Polyphenolic Compounds from Andean Berry (Vaccinium meridionale Swartz) and Derived Functional Benefits: A Systematic and Updated Review
Abstract
1. Introduction
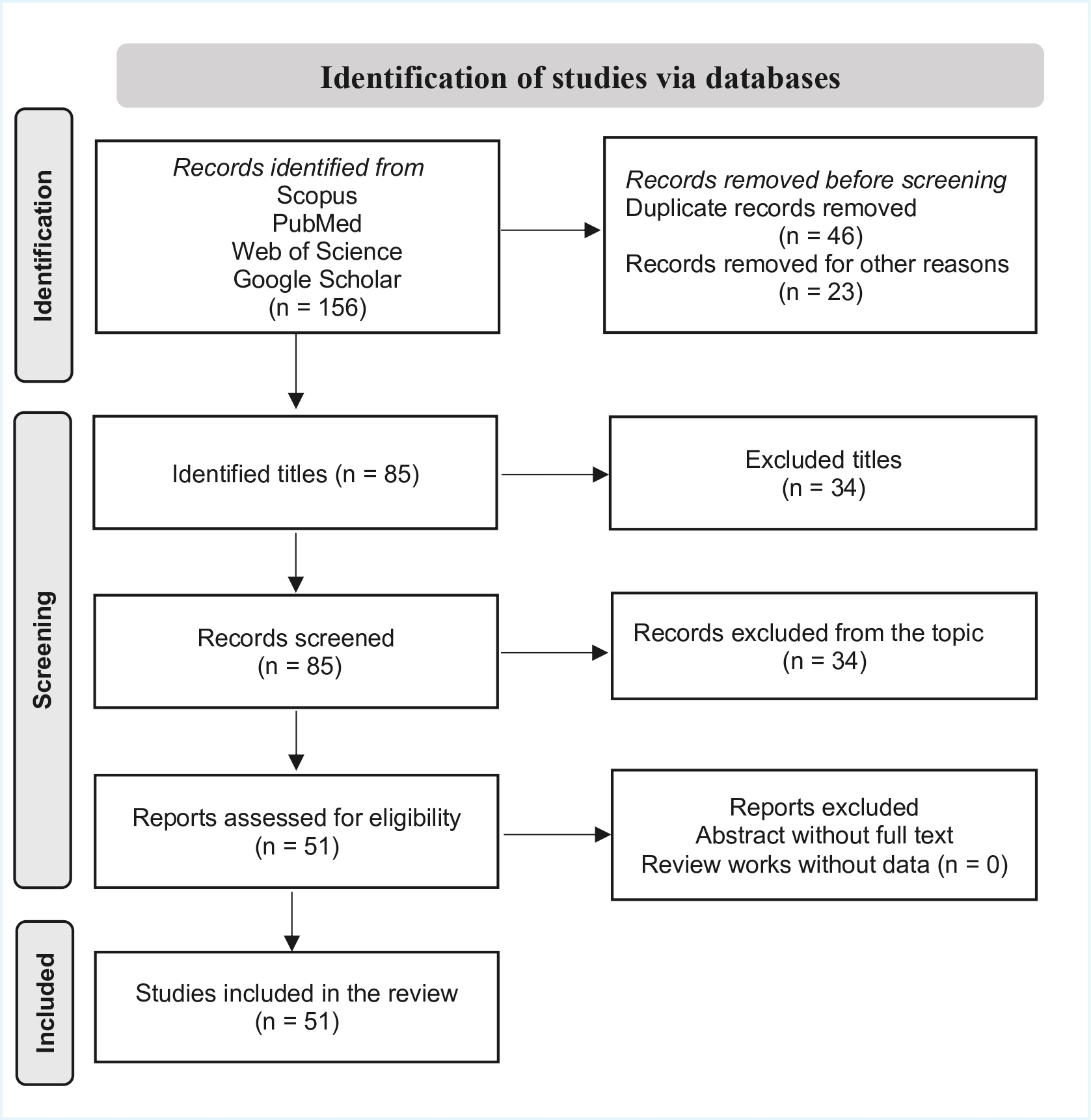
2. Materials and Methods
3. Polyphenolic Compounds’ Composition of Vaccinium meridionale
3.1. Overall Composition
| Phenolic Compound | Chemical Structure | References |
|---|---|---|
| Cyanidin-3-O-glucoside | 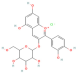 | [13,31,35,41] |
| Cyanidin-3-O-galactoside |  | [13,35,38] |
| Cyanidin-3-arabinoside |  | [13,41] |
| Chlorogenic acid |  | [13,35,42,43,44] |
| Caffeic acid |  | [13,35,43,45] |
| p-coumaric acid |  | [42,44,46] |
| Ferulic acid | 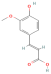 | [44,45] |
| Quercetin | 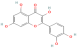 | [13,35,45,46] |
| Rutin |  | [44,45,46] |
3.2. Effect of Processing on the Content of Polyphenolic Compounds
| Sample Matrix | Processing Method | Total Phenolic Compounds | Total Monomeric Anthocyanins | Antioxidant Capacity | Impact on Selected Polyphenolic Compounds | References |
|---|---|---|---|---|---|---|
| Fruit | Unprocessed fruit | 609 ± 31 mg GAE/100 g | 200.60 ± 10.20 mg C3G/100 g | DPPH: 2404 ± 120 μM Trolox/100 g fresh fruit. ABTS: 8694 ± 435 μM Trolox/100 g fresh fruit. FRAP: 581 ± 29 mg ascorbic acid/100 g fresh fruit. | Chlorogenic acid, quercetin, and other flavonoids are present in significant concentrations. | [13,31] |
| Fruit | Lyophilization (freeze-drying) | 1046.01–2546 mg GAE/100 g | 82.64–150.70 mg C3G/100 g | ORAC: 41775.20 ± 6168.20 mmol Trolox/100 g lyophilized extract. FRAP: 5.10 mmol FeSO4/g. | Preserves most of the phenolic compounds but reduces the total monomeric anthocyanins content by 63.3%. | [49,50] |
| Fruit | Freezing | 1046.01 ± 26.95 mg GAE/100 g | 82.64 mg C3G/100 g | - | May slightly decrease phenolic compounds (including anthocyanins) compared to fresh fruit. | [31] |
| Extract | Aqueous extraction | 4409.78 ± 63.05 mg GAE/100 g | 106.57 mg C3G/100 g | - | Increased extraction of total phenolic compounds (including anthocyanins) improves the bioavailability of these compounds. | [44,52] |
| Extract | Maceration | 86 ± 4 mg GAE/100 g | -- | ABTS: 3.8 ± 0.30 µmol Trolox Eq/mL DPPH: 22.90 ± 5.40 µmol Trolox Eq/mL | Maceration can extract various phenolic compounds, and high levels of chlorogenic acid have been reported. | [49] |
| Fruit | Fruit juice | 2032.50 ± 41.70 mg GAE/L | 371.5 ± 20.1 mg C3G/L | ORAC: 24.00 µmol TE/g. | Improve the bioactivity of antioxidants; increase the concentration of vitamin C and other flavonoids. | [24] |
| Fruit | Concentration | 953.70 mg GAE/100 g | 1224 mg C3G/100 g | - | Concentrating may increase antioxidant activity but decrease the levels of heat-sensitive compounds. | [50] |
| Fruit | Osmotic dehydration | 692.70–47.40 mg GAE/100 g | - | ORAC (hydrophilic fraction): 11,490.80 ± 631.60 μmol TE/100 g FRAP: 4084.50 ± 106.1 μmol TE/100 g DPPH: 5731.60 ± 108.80 μmol TE/100 g | Decrease in polyphenolic compounds’ content, including anthocyanin concentration in the drying process | [51] |
| Fruit | Drying at different temperatures (40, 50, and 60 °C) | 3.87–47.40 mg GAE/100 g | 1.21–2.36 mg C3G/100 g | At 40°c, 24% of anthocyanins are retained, with a greater loss as the temperature increases. | [30] | |
| Vinegar | Aqueous/methanolic extract/HCL | 4409.78 ± 63.05 mg GAE/100 mL | 106.57 ± 1.43 mg C3G/100 mL | FRAP: 476.84 ± 18.81 µmol TE/100 mL. ORAC: 113,176.15 ± 4527.04 µmol TE/100 mL. DPPH: 128.03 ± 3.84 µmolTE/100 g Fresh Weight. | Prolonged process, moderate phenolic compounds content | [43] |
| Extract | Ethanolic extract | 83.98–167.90 mg GAE/100 g | 29.08–747.77 mg C3G/100 g | DPPH: 1143.68 ± 3.87 ug Trolox/g ABTS: 253.27 ± 12.57 g ascorbic acid/kg ORAC 472.25 ± 6.08 mg TE/g, FRAP 1.98 ± 0.02 mol ferric sulfate/kg | High levels of quercetin and cyanidin derivatives | [53] |
| Fruit | Freeze-dried juice | 9.80 ± 0.04 mg GAE/100 g | - | - | Contains condensed tannins and flavonoids. | [30,38] |
| Wine | Wine | 5794.30 ± 169.10 mg GAE/100 g | - | - | Contains a higher concentration of phenols when compared to V. myrtillus | [37] |
4. Biological Effects Evaluated on Vaccinium meridionale
4.1. Matrix Extracts
4.2. Food Matrices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABJ | Andean berry juice |
| 5-FU | 5-Fluorouracil. |
| 8-iso-PGF2α | 8-iso-prostaglandin F2α. |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid). |
| ACF | Aberrant crypt foci |
| Akt | Protein kinase. |
| AP-1 | Activator protein-1. |
| BMI | Body mass index. |
| C3G | Cyanidin-3-O-glucoside. |
| CAT | Catalase. |
| CCR1 | C-C chemokine receptor 1. |
| CCR5 | C-C- chemokine receptor 5. |
| CK | Creatine kinase |
| COX-2 | Cyclooxygenase-2 |
| DPPH | 2,2-diphenyl-1-picrylhydrazil. |
| eNOS | Endothelial nitric oxide synthase. |
| FRAP | Ferric reducing antioxidant power. |
| FW | Fresh weight. |
| GAE | Gallic acid equivalents. |
| GCSF | Granulocyte colony-stimulating factor. |
| GSH | Reduced glutathione. |
| HDL | High-density lipoprotein cholesterol. |
| HPLC | High-performance liquid chromatography. |
| hs-CRP | High sensitivity-C reactive protein |
| IC50 | Half-maximal inhibitory concentration. |
| ICAM-1 | Intercellular adhesion molecule 1. |
| iNOS | Inducible nitric oxide synthase. |
| IL-1R | Interleukin-1 receptor. |
| IL-1β | Interleukin-1β. |
| IL-6 | Interleukin-6. |
| LC50 | Half-maximal lethal concentration. |
| LDH | Lactate dehydrogenase. |
| LDL | Low-density lipoprotein cholesterol. |
| LPO | Lipid peroxidation. |
| LPS | Lipopolysaccharide. |
| LEU | Leuprolide. |
| MeSH | Medical Subject Headings. |
| MDA | Malondialdehyde. |
| MMP-9 | Matrix metalloproteinase-9 |
| NF-κB | Nuclear factor κB. |
| NO | Nitric oxide. |
| ORAC | Oxygen radical absorbance capacity. |
| OXA | Oxaliplatin. |
| Ox-LDL | Oxidized low-density lipoprotein cholesterol. |
| PPARγ | Proliferator-activated receptor gamma. |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROS | Reactive oxygen species. |
| SGOT | Serum glutamic oxaloacetic transaminase |
| SGPT | Serum glutamic pyruvic transaminase |
| SNP | Single-nucleotide polymorphism. |
| SOD | Superoxide dismutase. |
| TE | Trolox equivalents. |
| TEAC | Trolox equivalents antioxidant capacity. |
| TNF-α | Tumor necrosis factor α |
| TUNEL | TdT-mediated dUTP Nick-End labeling. |
| VCAM-1 | Vascular cell adhesion molecule-1 |
References
- Medina Cano, C.I.; Ligarreto Moreno, G.A.; Vargas Arcila, M.O. Proposal of Descriptors to Study the Variability of Vaccinium meridionale Swartz. Rev. Fac. Nac. Agron. Medellín 2023, 76, 10445–10455. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Franco Tobón, Y.N.; Agudelo, C.; Arango-Varela, S.S.; Rojano, B. Yahia, E.M., Ed.; Andean Berry (Vaccinium meridionale Swartz). In Fruit and Vegetable Phytochemicals; Wiley: Hoboken, NJ, USA, 2017; pp. 869–882. ISBN 978-1-119-15794-6. [Google Scholar]
- Magnitskiy, S. Native Plants from the Genus Vaccinium in Colombia and Their Potential Uses. A Review. Rev. Colomb. Cienc. Hortic. 2023, 17, e15503. [Google Scholar] [CrossRef]
- Becerra, A.D.; Quevedo-Rubiano, S.; Magnitskiy, S.; Lancheros, H.O. Revista Colombiana de Ciencias Hortícolas; Universidad Pedagógica y Tecnológica de Colombi: Tunja, Colombia, 2022; p. e15034. [Google Scholar]
- Medina-Cano, C.I.; Martínez-Bustamante, E.; López-Orozco, C.A. Phenological Scale for the Mortiño or Agraz (Vaccinium meridionale Swartz) in the High Colombian Andean Area. Rev. Fac. Nac. Agron. Medellín 2019, 72, 8897–8908. [Google Scholar] [CrossRef]
- Sepúlveda, J.; Rondón González, F.; Soto Sedano, J.C.; Velasco, G.P.; Mosquera, T.; Delgado, M.C.; Ligarreto Moreno, G.A.; Magnitskiy, S.; Miranda, Y.; Garzón Gutiérrez, L.N. SNP Analysis Reveals Bovel Insights into the Genetic Diversity of Colombian Vaccinium meridionale. Genes 2025, 16, 675. [Google Scholar] [CrossRef] [PubMed]
- Buitrago Guacaneme, C.M.; Rincón Soledad, M.C.; Balaguera López, H.E.; Ligarreto Moreno, G.A. Tipificación de Diferentes Estados de Madurez Del Fruto de Agraz (Vaccinium meridionale Swartz). Rev. Fac. Nac. Agron. Medellín 2015, 68, 7521–7531. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Polashock, J.J.; Rowland, L.J.; Ogden, E.; Luteyn, J.L. Fertile Intersectional Hybrids of 4× Andean Blueberry (Vaccinium meridionale) and 2× Lingonberry (V. Vitis-Idaea). HortScience 2022, 57, 525–531. [Google Scholar] [CrossRef]
- Quevedo-Rubiano, S.; Aranda-Camacho, Y.; Ligarreto-Moreno, G.A.; Magnitskiy, S. Characterization of the Localized Agri-Food System (SYAL) for the Andean Blueberry (Vaccinium meridionale Swartz) in the Boyaca Department, Colombia. Rev. Colomb. Cienc. Hortic. 2021, 15, e11593. [Google Scholar] [CrossRef]
- Hernández-Pérez, M.I.; Lobo-Arias, M.; Medina-Cano, C.I.; Cartagena-Valenzuela, J.R. Revista de la Facultad Nacional de Agronomía Medellín; Universidad Nacional de Colombia: Medellín, Colombia, 2012; pp. 6627–6635. [Google Scholar]
- Alsharairi, N.A. A Review with a Focus on Vaccinium-Berries-Derived Bioactive Compounds for the Treatment of Reproductive Cancers. Plants 2024, 13, 1047. [Google Scholar] [CrossRef]
- Prieto Martínez, A.; Coutiño Diaz, M.; Anaya Romero, L.; Ali Redha, A.; Zare, R.; Ventura Hernandez, S.; Prokopidis, K.; Clifford, T. Effects of Vaccinium Berries (Blueberries, Cranberries and Bilberries) on Oxidative Stress, Inflammation, Exercise Performance, and Recovery—A Systematic Review. Food Funct. 2024, 15, 444–459. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical Composition, Anthocyanins, Non-Anthocyanin Phenolics and Antioxidant Activity of Wild Bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Gaviria, C.A.; Ochoa, C.I.; Sánchez, N.Y.; Medina, C.I.; Lobo, M.; Galeano, P.L.; Mosquera, A.J.; Tamayo, A.; Lopera, Y.E.; Rojano, B.A. Ligarreto, G.A., Ed.; Propiedades Antioxidantes de Los Frutos de Agraz o Mortiño (Vaccinium meridionale Swartz). In Perspectivas del Cultivo de Agraz o Mortiño (Vaccinum meridionale Swartz) en la Zona Altoandina de Colombia; Gente Nueva Editorial: Bogotá, Colombia, 2009; pp. 93–112. [Google Scholar]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical Composition and Phenolic Compound Profile of Mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Polyphenolic Composition and Antioxidant Capacity of Extruded Cranberry Pomace. J. Agric. Food Chem. 2010, 58, 4037–4042. [Google Scholar] [CrossRef]
- Quintero-Castaño, V.D.; Vasco-Leal, J.F.; Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Castellanos-Galeano, F.; Álvarez-Barreto, C.; Bello-Pérez, L.A.; Cortés-Rodriguez, M.; Quintero-Castaño, V.D.; Vasco-Leal, J.F.; et al. Novel OSA—Modified Starch from Gros Michel Banana for Encapsulation of Andean Blackberry Concentrate: Production and Storage Stability. Starch—Stärke 2021, 73, 2000180. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Impact of Different Stages of Juice Processing on the Anthocyanin, Flavonol, and Procyanidin Contents of Cranberries. J. Agric. Food Chem. 2011, 59, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Zhang, B.; Wang, S. Berries and Their Active Compounds in Prevention of Age-Related Macular Degeneration. Antioxidants 2024, 13, 1558. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Escobar-Barranco, N.; Gil Archila, E.; Villamil, R.-A.; Bonilla, A. Chemical and Nutritional Value of Colombian Ericaceae with Edible Fruits. Food Chem. Adv. 2025, 8, 101045. [Google Scholar] [CrossRef]
- Vivas-Álzate, L.C.; Ciro-Velásquez, H.J.; Sepúlveda-Valencia, J.U.; Pérez Monterroza, E.J. Evaluating the Andean Blueberry (Vaccinium meridionale Swartz) Powder in the Preparation of Ice Cream: Improving the Antioxidant Capacity and the Total Phenolic Content. Rev. Fac. Nac. Agron. Medellín 2025, 78, 11015–11023. [Google Scholar] [CrossRef]
- Garzón, G.A.; Medina, J.L.; Montana, T.L.; Sánchez, M.; Novoa, C.F.; Gutiérrez, L. Utilization of Vaccinium meridionale S. Pomace as an Eco-friendly and Functional Colorant in Greek-style Yogurt. J. Food Sci. 2021, 86, 3896–3908. [Google Scholar] [CrossRef] [PubMed]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological Applications of Natural Colorants in Food Systems: A Review. Foods 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Akin, Z.; Ozcan, T. Functional Properties of Fermented Milk Produced with Plant Proteins. LWT 2017, 86, 25–30. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; Gonzalez de Mejia, E. Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium Floribundum and Aristotelia Chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Wan, C.; Langyan, S.; Echeverría, J.; Devkota, H.P.; Tewari, D.; Moosavi, M.A.; Ezzat, S.M.; Perez-Vazquez, A.; Fraga-Corral, M.; Cravotto, G.; et al. Edible Fruits and Berries as a Source of Functional Polyphenols: Current Scene and Future Perspectives. Phytochem. Rev. 2025, 24, 1197–1225. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Gaviria-Montoya, C.; Ochoa-Ospina, C.; Sánchez-Mesa, N.; Medina-Cano, C.; Lobo-Arias, M.; Mosquera-Martínez, A.; Tamayo-Tenorio, A.; Lopera-Pérez, Y.; Rojano, B. Actividad Antioxidante Inhibición de La Peroxidación Lipídica de Extractos de Frutos de Mortiño (Vaccinium meridionale Swartz). Boletín Latinoam. Caribe Plantas Med. Aromáticas 2009, 8, 519–528. [Google Scholar]
- Cerrato, A.; Piovesana, S.; Aita, S.E.; Cavaliere, C.; Felletti, S.; Laganà, A.; Montone, C.M.; Vargas-de-la-Cruz, C.; Capriotti, A.L. Detailed Investigation of the Composition and Transformations of Phenolic Compounds in Fresh and Fermented Vaccinium floribundum Berry Extracts by High-resolution Mass Spectrometry and Bioinformatics. Phytochem. Anal. 2022, 33, 507–516. [Google Scholar] [CrossRef]
- Howard, L.; Brownmiller, C.R.; Astrid Garzón, G. Monitoring Effects on Anthocyanins, Non-Anthocyanin Phenolics and ORACFL Values of Colombian Bilberry (V. Meridionale Swartz) during Pulping and Thermal Operations. Heliyon 2024, 10, e33504. [Google Scholar] [CrossRef]
- López-Vidaña, E.C.; Pilatowsky Figueroa, I.; Cortés, F.B.; Rojano, B.A.; Navarro Ocaña, A. Effect of Temperature on Antioxidant Capacity during Drying Process of Mortiño (Vaccinium meridionale Swartz). Int. J. Food Prop. 2017, 20, 294–305. [Google Scholar] [CrossRef]
- Garzón, G.A.; Soto, C.Y.; López, R.M.; Riedl, K.M.; Browmiller, C.R.; Howard, L. Phenolic Profile, in Vitro Antimicrobial Activity and Antioxidant Capacity of Vaccinium meridionale Swartz Pomace. Heliyon 2020, 6, e03845. [Google Scholar] [CrossRef]
- Estupiñan-Amaya, M.; Fuenmayor, C.A.; López-Córdoba, A. New Freeze-Dried Andean Blueberry Juice Powders for Potential Application as Functional Food Ingredients: Effect of Maltodextrin on Bioactive and Morphological Features. Molecules 2020, 25, 5635. [Google Scholar] [CrossRef]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Maldonado-Celis, M.E. Andean Berry (Vaccinium meridionale Swartz) Juice, in Combination with Aspirin, Displayed Antiproliferative and pro-Apoptotic Mechanisms in Vitro While Exhibiting Protective Effects against AOM-Induced Colorectal Cancer in Vivo. Food Res. Int. 2022, 157, 111244. [Google Scholar] [CrossRef]
- Lopera, Y.E.; Fantinelli, J.; González Arbeláez, L.F.; Rojano, B.; Ríos, J.L.; Schinella, G.; Mosca, S. Antioxidant Activity and Cardioprotective Effect of a Nonalcoholic Extract of Vaccinium meridionale Swartz during Ischemia-Reperfusion in Rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 516727. [Google Scholar] [CrossRef]
- Reißner, A.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and Physicochemical Properties of Dried Berry Pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Jurevičiūtė, I.; Keršienė, M.; Bašinskienė, L.; Leskauskaitė, D.; Jasutienė, I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods 2022, 11, 716. [Google Scholar] [CrossRef]
- Su, Z. Anthocyanins and Flavonoids of Vaccinium L. TOPHARMCJ 2012, 3, 7–37. Pharm. Crops 2012, 3, 7–37. [Google Scholar] [CrossRef]
- Zapata-Vahos, I.C.; Ochoa, S.; Maldonado, M.E.; Zapata-Zapata, A.D.; Rojano, B. Cytotoxic Effect and Antioxidant Activity of Andean Berry (Vaccinium meridionale Sw) Wine. J. Med. Plants Res. 2016, 10, 402–408. [Google Scholar] [CrossRef]
- Zapata, I.C.; Ochoa, S.; Alzate, A.F.; Zapata, A.D.; Rojano, B.A. Vinegar of Andean Berries (Vaccinium meridionale SW): Antioxidant and Antiproliferative Activity in Colon Cancer Cells SW480. Vitae 2020, 26, 135–147. [Google Scholar] [CrossRef]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Reyes-Dieck, C.; Yahia, E.M.; Maldonado-Celis, M.E. Antiproliferative Potential of Andean Berry (Vaccinium meridionale Swartz) Juice in Combination with Aspirin in Human SW480 Colon Adenocarcinoma Cells. J. Food Biochem. 2021, 45, e13760. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Campos-Vega, R.; Loarca-Piña, G.; Maldonado-Celis, M.E. Bioaccessibility during in Vitro Digestion and Antiproliferative Effect of Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice. J. Agric. Food Chem. 2018, 66, 7358–7366. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Lorenzo, J.M.; Zamuz, S.; Valdés, M.E.; Moreno, D.; Balcázar, M.C.G.; Fernández-Arias, J.M.; Reyes, J.F.; Franco, D. The Antioxidant Effect of Colombian Berry (Vaccinium meridionale Sw.) Extracts to Prevent Lipid Oxidation during Pork Patties Shelf-Life. Antioxidants 2021, 10, 1290. [Google Scholar] [CrossRef]
- Sequeda-Castañeda, L.; Barrera-Bugallo, A.; Celis, C.; Iglesias, J.; Morales, L. Evaluation of Antioxidant and Cytotoxic Activity of Extracts from Fruits in Fibroblastoma HT1080 Cell Lines: Four Fruits with Commercial Potential in Colombia. Emir. J. Food Agric. 2016, 28, 143. [Google Scholar] [CrossRef]
- Jia, H.; Jia, Y.; Ren, F.; Liu, H. Enhancing Bioactive Compounds in Plant-Based Foods: Influencing Factors and Technological Advances. Food Chem. 2024, 460, 140744. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Celis, M.E.; Arango-Varela, S.S.; Rojano, A.B. Free Radical Scavenging Capacity and Cytotoxic and Antiproliferative Effects of Vaccinium meridionale Sw. against Colon Cancer Cell Lines. Rev. Cuba. Plantas Med. 2014, 19, 172–184. [Google Scholar]
- Franco-Tobón, Y.N.; Rojano, B.A.; Alzate Arbeláez, A.F.; Morales Saavedra, D.M.; Maldonado-Celis, M.E. Efecto Del Tiempo de Almacenamiento Sobre Propiedades Fisicoquímicas y Antioxidantes de Productos Derivados Del Fruto Agraz (Vaccinium meridionale Swartz). Vitae 2016, 23, 184–193. [Google Scholar] [CrossRef]
- Gallego Peláez, E.; Maldonado Celis, M.E.; Posada Jhonson, L.G.; Gómez García, A.C.; Torres Camargo, D. Consumption of Osmo-Dehydrated Andean Berry (Vaccinium meridionale Swartz) Decreases Levels of pro-Inflammatory Biomarkers of Overweight and Obese Adults. Vitae 2021, 28, 343810. [Google Scholar] [CrossRef]
- Espinosa-Moncada, J.; Marín-Echeverri, C.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Aristizábal, J.C.; Blesso, C.N.; Fernandez, M.L.; Barona-Acevedo, J. Evaluation of Agraz Consumption on Adipocytokines, Inflammation, and Oxidative Stress Markers in Women with Metabolic Syndrome. Nutrients 2018, 10, 1639. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Vallejo, W.; Camargo, G.; Muñoz-Acevedo, A.; Quiñones, C.; Schott, E.; Zarate, X. Potential Use of an Anthocyanin-Rich Extract from Berries of Vaccinium Meridionale Swartz as Sensitizer for TiO2 Thin Films—An Experimental and Theoretical Study. J. Photochem. Photobiol. A Chem. 2019, 384, 112050. [Google Scholar] [CrossRef]
- Arango-Varela, S.S.; Torres-Camargo, D.; Reyes-Dieck, C.; Zapata-Londoño, M.B.; Maldonado-Celis, M.E. Aqueous Extract of Andean Berry Induces Apoptosis in Human Colon Cancer Cells without Mitochondrial Damage. J. Berry Res. 2021, 11, 377–393. [Google Scholar] [CrossRef]
- Zapata-Londoño, B.; Lozano-Casabianca, G.A.; Landázuri, P.; Arango-Varela, S.S.; Maldonado-Celis, M.E. Aqueous Extract of Andean Berry (Vaccinium meridionale Swartz) Promotes Antiproliferative Effect of 5-Fluorouracil and Leucovorin Treatment with or without Oxaliplatin and Inhibits Metastatic Potential in Colon Adenocarcinoma Cells. Rev. Colomb. Cancerol. 2023, 27, 433–442. [Google Scholar]
- Kristo, A.; Klimis-Zacas, D.; Sikalidis, A. Protective Role of Dietary Berries in Cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Hernández-Arriaga, A.M.; Rendón, J.C.; Campos-Vega, R.; Maldonado-Celis, M.E. Fermented Non-Digestible Fraction of Andean Berry (Vaccinium meridionale Swartz) Juice Induces Apoptosis in Colon Adenocarcinoma Cells. Prev. Nutr. Food Sci. 2020, 25, 272–279. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Zuñiga-Miranda, J.; Mayorga-Ramos, A.; Tejera, E.; Guamán, L.P. Exploring the Multifaceted Biological Activities of Anthocyanins Isolated from Two Andean Berries. Foods 2024, 13, 2625. [Google Scholar] [CrossRef]
- Deng, H.; Meng, X.; Xue, B.; Li, L. Unveiling the Antibacterial Potential of Anthocyanins—A Comprehensive Review on This Natural Plant Extract. Crit. Rev. Food Sci. Nutr. 2025, 65, 5417–5430. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Li, K.; Jing, H.; Zheng, L. In Vivo Therapeutic Effect of Vaccinium meridionale Swartz in Ischemia-Reperfusion Induced Male Albino Rats. J. Food Sci. 2018, 83, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Colorado, D.; Fernandez, M.; Orozco, J.; Lopera, Y.; Muñoz, D.L.; Acín, S.; Balcazar, N. Metabolic Activity of Anthocyanin Extracts Loaded into Non-Ionic Niosomes in Diet-Induced Obese Mice. Pharm. Res. 2020, 37, 152. [Google Scholar] [CrossRef]
- Lee-Martínez, S.N.; Luzardo-Ocampo, I.; Vergara-Castañeda, H.A.; Vasco-Leal, J.F.; Gaytán-Martínez, M.; Cuellar-Nuñez, M.L. Native Corn (Zea mays L., Cv. ‘Elotes Occidentales’) Polyphenols Extract Reduced Total Cholesterol and Triglycerides Levels, and Decreased Lipid Accumulation in Mice Fed a High-Fat Diet. Biomed. Pharmacother. 2024, 180, 117610. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Arango, S.; Cortés-mancera, F.; Rojano, B.; Maldonado-Celis, M.E.; Maldonado, M.E.; Carlos, D.A.; Sandra, A.; Fabián, C.-M.; Benjamín, R.; et al. Antiproliferative and Pro-Apoptotic Effects of Andean Berry Juice (Vaccinium meridionale Swartz) on Human Colon Adenocarcinoma SW480 Cells. J. Med. Plants Res. 2017, 11, 393–402. [Google Scholar] [CrossRef]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Maldonado-Celis, M.E.; Campos-Vega, R. Andean Berry (Vaccinium meridionale Swartz) Juice in Combination with Aspirin Modulated Anti-Inflammatory Markers on LPS-Stimulated RAW 264.7 Macrophages. Food Res. Int. 2020, 137, 109541. [Google Scholar] [CrossRef] [PubMed]
- Luzardo-Ocampo, I.; Campos-Vega, R.; Gonzalez de Mejia, E.; Loarca-Piña, G. Consumption of a Baked Corn and Bean Snack Reduced Chronic Colitis Inflammation in CD-1 Mice via Downregulation of IL-1 Receptor, TLR, and TNF-α Associated Pathways. Food Res. Int. 2020, 132, 109097. [Google Scholar] [CrossRef] [PubMed]
- Fantinelli, J.; González-Arbeláez, L.; Ciocci-Pardo, A.; Schinella, G.; Mosca Susana, M. Comparative Effects of Natural Products on Ischemia-Reperfusion Injury: Relation to Their “in Vitro” Antioxidant Capacity. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2016, 15, 151–163. [Google Scholar]
- Galvis-Pérez, Y.; Marín-Echeverri, C.; Franco Escobar, C.P.; Aristizábal, J.C.; Fernández, M.-L.; Barona-Acevedo, J. Comparative Evaluation of the Effects of Consumption of Colombian Agraz (Vaccinium meridionale Swartz) on Insulin Resistance, Antioxidant Capacity, and Markers of Oxidation and Inflammation, Between Men and Women with Metabolic Syndrome. BioResearch Open Access 2020, 9, 247–254. [Google Scholar] [CrossRef]
- Quintero-Quiroz, J.; Celis-Torres, A.; Ciro-Gómez, G.; Torres, J.; Corrales-García, L.; Rojas, J. Physicochemical Properties and Functional Characteristics of Ultrasound-Assisted Legume-Protein Isolates: A Comparative Study. J. Food Sci. Technol. 2022, 59, 1665–1676. [Google Scholar] [CrossRef]
- Mashhour, Z.M.; Barghchi, H.; Gheflati, A.; Mansouri, A.H.; Dehnavi, Z.; Khorasnchi, Z.; Binabaj, N.B.; Sahebanmaleki, M.; Moshari, J.; Nattagh-Eshtivani, E. Effects of Vaccinium meridionale Swartz (Agraz) Supplementation on Inflammatory and Oxidative Stress Markers in Patients with Metabolic Syndrome: A Systematic Review of Randomized Controlled Clinical Trials. Clin. Nutr. Res. 2025, 14, 230. [Google Scholar] [CrossRef]
- González, M.; Samudio, I.; Sequeda-Castañeda, L.G.; Celis, C.; Iglesias, J.; Morales, L. Cytotoxic and Antioxidant Capacity of Extracts from Vaccinium meridionale Swartz (Ericaceae) in Transformed Leukemic Cell Lines. J. Appl. Pharm. Sci. 2017, 7, 24–30. [Google Scholar] [CrossRef]
- Osorio, M.; Posada, L.; Martínez, E.; Estrada, V.; Quintana, G.; Maldonado, M.E.; Peresin, S.; Orozco, J.; Castro, C. Bacterial Nanocellulose Spheres Coated with Meta Acrylic Copolymer: Vaccinium meridionale Swartz Extract Delivery for Colorectal Cancer Chemoprevention. Food Hydrocoll. 2024, 147, 109310. [Google Scholar] [CrossRef]
- Zapata-Vahos, I.C.; Villacorta, V.; Maldonado-Celis, M.E.; Castro-Restrepo, D.; Rojano, B. Antioxidant and Cytotoxic Activity of Black and Green Tea from Vaccinium meridionale Swartz Leaves. J. Med. Plants Res. 2015, 9, 445–453. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Ceballos, N.; Gómez-García, A.; Maldonado-Celis, M.E. Andean Berry (Vaccinium meridionale Swartz) Juice Improves Plasma Antioxidant Capacity and IL-6 Levels in Healthy People with Dietary Risk Factors for Colorectal Cancer. J. Berry Res. 2018, 8, 251–261. [Google Scholar] [CrossRef]
- Quintero-Quiroz, J.; Galvis-Pérez, Y.; Galeano-Vásquez, S.; Marín-Echeverri, C.; Franco-Escobar, C.; Ciro-Gómez, G.; Núñez-Rangel, V.; Aristizábal-Rivera, J.C.; Barona-Acevedo, J. Physico-Chemical Characterization and Antioxidant Capacity of the Colombian Berry (Vaccinium meridionale Swartz) with a High-Polyphenol Content: Potential Effects in People with Metabolic Syndrome. Food Sci. Technol. 2019, 39, 573–582. [Google Scholar] [CrossRef]
- Marín-Echeverri, C.; Blesso, C.N.; Fernández, M.L.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Núñez-Rangel, V.; Aristizábal, J.C.; Barona-Acevedo, J. Effect of Agraz (Vaccinium meridionale Swartz) on High-Density Lipoprotein Function and Inflammation in Women with Metabolic Syndrome. Antioxidants 2018, 7, 185. [Google Scholar] [CrossRef]
| Bioactive Compounds | Concentration Range | References |
|---|---|---|
| Total monomeric anthocyanins (mg C3G/100 g) | 41.90–747.60 | [13,31,34,35,36] |
| Total phenolic compounds (mg GAE/100 g) | 141.20–1311.80 |
| Evaluated Matrix | Biological Model Used | Main Effects | References |
|---|---|---|---|
| In vitro studies | |||
| Vinegar from Vaccinium meridionale | SW480 colon cancer cells | Antioxidant capacity (↑ DPPH, ↑ FRAP, ↑ ORAC); Antiproliferative/cytotoxic effects (↓ cell viability, IC50 = 536 µg/mL). | [43] |
| Pomace (phenolic extract) | Bacteria: Staphylococcus aureus, E. coli | Antimicrobial activity (↑ inhibition zone vs. S. aureus > E. coli); Antioxidant capacity (↑ ORAC, ↑ ABTS). | [35] |
| Aqueous fruit extract (lyophilized) | SW480, SW620 colon cancer cell lines | Antioxidant (↑ total phenolic compounds, ↑ anthocyanins); cytotoxic/antiproliferative effects (↓ viability; IC50 ≈ 56–59 µg/mL). | [49] |
| Fermented non-digestible fraction of Andean berry juice | HT29 colon adenocarcinoma cells | Pro-apoptotic (↑ TUNEL positive cells, ↑ apoptosis markers); Antiproliferative (↓ viability, LC50 ≈ 24.7% v/v); Oxidative stress modulation (↓ SOD activity, ↑ 8-iso-PGF2α). | [57] |
| Fruit extracts (methanol) from several fruits, including V. meridionale | HT1080 fibroblasts | Antioxidant (↑ DPPH / ABTS / ORAC protection); Protective against rotenone-induced viability loss (↑ viability). | [47] |
| Alcoholic beverage extracts/dealcoholized fermented beverage | SW480 colon cancer cells | Antioxidant (↑ phenolics, anthocyanins); Antiproliferative (↓ viability; IC50 ≈ 139 µg/mL; Max inhibition 37.2% at 200 µg/mL). | [42] |
| Extracts (various: methanol, aqueous, juice) | OCI-AML3 and MOLT-4 leukemia cell lines | Antioxidant (↑ total phenolic compounds); Cytotoxic (↓ cell viability—modest vs. doxorubicin: ~23–24%) | [70] |
| Andean Berry Juice (ABJ)—digested/fermented fractions | SW480 colon cancer cells | Elevated bioaccessibility for gallic acid; Fermented fraction: strong antiproliferative effect (↓ viability, IC50 ≈ 19.3% v/v); markers. | [45] |
| Vaccinium meridionale extract adsorbed on bacterial nanocellulose | SW480, SW620 cancer cells and HaCaT keratinocytes | Cytotoxic (↓ viability in cancer lines; selectivity index shows less effect on HaCaT); Controlled release profile demonstrated (encapsulation protects in gastric pH). | [71] |
| Aqueous Andean berry extract | SW480, SW620 colon cancer cells | Antiproliferative (↓ viability; IC50 ≈ 19.2–23 mg/mL depending on cell line/time); Induced apoptosis (↑ Annexin V), cell-cycle arrest (S/G2-M or G0/G1 depending on the cell line); ROS modulation (↑ intracellular ROS, ↓ GSH). | [54] |
| Aqueous extract + 5-FU/leucovorin ± oxaliplatin | SW480 / SW620 colon cancer cells | Joint/co-adjuvant effect: ↓ viability and ↓ adhesion/migration/invasion in SW620; ↓ MMP-9 levels (↓ metastasis markers). | [55] |
| Andean Berry Juice + Aspirin | RAW 264.7 LPS-stimulated macrophages | Anti-inflammatory (↓ NO production, ↓ ROS); ↓ IL-1β, ↓ MCP-1, ↓ GCSF observed; predicted interactions with CCR1/CCR5/NF-κB (in silico). | [64] |
| SW480 colon cancer cells | Antiproliferative (↓ viability), modulation of ROS/GSH balance; epigenetic modulation noted as a potential mechanism. | [44] | |
| SW480 colon cancer cells | In vitro ↑ antiproliferative and pro-apoptotic effects (↑ apoptosis markers, ↑ cell cycle arrest). | [37] | |
| Andean Berry Juice | SW480 colon cancer cells | Antiproliferative / pro-apoptotic (↓ proliferation; IC50 ≈ 8% v/v); ↑ Caspase-3 activity; ↑ ROS; ↓ GSH (oxidative stress-mediated apoptosis). | [63] |
| Green and black tea from V. meridionale leaves (infusions) | SW480 colon cancer cells | Antioxidant (↑ TEAC/DPPH/ORAC); Antiproliferative (↓ viability; green tea IC50 ≈ 26.3 µg/mL, black tea ≈ 36 µg/mL). | [72] |
| In vivo studies | |||
| Andean Berry juice + Aspirin | Mice treated with AOM | Chemoprotective effects: preserved colonic architecture; ↓ aberrant crypt foci formation (↓ early markers of colon carcinogenesis). | [37] |
| V. meridionale anthocyanin-loaded niosomes (oral) | Diet-induced obesity (DIO) mouse model | Metabolic improvements: ↓ fasting glucose (↓), ↓ insulin resistance (↑ insulin sensitivity), ↓ body weight, and visceral fat; improved lipid markers. | [61] |
| Vaccinium meridionale Sw. extracts | Male albino Wistar rats, ischemia–reperfusion model | Cardioprotective / antioxidant: ↑ SOD and CAT (↑ antioxidant enzymes), ↓ lipid peroxidation (↓ MDA), ↑ eNOS and Akt expression (↑ protective signaling). | [60] |
| Fruit | Isolated rat hearts (Langendorff)/ex vivo perfusion | Mixed cardioprotective outcomes: V. meridionale shows antioxidant activity and protective effects in some protocols (↑ antioxidant capacity; variable cardioprotection vs. other extracts). | [66] |
| Clinical studies | |||
| Andean Berry Juice | Healthy volunteers with dietary CRC risk factors (250 mL/day for 14 days) | ↑ Plasma antioxidant capacity (↑ Trolox eq. mM); ↓ isoprostanes (↓ 8-iso); ↓ IL-6 (↓ pro-inflammatory cytokine). | [73] |
| Freeze-dried fruit. | Adults with cardiovascular risk factors | Increases in antioxidant capacity in serum (↑ DPPH scavenging in women); variable systemic phenol changes — some sex differences. | [74] |
| Osmo-dehydrated fruit. | Overweight/obese adults (35 g/day for 21 days) | ↓ Pro-inflammatory biomarkers (↓ IL-6, ↓ IL-1β, ↓ TNF-α); ↑ plasma antioxidant capacity; small decrease in BMI (non-robust). | [51] |
| Nectar | Women with MetS (human) | ↑ Serum antioxidant capacity (↑); ↓ urinary 8-OH-dG (↓ DNA oxidative damage); modest changes in inflammatory/adipocytokine markers. | [52] |
| Men and women with metabolic syndrome (human) | ↑ Antioxidant capacity; ↓hs-CRP (↓ inflammation); sex-specific improvements (women: ↓ insulin resistance; men: ↓ oxLDL). | [67] | |
| Reconstituted freeze-dried fruit in water (200 mL) | Men and women with MetS (human) | No significant change in HDL function or major inflammation markers overall; correlations observed (↑ PON1 correlated with ↑ cholesterol efflux). | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Polo, A.R.; Luzardo-Ocampo, I.; Navarro-Gallón, S.; Quijano, S.A.; Arango-Varela, S.S. Polyphenolic Compounds from Andean Berry (Vaccinium meridionale Swartz) and Derived Functional Benefits: A Systematic and Updated Review. Foods 2025, 14, 3861. https://doi.org/10.3390/foods14223861
Ramos-Polo AR, Luzardo-Ocampo I, Navarro-Gallón S, Quijano SA, Arango-Varela SS. Polyphenolic Compounds from Andean Berry (Vaccinium meridionale Swartz) and Derived Functional Benefits: A Systematic and Updated Review. Foods. 2025; 14(22):3861. https://doi.org/10.3390/foods14223861
Chicago/Turabian StyleRamos-Polo, Ana Rosa, Ivan Luzardo-Ocampo, Sandra Navarro-Gallón, Silvia A. Quijano, and Sandra Sulay Arango-Varela. 2025. "Polyphenolic Compounds from Andean Berry (Vaccinium meridionale Swartz) and Derived Functional Benefits: A Systematic and Updated Review" Foods 14, no. 22: 3861. https://doi.org/10.3390/foods14223861
APA StyleRamos-Polo, A. R., Luzardo-Ocampo, I., Navarro-Gallón, S., Quijano, S. A., & Arango-Varela, S. S. (2025). Polyphenolic Compounds from Andean Berry (Vaccinium meridionale Swartz) and Derived Functional Benefits: A Systematic and Updated Review. Foods, 14(22), 3861. https://doi.org/10.3390/foods14223861









