Screening and Identification of Antioxidant Peptides from Sea Cucumber Gonad Proteins and Their Activation of Superoxide Dismutase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation and Fractionation of Enzymatic Hydrolysates from Sea Cucumber Gonads
2.3. Determination of Molecular Weight Distribution of Peptide Fractions
2.4. Antioxidant Assays
2.4.1. DPPH Free Radical Scavenging Assay
2.4.2. ABTS Radical Scavenging Assay
2.4.3. SOD Activation Assay
2.5. Peptide Sequence Identification and Virtual Screening
2.5.1. Identification and Sequencing of Peptides by Liquid Chromatography–Tandem Mass Spectrometry
2.5.2. In Silico Screening of the Identified Peptides
2.6. Molecular Docking and Binding Mode Analysis
2.7. Molecular Dynamics (MD) Simulations
2.8. Statistical Analysis
3. Results
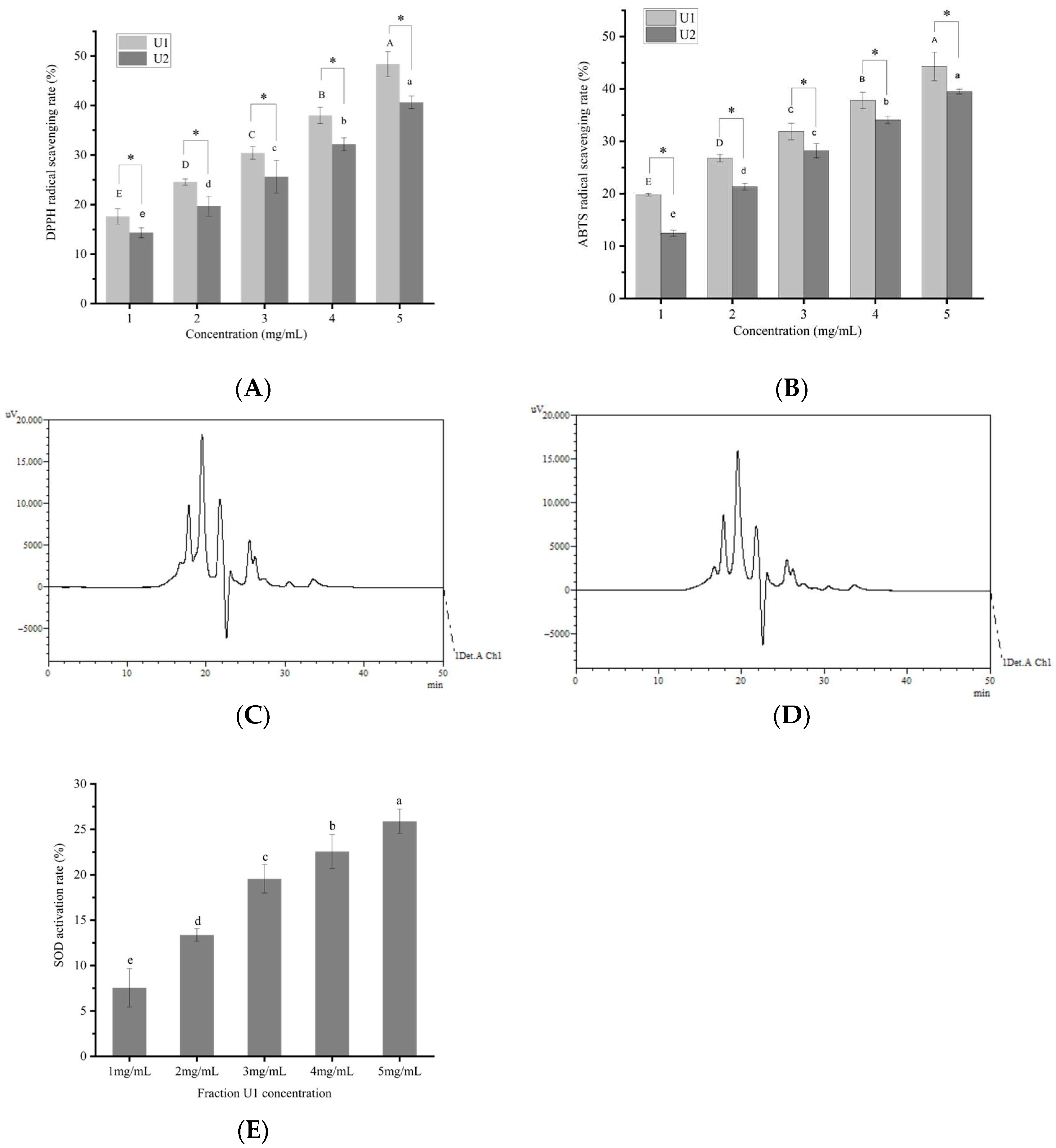
3.1. Antioxidant Activity of Sea Cucumber Gonadal Peptides and Fractionation
3.2. SOD Activation Rate of U1 Fraction
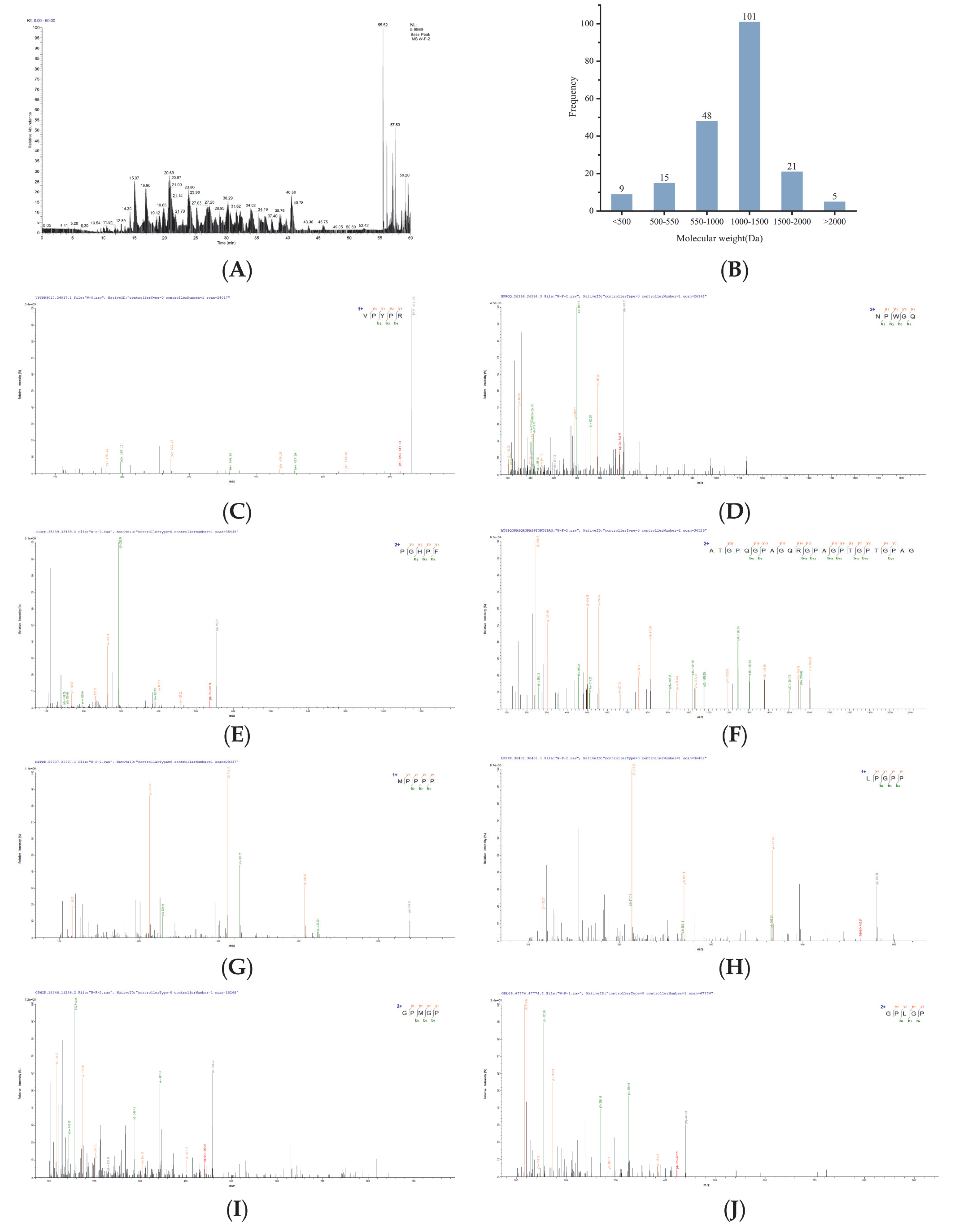
3.3. Identification of Antioxidant Peptides in U1 Fraction and In Silico Screening
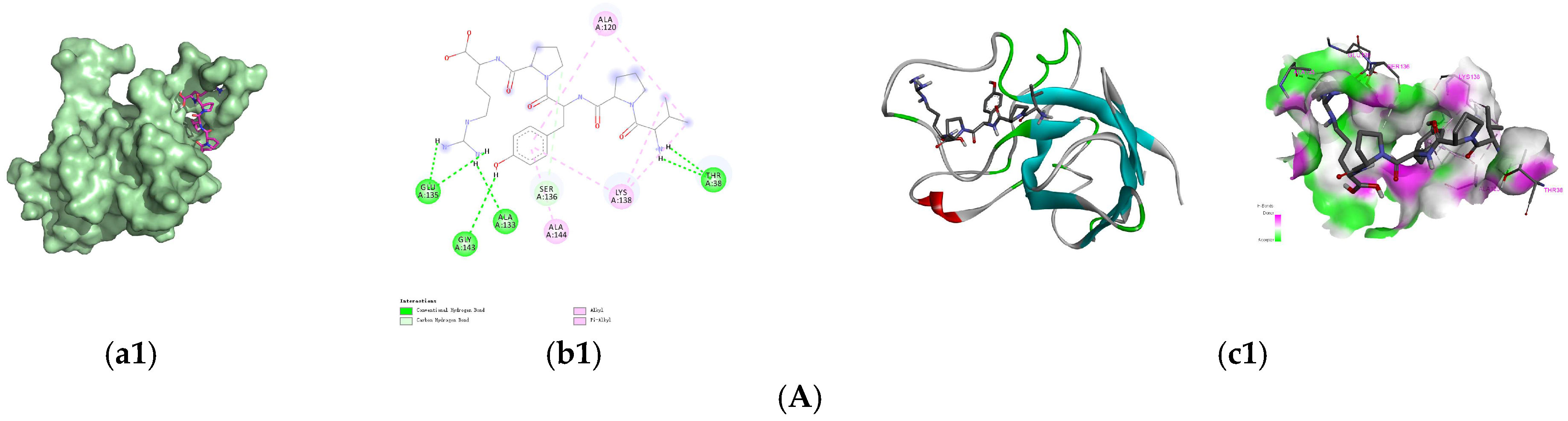
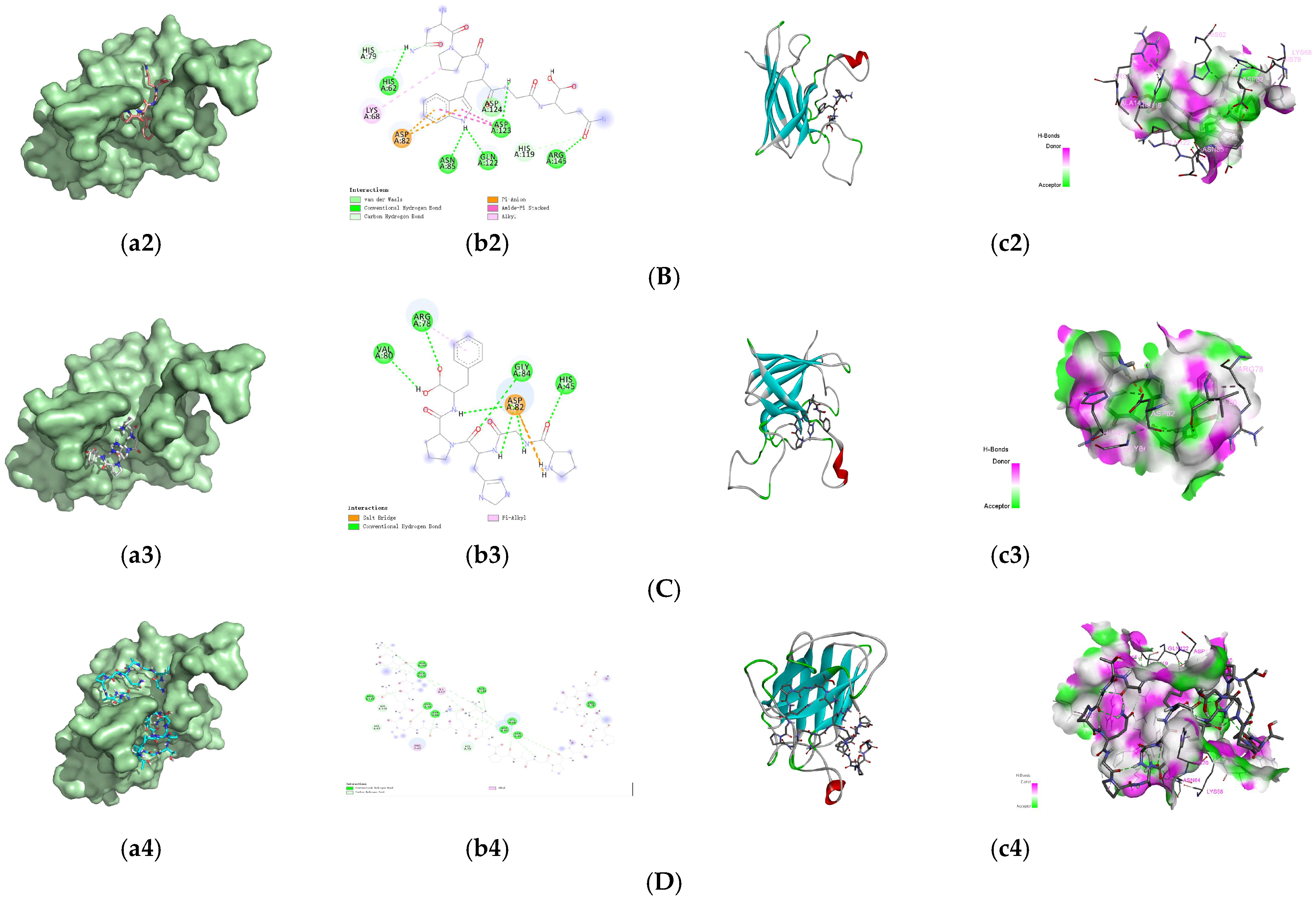
3.4. Molecular Docking of the Identified Peptides to SOD
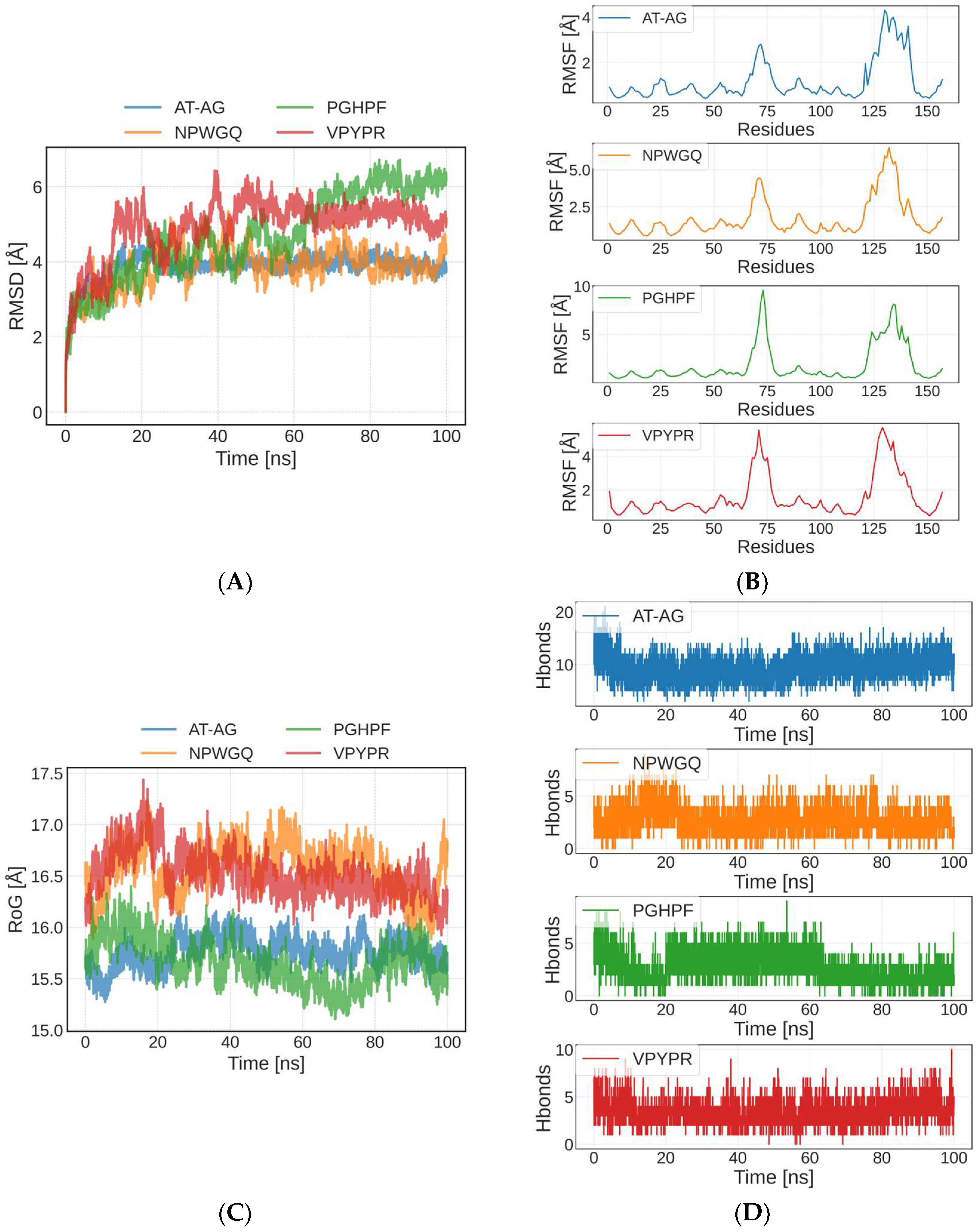
3.5. Molecular Dynamics Simulation of Peptide–SOD Binding
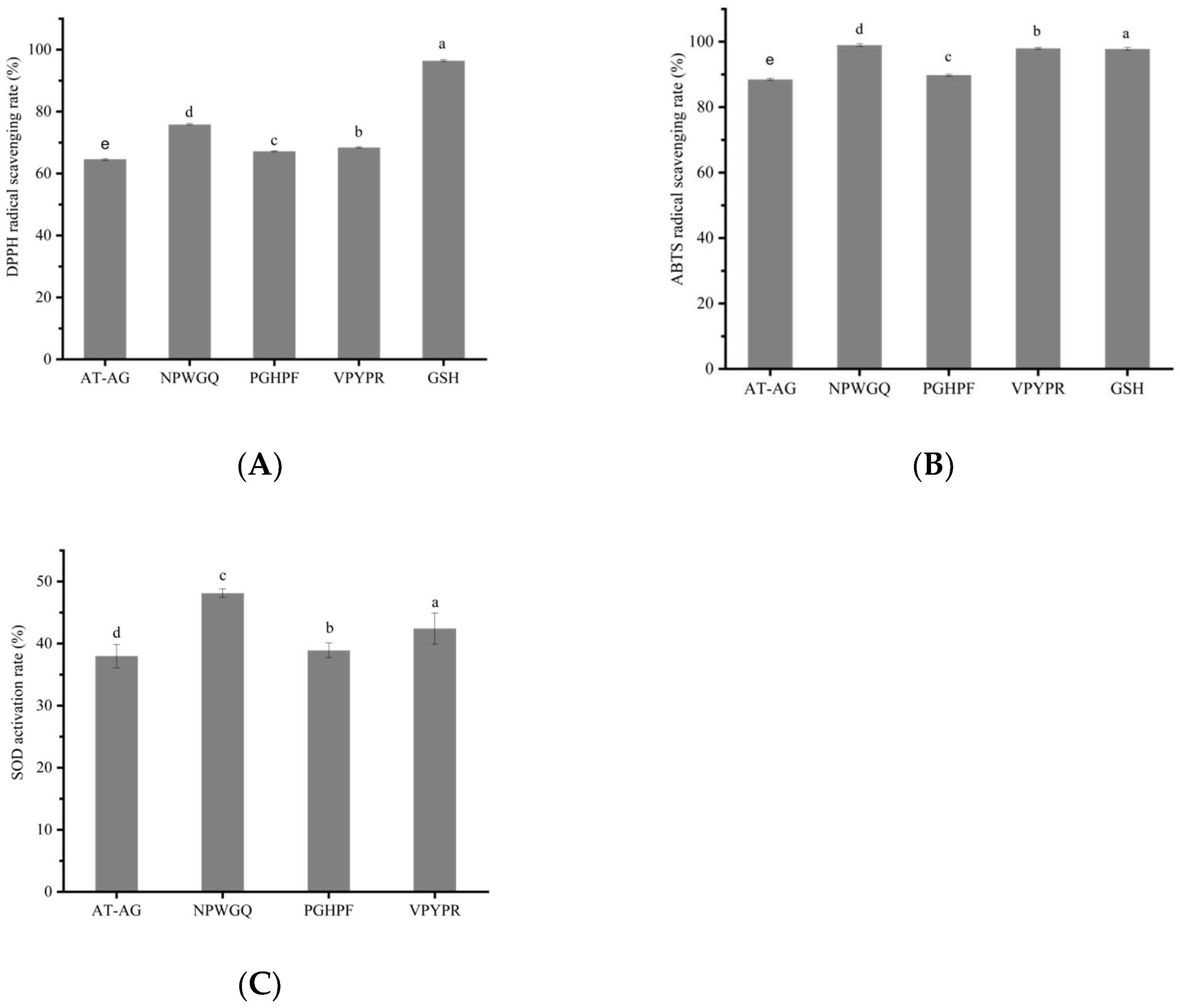
3.6. In Vitro Antioxidant Activity of the Screened Peptides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. Adaptive Homeostasis and the Free Radical Theory of Ageing. Free Radic. Biol. Med. 2018, 124, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative Effects and Mechanism Study of Bioactive Peptides from Defatted Walnut (Juglans regia L.) Meal Hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Z.; Wang, Y.; Li, Y.; Zheng, W.; Chai, Y.; Wei, G.; Huang, A. Identification and Characterization of Novel Antioxidant Peptides from Yunnan Dry-Cured Beef: A Combined in Silico and in Vitro Study. Food Chem. 2025, 477, 143485. [Google Scholar] [CrossRef] [PubMed]
- Song, A.Y.; Cheung, H. Seizing a Venue Linking Opportunity: China’s Strategy to Advance Its Sea Cucumber Interests in Global Environmental Governance. Mar. Policy 2024, 169, 106345. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, L.; Wang, S.; Zhao, M.; Liu, X. Anti-Diabetic and Anti-Hyperlipidemic Effects of Sea Cucumber (Cucumaria Frondosa) Gonad Hydrolysates in Type II Diabetic Rats. Food Sci. Hum. Wellness 2022, 11, 1614–1622. [Google Scholar] [CrossRef]
- Abdo, A.A.A.; Hou, Y.; Hassan, F.A.; Al-Sheraji, S.H.; Aleryani, H.; Alanazi, A.; Sang, Y. Antioxidant Potential and Protective Effect of Modified Sea Cucumber Peptides against H2O2-Induced Oxidative Damage in Vitro HepG2 Cells and in Vivo Zebrafish Model. Int. J. Biol. Macromol. 2024, 266, 131090. [Google Scholar] [CrossRef]
- Chen, G.; Ge, X.; Sun, Y.; Sui, W.; Jin, Y.; Geng, J.; Zhang, M.; Wu, T. Identification of Two Novel α-Amylase Inhibitory Activity Peptide from Russian Sea Cucumber Body Wallprotein Hydrolysate. Int. J. Biol. Macromol. 2025, 295, 139499. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Z.; Chen, Y.; Wu, T.; Fersht, V.; Jin, Y.; Meng, J.; Zhang, M. Sea Cucumber Peptides Inhibit the Malignancy of NSCLC by Regulating miR-378a-5p Targeted TUSC2. Food Funct. 2021, 12, 12362–12371. [Google Scholar] [CrossRef]
- Feng, J.; Wang, H.; Luo, X.; Zhang, L.; Zhou, P. Identification and Molecular Mechanism of the Anti-Inflammatory Effect of Sea Cucumber Peptides: Network Pharmacology, Molecular Docking and Animal Experiments. Int. J. Biol. Macromol. 2024, 279, 134958. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, Preparation, and Purification of Marine Bioactive Peptides. Biomed Res. Int. 2017, 2017, 9746720. [Google Scholar] [CrossRef]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive Oxygen Species in Normal and Tumor Stem Cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [CrossRef]
- Aparici, M.; Bravo, M.; Calama, E.; García-González, V.; Domènech, T.; Córdoba, M.; Roger, I.; Cortijo, J.; Góngora-Benítez, M.; Paradís-Bas, M.; et al. Pharmacological Characterization of a Novel Peptide Inhibitor of the Keap1-Nrf2 Protein-Protein Interaction. Biochem. Pharmacol. 2022, 204, 115226. [Google Scholar] [CrossRef]
- Yi, G.; Din, J.U.; Zhao, F.; Liu, X. Effect of Soybean Peptides against Hydrogen Peroxide Induced Oxidative Stress in HepG2 Cells via Nrf2 Signaling. Food Funct. 2020, 11, 2725–2737. [Google Scholar] [CrossRef]
- Han, A.; Liu, H.; Dai, Y.; Sun, S.; Ma, H. Screening of Umami Peptides from Fermented Grains by Machine Learning, Molecular Docking and Molecular Dynamics Simulation. Food Biosci. 2024, 62, 105536. [Google Scholar] [CrossRef]
- Liu, L.; Liang, J.; He, M.; Jiang, B.; Liu, J.; Wu, J.; Li, P.; Du, B. Pueraria Lobata-Derived Peptides Hold Promise as a Novel Antioxidant Source by Mitigating Ethanol-Induced Oxidative Stress in HepG2 Cells through Regulating the Keap1/Nrf2 Pathway. Food Chem. 2025, 490, 145014. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Zhao, Y.-Q.; Zhao, G.-X.; Chi, C.-F.; Wang, B. Antioxidant Peptides from Collagen Hydrolysate of Redlip Croaker (Pseudosciaena Polyactis) Scales: Preparation, Characterization, and Cytoprotective Effects on H2O2-Damaged HepG2 Cells. Mar. Drugs 2020, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Wells, G. Peptide and Small Molecule Inhibitors of the Keap1-Nrf2 Protein-Protein Interaction. Biochem. Soc. Trans. 2015, 43, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; He, X.; Chen, T.; Liu, J.; Luo, Z.; Sun, S.; Qin, D.; Huang, W.; Tang, Y.; Liu, C.; et al. Peptides Isolated from Yak Milk Residue Exert Antioxidant Effects through Nrf2 Signal Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 9426314. [Google Scholar] [CrossRef]
- Wen-Tao, C.H.E.N.; Zhang, Y.-Y.; Qiang, Q.; Zou, P.; Xu, Y.; Sun, C.; Badar, I.H. Characterizations and Molecular Docking Mechanism of the Interactions between Peptide FDGDF (Phe-Asp-Gly-Asp-Phe) and SOD Enzyme. Heliyon 2024, 10, e24515. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Chi, C.-F.; Ma, J.-H.; Luo, H.-Y.; Xu, Y. Purification and Characterisation of a Novel Antioxidant Peptide Derived from Blue Mussel (Mytilus Edulis) Protein Hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Xun, X.-M.; Yan, C.-H.; Yuan, Z.-X.; Zhang, Z.-A.; Herman, R.A.; Xu, Y.; Wu, Q.-Y.; Wang, J. 60Co-γ-Ray-Irradiated Edible Silkworm (Bombyx mori) Pupae-Assisted Protease Digestion: A Strategy for Obtaining Low Molecular Weight Bioactive Peptides. Sustain. Chem. Pharm. 2025, 44, 101941. [Google Scholar] [CrossRef]
- Nan, Y.; Mu, B.; Ge, C.; Chen, S.; Cui, M.; Li, H.; Zhao, C.; Wang, J.; Piao, C.; Li, G. Exploring the Novel Antioxidant Peptides in Low-Salt Dry-Cured Ham: Preparation, Purification, Identification and Molecular Docking. Food Chem. 2024, 446, 138697. [Google Scholar] [CrossRef]
- Mavric-Scholze, E.; Simijonović, D.; Avdović, E.; Milenković, D.; Šaćirović, S.; Ćirić, A.; Marković, Z. Comparative Analysis of Antioxidant Activity and Content of (Poly)Phenolic Compounds in Cabernet Sauvignon and Merlot Wines of Slovenian and Serbian Vineyards. Food Chem. X 2025, 25, 102108. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Liu, Y.; Zhang, J.; Fang, S.; Wang, C. Microwave-Assisted Extraction of Dandelion Root Polysaccharides: Extraction Process Optimization, Purification, Structural Characterization, and Analysis of Antioxidant Activity. Int. J. Biol. Macromol. 2025, 299, 139732. [Google Scholar] [CrossRef]
- Jangir, A.; Kumar Biswas, A.; Arsalan, A.; Faslu Rahman, C.K.; Swami, S.; Agrawal, R.; Bora, B.; Kumar Mendiratta, S.; Talukder, S.; Chand, S.; et al. Development of Superoxide Dismutase Based Visual and Spectrophotometric Method for Rapid Differentiation of Fresh and Frozen-Thawed Buffalo Meat. Food Chem. 2024, 444, 138659. [Google Scholar] [CrossRef]
- Borawska-Dziadkiewicz, J.; Darewicz, M.; Tarczyńska, A.S. Properties of Peptides Released from Salmon and Carp via Simulated Human-like Gastrointestinal Digestion Described Applying Quantitative Parameters. PLoS ONE 2021, 16, e0255969. [Google Scholar] [CrossRef]
- Mengli, Z.; Ji, L.; Cancan, L.; Yanan, Z.; Yuanyuan, Z.; Hanyu, G.; Yinghao, X. Exploration of Antioxidant Peptides from Crocodile (Crocodylus siamensis) Meat Using Modern Information Technology: Virtual-Screening and Antioxidant Mechanisms. Food Res. Int. Ott. Ont 2025, 202, 115789. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, C.; Niu, C.; Wang, J.; Zheng, F.; Li, Q. Identification and Virtual Screening of Novel Antioxidant Peptides from Brewing By-Products and Their Cytoprotective Effects against H2O2-Induced Oxidative Stress. Food Biosci. 2024, 58, 103686. [Google Scholar] [CrossRef]
- Du, Z.; Comer, J.; Li, Y. Bioinformatics Approaches to Discovering Food-Derived Bioactive Peptides: Reviews and Perspectives. TrAC Trends Anal. Chem. 2023, 162, 117051. [Google Scholar] [CrossRef]
- Manish, M.; Mishra, S.; Anand, A.; Subbarao, N. Computational Molecular Interaction between SARS-CoV-2 Main Protease and Theaflavin Digallate Using Free Energy Perturbation and Molecular Dynamics. Comput. Biol. Med. 2022, 150, 106125. [Google Scholar] [CrossRef]
- Oladimeji, A.O.; Mountessou, B.Y.G.; Penta, P.; Babatunde, D.D.; Akintemi, E.O.; Sridar, B.; Babu, K.S. Molecular Structure, Molecular Docking, Molecular Dynamics Simulation, and Drug Likeness Evaluation of 3,7-Dihydroxy-1,2-Dimethoxyxanthone for Its Anticancer Activity. J. Mol. Struct. 2025, 1319, 139359. [Google Scholar] [CrossRef]
- Gao, T.; Yan, R.; Fang, N.; He, L.; Duan, Z.; Wang, J.; Ye, L.; Hu, S.; Chen, Y.; Yuan, S.; et al. Alisol C 23-Acetate Might Be a Lead Compound of Potential Lipase Inhibitor from Alismatis Rhizoma: Screening, Identification and Molecular Dynamics Simulation. Int. J. Biol. Macromol. 2024, 278, 134878. [Google Scholar] [CrossRef] [PubMed]
- Murmu, S.; Aravinthkumar, A.; Singh, M.K.; Sharma, S.; Das, R.; Jha, G.K.; Prakash, G.; Rana, V.S.; Kaushik, P.; Farooqi, M.S. Identification of Potent Phytochemicals against Magnaporthe oryzae through Machine Learning Aided-Virtual Screening and Molecular Dynamics Simulation Approach. Comput. Biol. Med. 2025, 188, 109862. [Google Scholar] [CrossRef] [PubMed]
- Malekzada, M.F.; Mosawi, S.H.; Fani, N.; Nazir, S. Integrating Molecular Docking and Molecular Dynamics Simulation Approaches for Investigation of the Affinity and Interactions of the Curcumin with Phosphatase and Tensin Homolog (PTEN) and Mutated PTEN. J. Mol. Struct. 2024, 1318, 139306. [Google Scholar] [CrossRef]
- Bakulin, I.K.; Kopanichuk, I.V.; Kondratyuk, N.D. Molecular-Level Insights to Structure and Hydrogen Bonds Network of 1,4-Dioxane Aqueous Solution. J. Mol. Liq. 2024, 393, 123523. [Google Scholar] [CrossRef]
- Du, Z.; Shan, Y.; Wang, H. Influence of Thermostat on Droplet Spreading in Molecular Dynamics Simulations. J. Mol. Liq. 2024, 396, 123936. [Google Scholar] [CrossRef]
- Jegani, K.T.; Balde, A.; Nazeer, R.A. A Review on Anti-Inflammatory and Antioxidant Peptides Derived from Marine Organisms: Mechanism of Action and Therapeutic Applications. Food Biosci. 2025, 63, 105745. [Google Scholar] [CrossRef]
- Liebana, C.; de los Ángeles Pereira, N.; Fernández-Gimenez, A.V.; Granone, L.I.; Fangio, M.F. Antioxidant and Functional Properties of Bioactive Peptide Fractions Derived from Shrimp (Pleoticus Muelleri) Waste Hydrolysates. Biocatal. Agric. Biotechnol. 2025, 67, 103639. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An Insight on Superoxide Dismutase (SOD) from Plants for Mammalian Health Enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant Protein-Derived Antioxidant Peptides: Isolation, Identification, Mechanism of Action and Application in Food Systems: A Review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Garzón, A.G.; Veras, F.F.; Brandelli, A.; Drago, S.R. Purification, Identification and in Silico Studies of Antioxidant, Antidiabetogenic and Antibacterial Peptides Obtained from Sorghum Spent Grain Hydrolysate. LWT 2022, 153, 112414. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Jia, N.; Zhang, W.; Hou, H.; Xue, C.; Wang, Y. Identification of Three Novel Antioxidative Peptides from Auxenochlorella Pyrenoidosa Protein Hydrolysates Based on a Peptidomics Strategy. Food Chem. 2022, 375, 131849. [Google Scholar] [CrossRef] [PubMed]
- Ranathunga, S.; Rajapakse, N.; Kim, S.-K. Purification and Characterization of Antioxidative Peptide Derived from Muscle of Conger Eel (Conger Myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Jung, W.-K.; Kim, S.-K. Free Radical Scavenging Activity of a Novel Antioxidative Peptide Purified from Hydrolysate of Bullfrog Skin, Rana Catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Pan, F.; Li, J.; Dou, R.; Wang, X.; Wang, Y.; He, Y.; Wang, S.; Cai, S. In Silico Analysis of Novel Dipeptidyl Peptidase-IV Inhibitory Peptides Released from Macadamia Integrifolia Antimicrobial Protein 2 (MiAMP2) and the Possible Pathways Involved in Diabetes Protection. Curr. Res. Food Sci. 2021, 4, 603–611. [Google Scholar] [CrossRef]
- Quan, Y.; Ren, H.; Liu, S.; Zhao, X.; Hao, J. Identification and Molecular Mechanism of Novel Antioxidant Peptides from Tiger Nut (Cyperus Esculentus L.). Food Biosci. 2025, 63, 105738. [Google Scholar] [CrossRef]
- Summart, R.; Imsoonthornruksa, S.; Yongsawatdigul, J.; Ketudat-Cairns, M.; Udomsil, N. Characterization and Molecular Docking of Tetrapeptides with Cellular Antioxidant and ACE Inhibitory Properties from Cricket (Acheta Domesticus) Protein Hydrolysate. Heliyon 2024, 10, e35156. [Google Scholar] [CrossRef]
- Wen, J.; Sui, Y.; Shi, J.; Xiong, T.; Cai, F.; Gao, X.; Mei, X. Binding Interaction between Rice Bran Albumin and Sweet Potato Leaves Polyphenol: Multi-Spectroscopic and Simulated Molecular Docking Analysis. Int. J. Biol. Macromol. 2025, 314, 144319. [Google Scholar] [CrossRef]
- Cui, W.; Xie, Y.; Zhang, Y.; Su, X.; Cui, T.; Chen, X.; Wang, Z.; Xu, F.; Zhou, H.; Xu, B. Antioxidant Potential of Peptides from Poultry Hemoglobin via Probiotic-Assisted Hydrolysis: Deciphering Mechanisms at the Cellular Level and through Molecular Dynamics Simulations. Food Res. Int. Ott. Ont 2025, 204, 115953. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Identification of Polyphenols from Broussonetia Papyrifera as SARS-CoV-2 Main Protease Inhibitors Using in Silico Docking and Molecular Dynamics Simulation Approaches. J. Biomol. Struct. Dyn. 2021, 39, 6747–6760. [Google Scholar] [CrossRef]
- Xin, X.-Y.; Ruan, C.-H.; Liu, Y.-H.; Jin, H.-N.; Park, S.-K.; Hur, S.-J.; Li, X.-Z.; Choi, S.-H. Identification of Novel Antioxidant and Anti-Inflammatory Peptides from Bovine Hemoglobin by Computer Simulation of Enzymolysis, Molecular Docking and Molecular Dynamics. Curr. Res. Food Sci. 2024, 9, 100931. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, W.; Xu, G.; Zhao, W.; Yu, Z. Valorization of Crocodile Head for Anti-Inflammatory Peptides: In Silico Screening and Cellular Validation. Food Res. Int. Ott. Ont 2025, 211, 116457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Li, W.; Han, W. Antioxidant Activity of Soybean Peptides and Keap1 Protein: A Combined in Vitro and in Silico Analysis. LWT 2024, 212, 117019. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Yang, J.; Zhou, X.; Li, W.; Han, W. Screening and Characterization of Antioxidant Peptides from Ganoderma Lucidum: Virtual Prediction, Experimental Validation, and Mechanistic Insights. Food Biosci. 2025, 69, 106878. [Google Scholar] [CrossRef]
- Tan, Z.; Song, J.; Zhang, T. Virtual Screening, Molecular Docking, and Molecular Dynamics Simulation Studies on the Hypoglycemic Function of Oat Peptides. J. Mol. Graph. Model. 2024, 133, 108869. [Google Scholar] [CrossRef]
- Chen, L.; Nie, M.; Yang, J.; Zhang, W.; Hsiang, T.; Jiang, Y.; Xie, B.; Chen, B. Structural Identification and Molecular Interaction Modeling Analysis of Antioxidant Activity Selenium-Enriched Peptides from Selenium-Enriched Pleurotus Eryngii. Antioxidants 2025, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, S.; Ebrahimpour, A.; Zarei, M.; Abdul Hamid, A.; Saari, N. Alcalase-Generated Proteolysates of Stone Fish (Actinopyga Lecanora) Flesh as a New Source of Antioxidant Peptides. Int. J. Food Prop. 2018, 21, 1541–1559. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Li, W.-J.; Zhang, J.-J.; Liu, Z.-Y.; Li, Y.; Liu, C.; Qin, S. The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis in Vitro. Mar. Drugs 2022, 20, 696. [Google Scholar] [CrossRef]
- Zhong, C.; Sun, L.-C.; Yan, L.-J.; Lin, Y.-C.; Liu, G.-M.; Cao, M.-J. Production, Optimisation and Characterisation of Angiotensin Converting Enzyme Inhibitory Peptides from Sea Cucumber (Stichopus Japonicus) Gonad. Food Funct. 2018, 9, 594–603. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, L.; Ma, C.; Zhang, S.; Zhong, L.; Lin, S. Characterization of a Synergistic Antioxidant Synthetic Peptide from Sea Cucumber and Pine Nut. J. Food Sci. Technol. 2022, 59, 2306–2317. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.-G.; Kim, S.-K. Investigation of Jumbo Squid (Dosidicus Gigas) Skin Gelatin Peptides for Their in Vitro Antioxidant Effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Fan, X.; Zhang, T.; Wang, H.; Ye, K. Novel Antioxidant Peptides from Bovine Blood: Purification, Identification and Mechanism of Action. LWT 2024, 205, 116499. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and Identification of Antioxidant Peptides from Enzymatically Hydrolyzed Rice Bran Protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Galland, F.; de Espindola, J.S.; Sacilotto, E.S.; Almeida, L.G.V.C.; Morari, J.; Velloso, L.A.; Dos Santos, L.D.; Rossini, B.C.; Bertoldo Pacheco, M.T. Digestion of Whey Peptide Induces Antioxidant and Anti-Inflammatory Bioactivity on Glial Cells: Sequences Identification and Structural Activity Analysis. Food Res. Int. 2024, 188, 114433. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of Specific Amino Acid and Secondary Structure to the Antioxidant Property of Corn Gluten Proteins. Food Res. Int. 2018, 105, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Ndayiragije, E.; Caumul, P.; Joondan, N.; Akerman, M.P.; Bhowon, M.G.; Jhaumeer-Laulloo, S. Radical Scavenging Abilities of L-Tyrosine and L-DOPA Schiff Bases and Their Fluorescence Binding Studies and Molecular Docking Interactions with Bovine Serum Albumin. J. Mol. Struct. 2023, 1284, 135352. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Wu, T.-K.; Fang, T.J. Antioxidant Properties of a New Antioxidative Peptide from Algae Protein Waste Hydrolysate in Different Oxidation Systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Lv, L. Purification and Identification of Antioxidant Peptides from Egg White Protein Hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, C.; Li, H.; Ma, X.; Li, J.; Chi, X.; Liu, Z. Proline Inhibits Postharvest Physiological Deterioration of Cassava by Improving Antioxidant Capacity. Phytochemistry 2024, 224, 114143. [Google Scholar] [CrossRef]
- Yang, W.; Hao, X.; Zhang, X.; Zhang, G.; Li, X.; Liu, L.; Sun, Y.; Pan, Y. Identification of Antioxidant Peptides from Cheddar Cheese Made with Lactobacillus helveticus. LWT 2021, 141, 110866. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, M.; Sun, L. The Iron-Cofactored Superoxide Dismutase of Edwardsiella Tarda Inhibits Macrophage-Mediated Innate Immune Response. Fish Shellfish Immunol. 2010, 29, 972–978. [Google Scholar] [CrossRef]
- Wuerges, J.; Lee, J.-W.; Yim, Y.-I.; Yim, H.-S.; Kang, S.-O.; Djinovic Carugo, K. Crystal Structure of Nickel-Containing Superoxide Dismutase Reveals Another Type of Active Site. Proc. Natl. Acad. Sci. USA 2004, 101, 8569–8574. [Google Scholar] [CrossRef] [PubMed]
- Arockiaraj, J.; Palanisamy, R.; Bhatt, P.; Kumaresan, V.; Gnanam, A.J.; Pasupuleti, M.; Kasi, M. A Novel Murrel Channa Striatus Mitochondrial Manganese Superoxide Dismutase: Gene Silencing, SOD Activity, Superoxide Anion Production and Expression. Fish Physiol. Biochem. 2014, 40, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, V.; Gnanam, A.J.; Pasupuleti, M.; Arasu, M.V.; Al-Dhabi, N.A.; Harikrishnan, R.; Arockiaraj, J. Comparative Analysis of CsCu/ZnSOD Defense Role by Molecular Characterization: Gene Expression-Enzyme Activity-Protein Level. Gene 2015, 564, 53–62. [Google Scholar] [CrossRef]
- Guo, Y.; Gong, P.; Qian, Y.; Liu, H.; Yu, B.; Qi, J. Rapid Screening and Identification of Superoxide Dismutase Activators from Traditional Chinese Medicines Based on Affinity Ultrafiltration Mass Chromatography Combined with Molecular Docking. J. Chromatogr. A 2023, 1710, 464408. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Jafari, S. Cell Penetrating Peptides: A Concise Review with Emphasis on Biomedical Applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Guo, Z.; Tan, T.; Zhang, Y. Novel Tyrosinase Inhibitory Peptide with Free Radical Scavenging Ability. J. Enzyme Inhib. Med. Chem. 2019, 34, 1633–1640. [Google Scholar] [CrossRef]
- Bergwik, J.; Kristiansson, A.; Larsson, J.; Ekström, S.; Åkerström, B.; Allhorn, M. Binding of the Human Antioxidation Protein A1-Microglobulin (A1M) to Heparin and Heparan Sulfate. Mapping of Binding Site, Molecular and Functional Characterization, and Co-Localization in Vivo and in Vitro. Redox Biol. 2021, 41, 101892. [Google Scholar] [CrossRef]
| Item | Value |
|---|---|
| Enzyme | Unspecific |
| Max Missed Cleavages | 0 |
| Precursor Tolerance (Main search) | 4.5 ppm |
| Precursor Tolerance (First search) | 20 ppm |
| MS/MS Tolerance | 20 ppm |
| Fixed modifications | - |
| Variable modifications | Oxidation (M), Acetyl (Protein N-term) |
| Database | uniprot-Haliotis discus hannai (Japanese abalone) [42344]-314-20240620.fasta; uniprot-Apostichopus (genus) [307971]-30561- 20240620.fasta |
| Database pattern | Target-Reverse |
| PSM FDR | 0.01 |
| Protein FDR | 0.01 |
| Site FDR | 0.01 |
| Molecular Weight Range (Da) | Proportion of Fraction U1 (%) | Proportion of Fraction U2 (%) |
|---|---|---|
| >2000 | 6.439 | 36.233 |
| 1000~2000 | 26.090 | 49.122 |
| 500~1000 | 60.615 | 10.247 |
| 200~500 | 6.856 | 4.398 |
| Peptide | PeptideRanker Score | Toxicity | Mass | PL | Free Radical Scavenging Ability |
|---|---|---|---|---|---|
| VPYPR | 0.641232 | Non-toxic | 630.74 | 9.81 | 0.55691272 |
| ATGPQGPAGQRGPAGPTGPTGPAG | 0.562816 | Non-toxic | 2057.18 | 10.9 | 0.54368335 |
| PGHPF | 0.946647 | Non-toxic | 553.61 | 8.26 | 0.58514124 |
| NPWGQ | 0.832775 | Non-toxic | 600.62 | 3.28 | 0.53533584 |
| MPPPP | 0.959959 | Non-toxic | 537.67 | 3.57 | 0.57264793 |
| GPMGP | 0.929226 | Non-toxic | 457.55 | 3.82 | 0.52304268 |
| GPLGP | 0.841507 | Non-toxic | 439.51 | 3.82 | 0.50645763 |
| LPGPP | 0.887134 | Non-toxic | 479.57 | 3.82 | 0.54216856 |
| Peptide | Binding Affinity (kcal/mol) |
|---|---|
| VPYPR | −7.6 |
| ATGPQGPAGQRGPAGPTGPTGPAG | −7.5 |
| PGHPF | −7.4 |
| NPWGQ | −7.2 |
| MPPPP | −6.7 |
| GPMGP | −6.4 |
| GPLGP | −6.4 |
| LPGPP | −6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, J.; Deng, Y.; Wang, Y.; Dou, P.; Fan, H.; Zeng, X.; Fan, X.; Zhang, L.; Liu, H.; et al. Screening and Identification of Antioxidant Peptides from Sea Cucumber Gonad Proteins and Their Activation of Superoxide Dismutase. Foods 2025, 14, 3848. https://doi.org/10.3390/foods14223848
Zhang Z, Wang J, Deng Y, Wang Y, Dou P, Fan H, Zeng X, Fan X, Zhang L, Liu H, et al. Screening and Identification of Antioxidant Peptides from Sea Cucumber Gonad Proteins and Their Activation of Superoxide Dismutase. Foods. 2025; 14(22):3848. https://doi.org/10.3390/foods14223848
Chicago/Turabian StyleZhang, Zhiqin, Jingxuan Wang, Yongke Deng, Yugui Wang, Peipei Dou, Hongbing Fan, Xiangquan Zeng, Xinguang Fan, Lili Zhang, Haimei Liu, and et al. 2025. "Screening and Identification of Antioxidant Peptides from Sea Cucumber Gonad Proteins and Their Activation of Superoxide Dismutase" Foods 14, no. 22: 3848. https://doi.org/10.3390/foods14223848
APA StyleZhang, Z., Wang, J., Deng, Y., Wang, Y., Dou, P., Fan, H., Zeng, X., Fan, X., Zhang, L., Liu, H., & Zhao, Q. (2025). Screening and Identification of Antioxidant Peptides from Sea Cucumber Gonad Proteins and Their Activation of Superoxide Dismutase. Foods, 14(22), 3848. https://doi.org/10.3390/foods14223848









