Comparison of Alcohol-Induced Hepatoprotective Effects of the High Fischer Ratio Oligopeptides with/Without Half Substitution by Pueraria lobata
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of HFOPs
2.3. Evaluation of In Vitro Activity of HFOPs, PL, and HFOPs + PL(1:1) on Hepatoprotective Against Alcohol-Induced Injury and Hepatoprotection
2.3.1. Preparation of HFOPs, PL, and HFOPs + PL(1:1) Solutions
2.3.2. Determination of ADH Activity
2.3.3. Determination of SOD Activity
2.3.4. Determination of the Scavenging Capacity of Hydroxyl Radicals, DPPH Radicals, and ABTS Radicals
2.4. Protective Effect of HFOPs, PL, and HFOPs + PL(1:1) on Alcohol-Injured HepG2 Cells
2.4.1. HepG2 Cell Viability
2.4.2. Alcohol-Injured HepG2 Cell Viability
2.4.3. Effects of HFOPs, PL, and HFOPs + PL(1:1) on Liver Function Indicators, Antioxidant Activity, and Inflammatory Factors in Alcohol-Injured HepG2 Cells
2.5. In Vivo Experiment in Mice
2.5.1. Grouping and Model Establishment of Animal Experiment
2.5.2. Determination of Drunkenness and Sobriety Time in Mice
2.5.3. Determination of Liver Index
2.5.4. Determination of Serum Indexes
2.5.5. Determination of Biochemical Parameters in Liver Tissue
2.6. Statistical Analysis
3. Results
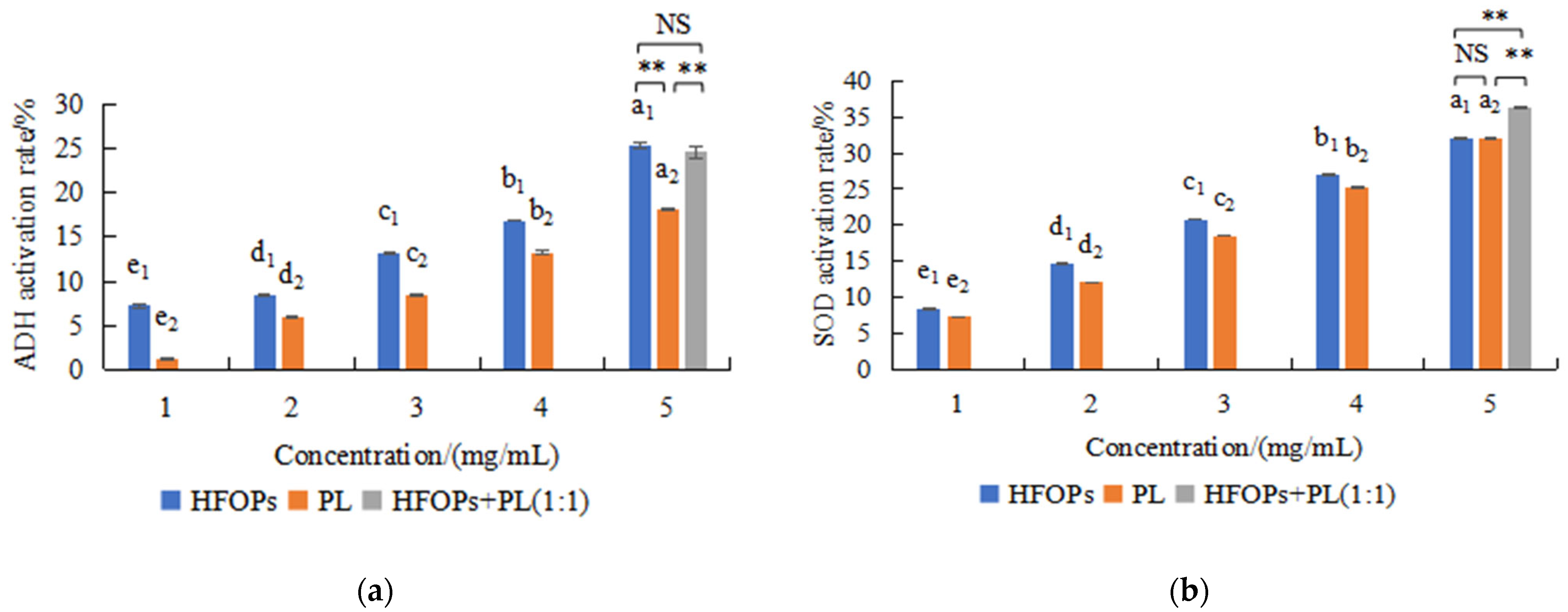
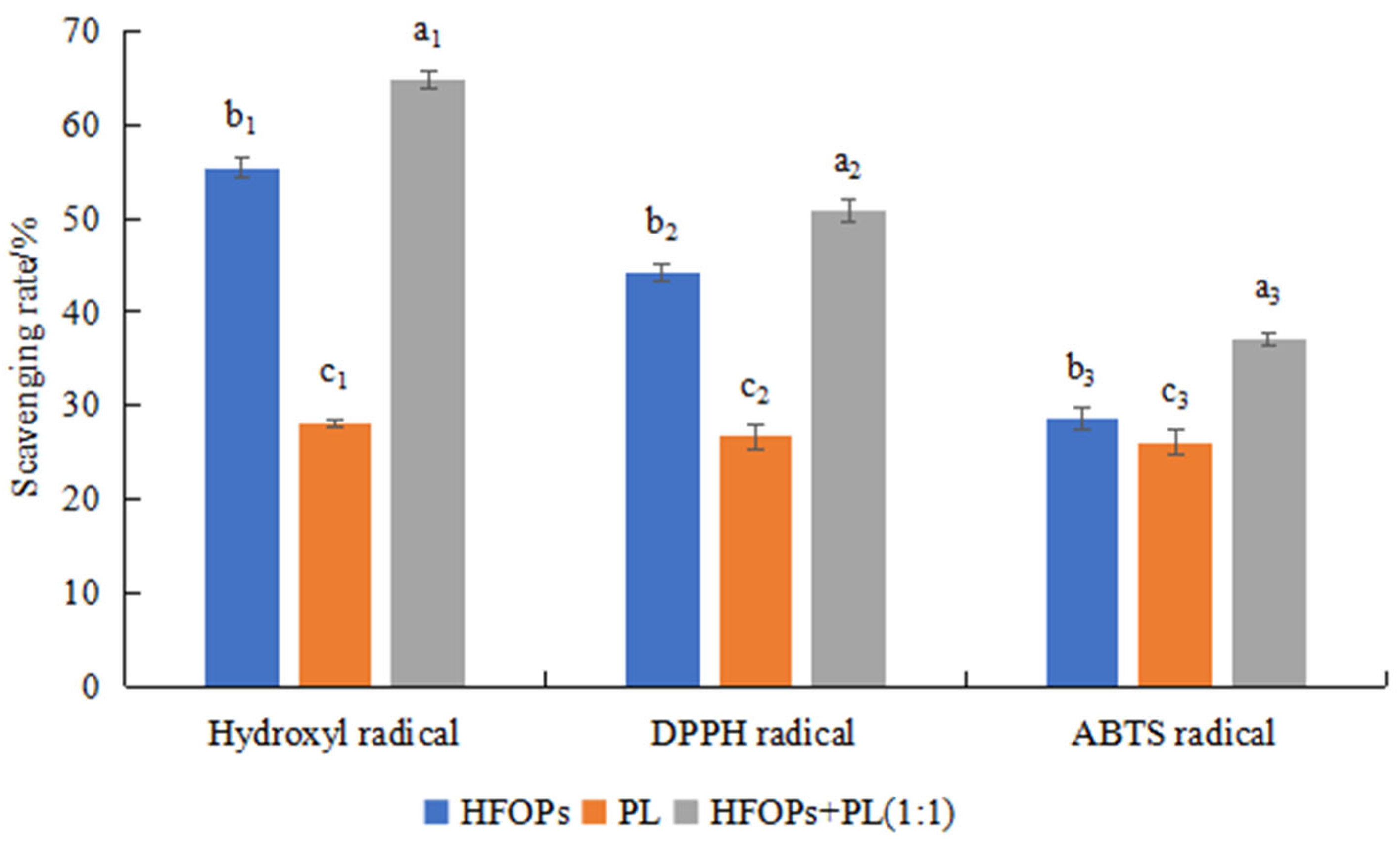
3.1. Effects of HFOPs, PL, and HFOPs+PL(1:1) on ADH and Antioxidant Activities In Vitro
3.2. Protective Effects of HFOPs, PL, and HFOPs + PL(1:1) on Alcohol-Injured HepG2 Cells
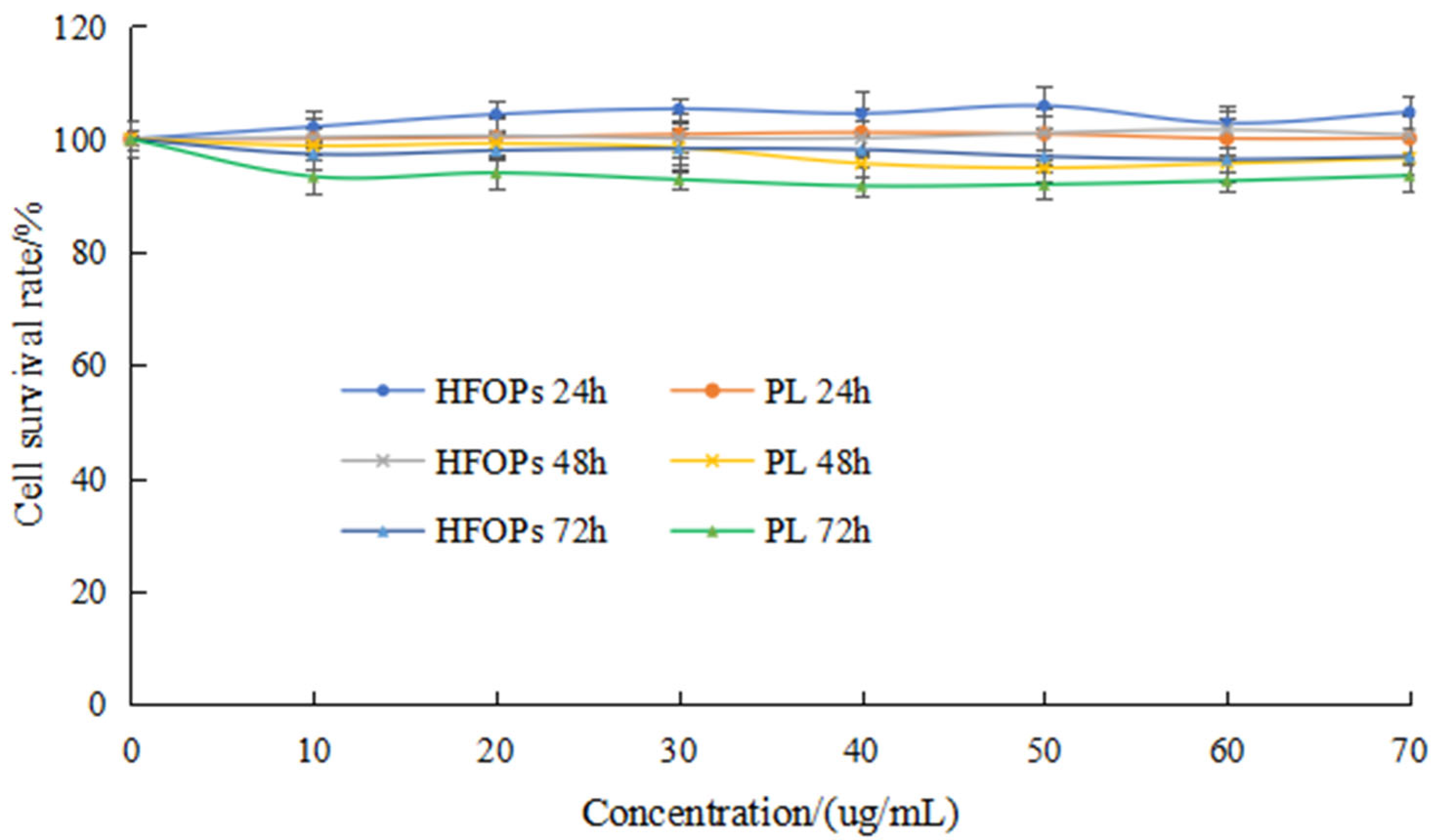
3.2.1. Effects of HFOPs and PL on HepG2 Cell Viability
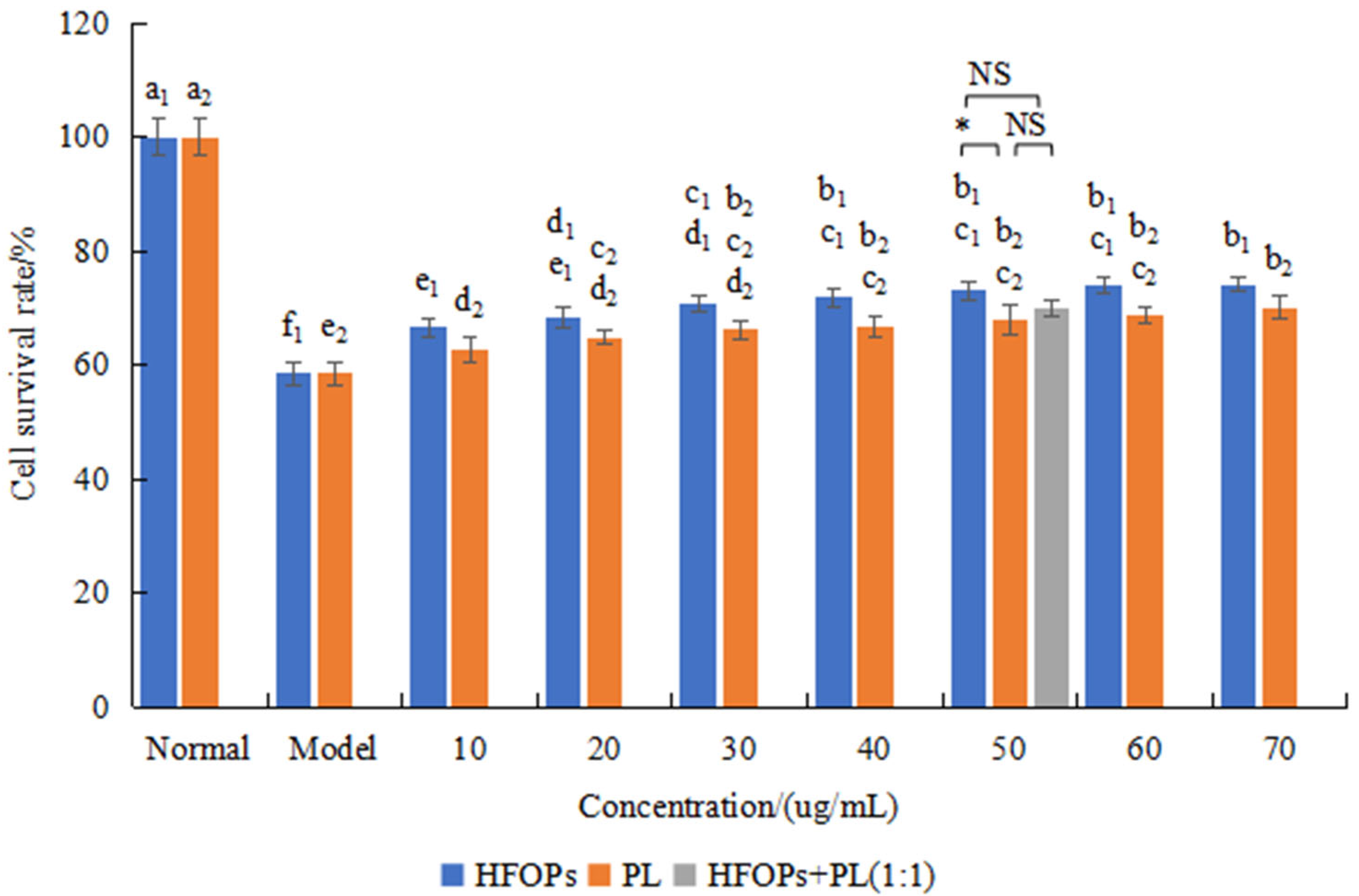
3.2.2. Effects of HFOPs, PL, and HFOPs + PL(1:1) on Alcohol-Injured HepG2 Cell Viability
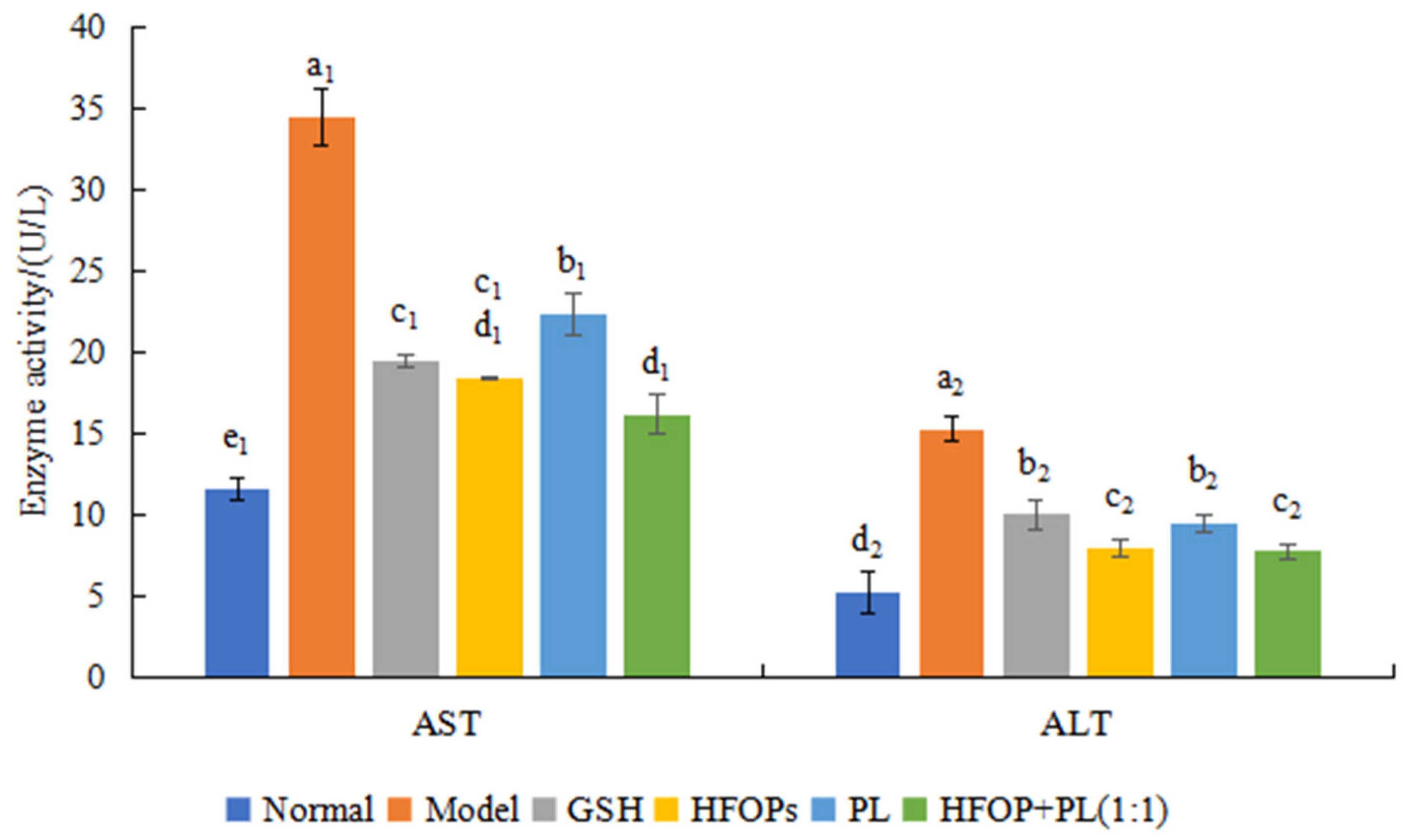
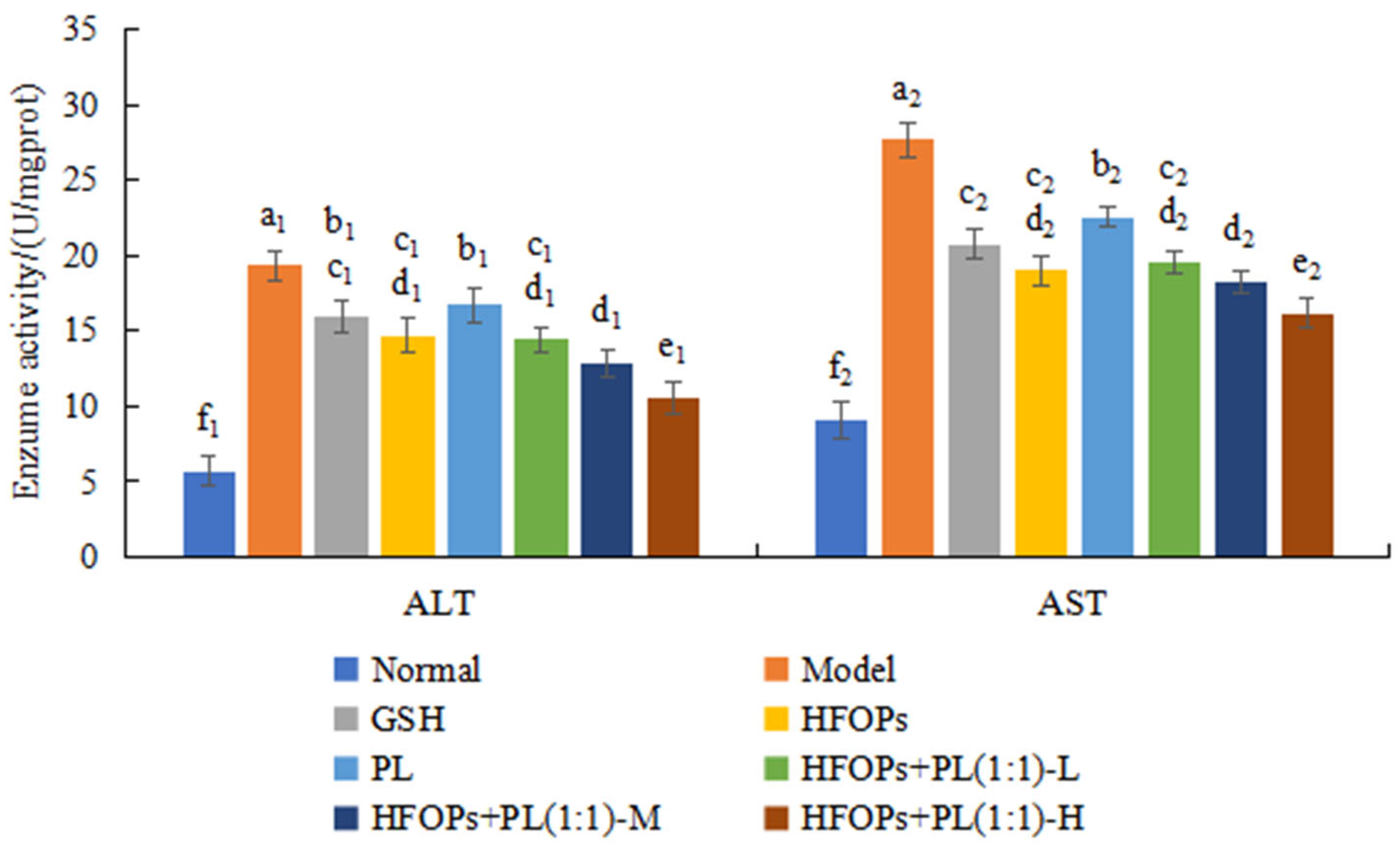
3.2.3. Effects of HFOPs, PL, and HFOPs + PL(1:1) on the AST and ALT in Alcohol-Injured HepG2 Cells
3.2.4. Effects of HFOPs, PL, and HFOPs + PL(1:1) on the Antioxidant Activity in Alcohol-Injured HepG2 Cells
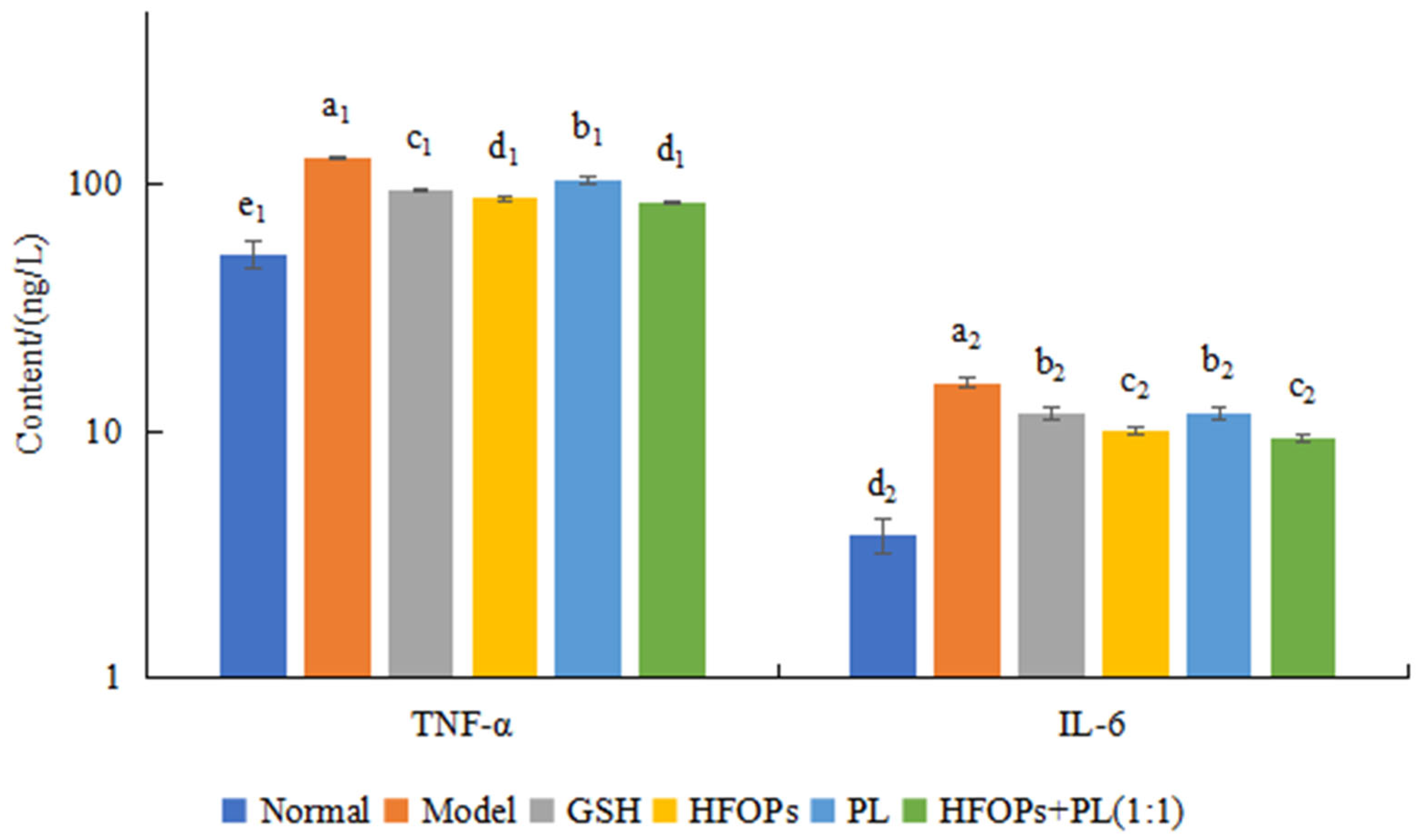
3.2.5. Effects of HFOPs, PL, and HFOPs + PL(1:1) on TNF-α and IL-6 Content in Alcohol-Injured HepG2 Cells
3.3. Protective Effect of HFOPs, PL, and HFOPs+PL(1:1) on Acute Alcoholic Liver Injury in Mice
3.3.1. Effects of HFOPs, PL, and HFOPs + PL(1:1) on the Drunkenness Time, Sobriety Time, and Liver in Drunken Mice
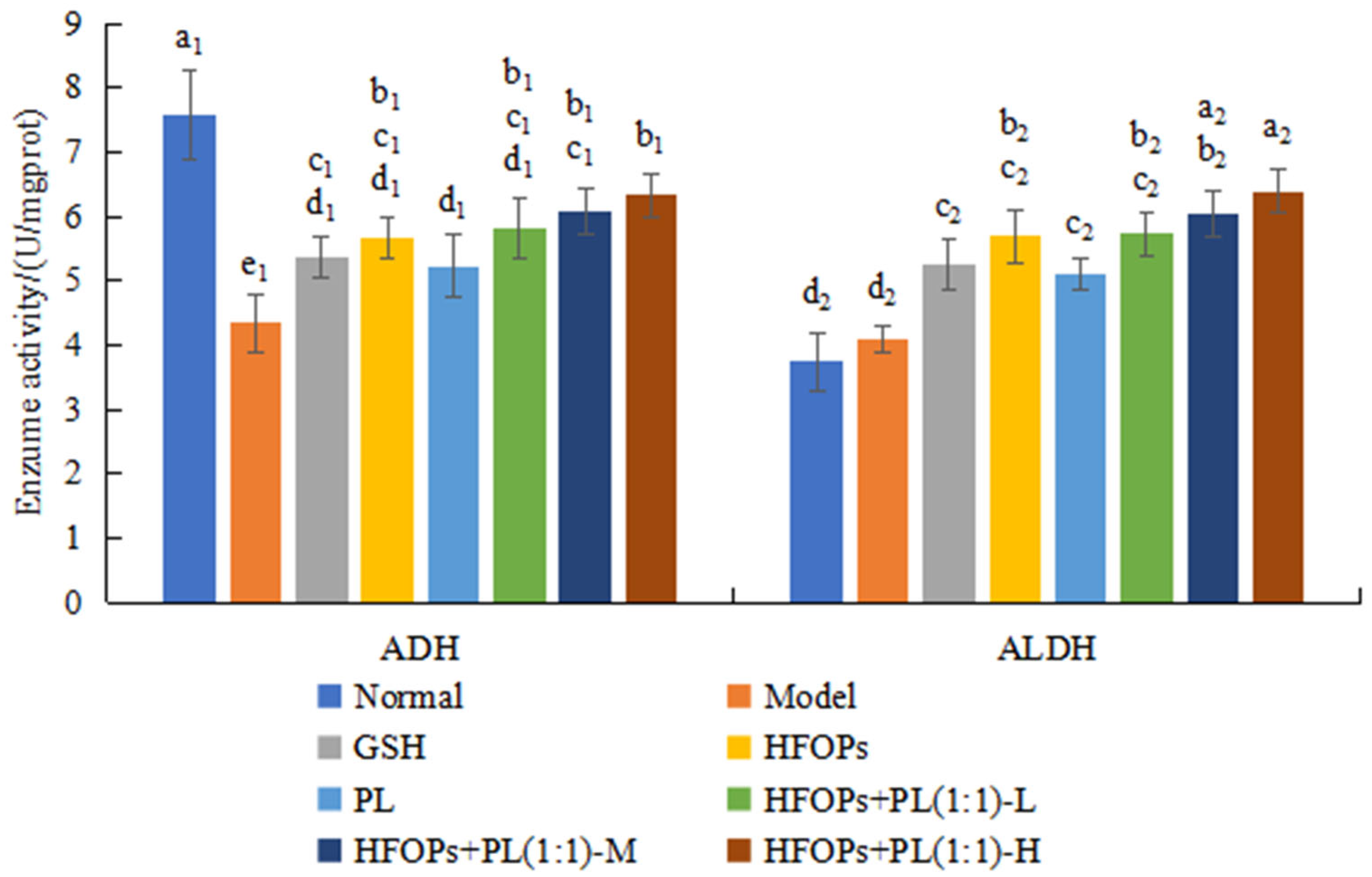
3.3.2. Effects of HFOPs, PL, and HFOPs + PL(1:1) on ADH and ALDH in the Liver of Mice with Acute Alcoholic Liver Injury
3.3.3. Effects of HFOPs, PL, and HFOPs + PL(1:1) on Serum ALT and AST in Mice with Acute Alcoholic Liver Injury
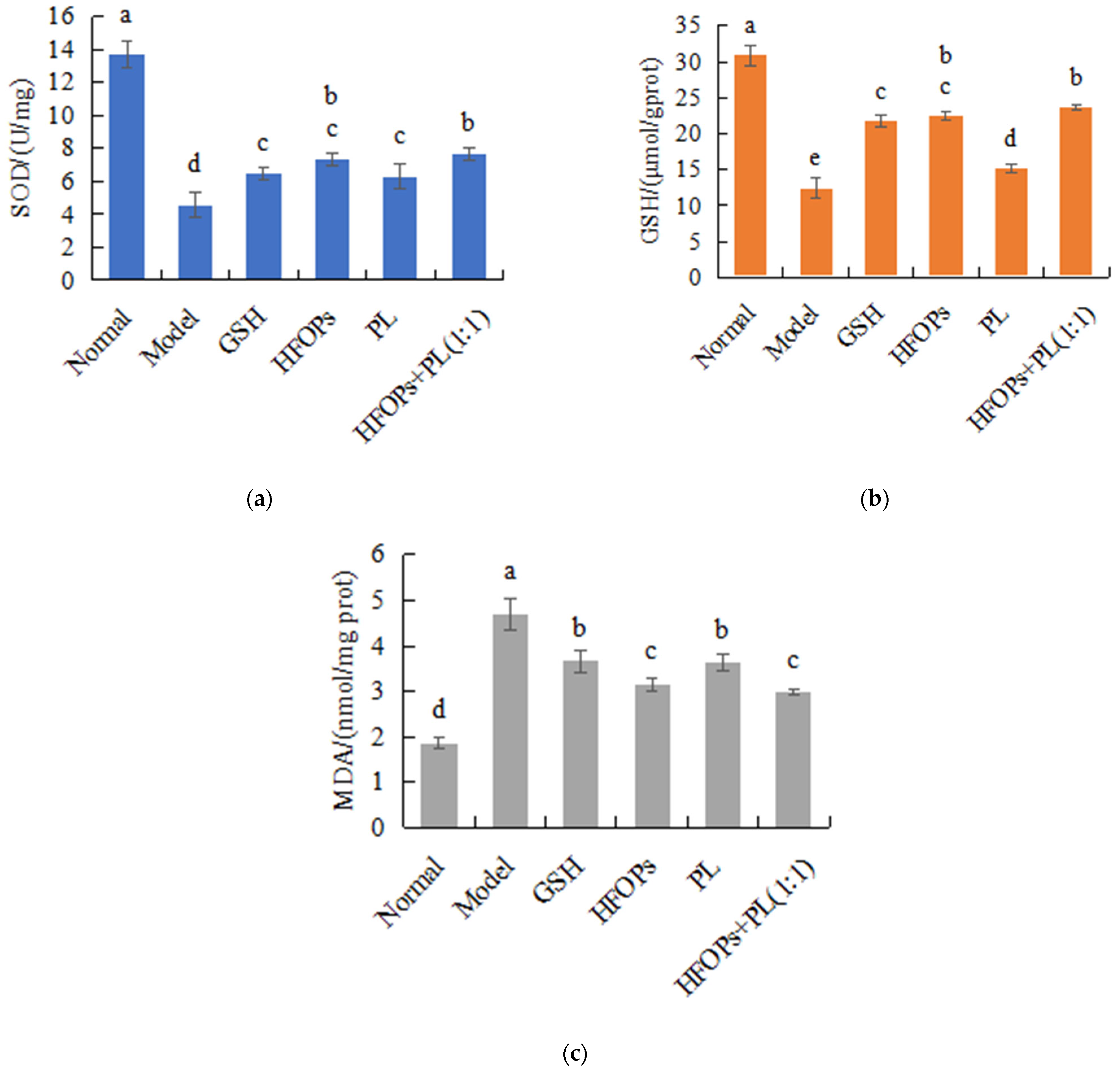
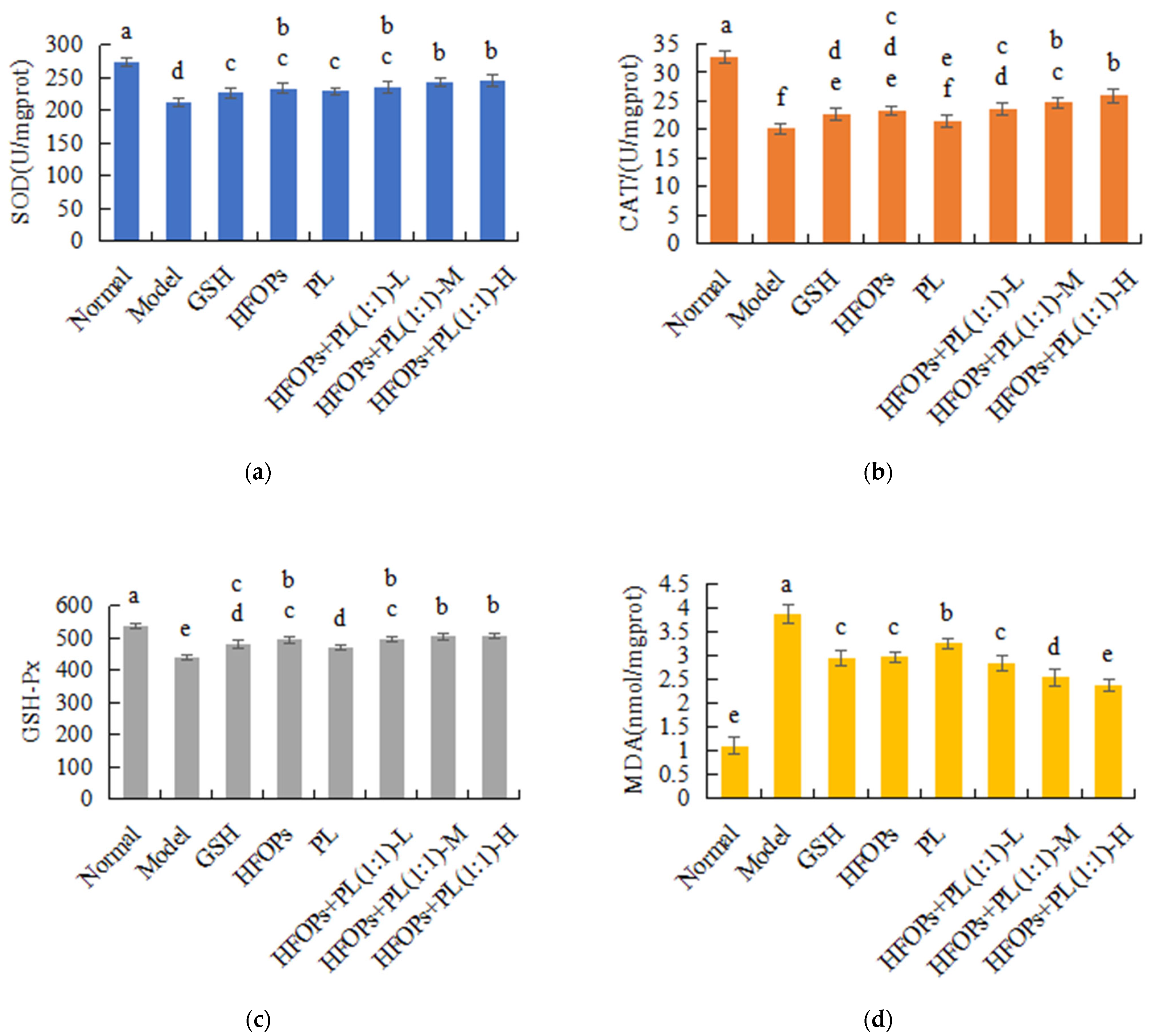
3.3.4. Effects of HFOPs, PL, and HFOPs + PL(1:1) on Antioxidant Enzyme Activity and MDA Content in the Liver of Mice with Acute Alcoholic Liver Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchanan, R.; Sinclair, J.M.A. Alcohol Use Disorder and the Liver. Addiction 2021, 116, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhao, M.; Zhou, F.; Gallego, M.; Gao, J.; Toldrá, F.; Mora, L. Isolation and Identification of Alcohol Dehydrogenase Stabilizing Peptides from Alcalase Digested Chicken Breast Hydrolysates. J. Funct. Foods 2020, 64, 103617. [Google Scholar] [CrossRef]
- Rosato, V.; Abenavoli, L.; Federico, A.; Masarone, M.; Persico, M. Pharmacotherapy of Alcoholic Liver Disease in Clinical Practice. Int. J. Clin. Pract. 2016, 70, 119–131. [Google Scholar] [CrossRef]
- Zhao, H.; Wen, L.; Wang, C.; Talab, A.; Qiu, J.; Xue, C.; Wang, Y.; Zhang, T. Sea Cucumber Saponins Reduce Blood Alcohol and Acetaldehyde Concentration by Inhibiting Alcohol Absorption and Regulating Alcohol Metabolism Enzyme Activity. Food Biosci. 2025, 63, 105742. [Google Scholar] [CrossRef]
- Kakazu, E.; Sano, A.; Morosawa, T.; Inoue, J.; Ninomiya, M.; Iwata, T.; Nakamura, T.; Takai, S.; Sawada, S.; Katagiri, H.; et al. Branched Chain Amino Acids Are Associated with the Heterogeneity of the Area of Lipid Droplets in Hepatocytes of Patients with Non-alcoholic Fatty Liver Disease. Hepatol. Res. 2019, 49, 860–871. [Google Scholar] [CrossRef]
- Dong, X.-M.; Suo, S.-K.; Wang, Y.-M.; Zeng, Y.-H.; Chi, C.-F.; Wang, B. High Fischer Ratio Oligopeptides from Antarctic Krill: Ameliorating Function and Mechanism to Alcoholic Liver Injury through Regulating AMPK/Nrf2/IκBα Pathways. J. Funct. Foods 2024, 122, 106537. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Wang, L.; Ou, X.; Huang, J. High Fischer Ratio Oligopeptides in Food: Sources, Functions and Application Prospects. J. Future Foods 2024, 4, 128–134. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Li, X.; Wang, B. High Fischer Ratio Oligopeptides Determination from Antartic Krill: Preparation, Peptides Profiles, and in Vitro Antioxidant Activity. J. Food Biochem. 2019, 43, e12827. [Google Scholar] [CrossRef]
- Qin, Y.; Cheng, M.; Fan, X.; Shao, X.; Wang, C.; Jiang, H.; Zhang, X. Preparation and Antioxidant Activities of High Fischer’s Ratio Oligopeptides from Goat Whey. Food Sci. Anim. Resour. 2022, 42, 800–815. [Google Scholar] [CrossRef]
- Xi, C.; Zhang, M.; Li, B.; Meng, X.; Xu, S.; Du, H.; Wang, X.; Xu, J.; Ke, H.; Cui, Y.; et al. Metabolomics of the Anti-Inflammatory Effect of Pueraria Lobata and Pueraria Lobata Var. Thomsonii in Rats. J. Ethnopharmacol. 2023, 306, 116144. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, G.; Shang, M.; Wang, Y.; Xia, C.; Duan, B.; Xu, L. Extraction, Structural-Activity Relationships, Bioactivities, and Application Prospects of Pueraria Lobata Polysaccharides as Ingredients for Functional Products: A Review. Int. J. Biol. Macromol. 2023, 243, 125210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Peng, D.; Li, W.; Chen, S.; Liu, B.; Huang, P.; Wu, J.; Du, B.; Li, P. Probiotic-Fermented Pueraria Lobata (Willd.) Ohwi Alleviates Alcoholic Liver Injury by Enhancing Antioxidant Defense and Modulating Gut Microbiota. J. Sci. Food Agric. 2022, 102, 6877–6888. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Luo, C.; Zhang, X.; Liu, Y.; Wang, Z.; Cacciamani, P.; Shi, J.; Cui, Y.; Wang, C.; Sinha, B.; et al. Tartary Buckwheat Extract Alleviates Alcohol-Induced Acute and Chronic Liver Injuries through the Inhibition of Oxidative Stress and Mitochondrial Cell Death Pathway. Am. J. Transl. Res. 2020, 12, 70–89. [Google Scholar]
- Zheng, S.-L.; Wang, Y.-Z.; Zhao, Y.-Q.; Chi, C.-F.; Zhu, W.-Y.; Wang, B. High Fischer Ratio Oligopeptides from Hard-Shelled Mussel: Preparation and Hepatoprotective Effect against Acetaminophen-Induced Liver Injury in Mice. Food Biosci. 2023, 53, 102638. [Google Scholar] [CrossRef]
- Wang, S.P.; Althoff, D.M. Different Genetic Basis for Alcohol Dehydrogenase Activity and Plasticity in a Novel Alcohol Environment for Drosophila Melanogaster. Heredity 2020, 125, 101–109. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Zhang, C.; Li, S.; Yang, Q.; Zhang, J.; Gong, Z.; Han, J.; Jia, L. Antioxidant Activity and Protective Effects of Enzyme-Extracted Oudemansiella Radiata Polysaccharides on Alcohol-Induced Liver Injury. Molecules 2018, 23, 481. [Google Scholar] [CrossRef]
- Li, G.; Zhan, J.; Hu, L.; Yuan, C.; Takaki, K.; Ying, X.; Hu, Y. Identification of a New Antioxidant Peptide from Porcine Plasma by in Vitro Digestion and Its Cytoprotective Effect on H2O2 Induced HepG2 Model. J. Funct. Foods 2021, 86, 104679. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Chen, Y.; Sun, H.; Liu, Y. Preparation and Characterization of Lipophilic Antioxidative Peptides Derived from Mung Bean Protein. Food Chem. 2022, 395, 133535. [Google Scholar] [CrossRef]
- Hu, X.; Pan, C.; Cai, M.; Li, L.; Yang, X.; Xiang, H.; Chen, S. Novel Antioxidant Peptides from Grateloupia Livida Hydrolysates: Purification and Identification. Foods 2022, 11, 1498. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse Model of Chronic and Binge Ethanol Feeding (the NIAAA Model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Chen, X.; Cai, F.; Guo, S.; Ding, F.; He, Y.; Wu, J.; Liu, C. Protective Effect of Flos Puerariae Extract Following Acute Alcohol Intoxication in Mice. Alcohol. Clin. Exp. Res. 2014, 38, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Deng, Y.; Chen, L.; Zheng, Z.; Ming, Y. Protective Effect of Corilagin on Alcoholic Liver Injury. Nat. Prod. Commun. 2024, 19, 1–10. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Triterpenoids from Fruits of Sorbus Pohuashanensis Inhibit Acetaminophen-Induced Acute Liver Injury in Mice. Biomed. Pharmacother. 2019, 109, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, Y.; Zhang, Y.; Dong, J.; Jiang, S.; Tang, Y. DHA-Enriched Phosphatidylserine Ameliorates Cyclophosphamide-Induced Liver Injury via Regulating the Gut-Liver Axis. Int. Immunopharmacol. 2024, 140, 112895. [Google Scholar] [CrossRef]
- Xie, L.; Huang, W.; Li, J.; Chen, G.; Xiao, Q.; Zhang, Y.; He, H.; Wang, Q.; He, J. The Protective Effects and Mechanisms of Modified Lvdou Gancao Decoction on Acute Alcohol Intoxication in Mice. J. Ethnopharmacol. 2022, 282, 114593. [Google Scholar] [CrossRef]
- Chinnappan, R.; Mir, T.A.; Alsalameh, S.; Makhzoum, T.; Adeeb, S.; Al-Kattan, K.; Yaqinuddin, A. Aptasensors Are Conjectured as Promising ALT and AST Diagnostic Tools for the Early Diagnosis of Acute Liver Injury. Life 2023, 13, 1273. [Google Scholar] [CrossRef]
- Church, R.J.; Watkins, P.B. The Challenge of Interpreting Alanine Aminotransferase Elevations in Clinical Trials of New Drug Candidates. Clin. Transl. Sci. 2021, 14, 434–436. [Google Scholar] [CrossRef]
- Wang, L.-C.; Kuo, I.-U.; Tsai, T.-Y.; Lee, C.-L. Antrodia camphorata-Fermented Product Cultured in Deep Ocean Water Has More Liver Protection against Thioacetamide-Induced Fibrosis. Appl. Microbiol. Biotechnol. 2013, 97, 9955–9967. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, S.; Feng, M.; Wang, L.; Gao, F. Hepatoprotective Effect of Albumin Peptide Fractions from Corn Germ Meal against Alcohol-Induced Acute Liver Injury in Mice. Foods 2023, 12, 1183. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.-J.; Wang, X.-M.; Zhao, G.-H.; Cai, D.; Yu, J.-H.; Yin, F.-W.; Zhou, D.-Y. Preparation and Hepatoprotective Activities of Peptides Derived from Mussels (Mytilus edulis) and Clams (Ruditapes philippinarum). Mar. Drugs 2022, 20, 719. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hao, Y.-T.; Zhu, N.; Liu, X.-R.; Mao, R.-X.; Kang, J.-W.; Hou, C.; Zhang, T.; Li, Y. Walnut (Juglans regia L.) Oligopeptides Alleviate Alcohol-Induced Acute Liver Injury through the Inhibition of Inflammation and Oxidative Stress in Rats. Nutrients 2023, 15, 2210. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Luo, H.; Jin, Y.; Shu, Y.; Wen, C.; Qin, T.; Yang, X.; Ma, L.; Liu, Y.; You, Y.; et al. Asperosaponin VI Protects Alcohol-Induced Hepatic Steatosis and Injury via Regulating Lipid Metabolism and ER Stress. Phytomedicine 2023, 121, 155080. [Google Scholar] [CrossRef]
- Wu, L.; Li, W.; Chen, G.; Yang, Z.; Lv, X.; Zheng, L.; Sun, J.; Ai, L.; Sun, B.; Ni, L. Ameliorative Effects of Monascin from Red Mold Rice on Alcoholic Liver Injury and Intestinal Microbiota Dysbiosis in Mice. Food Biosci. 2022, 50, 102079. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, Y.; Zhao, J.; He, H.; Hou, T. Selenium-Biofortified Corn Peptides: Attenuating Concanavalin A—Induced Liver Injury and Structure Characterization. J. Trace Elem. Med. Biol. 2019, 51, 57–64. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, Y.; Chen, X.; Zhang, M.; Hu, Z.; Chen, L.; Huang, J. High Fischer Ratio Oligopeptides of Gluten Alleviate Alcohol-Induced Liver Damage by Regulating Lipid Metabolism and Oxidative Stress in Rats. Foods 2024, 13, 436. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, M.; Hemar, Y. Study of the in Vitro Properties of Oligopeptides from Whey Protein Isolate with High Fisher’s Ratio and Their Ability to Prevent Allergic Response to β-Lactoglobulin in Vivo. Food Chem. 2023, 405, 134841. [Google Scholar] [CrossRef]
- Ma, J.-Q.; Ding, J.; Zhang, L.; Liu, C.-M. Ursolic Acid Protects Mouse Liver against CCl4-Induced Oxidative Stress and Inflammation by the MAPK/NF-κB Pathway. Environ. Toxicol. Pharmacol. 2014, 37, 975–983. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Xu, G.-L.; Jia, W.-D.; Han, S.-J.; Ren, W.-H.; Wang, W.; Liu, W.-B.; Zhang, C.-H.; Chen, H. Estrogen Suppresses Metastasis in Rat Hepatocellular Carcinoma through Decreasing Interleukin-6 and Hepatocyte Growth Factor Expression. Inflammation 2012, 35, 143–149. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Xia, Y.; Li, X.; Chen, T.; Yan, J.; Wang, Y. Alteration of Liver Immunity by Increasing Inflammatory Response during Co-Administration of Methamphetamine and Atazanavir. Immunopharmacol. Immunotoxicol. 2020, 42, 237–245. [Google Scholar] [CrossRef]
- Ren, J.; Li, S.; Song, C.; Sun, X.; Liu, X. Black Soybean-Derived Peptides Exerted Protective Effect against Alcohol-Induced Liver Injury in Mice. J. Funct. Foods 2021, 87, 104828. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective Effect of Kaempferol against Alcoholic Liver Injury in Mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef]
| Groups | Sample Concentration/(mg·mL−1) | Dose Administered/(mg·kg−1 bw) | Alcohol Dose/(mL·10g−1 bw) | Drunkenness Time/min | Sobriety Time/min | Liver Index/% |
|---|---|---|---|---|---|---|
| Normal | - | - | - | - | - | 4.05 ± 0.09 e |
| Model | - | - | 0.09 | 10.13 ± 3.48 d | 65.38 ± 6.16 a | 5.23 ± 0.16 a |
| GSH | 5 | 50 | 0.09 | 14.13 ± 4.05 bcd | 58.13 ± 4.97 ab | 4.47 ± 0.12 bcd |
| HFOPs | 40 | 400 | 0.09 | 21.75 ± 4.33 ab | 48.88 ± 5.67 bcd | 4.49 ± 0.13 bcd |
| PL | 40 | 400 | 0.09 | 12.25 ± 4.68 cd | 52.25 ± 7.59 bc | 4.72 ± 0.21 bc |
| HFOPs + PL(1:1)-L | 20 + 20 | 200 + 200 | 0.09 | 19.63 ± 3.50 abc | 49.88 ± 7.68 bcd | 4.76 ± 0.14 b |
| HFOPs + PL(1:1)-M | 40 + 40 | 400 + 400 | 0.09 | 22.25 ± 6.54 ab | 44.75 ± 5.29 cd | 4.39 ± 0.28 cd |
| HFOPs + PL(1:1)-H | 60 + 60 | 600 + 600 | 0.09 | 25.13 ± 5.38 a | 39.63 ± 4.81 d | 4.34 ± 0.23 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Zhao, Q.; Chen, N.; Zhang, Z.; Wang, J.; Fan, H.; Liu, H.; Zhang, L. Comparison of Alcohol-Induced Hepatoprotective Effects of the High Fischer Ratio Oligopeptides with/Without Half Substitution by Pueraria lobata. Foods 2025, 14, 3859. https://doi.org/10.3390/foods14223859
Deng Y, Zhao Q, Chen N, Zhang Z, Wang J, Fan H, Liu H, Zhang L. Comparison of Alcohol-Induced Hepatoprotective Effects of the High Fischer Ratio Oligopeptides with/Without Half Substitution by Pueraria lobata. Foods. 2025; 14(22):3859. https://doi.org/10.3390/foods14223859
Chicago/Turabian StyleDeng, Yongke, Qin Zhao, Na Chen, Zhiqin Zhang, Jingxuan Wang, Hongbing Fan, Haimei Liu, and Lili Zhang. 2025. "Comparison of Alcohol-Induced Hepatoprotective Effects of the High Fischer Ratio Oligopeptides with/Without Half Substitution by Pueraria lobata" Foods 14, no. 22: 3859. https://doi.org/10.3390/foods14223859
APA StyleDeng, Y., Zhao, Q., Chen, N., Zhang, Z., Wang, J., Fan, H., Liu, H., & Zhang, L. (2025). Comparison of Alcohol-Induced Hepatoprotective Effects of the High Fischer Ratio Oligopeptides with/Without Half Substitution by Pueraria lobata. Foods, 14(22), 3859. https://doi.org/10.3390/foods14223859







