Peptides from ‘Vaina Morada’ Black Bean Inhibit α-Amylase and α-Glucosidase: A Combined In Silico–In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Silico and Omics Approaches
2.2.1. In Silico Digestion of Phaseolus vulgaris L. Storage Proteins
2.2.2. Physicochemical Characterization of Peptides
2.2.3. Computational Docking
2.3. Protein Extraction
2.4. Enzymatic Hydrolysis
2.5. Electrophoresis Profile Assay
2.6. Degree of Hydrolysis
2.7. Antioxidant Capacity Assays
2.7.1. ABTS Assay
2.7.2. DPPH Assay
2.8. Biological Activity Assays
2.8.1. α-Amylase Inhibition
2.8.2. α-Glucosidase Inhibition
2.9. Statistical Analysis
3. Results
3.1. Peptide Sequencing and Predicted Biological Potential
Computational Docking
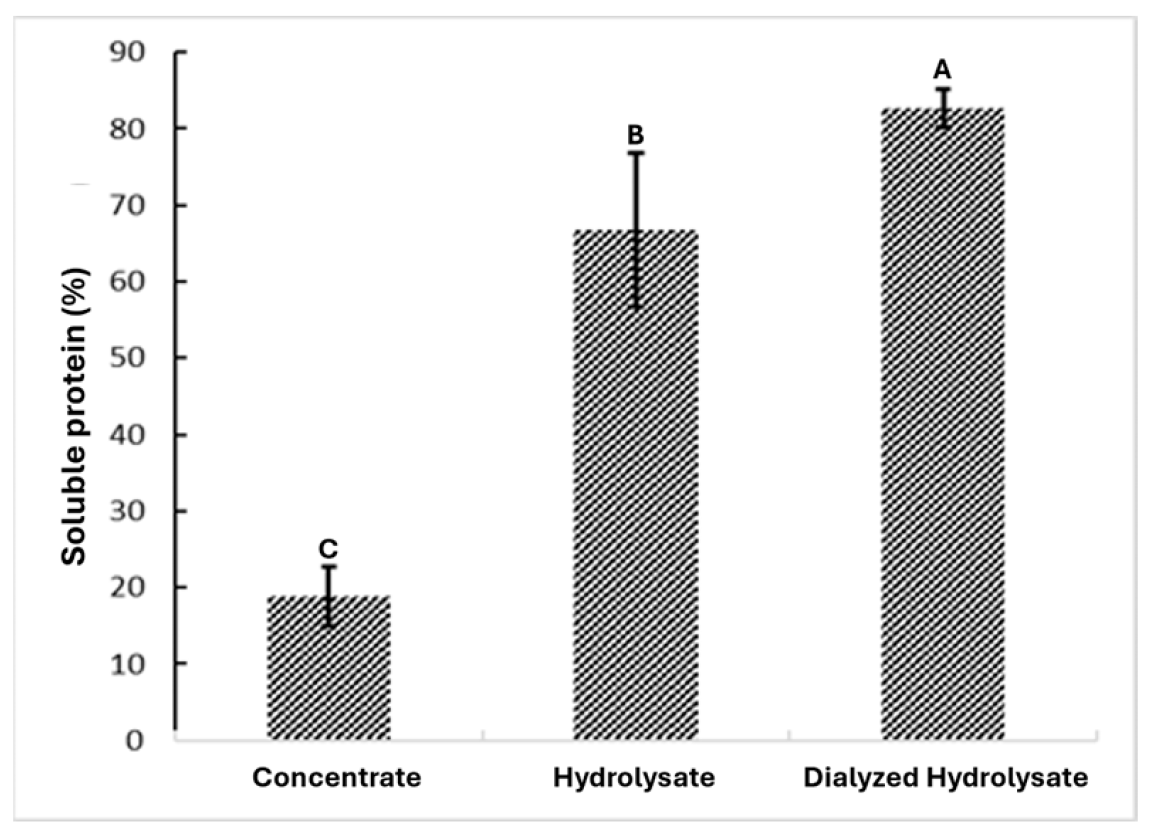
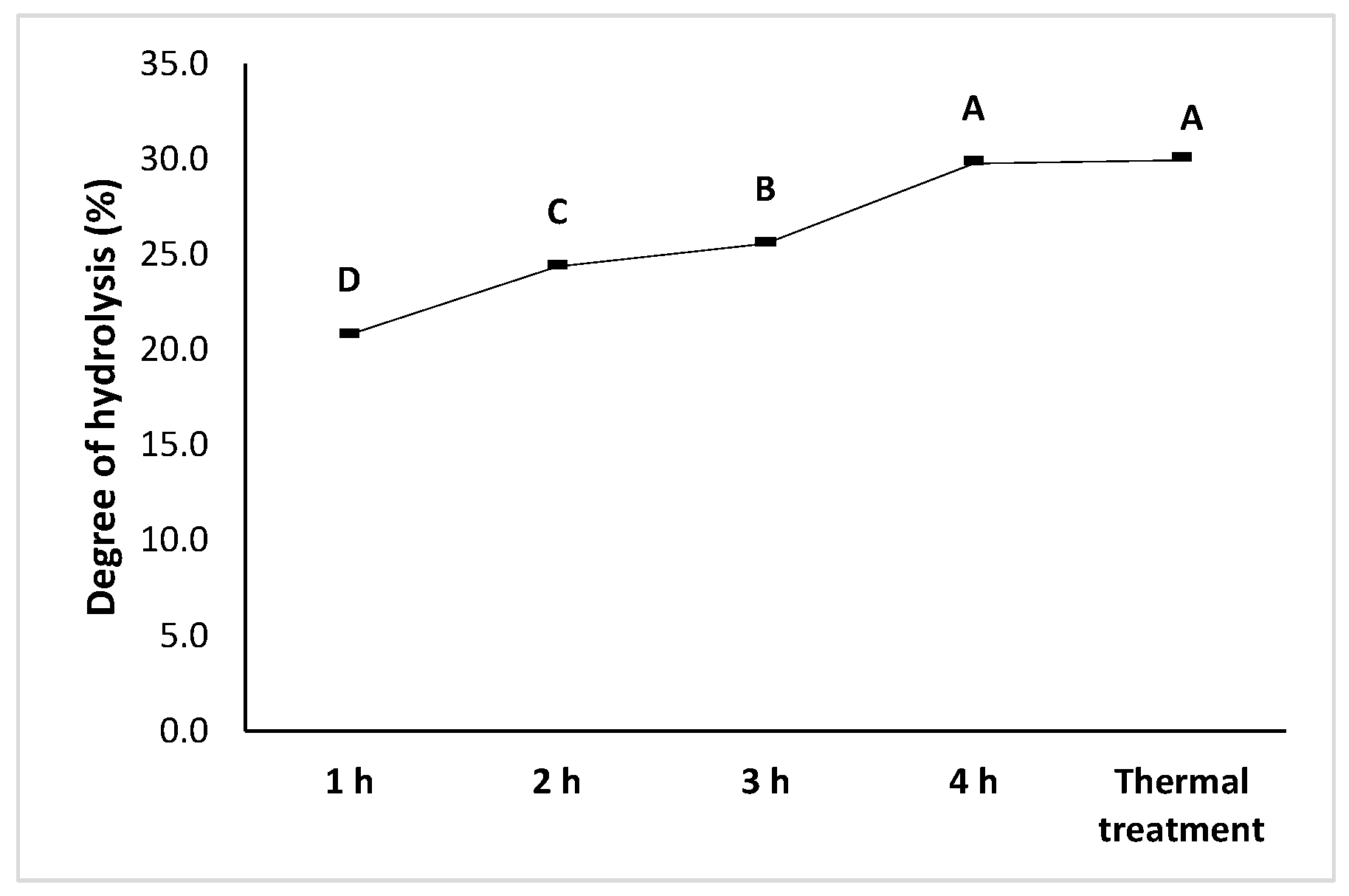
3.2. Protein Hydrolysate Characterization
3.2.1. Protein Concentration in the Hydrolysate Obtention Process
3.2.2. Protein Profile
3.2.3. Degree of Hydrolysis
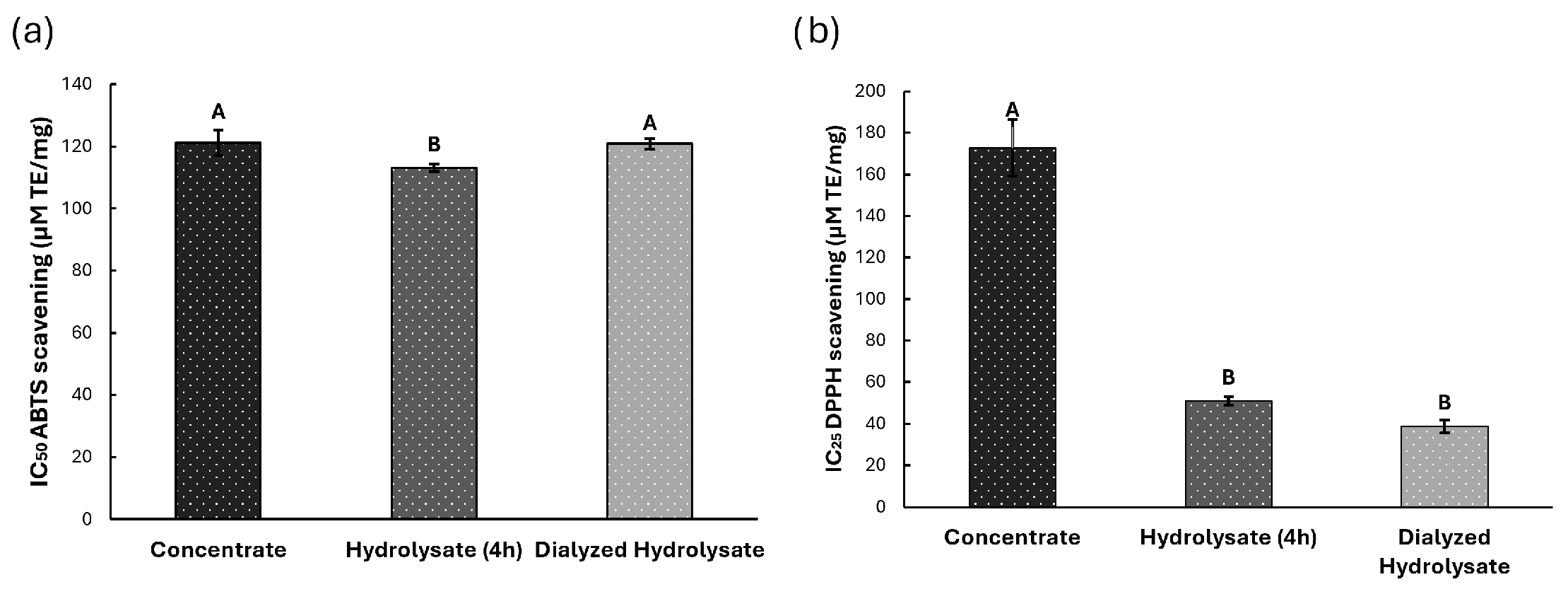
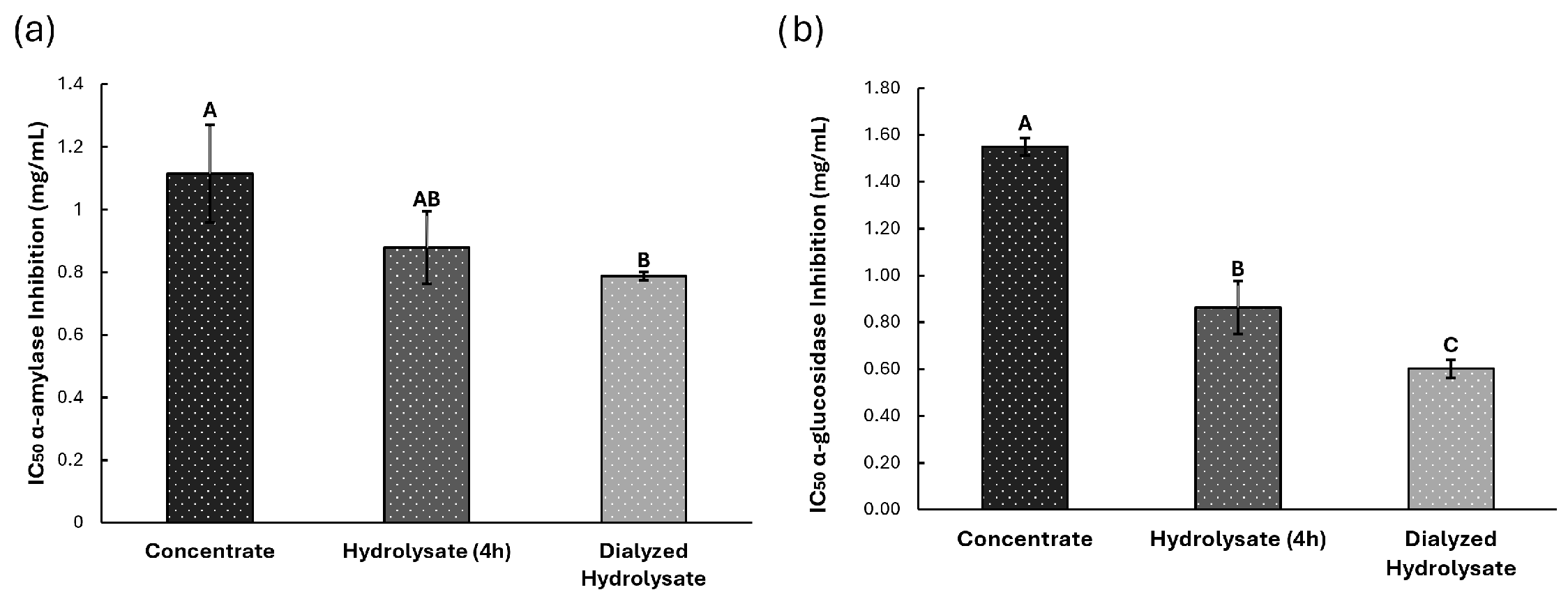
3.3. Biological Potential
3.3.1. Antioxidant Potential
3.3.2. Antidiabetes Potential
4. Discussion
4.1. Computational Modeling of Bioactive Peptide Interactions in Food Matrices: Molecular Docking and Stability Analysis
4.2. Protein Hydrolysate Characterization and Biological Potential: Correlating Hydrolysis Efficiency, Antioxidant Activity, and α-Amylase/α-Glucosidase Inhibition in Black Bean “Vaina Morada” Peptides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orona-Tamayo, D.; Valverde, M.E.; Paredes-López, O. Péptidos bioactivos de cultivos alimentarios seleccionados de Latinoamérica: Un enfoque nutracéutico y molecular. Crit. Rev. Food Sci. Nutr. 2018, 59, 1949–1975. [Google Scholar] [CrossRef]
- Alcázar-Valle, M.; García-Morales, S.; Mojica, L.; Morales-Hernández, N.; Sánchez-Osorio, E.; Flores-López, L.; Lugo-Cervantes, E. Nutritional, Antinutritional Compounds and Nutraceutical Significance of Native Bean Species (Phaseolus spp.) of Mexican Cultivars. Agriculture 2021, 11, 1031. [Google Scholar] [CrossRef]
- Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef] [PubMed]
- Cid-Gallegos, M.S.; de las Mercedes Gómez y Gómez, Y.; Corzo-Ríos, L.J.C.-R.; Sanchez-Chino, X.M.; Moguel-Concha, D.; Borges-Martínez, E.; Jiménez-Martínez, C. Potencial Nutricional y Bioactivo de Frijol (Phaseolus vulgaris) en la Salud Humana. Investig. Desarro. Cienc. Tecnol. Aliment. 2023, 8, 309–318. [Google Scholar] [CrossRef]
- Mendoza Jimenez, Y.L.; Eusebio Moreno, J.C.; Alvarez Garcia, R.; Abreu Corona, A.; Vargas Hernandez, G.; Tellez Jurado, A.; Tovar Jimenez, X. Actividad Antioxidante de los Hidrolizados Proteicos del Frijol Común (Phaseolus vulgaris) cv Negro Primavera-28 y Flor de Durazno. Repositorio Institucional UAM 2018. Available online: http://ilitia.cua.uam.mx:8080/jspui/handle/123456789/425 (accessed on 14 December 2024).
- Oso, A.A.; Ashafa, A.O. Nutritional Composition of Grain and Seed Proteins. In Grain and Seed Proteins Functionality; Jiménez-López, J.C., Ed.; IntechOpen: London, UK, 2021; pp. 31–50. [Google Scholar] [CrossRef]
- Flores-Medellín, S.A.; Camacho-Ruiz, R.M.; Guízar-González, C.; Rivera-Leon, E.A.; Llamas-Covarrubias, I.M.; Mojica, L. Protein Hydrolysates and Phenolic Compounds from Fermented Black Beans Inhibit Markers Related to Obesity and Type-2 Diabetes. Legume Sci. 2021, 3, e64. [Google Scholar] [CrossRef]
- Del Carmen, M.; Guerrero, G. Caracterización Estructural y Funcional de Dos Metalo-Carboxipeptidasas de la Familia M14 con Especificidad de Sustrato Tipo Acídico: Carboxipeptidasa Citosólica 6 y Carboxipeptidasa O Humanas; Universitat Autònoma de Barcelona: Barcelona, Spain, 2017. [Google Scholar]
- Olatunde, O.O.; Owolabi, I.O.; Fadairo, O.S.; Ghosal, A.; Coker, O.J.; Soladoye, O.P.; Aluko, R.E.; Bandara, N. Enzymatic Modification of Plant Proteins for Improved Functional and Bioactive Properties. Food Bioprocess Technol. 2023, 16, 1216–1234. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá, F. Advanced Enzymatic Hydrolysis of Food Proteins for the Production of Bioactive Peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel Technologies for the Production of Bioactive Peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Hou, D.; Feng, Q.; Tang, J.; Shen, Q.; Zhou, S. An Update on Nutritional Profile, Phytochemical Compounds, Health Benefits, and Potential Applications in the Food Industry of Pulses Seed Coats: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1960–1982. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive Peptides from Germinated Soybean with Antidiabetic Potential by Inhibition of Dipeptidyl Peptidase-IV, α-Amylase, and α-Glucosidase Enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- do Evangelho, J.A.; Vanier, N.L.; Pinto, V.Z.; De Berrios, J.J.; Dias, A.R.G.; da Rosa Zavareze, E. Black Bean (Phaseolus vulgaris L.) Protein Hydrolysates: Physicochemical and Functional Properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Poblete-Aro, C.; Russell-Guzmán, J.; Parra, P.; Soto-Muñoz, M.; Villegas-González, B.; Cofré-Bola-Dos, C.; Herrera-Valenzuela, T. Efecto del Ejercicio Físico sobre Marcadores de Estrés Oxidativo en Pacientes con Diabetes Mellitus Tipo 2. Rev. Méd. Chile 2018, 146, 362–372. [Google Scholar] [CrossRef]
- Rodríguez Núñez, A.; Calá Fernández, J.; Vadell, H.C.; Ángel, M.; Deler, M. Oxidative Stress Markers in Patients with Metabolic Syndrome. Rev. Enfermedades Transm. Finlay 2021, 11, 23–30. [Google Scholar]
- Mojica, L.; Gonzalez de Mejia, E. Peptides in Common Bean Protein Hydrolysates Inhibit Molecular Target Enzymes in Type-2 Diabetes. FASEB J. 2015, 29, 607.11. [Google Scholar] [CrossRef]
- Atkinson, T.; Barrett, T.D.; Cameron, S.; Guloglu, B.; Greenig, M.; Tan, C.B.; Laterre, A. Protein Sequence Modelling with Bayesian Flow Networks. Nat. Commun. 2025, 16, 3197. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Hu, S.; Wu, C.; Zhang, Y.; Ma, L.; Li, X.; Li, D. Exploration of the Structural Features and Anti-Oxidative Activity of Whey Protein Hydrolysates Produced by Lactiplantibacillus plantarum. Food Res. Int. 2025, 211, 116375. [Google Scholar] [CrossRef] [PubMed]
- Gokul, K.; Samad, R.; Thomas, A.M.; Antony, S.P. Unveiling Thymosin Antimicrobial Peptide from Pearl Spot, Etroplus suratensis: Molecular Characterization, Phylogenetic Analysis, and Functional Implications. Ecol. Genet. Genom. 2024, 31, 100253. [Google Scholar] [CrossRef]
- Patil, S.; Joshi, S.; Kumar, V. Identifying the Role of Casparian Strip Membrane Proteins in Rice under Salinity Stress. Gene Rep. 2025, 39, 102192. [Google Scholar] [CrossRef]
- Albert, S. Discovery of Potent Inhibitors from Traditional Rice Varieties Targeting α-Amylase: A Computational Approach and In Vitro Validation. J. Biosci. 2025, 50, 21. [Google Scholar] [CrossRef]
- Alp, M.; Misturini, A.; Sastre, G.; Gálvez-Llompart, M. Drug Screening of α-Amylase Inhibitors as Candidates for Treating Diabetes. J. Cell. Mol. Med. 2023, 27, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Riyaphan, J.; Pham, D.C.; Leong, M.K.; Weng, C.F. In Silico Approaches to Identify Polyphenol Compounds as α-Glucosidase and α-Amylase Inhibitors against Type-II Diabetes. Biomolecules 2021, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghulikah, H.A.; Mughal, E.U.; Elkaeed, E.B.; Naeem, N.; Nazir, Y.; Alzahrani, A.Y.A.; Shah, S.W.A. Discovery of Chalcone Derivatives as Potential α-Glucosidase and Cholinesterase Inhibitors: Effect of Hyperglycemia in Paving a Path to Dementia. J. Mol. Struct. 2023, 1275, 134658. [Google Scholar] [CrossRef]
- Alfaro-Diaz, A.; Urías-Silvas, J.E.; Loarca-Piña, G.; Gaytan-Martínez, M.; Prado-Ramirez, R.; Mojica, L. Techno-Functional Properties of Thermally Treated Black Bean Protein Concentrate Generated through Ultrafiltration Process. LWT 2021, 136, 110296. [Google Scholar] [CrossRef]
- Lu, Y.; Fu, T.J. Performance of commercial colorimetric assays for total soluble protein quantification in heat-treated milk samples. Food Anal. Methods 2020, 13, 1337–1345. [Google Scholar] [CrossRef]
- Mojica, L.; De Mejía, E.G. Optimization of Enzymatic Production of Antidiabetic Peptides from Black Bean (Phaseolus vulgaris L.) Proteins, Their Characterization and Biological Potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Galanopoulos, M.; Sismour, E.; Ren, S.; Zhang, B. Effect of enzymatic hydrolysis with endo- and exo-proteases on the secondary structure, functional and antioxidant properties of chickpea protein hydrolysates. J. Food Meas. Charact. 2020, 14, 343–352. [Google Scholar] [CrossRef]
- Fonseca-Hernández, D.; Lugo-Cervantes, E.D.C.; Escobedo-Reyes, A.; Mojica, L. Black Bean (Phaseolus vulgaris L.) Polyphenolic Extract Exerts Antioxidant and Antiaging Potential. Molecules 2021, 26, 6716. [Google Scholar] [CrossRef]
- Chemjong, K. Study of α-amylase inhibitory activity and antimicrobial activity of local tea Camellia sinensis (L.) Kuntze, leaves of Buddleja asiatica Lour., and roots of Polygala arillata Buch.-Ham. ex D. Don. Ph.D. Thesis, Tribhuvan University, Department of Chemistry, Kathmandu, Nepal, 2019. [Google Scholar]
- Elya, B.; Handayani, R.; Sauriasari, R.; Hasyyati, U.S.; Permana, I.T.; Permatasari, Y.I. Antidiabetic Activity and Phytochemical Screening of Extracts from Indonesian Plants by Inhibition of Alpha Amylase, Alpha Glucosidase and Dipeptidyl Peptidase IV. Pak. J. Biol. Sci. 2015, 18, 279. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Millan-Linares, M.C.; Montserrat-De-La-Paz, S. Strengths and Limitations of in Silico Tools to Assess Physicochemical Properties, Bioactivity, and Bioavailability of Food-Derived Peptides. Trends Food Sci. Technol. 2023, 138, 433–440. [Google Scholar] [CrossRef]
- Hu, K.; Huang, H.; Li, H.; Wei, Y.; Yao, C. Legume-derived bioactive peptides in type 2 diabetes: Opportunities and challenges. Nutrients 2023, 15, 1096. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, C.; Wang, Y.; Zhang, X.; Qi, B. Physicochemical, Structural, Foaming, and Rheological Properties of Soybean Peptide Aggregates Obtained from Enzymatic By-Products. Food Hydrocoll. 2025, 164, 111224. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Zhou, D.; Li, Y.; Wang, Y.; Xu, S.C.; Zhao, X.H. The Triglyceride-Glucose Index Predicts Coronary Artery Disease Severity and Cardiovascular Outcomes in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Dis. Markers 2019, 2019, 6891537. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, H.; Wen, Y.; Liu, Y.; Wang, J.; Sun, B. Molecular Mechanism for the α-Glucosidase Inhibitory Effect of Wheat Germ Peptides. J. Agric. Food Chem. 2021, 69, 15231–15239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chi, H.; Ma, S.; Zhao, L.; Cai, S. Identification of Novel α-Glucosidase Inhibitory Peptides in Rice Wine and Their Antioxidant Activities Using In Silico and In Vitro Analyses. LWT 2023, 178, 114629. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Wang, H.; Yang, J.; Li, L.; Zhu, J.; Liu, Y. Virtual screening technology for two novel peptides in soybean as inhibitors of α-amylase and α-glucosidase. Foods 2023, 12, 4387. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Luna-Vital, D.; de Mejia, E.G. Identification and Comparison of Peptides from Chickpea Protein Hydrolysates Using Either Bromelain or Gastrointestinal Enzymes and Their Relationship with Markers of Type 2 Diabetes and Bitterness. Nutrients 2020, 12, 3843. [Google Scholar] [CrossRef]
- Quintero-Soto, M.F.; Chávez-Ontiveros, J.; Garzón-Tiznado, J.A.; Salazar-Salas, N.Y.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; López-Valenzuela, J.A. Characterization of peptides with antioxidant activity and antidiabetic potential obtained from chickpea (Cicer arietinum L.) protein hydrolysates. J. Food Sci. 2021, 86, 2962–2977. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp.). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, Biological Activities, and Applications in Foods and Health Benefits. A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Galván, M.; Pacheco-Hernández, Y.; Lozoya-Gloria, E.; Villa-Ruano, N. Dietary Natural Products as Inhibitors of α-Amylase and α-Glucosidase: An Updated Review of Ligand-Receptor Correlations Validated by Docking Studies. Food Biosci. 2024, 62, 105456. [Google Scholar] [CrossRef]
- Santamaria, L.; Pajak, A.; House, J.D.; Marsolais, F. Identification and Characterization of a Pepsin- and Chymotrypsin-Resistant Peptide in the α Subunit of the 11S Globulin Legumin from Common Bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2024, 72, 14844–14850. [Google Scholar] [CrossRef] [PubMed]
- Ohara, A.; Cason, V.G.; Nishide, T.G.; Miranda de Matos, F.; de Castro, R.J.S. Improving the Antioxidant and Antidiabetic Properties of Common Bean Proteins by Enzymatic Hydrolysis Using a Blend of Proteases. Biocatal. Biotransform. 2021, 39, 100–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Romero, H.M. Exploring the Structure-Function Relationship of Great Northern and Navy Bean (Phaseolus vulgaris L.) Protein Hydrolysates: A Study on the Effect of Enzymatic Hydrolysis. Int. J. Biol. Macromol. 2020, 162, 1516–1525. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, L.; Feng, S.; Liu, F.; Luo, Y. Mechanistic Study on the Nanocomplexation between Curcumin and Protein Hydrolysates from Great Northern Bean (Phaseolus vulgaris L.) for Delivery Applications in Functional Foods. LWT 2021, 139, 110572. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Sun, H.; Du, M.; Qiu, J.; Tang, M.; Sun, X.; Zhu, B. Antioxidative Peptides from Proteolytic Hydrolysates of False Abalone (Volutharpa ampullacea perryi): Characterization, Identification, and Molecular Docking. Mar. Drugs 2019, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- González-Osuna, M.F.; Torres-Arreola, W.; Márquez-Ríos, E.; Wong-Corral, F.J.; Lugo-Cervantes, E.; Rodríguez-Figueroa, J.C.; García-Sánchez, G.; Ezquerra-Brauer, J.M.; Soto-Valdez, H.; Castillo, A.; et al. Antioxidant Activity of Peptide Fractions from Chickpea Globulin Obtained by Pulsed Ultrasound Pretreatment. Horticulturae 2023, 9, 415. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X. Purification, Identification and Evaluation of Antioxidant Peptides from Pea Protein Hydrolysates. Molecules 2023, 28, 2952. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Ai, Z.; Zhang, Y.; Tan, C.P.; Liu, Y. Exploring the Antioxidant and Structural Properties of Black Bean Protein Hydrolysate and Its Peptide Fractions. Front. Nutr. 2022, 9, 884537. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dong, Q.; Yu, C.; Chen, H.; Zhao, Y.; Zhang, B.; Yu, P.; Chen, M. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants 2024, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, G.; Melchior, S.; Plazzotta, S.; Calligaris, S.; Innocente, N. Effect of Enzymatic Hydrolysis with Alcalase or Protamex on Technological and Antioxidant Properties of Whey Protein Hydrolysates. Food Res. Int. 2024, 188, 114499. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive Proteins and Phytochemicals from Legumes: Mechanisms of Action Preventing Obesity and Type-2 Diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Aragón, P.N.; Segura-Campos, M.; Negrete-León, E.; Acevedo-Fernández, J.J.; Betancur-Ancona, D.; Chel-Guerrero, L.; Castañeda-Corral, G. Protein Hydrolysates and Ultrafiltered <1 KDa Fractions from Phaseolus lunatus, Phaseolus vulgaris and Mucuna pruriens Exhibit Antihyperglycemic Activity, Intestinal Glucose Absorption and α-Glucosidase Inhibition with No Acute Toxicity in Rodents. J. Sci. Food Agric. 2019, 99, 587–595. [Google Scholar] [CrossRef]
- Sarteshnizi, R.A.; Sahari, M.A.; Gavlighi, H.A.; Regenstein, J.M.; Nikoo, M.; Udenigwe, C.C. Influence of Fish Protein Hydrolysate-Pistachio Green Hull Extract Interactions on Antioxidant Activity and Inhibition of α-Glucosidase, α-Amylase, and DPP-IV Enzymes. LWT 2021, 142, 111019. [Google Scholar] [CrossRef]
- Abdulrahman, B.O.; Bala, M.; Bello, O.M. Evaluation of in vitro antioxidant and antidiabetic potential of extracts from Phaseolus vulgaris L. seeds (black turtle beans). Funct. Food Sci. 2021, 1, 23–38. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Piyaphan, P.; Khamthong, S.; Unkam, M.; Silprasit, K. Comparison of FTIR fingerprint, phenolic content, antioxidant and anti-glucosidase activities among Phaseolus vulgaris L., Arachis hypogaea L. and Plukenetia volubilis L. Electron. J. Biotechnol. 2023, 61, 14–23. [Google Scholar] [CrossRef]
- Abeer, M.M.; Trajkovic, S.; Brayden, D.J. Measuring the Oral Bioavailability of Protein Hydrolysates Derived from Food Sources: A Critical Review of Current Bioassays. Biomed. Pharmacother. 2021, 144, 112275. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-Derived Non-Phenolic α-Amylase and α-Glucosidase Inhibitors for Controlling Starch Digestion Rate and Guiding Diabetes-Friendly Recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]


| Parental Protein | Peptide Sequence | Net Charge | Mass (Da) | Bioactive Sequence | Biological Potential | Isoelectric Point (Ip) | Hydrophobicity (Kcal × mol−1) | Instability Index | Estimated Half-Life (h) | Affinity (kcal/mol) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Amylase | α-Glucosidase | ||||||||||
| Phaseolin | VDGHHHQQEQQKGS | −1 | 1613.7274 | GH, KG, GS, QK, DG, HH, GHH, HHH, QE, QQ, VD | ACE, Antioxidative, DPP-IV | 6.35 | 30.34 | 47.51 | 10–100 | −8.2 | −7.0 |
| EEEGQQEEGQQEG | −6 | 1475.5628 | GQ, EG, EEE, EE, QE, QQ | ACE, Stimulating, Neuropeptide, DPP-IV | 2.76 | 36.21 | 143.72 | 1–10 | −6.7 | −8.3 | |
| HQQEQQKGRKGAF | 2 | 1540.7837 | AF, GA, GR, KG, QK, GA, QE, QQ, RK | ACE, DPP-IV | 10.58 | 25.44 | 48.9 | 3.5–10 | −9.1 | −8.2 | |
| GINANNNNRNL | 1 | 1212.5943 | GI, IN, NA, NL, NN, NR, RN | ACE, DPP-IV, Renin inhibitor | 11.13 | 14.09 | 12.59 | 10–30 | −8.7 | −9.7 | |
| DNQKIPAGTIF | 0 | 1202.6276 | IPA, IP, IF, AG, GT, QK, PA, DN, KI, NQ, TI | ACE, DPP-IV | 6.77 | 14.05 | 1.69 | 1.1–10 | −8.1 | −9.9 | |
| VGPKGNKETL | 1 | 1041.5801 | GP, VG, KG, NK, KE, VGP, ET, PK, TL | Antiamnestic, ACE, DPP-IV, Antithrombotic, Regulating | 9.93 | 18.96 | 7.21 | 10–100 | −8.4 | −7.8 | |
| KQDNTIGNEF | −1 | 1164.5394 | IG, EF, DN, NE, NT, QD, TI | ACE, DPP-IV, Renin inhibitor, CaMPDE, Hypolipidemic | 4 | 19.01 | 34.96 | 0.03–1.3 | −7.4 | −9.1 | |
| VNNPQIHEF | −1 | 1096.5286 | PQ, EF, NP, HE, IH, NN, QI, VN | ACE, DPP-IV, Renin, CaMPDE, Hypolipidemic, DPP-III | 5.06 | 13.18 | 40.8 | 10–100 | −9.3 | −10 | |
| VNPDPKEDL | −2 | 1025.5013 | KE, VNP, DL, NP, KE, PK, VN | ACE, DPP-IV | 3.69 | 21.03 | 18.71 | 10–100 | −8.8 | −8.9 | |
| VKPDDRREY | 0 | 1176.587 | VK, RR, EY, KP, VKP, DR | ACE, Antibacterial, Neuropeptide, Antioxidative, Haemolytic, Antiviral, DPP-IV, Leucyltransferase | 6.95 | 24.2 | 44.2 | 10–100 | −8.3 | −9.1 | |
| PQQADAEL | −2 | 870.407 | DA, PQ, AEL, EL, AD, AE, QA, QQ | ACE, Antioxidative, DPP-IV, α-glucosidase, DPP-III | 2.98 | 16.6 | 46.29 | 20 | −7.7 | −9.0 | |
| TQGDNPIF | −1 | 890.4121 | IF, QG, GD, TQ, NP, DN, PI | ACE, DPP-IV | 3.05 | 11.87 | −4.28 | 7.2–20 | −8.4 | −9.2 | |
| IEMKEGAL | −1 | 889.4564 | GA, EG, IE, KE, AL, MK | ACE, DPP-IV | 4.09 | 16.57 | 53.06 | 0.3–20 | −6.8 | −7.6 | |
| VNEGEAH | −2 | 754.3236 | GE, EG, EA, AH, NE, VN | ACE, Antioxidative, DPP-IV, alpha-glucosidase, DPP-III | 4.07 | 19.53 | −14.33 | 10–100 | −6.7 | −7.8 | |
| TERTDNS | −1 | 821.3504 | TE, ER, DN, TD | ACE, DPP-IV | 4 | 18.79 | 36.09 | 7.2–20 | −7.8 | −8.9 | |
| VAIKATS | 1 | 688.4107 | AI, KA, VA, AT, TS | ACE, DPP-IV, DPP-III | 10.14 | 10.83 | −3.56 | 10–100 | −6.2 | −7.8 | |
| REEEES | −3 | 777.313 | EEE, EE, ES | Stimulating, DPP-IV | 3.62 | 24.69 | 203.43 | 0.02–1 | −7.0 | −7.8 | |
| TEAQQS | −1 | 662.2862 | EA, TE, QQ, QS | ACE, DPP-IV, Alpha-glucosidase | 3.13 | 14.28 | 145.77 | 7.2–20 | −5.8 | −6.8 | |
| EQIEEL | −3 | 759.3638 | IE, EE, EL, QI | ACE, Stimulating, Antioxidative, DPP-IV | 2.93 | 17.19 | 168 | 0.3–10 | −7.2 | −7.7 | |
| VIPAAY | 0 | 632.3523 | IPA, AY, IP, AA, PA, VI | ACE, Antioxidative, Hypotensive, DPP-IV | 5.45 | 6.75 | 35.63 | 10–100 | −8.5 | −9.7 | |
| AGKTDN | 0 | 604.2808 | AG, GK, DN, KT, TD | ACE, DPP-IV | 6.76 | 17.09 | −5.82 | 4.4–20 | −7.3 | −8.4 | |
| MMRAR | 2 | 663.33 | RA, AR, MM, MR | ACE, Antioxidative, AU-MP, DPP-IV | 12.49 | 10.68 | −12.84 | 10–30 | −6.0 | −7.1 | |
| QDNPF | −1 | 619.2594 | NP, DN, PF, QD | DPP-IV, ACE2 | 3.05 | 11.59 | 79.28 | 0.1–10 | −7.8 | −9.1 | |
| KPETL | 0 | 586.3316 | KP, ET, TL, PE | ACE, Antioxidative, DPP-IV, alpha-glucosidase, DPP-III | 6.53 | 13.47 | 27.68 | 0.03–1.3 | −6.1 | −7.2 | |
| RIIQL | 1 | 641.4213 | II, IQ, QL, RI | Stimulating, DPP-IV | 10.73 | 6.99 | 8 | 0.02–1 | −7.6 | −8.5 | |
| EEINR | −1 | 659.3229 | EI, EE, IN, NR | ACE, Stimulating, DPP-IV, Renin | 4.08 | 16.7 | 111.72 | 0.3–10 | −7.6 | −8.3 | |
| VNIDS | −1 | 546.2641 | VN | DPP-IV | 3.05 | 11.27 | 134.4 | 10–100 | −7.7 | −9.0 | |
| KHAKS | 2 | 569.3277 | HA, KH, KS | DPP-IV | 10.57 | 16.79 | 8 | 0.03–1.3 | −5.9 | −7.3 | |
| IGRAL | 1 | 528.3375 | RA, IG, GR, AL | ACE, AUMP, DPP-IV | 11.12 | 8.99 | 8 | 0.3–20 | −6.9 | −7.7 | |
| INKQS | 1 | 588.3222 | NK, IN, QS | ACE, DPP-IV | 10.15 | 11.66 | 190.6 | 0.3–20 | −6.7 | −7.4 | |
| KNQY | 1 | 551.2696 | NQ, QY | DPP-IV | 9.48 | 11.61 | - | - | −6.7 | −7.8 | |
| GHIR | 1 | 481.2755 | IR, HIR, GH, HI | ACE, Antioxidative, DPP-IV, CaMPDE, Renin | 11.13 | 12.07 | - | - | −5.8 | −7.4 | |
| DQQS | −1 | 476.1861 | DQ, QQ, QS | DPP-IV | 3.05 | 13.54 | - | - | −5.7 | −7.2 | |
| KHIL | 1 | 509.3317 | IL, HI, KH | ACE, Stimulating, Neuropeptide, DPP-IV | 9.8 | 10.66 | - | - | −5.9 | −6.5 | |
| VPHY | 0 | 514.2533 | HY, VP, PH, PHY | ACE, Antioxidative, Anti-inflammatory, DPP-IV | 7.8 | 9.2 | - | - | −8.7 | −8.7 | |
| DGKD | −1 | 433.1803 | GK, DG, KD | ACE, Antioxidative | 3.91 | 19.13 | - | - | −6.4 | −7.4 | |
| VMKL | 1 | 489.2976 | KL, VM, MK | ACE, DPP-IV | 10.14 | 8.32 | - | - | −6.2 | −7.5 | |
| RAEL | 0 | 487.2747 | RA, AEL, EL, AE | ACE, Antioxidative, AUMP, DPP-IV | 6.51 | 12.59 | - | - | −6.5 | −8.3 | |
| Lectin | GINEGNTETND | −3 | 1162.4722 | GI, EG, TE, ET, IN, ND, NE, TN, NT | ACE, DPP-IV | 2.74 | 23.03 | −10.22 | 10–30 | −9.0 | −9.8 |
| DPKQRHIGID | 0 | 1177.6186 | IG, GI, RHI, DP, HI, PK, RH | ACE, Antioxidative, DPP-IV | 7.92 | 21.94 | 87.39 | 0.03–10 | −8.7 | −9.2 | |
| VNDNGEPTL | −2 | 957.4389 | GEP, GE, NG, PT, EP, DN, ND, TL, VN | ACE, DPP-IV, DPP-III | 2.98 | 16.7 | 5.21 | 10–100 | −10.0 | −11.8 | |
| QPKTNAGL | 1 | 827.4488 | GL, AG, QP, KT, NA, PK, TN | ACE, DPP-IV | 9.84 | 13.11 | 14.04 | 0.1–10 | −8.2 | −8.6 | |
| VNGENAE | −2 | 731.3076 | GE, NG, AE, NA, VN | ACE, DPP-IV, DPP-III | 2.92 | 18.05 | −23.67 | 10–100 | −7.4 | −9.7 | |
| APIQIW | 0 | 726.4053 | IW, AP, IQ, PI, QI | ACE, DPP-IV | 5.71 | 4.98 | 40.43 | 4.4–20 | −9.4 | −9.9 | |
| DNTTGA | −1 | 577.2336 | GA, TG, DN, NT, TT | ACE, DPP-IV | 3.13 | 14.54 | −34.12 | 0.03–10 | −8.1 | −8.9 | |
| GPADGL | −1 | 528.2536 | GP, GPA, GL, DG, DGL, PA, AD | Antiamnestic, ACE, DPP-IV, Antithrombotic, Regulating, Alpha-glucosidase | 3.12 | 13.23 | 26.28 | 10–30 | −7.3 | −7.7 | |
| DTCINL | −1 | 677.3044 | IN, NL | DPP-IV | 3.12 | 10.25 | −16.72 | 0.03–10 | −7.3 | −8.3 | |
| IKTTPW | 1 | 744.4158 | TP, PW, KT, TT | ACE, Antioxidative, DPP-IV | 10.15 | 8.13 | −10.62 | 0.3–20 | −8.6 | −9.6 | |
| DGTTS | −1 | 479.1857 | GT, DG, TS, TT | ACE, DPP-IV | 3.05 | 13.65 | −8.98 | 0.03–10 | −6.2 | −7.0 | |
| NETNL | −1 | 589.2699 | ET, NE, NL, TN | DPP-IV | 3.2 | 12.23 | −22.06 | 0.03–10 | −7.3 | −7.7 | |
| QRDAT | 0 | 589.2812 | DA, AT | ACE, DPP-IV, DPP-III | 6.48 | 14.87 | 8 | 0.1–10 | −7.0 | −8.1 | |
| VPNNS | 0 | 529.2489 | VP, NN, PN | ACE, DPP-IV | 5.45 | 9.74 | 46.52 | 10–100 | −7.2 | −9.2 | |
| DNGTY | −1 | 568.2122 | GT, NG, TY, DN | ACE, Antioxidativem DPP-IV | 3.05 | 13.08 | −39.04 | 0.03–10 | −7.3 | −8.4 | |
| NQIL | 0 | 486.2794 | IL, NQ, QI | ACE, Stimulating, Neuropeptide, DPP-IV | 5.36 | 7.15 | - | - | −6.4 | −7.7 | |
| NAHT | 0 | 441.1967 | AH, HT, NA | ACE, Antioxidative, DPP-IV | 7.38 | 11.83 | - | - | −5.8 | −7.9 | |
| QKTS | 1 | 462.2431 | QK, KT, TS | ACE, DPP-IV | 9.84 | 12.18 | - | - | −6.5 | −7.6 | |
| KGQL | 1 | 444.2689 | KG, GQ, QL | ACE, Neuropeptide, DPP-IV | 9.8 | 11.37 | - | - | −5.2 | −7.0 | |
| GRAF | 1 | 449.2381 | AF, RA, GR | ACE, AUMP, DPP-IV | 11.13 | 9.65 | - | - | −7.2 | −7.8 | |
| a-Amilasa inhibitor | TMNIRTHRQANS | 2 | 1427.7032 | IR, HR, MN, QA, TH, TM | ACE, Antioxidative, DPP-IV, CaMPDE, Renin | 12.48 | 15.99 | 61.83 | 7.2–20 | −9.1 | −6.8 |
| APIQIRDS | 0 | 898.4858 | IR, AP, IQ, PI | ACE, Antioxidative, DPP-IV, CaMPDE, Renin | 6.79 | 12.98 | 56.9 | 4.4–20 | −7.8 | −8.9 | |
| VNNNDIKS | 0 | 902.4444 | KS, ND, NN, VN | DPP-IV | 6.71 | 15.77 | −1.86 | 10–100 | −8.1 | −9.3 | |
| DGQNAE | −2 | 632.2394 | GQ, DG, AE, NA | ACE, Neuropeptide, DPP-IV | 2.87 | 18.44 | 8.33 | 0.03–10 | −6.6 | −7.6 | |
| MIMAS | 0 | 551.2439 | MA, AS, IM, MI | DPP-IV | 5.4 | 6.4 | 32.68 | 10–30 | −5.6 | −7.2 | |
| ATETS | −1 | 507.2169 | TE, AT, ET, TS | ACE, DPP-IV | 3.13 | 12.99 | 46.52 | 4.4–20 | −6.9 | −8.3 | |
| IIDAF | −1 | 577.3102 | AF, DA, II | ACE, Stimulating, DPP-IV, DPP-III | 3.05 | 8.09 | 8 | 0.3–20 | −7.9 | −10.0 | |
| NKTNL | −1 | 588.3222 | NK, KT, NL, TN | ACE, DPP-IV | 9.63 | 11.4 | 25.3 | 0.03–10 | −7.4 | −8.1 | |
| QGDAT | −1 | 490.2017 | DA, QG, GD, AT | ACE, DPP-IV, DPP-III | 3 | 14.21 | 8 | 0.1–10 | −7.1 | −9.2 | |
| VQPES | −1 | 558.2641 | QP, ES, VQ, PE | ACE, DPP-IV, Alpha-glucosidase, DPP-III | 3.13 | 12.44 | 119.8 | 10–100 | −7.6 | −8.8 | |
| VRITY | 1 | 650.3741 | VR, TY, RI | ACE, Antioxidative, DPP-IV | 9.91 | 7.67 | 8 | 10–100 | −8.1 | −9.8 | |
| KDQKS | 1 | 604.3171 | QK, KD, DQ, KS | ACE, Antioxidative, DPP-IV | 9.63 | 18.37 | 8 | 0.03–1.3 | −5.8 | −7.1 | |
| HANS | 0 | 427.1811 | HA | DPP-IV | 7.69 | 12.04 | - | - | −6.4 | −7.5 | |
| NGNL | 0 | 416.2014 | NG, NL | ACE, DPP-IV | 5.36 | 9.5 | - | - | −6.5 | −8.2 | |
| TTGN | 0 | 391.1698 | TG, TT | ACE, DPP-IV | 5.32 | 10.4 | - | - | −7.1 | −7.6 | |
| DTNF | −1 | 495.1959 | NF, TN | ACE, DPP-IV | 3.05 | 10.93 | - | - | −8.1 | −9.1 | |
| KGDT | 0 | 419.201 | KG, GD | ACE, DPP-IV | 6.44 | 15.74 | - | - | −5.4 | −7.1 | |
| VHDY | −1 | 532.2275 | DY, HD, VH | ACE, Regulating, DPP-IV | 4.98 | 12.7 | - | - | −8.3 | −10.3 | |
| TGKS | 1 | 391.2061 | GK, TG, KS | ACE, DPP-IV | 9.82 | 12.56 | - | - | −6.0 | −6.5 | |
| ETHD | −2 | 500.1861 | ET, HD, TH | DPP-IV | 3.99 | 17.75 | - | - | −8.2 | −9.6 | |
| NKIL | 1 | 486.3157 | NK, IL, KI | ACE, Stimulating, Neuropeptide, DPP-IV | 9.63 | 9.18 | - | - | −4.0 | −4.4 | |
| Arcelin | NKPDDPEAHI | −2 | 1134.5289 | EA, NK, KP, AH, KP, DP, HI, PE | ACE, Antioxidative, DPP-IV, DPP-II, Alpha-glucosidase | 4.26 | 24.45 | 70.43 | 0.03–10 | −9.3 | −9.6 |
| NQIEIDMNS | −2 | 1062.4636 | EI, IE, DM, MN, NQ, QI | ACE, DPP-IV | 2.92 | 15.19 | 70.73 | 0.03–10 | −9.7 | −8.8 | |
| VRGNGDPT | 0 | 814.3922 | GD, NG, VR, PT, RG, DP | ACE, DPP-IV, Leucyltransferase | 6.74 | 16.43 | −31.26 | 10–100 | −7.7 | −9.4 | |
| EPKRKDY | 1 | 934.4858 | KR, DY, KD, EP, PK, RK | ACE, Regulating, DPP-IV, Antioxidative | 9.58 | 22.01 | 82.66 | 0.3–10 | −7.2 | −8.0 | |
| GTKCNF | 1 | 668.2943 | GT, NF, TK | ACE, DPP-IV | 9 | 11.22 | −30.87 | 10–30 | −8.5 | −10.2 | |
| QHTTS | 0 | 572.2547 | HT, QH, TS, TT | DPP-IV | 7.59 | 11.96 | −7.08 | 0.1–10 | −7.4 | −8.4 | |
| THANS | 0 | 528.2286 | HA, TH | DPP-IV | 7.57 | 12.29 | 8 | 7.2–20 | −7.2 | −8.4 | |
| DTNKL | 0 | 589.3062 | NKL, KL, NK, TN | ACE, DPP-IV | 6.77 | 14.19 | −21.74 | 0.03–10 | −7.1 | −8.6 | |
| QGDAS | −1 | 476.1861 | DA, QG, GD, AS | ACE, DPP-IV, DPP-III | 3.05 | 14.42 | 8 | 0.1–10 | −6.6 | −8.4 | |
| MGRAF | 1 | 580.2784 | AF, RA, GR, MG | ACE, AUMP, DPP-IV | 10.88 | 8.98 | 8 | 0.3–20 | −7.0 | −8.2 | |
| TTGKL | 1 | 518.3055 | GK, TG, KL, TT | ACE, DPP-IV | 9.82 | 11.1 | −42.94 | 7.2–20 | −6.2 | −8.0 | |
| NNENS | −1 | 576.2133 | NEN, NE, NN | Antioxidative, DPP-IV | 3.13 | 14.54 | 8 | 0.03–10 | −7.4 | −8.2 | |
| VARES | 0 | 560.291 | AR, VA, ES | ACE, DPP-IV | 6.84 | 13.84 | 46.52 | 10–100 | −7.5 | −8.4 | |
| VHMEK | 0 | 642.315 | ME, EK, VH | ACE, DPP-IV | 7.86 | 15.53 | 8 | 10–100 | −6.7 | −8.2 | |
| DPTS | −1 | 418.1694 | PT, DP, TS | ACE, DPP-IV | 3.05 | 12.39 | - | - | −6.8 | −7.5 | |
| KNNL | 1 | 487.2747 | NL, NN | DPP-IV | 9.8 | 11.15 | - | - | −6.8 | −8.2 | |
| NGEK | 0 | 446.2119 | GE, NG, EK | ACE, DPP-IV, DPP-III | 6.38 | 16.33 | - | - | −6.4 | −7.3 | |
| IRPY | 1 | 547.311 | IR, IRP, RP, RPY, PY | ACE, Antioxidative, DPP-IV, CaMPDE, Renin | 9.92 | 8.02 | - | - | −8.5 | −10.0 | |
| Acarbose | −7.1 | −8 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Lozano, F.; Contreras, J.; Alfaro-Diaz, A.; Luna-Vital, D.A.; Mathis, A.C.G.; Urías-Silvas, J.E.; Mojica, L. Peptides from ‘Vaina Morada’ Black Bean Inhibit α-Amylase and α-Glucosidase: A Combined In Silico–In Vitro Study. Foods 2025, 14, 3847. https://doi.org/10.3390/foods14223847
Ramirez-Lozano F, Contreras J, Alfaro-Diaz A, Luna-Vital DA, Mathis ACG, Urías-Silvas JE, Mojica L. Peptides from ‘Vaina Morada’ Black Bean Inhibit α-Amylase and α-Glucosidase: A Combined In Silico–In Vitro Study. Foods. 2025; 14(22):3847. https://doi.org/10.3390/foods14223847
Chicago/Turabian StyleRamirez-Lozano, Filiberto, Jonhatan Contreras, Arturo Alfaro-Diaz, Diego Armando Luna-Vital, Anne C. Gschaedler Mathis, Judith Esmeralda Urías-Silvas, and Luis Mojica. 2025. "Peptides from ‘Vaina Morada’ Black Bean Inhibit α-Amylase and α-Glucosidase: A Combined In Silico–In Vitro Study" Foods 14, no. 22: 3847. https://doi.org/10.3390/foods14223847
APA StyleRamirez-Lozano, F., Contreras, J., Alfaro-Diaz, A., Luna-Vital, D. A., Mathis, A. C. G., Urías-Silvas, J. E., & Mojica, L. (2025). Peptides from ‘Vaina Morada’ Black Bean Inhibit α-Amylase and α-Glucosidase: A Combined In Silico–In Vitro Study. Foods, 14(22), 3847. https://doi.org/10.3390/foods14223847








