||||||
| ZA-LI | ME-LI | DN-LI | TH-LI | HA-LI | BA-LI | KH-LI | BS-LI | |||||
| 1 | 2-methyltetrahydrofuran-3-one | C5H8O2 | Sweet/bready/buttery | 14.64 | 0.19 | 0.13 | 0.15 | 0.24 | 0.21 | 0.16 | 0.15 | 0.15 |
| 2 | 2-Methylpyrazine | C5H6N2 | Nutty/cocoa/roasted | 14.73 | 0.59 | 0.72 | 0.51 | 1.03 | 0.95 | 1.10 | 0.35 | 1.76 |
| 3 | Acetoin | C4H8O2 | Sweet/buttery/creamy | 15.29 | 0.29 | 0.39 | 0.17 | 0.25 | 0.89 | 1.02 | 0.71 | 0.39 |
| 4 | 2,5-Dimethylpyrazine | C6H8N2 | Nutty/peanut/musty/earthy | 16.54 | 0.05 | 0.09 | 0.05 | 0.10 | 0.10 | 0.11 | 0.03 | 0.24 |
| 5 | 2,6-Dimethylpyrazine | C6H8N2 | Chocolate/nutty/roasted | 16.72 | 0.13 | 0.16 | 0.12 | 0.24 | 0.24 | 0.26 | 0.10 | 0.49 |
| 6 | 2-Ethylpyrazine | C6H8N2 | Nutty/roasted/cocoa/coffee | 16.89 | 0.09 | 0.12 | 0.08 | 0.16 | 0.13 | 0.17 | 0.06 | 0.31 |
| 7 | 2,3-dimethylpyrazine | C6H8N2 | Nutty/cocoa/coffee/walnut | 17.26 | 0.03 | 0.03 | 0.02 | 0.03 | 0.05 | 0.07 | 0.05 | 0.10 |
| 8 | 2-Ethyl-6-methylpyrazine | C7H10N2 | Roasted potato | 18.47 | 0.05 | 0.07 | 0.05 | 0.10 | 0.08 | 0.11 | 0.05 | 0.21 |
| 9 | Acetic acid | C2H4O2 | Sour/overripe fruit | 20.28 | 8.42 | 5.93 | 8.61 | 8.76 | 8.09 | 6.82 | 3.25 | 7.21 |
| 10 | Furfural | C5H4O2 | Sweet/woody/bready/caramel | 20.64 | 11.16 | 5.67 | 10.18 | 12.84 | 13.98 | 7.72 | 5.91 | 4.49 |
| 11 | 2-Furyl methyl ketone | C6H6O2 | Sweet/balsamic/almond/cocoa | 21.83 | 1.92 | 1.46 | 1.57 | 2.67 | 2.30 | 1.63 | 0.92 | 2.05 |
| 12 | 2-Acetoxymethylfuran | C7H8O3 | Sweet/fruity/banana/horseradish | 22.76 | 0.38 | 0.21 | 0.26 | 0.38 | 0.20 | 0.23 | 0.22 | 0.30 |
| 13 | 2,3-Butanediol | C4H10O2 | Fruity/creamy/buttery | 22.93 | 0.04 | 0.69 | 0.03 | 0.02 | 0.19 | 0.42 | 0.16 | 1.45 |
| 14 | 2-Furanmethanol | C5H6O2 | Sweet/bready/coffee | 26.08 | 0.12 | 0.14 | 0.10 | 0.14 | 0.11 | 0.14 | 0.03 | 0.17 |
| 15 | 2(5H)-Furanone | C4H4O2 | Buttery | 28.33 | 0.24 | 0.28 | 0.20 | 0.36 | 0.29 | 0.29 | 0.13 | 0.46 |
| 16 | Nonanal | C9H18O | Rose/orris/orange peel/fatty | 18.66 | 0.02 | 0.02 | 0.04 | 0.04 | 0.04 | 0.02 | 0.01 | 0.01 |
| 17 | 5-Methylfurfuryl alcohol | C6H8O2 | Caramellic/roasted/sweet/nutty | 27.66 | 0.02 | 0.04 | 0.01 | 0.02 | 0.02 | 0.03 | - | 0.06 |
| 18 | 2,3,5,6-Tetramethylpyrazine | C8H12N2 | Nutty/musty/chocolate/coffee | 21.06 | - | - | - | - | - | 0.03 | 0.02 | 0.03 |
| 19 | Ethyl octanoate | C10H20O2 | Fruity/winey/waxy/sweet | 19.93 | - | 0.01 | - | - | - | - | - | 0.05 |
| 20 | 2-Methoxy-4-vinylphenol | C7H8O2 | Spicy/peppery/smoky/woody | 30.93 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.02 |
| 21 | Benzaldehyde | C7H6O | Sharp/bitter/almond | 22.30 | 0.06 | 0.03 | 0.03 | 0.05 | 0.05 | 0.03 | 0.02 | 0.03 |
| 22 | Dimethyl trisulfide | C2H6S3 | Sulfurous/cooked onion/savory | 18.18 | 0.01 | 0.01 | 0.01 | 0.01 | - | 0.01 | - | 0.01 |
| 23 | Styrene | C8H8 | Sweet/balsamic/floral/plastic | 14.38 | 0.03 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 |
| 24 | Maltol | C6H6O3 | Sweet/caramellic/bread baked | 33.36 | 0.11 | 0.13 | 0.08 | 0.21 | 0.13 | 0.12 | 0.04 | 0.34 |
| 25 | Phenylethyl Alcohol | C8H10O | Floral/rose | 32.18 | 0.01 | 0.04 | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | 0.11 |
| No. | Compound Name | Molecular Formula | Aroma | RT * (min) | Concentration (μg/g) | |||||||
| Medium Roasting | ||||||||||||
| ZA-ME | ME-ME | DN-ME | TH-ME | HA-ME | BA-ME | KH-ME | BS-ME | |||||
| 1 | 2-methyltetrahydrofuran-3-one | C5H8O2 | Sweet/bready/buttery | 14.64 | 0.13 | 0.14 | 0.11 | 0.16 | 0.13 | 0.18 | 0.12 | 0.18 |
| 2 | 2-Methylpyrazine | C5H6N2 | Nutty/cocoa/roasted | 14.73 | 0.20 | 0.33 | 0.17 | 0.23 | 0.22 | 0.46 | 0.18 | 0.66 |
| 3 | Acetoin | C4H8O2 | Sweet/buttery/creamy | 15.29 | 0.15 | 0.16 | 0.07 | 0.09 | 0.94 | 0.86 | 1.22 | 0.20 |
| 4 | 2,5-Dimethylpyrazine | C6H8N2 | Nutty/peanut/musty/earthy | 16.54 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.05 | 0.01 | 0.07 |
| 5 | 2,6-Dimethylpyrazine | C6H8N2 | Chocolate/nutty/roasted | 16.72 | 0.05 | 0.08 | 0.05 | 0.07 | 0.06 | 0.13 | 0.05 | 0.20 |
| 6 | 2-Ethylpyrazine | C6H8N2 | Nutty/roasted/cocoa/coffee | 16.89 | 0.04 | 0.06 | 0.03 | 0.04 | 0.03 | 0.08 | 0.03 | 0.13 |
| 7 | 2,3-dimethylpyrazine | C6H8N2 | Nutty/cocoa/coffee/walnut | 17.26 | 0.01 | 0.02 | - | 0.01 | 0.02 | 0.05 | 0.02 | 0.04 |
| 8 | 2-Ethyl-6-methylpyrazine | C7H10N2 | Roasted potato | 18.47 | 0.03 | 0.04 | 0.02 | 0.03 | 0.03 | 0.06 | 0.02 | 0.11 |
| 9 | Acetic acid | C2H4O2 | Sour/overripe fruit | 20.28 | 4.21 | 4.91 | 3.97 | 4.43 | 4.05 | 5.48 | 3.57 | 5.55 |
| 10 | Furfural | C5H4O2 | Sweet/woody/bready/caramellic | 20.64 | 7.11 | 6.71 | 7.30 | 8.71 | 8.67 | 7.50 | 6.36 | 4.79 |
| 11 | 2-Furyl methyl ketone | C6H6O2 | Sweet/balsamic/almond/cocoa | 21.83 | 0.94 | 1.04 | 0.78 | 1.12 | 0.94 | 1.23 | 0.84 | 1.28 |
| 12 | 2-Acetoxymethylfuran | C7H8O3 | Sweet/fruity/banana/horseradish | 22.76 | 0.27 | 0.21 | 0.17 | 0.25 | 0.13 | 0.24 | 0.18 | 0.28 |
| 13 | 2,3-Butanediol | C4H10O2 | Fruity/creamy/buttery | 22.93 | 0.02 | 0.22 | 0.03 | 0.01 | 0.24 | 0.21 | 0.08 | 0.74 |
| 14 | 2-Furanmethanol | C5H6O2 | Sweet/bready/coffee | 26.08 | 0.03 | 0.05 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.05 |
| 15 | 2(5H)-Furanone | C4H4O2 | Buttery | 28.33 | 0.09 | 0.13 | 0.08 | 0.12 | 0.12 | 0.15 | 0.08 | 0.19 |
| 16 | Nonanal | C9H18O | Rose/orris/orange peel/fatty | 18.66 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | - | 0.01 |
| 17 | 5-Methylfurfuryl alcohol | C6H8O2 | Caramellic/roasted/sweet/nutty | 27.66 | - | - | - | - | - | - | - | - |
| 18 | 2,3,5,6-Tetramethylpyrazine | C8H12N2 | Nutty/musty/chocolate/coffee | 21.06 | - | - | - | - | - | 0.02 | - | 0.01 |
| 19 | Ethyl octanoate | C10H20O2 | Fruity/winey/waxy/sweet | 19.93 | - | 0.02 | - | - | - | - | - | 0.05 |
| 20 | 2-Methoxy-4-vinylphenol | C7H8O2 | Spicy/peppery/smoky/woody | 30.93 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
| 21 | Benzaldehyde | C7H6O | Sharp/bitter/almond | 22.30 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 |
| 22 | Dimethyl trisulfide | C2H6S3 | Sulfurous/cooked onion/savory | 18.18 | - | 0.01 | - | - | - | - | - | 0.01 |
| 23 | Styrene | C8H8 | Sweet/balsamic/floral/plastic | 14.38 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 |
| 24 | Maltol | C6H6O3 | Sweet/caramellic/bread baked | 33.36 | 0.03 | 0.04 | 0.02 | 0.04 | 0.04 | 0.05 | 0.02 | 0.07 |
| 25 | Phenylethyl Alcohol | C8H10O | Floral/rose | 32.18 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 |
| No. | Compound Name | Molecular Formula | Aroma | RT * (min) | Concentration (μg/g) | |||||||
| Dark Roasting | ||||||||||||
| ZA-DA | ME-DA | DN-DA | TH-DA | HA-DA | BA-DA | KH-DA | BS-DA | |||||
| 1 | 2-methyltetrahydrofuran-3-one | C5H8O2 | Sweet/bready/buttery | 14.64 | 0.11 | 0.19 | 0.11 | 0.15 | 0.14 | 0.15 | 0.11 | 0.15 |
| 2 | 2-Methylpyrazine | C5H6N2 | Nutty/cocoa/roasted | 14.73 | 0.14 | 0.28 | 0.14 | 0.19 | 0.19 | 0.35 | 0.14 | 0.61 |
| 3 | Acetoin | C4H8O2 | Sweet/buttery/creamy | 15.29 | 0.12 | 0.15 | 0.07 | 0.09 | 0.59 | 0.71 | 0.73 | 0.14 |
| 4 | 2,5-Dimethylpyrazine | C6H8N2 | Nutty/peanut/musty/earthy | 16.54 | 0.01 | 0.04 | 0.04 | 0.02 | 0.02 | 0.03 | 0.04 | 0.06 |
| 5 | 2,6-Dimethylpyrazine | C6H8N2 | Chocolate/nutty/roasted | 16.72 | 0.04 | 0.09 | 0.04 | 0.05 | 0.05 | 0.10 | 0.04 | 0.19 |
| 6 | 2-Ethylpyrazine | C6H8N2 | Nutty/roasted/cocoa/coffee | 16.89 | 0.02 | 0.05 | 0.02 | 0.04 | 0.03 | 0.06 | 0.02 | 0.11 |
| 7 | 2,3-dimethylpyrazine | C6H8N2 | Nutty/cocoa/coffee/walnut | 17.26 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.05 | 0.02 | 0.04 |
| 8 | 2-Ethyl-6-methylpyrazine | C7H10N2 | Roasted potato | 18.47 | 0.02 | 0.04 | 0.02 | 0.02 | 0.02 | 0.05 | 0.02 | 0.10 |
| 9 | Acetic acid | C2H4O2 | Sour/overripe fruit | 20.28 | 2.97 | 3.71 | 3.78 | 3.58 | 3.38 | 3.25 | 3.21 | 3.73 |
| 10 | Furfural | C5H4O2 | Sweet/woody/bready/caramellic | 20.64 | 6.30 | 5.91 | 7.68 | 8.81 | 8.57 | 5.91 | 7.54 | 4.43 |
| 11 | 2-Furyl methyl ketone | C6H6O2 | Sweet/balsamic/almond/cocoa | 21.83 | 0.74 | 0.91 | 0.82 | 1.00 | 0.90 | 0.92 | 0.94 | 1.18 |
| 12 | 2-Acetoxymethylfuran | C7H8O3 | Sweet/fruity/banana/horseradish | 22.76 | 0.27 | 0.36 | 0.24 | 0.27 | 0.17 | 0.22 | 0.27 | 0.31 |
| 13 | 2,3-Butanediol | C4H10O2 | Fruity/creamy/buttery | 22.93 | 0.02 | 0.28 | 0.02 | - | 0.13 | 0.16 | 0.06 | 0.64 |
| 14 | 2-Furanmethanol | C5H6O2 | Sweet/bready/coffee | 26.08 | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 0.04 |
| 15 | 2(5H)-Furanone | C4H4O2 | Buttery | 28.33 | 0.10 | 0.12 | 0.11 | 0.14 | 0.14 | 0.13 | 0.11 | 0.19 |
| 16 | Nonanal | C9H18O | Rose/orris/orange peel/fatty | 18.66 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
| 17 | 5-Methylfurfuryl alcohol | C6H8O2 | Caramellic/roasted/sweet/nutty | 27.66 | - | - | - | - | - | - | - | - |
| 18 | 2,3,5,6-Tetramethylpyrazine | C8H12N2 | Nutty/musty/chocolate/coffee | 21.06 | - | - | - | - | - | 0.02 | - | - |
| 19 | Ethyl octanoate | C10H20O2 | Fruity/winey/waxy/sweet | 19.93 | - | 0.01 | - | - | - | - | - | 0.08 |
| 20 | 2-Methoxy-4-vinylphenol | C7H8O2 | Spicy/peppery/smoky/woody | 30.93 | 0.02 | 0.05 | 0.02 | 0.01 | 0.01 | 0.03 | 0.03 | 0.02 |
| 21 | Benzaldehyde | C7H6O | Sharp/bitter/almond | 22.30 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 22 | Dimethyl trisulfide | C2H6S3 | Sulfurous/cooked onion/savory | 18.18 | - | 0.01 | - | - | - | - | - | - |
| 23 | Styrene | C8H8 | Sweet/balsamic/floral/plastic | 14.38 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 24 | Maltol | C6H6O3 | Sweet/caramellic/bread baked | 33.36 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | 0.04 | 0.02 | 0.06 |
| 25 | Phenylethyl Alcohol | C8H10O | Floral/rose | 32.18 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.06 |
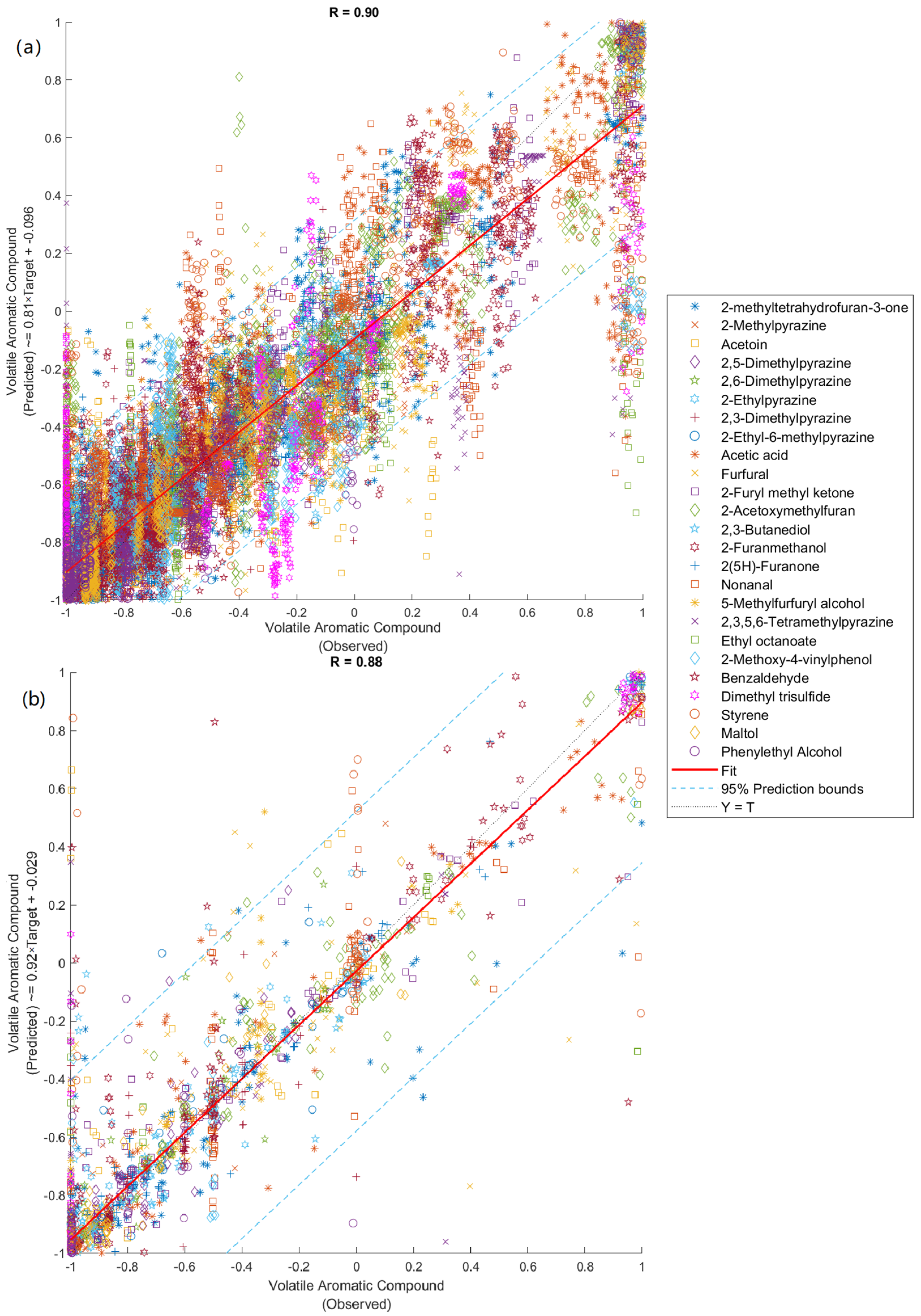
3.5. Machine Learning Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souda, B.; Rami, R.; Jalloul, B.; Mohamed, D. Roasted date palm seeds (Phoenix dactylifera) as an alternative coffee: Chemical composition and bioactive properties. Biomass Convers. Biorefin. 2022, 12, 3771–3781. [Google Scholar] [CrossRef]
- Shi, L.; de Souza, T.S.P.; Ahmadi, F.; Imran, A.; Dunshea, F.R.; Barrow, C.; Suleria, H.A.R. Valorization of Date Fruit (Phoenix dactylifera L.) Processing Waste and By-Products: A Review. Appl. Sci. 2023, 13, 12315. [Google Scholar] [CrossRef]
- Niazi, S.; Khan, I.M.; Rasheed, S.; Niazi, F.; Shoaib, M.; Raza, H.; Iqbal, M.W. An overview: Date palm seed coffee, a functional beverage. Int. J. Public Health 2017, 2, 18–25. [Google Scholar]
- Ghnimi, S.; Almansoori, R.; Jobe, B.; Hassan, M.; Afaf, K. Quality evaluation of coffee-like beverage from date seeds (Phoenix dactylifera L.). J. Food Process. Technol. 2015, 6, 1000525. [Google Scholar] [CrossRef]
- Hu, G.; Peng, X.; Gao, Y.; Huang, Y.; Li, X.; Su, H.; Qiu, M. Effect of roasting degree of coffee beans on sensory evaluation: Research from the perspective of major chemical ingredients. Food Chem. 2020, 331, 127329. [Google Scholar] [CrossRef]
- Choi, S.; Jung, S.; Ko, K.S. Effects of Coffee Extracts with Different Roasting Degrees on Antioxidant and Anti-Inflammatory Systems in Mice. Nutrients 2018, 10, 363. [Google Scholar] [CrossRef]
- Ismail, W.M.; Zayed, A.; Ramadan, N.S.; Sakna, S.T.; Farag, M.A. GC-MS based nutritional and aroma profiling of date palm seeds collected from different Egyptian cultivars for valorization purposes. Sci. Rep. 2025, 15, 16531. [Google Scholar] [CrossRef]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Rahman, R.A.; Chin, N.L.; Mousa, E.; Chang, L.S. Effect of the Roasting Conditions on the Physicochemical, Quality and Sensory Attributes of Coffee-Like Powder and Brew from Defatted Palm Date Seeds. Foods 2019, 8, 61. [Google Scholar] [CrossRef]
- Shi, L.; Ghafoor, K.; Viejo, C.G.; Jara, S.A.F.; Ahmadi, F.; Suleria, H.A. Impact of Roasting Temperature on Antioxidant Activities and Characterization of Polyphenols in Date Seed Beverages from Different Cultivars. J. Food Sci. 2025, 90, e70242. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Viejo, C.G.; Fuentes, S.; Dunshea, F.R.; Suleria, H.A. Evaluation of spontaneous fermentation impact on the physicochemical properties and sensory profile of green and roasted arabica coffee by digital technologies. Food Res. Int. 2024, 176, 113800. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.; Howell, K.; Dunshea, F.R. Assessment of beer quality based on foamability and chemical composition using computer vision algorithms, near infrared spectroscopy and machine learning algorithms. J. Sci. Food Agric. 2018, 98, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C. The Effect of Bubble Formation Within Carbonated Drinks on the Brewage Foamability, Bubble Dynamics and Sensory Perception by Consumers; The University of Melbourne: Melbourne, Australia, 2020. [Google Scholar]
- Gonzalez Viejo, C.; Tongson, E.; Fuentes, S. Integrating a low-cost electronic nose and machine learning modelling to assess coffee aroma profile and intensity. Sensors 2021, 21, 2016. [Google Scholar] [CrossRef]
- Gonzalez Viejo, G.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A. Impact of roasting on the phenolic and volatile compounds in coffee beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Emerging technologies based on artificial intelligence to assess the quality and consumer preference of beverages. Beverages 2019, 5, 62. [Google Scholar] [CrossRef]
- Yousif, A.K.; Alghzawi, H. Processing and characterization of carob powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- Babiker, E.E.; Atasoy, G.; Özcan, M.M.; Juhaimi, F.A.; Ghafoor, K.; Ahmed, I.A.M.; Almusallam, I.A. Bioactive compounds, minerals, fatty acids, color, and sensory profile of roasted date (Phoenix dactylifera L.) seed. J. Food Process. Preserv. 2020, 44, e14495. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Jioe, I.P.J. The Analysis of Chlorogenic Acid and Caffeine Content and Its Correlation with Coffee Bean Color under Different Roasting Degree and Sources of Coffee (Coffea arabica Typica). Processes 2021, 9, 2040. [Google Scholar] [CrossRef]
- Noda, K.; Amano, Y.; Shimamura, Y.; Murata, M. Distribution of pyrrolothiazolate, a pigment formed through the Maillard reaction between cysteine and glucose, in foods and beverages and some of its properties. Food Sci. Technol. Res. 2020, 26, 735–742. [Google Scholar] [CrossRef]
- Ciurczak, E.W.; Igne, B.; Workman, J., Jr.; Burns, D.A. Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Wu, H.; Viejo, C.G.; Fuentes, S.; Dunshea, F.R.; Suleria, H.A.R. The Impact of Wet Fermentation on Coffee Quality Traits and Volatile Compounds Using Digital Technologies. Fermentation 2023, 9, 68. [Google Scholar] [CrossRef]
- Silva, C.F.; Schwan, R.F.; Dias, Ë.S.; Wheals, A.E. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000, 60, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Otify, A.M.; El-Sayed, A.M.; Michel, C.G.; ElShebiney, S.A.; Ehrlich, A.; Wessjohann, L.A. Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics. Molecules 2019, 24, 3377. [Google Scholar] [CrossRef] [PubMed]
- Gruczyńska, E.; Kowalska, D.; Kozłowska, M.; Majewska, E.; Tarnowska, K. Furan in roasted, ground and brewed coffee. Rocz. Panstw. Zakl. Hig. 2018, 69, 111–118. [Google Scholar]
- Doughty, A.; Mackie, J.C.; Palmer, J.M. Kinetics of the thermal decomposition and isomerisation of pyrazine (1,4 diazine). Symp. (Int.) Combust. 1994, 25, 893–900. [Google Scholar] [CrossRef]
- Diviš, P.; Pořízka, J.; Kříkala, J. The effect of coffee beans roasting on its chemical composition. Slovak J. Food Sci. Potravin. 2019, 13, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Cho, S.-M.; Choi, J.-H.; Jeong, H.; Lee, S.M.; Koo, B.; Choi, I.-G. A Simultaneous Conversion and Extraction of Furfural from Pentose in Dilute Acid Hydrolysate of Quercus mongolica Using an Aqueous Biphasic System. Appl. Sci. 2021, 11, 163. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Z.; Gao, N.; Zhang, Y.; Wang, M.; Ren, G.; Song, B.; Liang, Q.; Bao, Y.; Tan, H.; et al. Effects of sucrose degradation product furfural on cyanidin-3-O-glucoside: Mechanism of action, stability, and identification of products in sugar solutions. Food Res. Int. 2023, 168, 112788. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, P.; Luo, P.; Wu, P. Occurrence of Furfural and Its Derivatives in Coffee Products in China and Estimation of Dietary Intake. Foods 2023, 12, 200. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Powers, S.; Elmore, J.; Briddon, A.; Mottram, D.; Halford, N. Evidence for the complex relationship between free amino acid and sugar concentrations and acrylamide-forming potential in potato. Ann. Appl. Biol. 2014, 164, 286–300. [Google Scholar] [CrossRef]
- The Good Scents Company. TGSC Information System—Odor and Flavor Database. Available online: https://www.thegoodscentscompany.com/search.php (accessed on 9 June 2025).


| Cultivar | Light Roasted (LI) | Medium Roasted (ME) | Dark Roasted (DA) |
|---|---|---|---|
| Zahidi | ZA-LI | ZA-ME | ZA-DA |
| Medjool | ME-LI | ME-ME | ME-DA |
| Deglet nour | DN-LI | DN-ME | DN-DA |
| Thoory | TH-LI | TH-ME | TH-DA |
| Halawi | HA-LI | HA-ME | HA-DA |
| Barhee | BA-LI | BA-ME | BA-DA |
| Khadrawy | KH-LI | KH-ME | KH-DA |
| Bau Strami | BS-LI | BS-ME | BS-DA |
| Sample Name | Zahidi | Medjool | Deglet Nour | Thoory | Halawi | Barhee | Khadrawy | Bau Strami | |
|---|---|---|---|---|---|---|---|---|---|
| Roasting Level | pH | Average | |||||||
| Light | ZA-LI | ME-LI | DN-LI | TH-LI | HA-LI | BA-LI | KH-LI | BS-LI | |
| 6.22 ± 0.01 aA | 5.72 ± 0.01 cA | 5.38 ± 0.05 dA | 5.89 ± 0.03 bA | 5.90 ± 0.03 bA | 5.95 ± 0.03 bA | 5.49 ± 0.05 cA | 5.95 ± 0.02 bA | 5.95 ± 0.05 | |
| Medium | ZA-ME | ME-ME | DN-ME | TH-ME | HA-ME | BA-ME | KH-ME | BS-ME | |
| 4.30 ± 0.02 aB | 4.37 ± 0.02 aB | 3.71 ± 0.01 cB | 3.77 ± 0.02 cB | 4.03 ± 0.02 bB | 4.04 ± 0.05 bB | 3.79 ± 0.02 cB | 3.94 ± 0.03 bB | 3.99 ± 0.05 | |
| Dark | ZA-DA | ME-DA | DN-DA | TH-DA | HA-DA | BA-DA | KH-DA | BS-DA | |
| 3.97 ± 0.01 aC | 3.25 ± 0.01 cC | 3.76 ± 0.06 abB | 3.93 ± 0.06 abB | 3.30 ± 0.02 cC | 3.87 ± 0.02 abC | 3.39 ± 0.09 cB | 3.74 ± 0.06 bB | 3.65 ± 0.06 | |
| Sample | Light Roasted | Medium Roasted | Dark Roasted | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | ||
| Powder | Zahidi | 46.33 ± 1.12 ab | 15.07 ± 0.49 a | 22.17 ± 0.21 ab | 20.53 ± 6.70 a | 12.40 ± 2.72 a | 14.40 ± 4.46 a | 20.67 ± 0.83 ab | 10.73 ± 0.32 bcd | 12.67 ± 0.38 abc |
| Medjool | 43.80 ± 7.24 ab | 14.73 ± 0.29 a | 21.30 ± 2.25 ab | 27.13 ± 1.89 a | 15.20 ± 0.92 a | 18.33 ± 1.04 a | 20.67 ± 1.86 ab | 13.47 ± 0.46 a | 14.97 ± 0.14 a | |
| Deglet nour | 45.57 ± 0.75 ab | 14.87 ± 0.83 a | 22.40 ± 0.36 ab | 21.03 ± 3.10 a | 12.17 ± 1.15 a | 14.47 ± 1.80 a | 18.03 ± 1.91 b | 9.37 ± 0.45 de | 11.20 ± 0.36 c | |
| Thoory | 51.90 ± 0.62 a | 14.43 ± 0.29 a | 23.10 ± 0.20 a | 22.20 ± 1.21 a | 12.50 ± 0.50 a | 14.83 ± 0.80 a | 18.20 ± 0.99 ab | 9.17 ± 0.45 e | 10.80 ± 0.56 c | |
| Halawi | 43.30 ± 1.49 ab | 13.07 ± 0.23 ab | 19.73 ± 0.57 ab | 23.40 ± 3.54 a | 13.37 ± 1.76 a | 16.10 ± 2.36 a | 16.87 ± 1.25 b | 10.83 ± 0.15 bcd | 11.30 ± 0.62 c | |
| Barhee | 31.23 ± 3.96 c | 11.43 ± 1.11 b | 16.13 ± 2.05 c | 24.73 ± 3.06 a | 14.10 ± 1.31 a | 16.87 ± 1.89 a | 18.97 ± 0.99 ab | 10.03 ± 0.49 cde | 11.73 ± 0.58 c | |
| Khadrawy | 39.43 ± 2.91 bc | 13.80 ± 0.89 a | 19.43 ± 1.36 bc | 24.80 ± 1.50 a | 13.53 ± 0.55 a | 15.90 ± 0.70 a | 16.87 ± 1.32 b | 11.27 ± 1.17 bc | 12.33 ± 0.99 bc | |
| Bau Strami | 46.23 ± 0.93 ab | 14.37 ± 0.45 a | 21.93 ± 0.32 ab | 23.67 ± 2.90 a | 13.70 ± 1.31 a | 16.53 ± 1.80 a | 22.83 ± 0.21 a | 11.80 ± 0.11 b | 14.67 ± 0.15 a | |
| Average | 43.48 ± 6.38 | 13.97 ± 1.30 | 20.78 ± 2.39 | 23.44 ± 3.51 | 13.37 ± 1.54 | 15.93 ± 2.22 | 19.14 ± 2.44 | 10.83 ± 1.43 | 12.46 ± 1.69 | |
| ΔE | 20.62 | 6.08 | ||||||||
| Beverage | Zahidi | 52.67 ± 1.83 a | 5.13 ± 0.32 a | −10.33 ± 1.01 a | 44.70 ± 5.09 b | 4.77 ± 0.32 a | 10.20 ± 4.10 ab | 48.20 ± 0.40 c | 3.47 ± 0.21 a | 6.50 ± 1.25 bc |
| Medjool | 58.60 ± 8.79 a | 3.30 ± 2.08 ab | −15.37 ± 7.29 a | 51.97 ± 1.65 ab | 1.33 ± 0.50 cd | −5.37 ± 3.83 c | 31.93 ± 1.92 e | 2.03 ± 0.38 ab | 19.03 ± 1.47 a | |
| Deglet nour | 53.53 ± 1.70 a | 3.13 ± 0.58 ab | −12.70 ± 0.80 a | 46.63 ± 1.41 ab | 2.63 ± 0.29 b | 8.63 ± 1.89 ab | 48.83 ± 0.67 bc | 1.50 ± 0.10 ab | 1.10 ± 0.10 c | |
| Thoory | 50.90 ± 4.61 a | 4.00 ± 0.17 ab | −11.30 ± 1.21 a | 48.27 ± 1.32 ab | 1.00 ± 0.36 d | 3.80 ± 1.30 b | 55.80 ± 2.02 a | 0.80 ± 0.44 ab | −9.07 ± 2.29 d | |
| Halawi | 54.10 ± 1.40 a | 2.53 ± 0.32 b | −15.53 ± 1.60 a | 47.43 ± 2.32 ab | 2.17 ± 0.50 bc | 12.80 ± 2.79 a | 41.77 ± 2.15 d | 0.63 ± 2.75 ab | 11.47 ± 1.41 ab | |
| Barhee | 55.07 ± 3.27 a | 2.27 ± 0.29 b | −18.20 ± 3.63 a | 46.67 ± 2.89 ab | −0.77 ± 0.23 e | 5.80 ± 3.24 ab | 53.37 ± 0.93 ab | −0.40 ± 0.46 b | 2.50 ± 1.06 c | |
| Khadrawy | 52.63 ± 4.96 a | 3.63 ± 0.32 ab | −12.40 ± 1.75 a | 54.77 ± 3.25 a | 0.67 ± 0.50 d | −9.13 ± 1.21 c | 41.30 ± 2.10 d | 1.13 ± 0.87 ab | 12.53 ± 1.93 ab | |
| Bau Strami | 49.47 ± 1.36 a | 3.47 ± 0.57 ab | −11.57 ± 0.80 a | 46.80 ± 3.75 ab | 2.12 ± 0.06 bc | 7.87 ± 4.04 ab | 47.90 ± 2.01 c | 2.37 ± 0.31 ab | 9.17 ± 2.32 bc | |
| Average | 53.37 ± 4.41 | 3.43 ± 1.09 | −13.43 ± 3.62 | 48.40 ± 4.02 | 1.74 ± 1.58 | 4.33 ± 7.78 | 46.77 ± 6.87 | 1.44 ± 1.44 | 6.65 ± 8.56 | |
| ΔE | 18.51 | 2.86 | ||||||||
| Stage | Samples | Accuracy | Error | Performance (MSE) |
|---|---|---|---|---|
| Model 1: Inputs: NIR; Targets: type of date seed beverage | ||||
| Training | 151 | 100% | 0% | 2.33 × 10−10 |
| Testing | 65 | 96.9% | 3.1% | 0.11 |
| Overall | 216 | 99.1% | 0.9% | - |
| Model 2: Inputs: electronic nose; Targets: type of date seed beverage | ||||
| Training | 504 | 100% | 0% | 1.42 × 10−9 |
| Testing | 216 | 92.6% | 7.4% | 0.11 |
| Overall | 720 | 97.8% | 2.2% | - |
| Stage | Samples | Observations | R | Slope | Performance (MSE) |
|---|---|---|---|---|---|
| Model 3: Inputs: NIR; Targets: volatile aromatic compounds | |||||
| Training | 50 | 1250 | 0.99 | 0.87 | 0.006 |
| Testing | 22 | 550 | 0.67 | 0.80 | 0.240 |
| Overall | 72 | 1800 | 0.88 | 0.92 | - |
| Model 4: Inputs: electronic nose; Targets: volatile aromatic compounds | |||||
| Training | 504 | 12,600 | 0.90 | 0.81 | 0.051 |
| Testing | 216 | 5400 | 0.88 | 0.79 | 0.063 |
| Overall | 720 | 18,000 | 0.90 | 0.81 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Wu, H.; Ghafoor, K.; Gonzalez Viejo, C.; Fuentes, S.; Ahmadi, F.; Suleria, H.A.R. Impacts of Roasting Intensity and Cultivar on Date Seed Beverage Quality Traits and Volatile Compounds Using Digital Technologies. Foods 2025, 14, 3902. https://doi.org/10.3390/foods14223902
Shi L, Wu H, Ghafoor K, Gonzalez Viejo C, Fuentes S, Ahmadi F, Suleria HAR. Impacts of Roasting Intensity and Cultivar on Date Seed Beverage Quality Traits and Volatile Compounds Using Digital Technologies. Foods. 2025; 14(22):3902. https://doi.org/10.3390/foods14223902
Chicago/Turabian StyleShi, Linghong, Hanjing Wu, Kashif Ghafoor, Claudia Gonzalez Viejo, Sigfredo Fuentes, Farhad Ahmadi, and Hafiz A. R. Suleria. 2025. "Impacts of Roasting Intensity and Cultivar on Date Seed Beverage Quality Traits and Volatile Compounds Using Digital Technologies" Foods 14, no. 22: 3902. https://doi.org/10.3390/foods14223902
APA StyleShi, L., Wu, H., Ghafoor, K., Gonzalez Viejo, C., Fuentes, S., Ahmadi, F., & Suleria, H. A. R. (2025). Impacts of Roasting Intensity and Cultivar on Date Seed Beverage Quality Traits and Volatile Compounds Using Digital Technologies. Foods, 14(22), 3902. https://doi.org/10.3390/foods14223902









